Abstract
Background: The protein of Niemann-pick type C1 (NPC1) gene promotes the egress of cholesterol from late endosomes and lysosomes to other cellular compartments and contributes to a process known as reverse cholesterol transport. This study aimed to examine whether promoter methylation of NPC1 is associated with risk of cardiovascular disease (CVD). Methods: Fifty CVD patients and 50 healthy subjects as the control group were recruited in this study. Promoter methylation of NPC1 gene was defined using a nested-methylation specific polymerase chain reaction method. Statistical analyses were done using the chi-square, t-test or ANOVA tests. Results: Our study showed that the frequency of semi-methylated promoter (methylated/unmethylated status) was significantly higher in CVD patients than that in controls (OR = 6.521, 95% CI = 2.211-19.215, P = 0.008). However, a completely methylated promoter (methylated/methylated status) was not detected in any subjects in either of the two groups tested. Additionally, the analysis of clinical data according to the methylation status of NPC1 gene demonstrated that serum levels of total cholesterol, total triglycerides, high low-density lipoprotein cholesterol (LDL-C) and low high-density lipoprotein cholesterol (HDL-C) are influenced by NPC1 methylation, so that subjects with a completely unmethylated promoter (Unmethylated/unmethylated status) held lower levels of total triglycerides, total cholesterol, LDL-C and higher levels of HDL-C. Conclusion: Our findings propose that the NPC1 promoter methylation is a probable mechanism that can result in reduced/impaired NPC1 expression/activity and may thus contribute to progression of CVD.
Key Words: Niemann-pick type C1 (NPC1), Promoter methylation, cardiovascular disease
INTRODUCTION
Cardiovascular disease (CVD), the most important cause of morbidity and mortality, remains a major public health challenge. Obesity, hypertension, insulin resistance and dyslipidaemia have been characterized as the most crucial underlying risk factors associated with higher incidence of major cardiovascular events [1]. The clustering of these risk factors within an individual is known as metabolic syndrome, that their combinations intensify the risk of CVD and diabetes [2]. Dyslipidaemia is defined as abnormality in the serum levels of lipids, including hypolipidemia or hyperlipidemia. Aberrant serum lipid profiles may include hypercholesterolemia, hypertriglyceridemia, high low-density lipoprotein cholesterol (LDL-C) and low high-density lipoprotein cholesterol (HDL-C) [3].
Cholesterol is an abundant metabolite in mammalian tissues that plays substantial roles in several biological systems. However, immoderate cholesterol termed hypercholesterolemia can result in pathological conditions, such as atherosclerotic CVD [4], coronary heart disease [5], Alzheimer’s disease [6], renal dysfunction [7], coronary artery and cerebral vascular disease [6]. Cells acquire cholesterol through endogenous synthesis as well as through the uptake of exogenous sources of cholesterol, particularly LDL. LDL are brought into the cell via receptor-mediated endocytosis and are delivered to the late endosome/lysosome, where cholesterol esters within the core of the LDL particle are hydrolyzed by acid lipase [8]. Unesterified cholesterol/phospholipid complexes exit the late endosome/lysosome, apparently through a Niemann-Pick type C1/2 (NPC1/NPC2)-dependent mechanism [9].
NPC1 is a transmembrane protein containing a sterol-sensitive domain that participates in cholesterol trafficking from the late endosome/lysosome to the plasma membrane [9, 10]. In human, the NPC1 is ubiquitously expressed in all tissues with highest expression in the liver and its expression seems to be regulated by sterol metabolism. The most prominent biochemical feature of NPC1-deficient cells is an excessive storage of unesterified cholesterol [11]. In addition to cholesterol, a variety of other lipids, such as sphingomyelin, sphingosine, bis(monoacylglycero) phosphate, and complex glycosphingolipids, particularly the gangliosides GM2 and GM3 are gathered in the late endosome/lysosome of NPC-deficient cells [8]. The efflux process for the removal of lipids from macrophage foam cells is critical for the maintenance of lipid homeostasis and the development of atherosclerosis. Monocyte/ macrophage lineage expresses high level of NPC1 and deletion of Npc1 in macrophages is primarily responsible for the accelerated atherosclerosis [12].
Moreover, NPC1 deficient subjects show decreased plasma HDL-C levels. HDL-C, a complex of Apo-a lipoproteins, has anti-oxidative, anti-proliferative, anti-thrombotic, and anti-inflammatory properties. It has been shown that HDL-C has protective roles against CVD by mediating the process of reverse cholesterol transport, which involves the transfer of excess cholesterol from macrophages in the peripheral tissues through the blood stream to the liver [13]. Low HDL-C concentrations have been consistently associated with increased risk for all forms of atherosclerotic disease [14] and its clinical sequelae [15], including myocardial infarction [16], stroke [17] and sudden death [3, 18].
Hypermethylation of the cytosine within cytosine phosphate guanine islands in a gene promoter is the best known epigenetic variation. It leads to gene silencing, which is involved in the pathogenesis of various diseases [19].
In this study, we aimed to evaluate the influence of NPC1 promoter methylation on susceptibility to CVD by comparing the methylation status of this gene in CVD patients and healthy subjects.
MATERIALS AND METHODS
Patients and clinical data collection . A total of 100 subjects including 50 CVD patients and 50 healthy individuals were recruited for the methylation analysis of NPC1 gene. The CVD patients were defined as the presence of recognized myocardial infarction and coronary insufficiency (unstable angina with demonstrated ischemic electrocardiographic changes). Blood samples were collected after a 12-hour overnight fast before cardiovascular procedures. At the time of enrollment, participants completed questionnaires on race/ethnic status, demographics, history of cigarette smoking, medical history and medications. In addition, use of lipid-lowering drugs (LLD), family history of hypertension, diabetes mellitus, serum levels of total cholesterol of plasma, total triglycerides, LDL-C and HDL-C were recorded (Table 1). Hypertension was determined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg or therapy for hypertension [20]. Diabetes mellitus was diagnosed as a fasting blood glucose level >126 mg/dl or current use of hypoglycemic agents [21]. Total cholesterol, total triglycerides, HDL-C and LDL-C levels were measured as mg/dl using a standard enzymatic kit (Pars Azmoon Co., Iran). The study was approved by the institutional review board of cardiovascular institute, and the participating hospitals. All subjects provided their written informed consent and were self-reported as Zahedan University of Medical Sciences (Zahedan, Iran).
Table 1.
Clinical and biochemical data from cardiovascular and healthy subjects
| Parameters | CVD patients | Control subjects | P |
|---|---|---|---|
| n | 50 | 50 | |
| Age (years) | 60.16 ± 12.54 | 59.54 ± 11.71 | 0.805 |
| Men n (%) | 28 (56.0) | 20 (40.0) | 0.109 |
| Use of LLD n (%) | 19 (38) | 0 (0.0) | <0.0001 |
| Ethnicity Fars Balouch |
48 (96.0) 2 (4.0) |
35 (70.0) 15 (30.0) |
0.001 |
| Current smoking n (%) | 23 (46) | 0 (0.0) | <0.0001 |
| History of CVD n (%) | 20 (40.0) | 4 (8.0) | <0.0001 |
| History of hypertension n (%) | 8 (16) | 0 (0.0) | 0.003 |
| History of diabetes n (%) | 19 (38) | 28 (56.0) | <0.0001 |
| Triglyceride level (mg/dl) | 202.52 ± 36.56 | 138.82 ± 46.59 | <0.0001 |
| Cholesterol level (mg/dl) | 218.43 ± 35.28 | 182.02 ± 30.45 | <0.0001 |
| LDL-cholesterol (mg/dl) | 130.54 ± 30.42 | 120.02 ± 33.48 | 0.114 |
| HDL-cholesterol (mg/dl) | 37.24 ± 6.14 | 47.41 ± 9.05 | <0.0001 |
CVD, cardiovascular disease; LLD, Lipid-lowering drugs
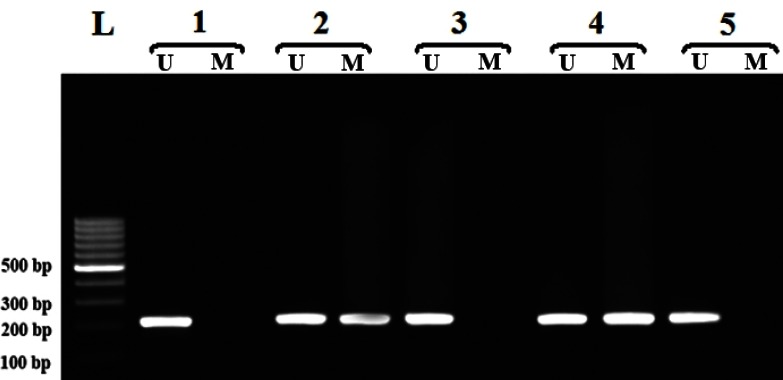
DNA preparation, sodium bisulfite modification and methylation specific PCR (MSP) amplification of Niemann-pick type C1 promoter. Blood samples were collected in EDTA containing tubes and genomic DNA was isolated from peripheral blood leukocytes as described previously [22]. The DNA samples were treated with sodium bisulfite, which converts unmethylated C to U, but once methylation occurs at the C residues, they will withstand the treatment. All extracted genomic DNA were subjected to bisulfite conversion using a MOD50 kit (Sigma-Aldrich). In the current study, we developed nested-MSP for the detection of NCP1 promoter methylation, which increases MSP sensitivity to detect the hypermethylated promoter [23]. The primers used for the first stage of the MSP distinguish the bisulfite-modified template but not methylated and unmethylated templates. The cycling conditions for the stage 1 of the nested-MSP were: 95°C for 5 min; 30 cycles of 95°C for 45 s, 62°C for 30 s, 72°C for 30 s and a final extension at 72°C for 10 min. The PCR product of the first stage (461 bp) was diluted 1:200 and subjected to the second stage of the nested-MSP using two pairs of primers, one specific for the methylated alleles and one specific for the unmethylated ones. Primer sequences of the first and second stages of the nested-MSP have been all listed in Table 2. The PCR conditions for the second stage of the nested-MSP were: complete denaturation of DNA at 95°C for 5 min and 30 cycles involving denaturation at 95°C for 30 s, annealing at 63°C for 30 s, extension at 72°C for 45 s and a final extension at 72°C for 10 min. The PCR products were assessed by electro-phoresis in 1.5% agarose gels stained with 0.5 μg/ml ethidium bromide, and a photo showing different methylation patterns was taken (Fig. 1). The amplicon sizes for the methylated and unmethylated alleles were 216 bp and 217 bp, respectively.
Table 2.
Primer sequence for the methylation analysis of NPC1 promoter
| Primers | Sequence (5´ to 3´) | Amplicons size (bp) |
|---|---|---|
| F | TAGTTGGGTTAGTAGGTGG | 461 |
| R | TTACCCTTCTCCTCAACC | |
| UF | GTTTTGGGTTTTGTTAGAAAGTGTT | 217 |
| UR | AACCATAATACTTTCCCTTTTCATC | |
| MF | TTCGGGTTTTGTTAGAAAGTGTC | 216 |
| MR | TAACCGTAATACTTTCCCTTTTCG |
Clinical characteristics of age, LDL-C, HDL-C, total cholesterol and total triglycerides values are given as mean ± SD and other values as number and percent of individuals. F, forward; R: reverse; U, unmethylated; M, methylated
Fig. 1.
Different methylation patterns of the NPC1 in cardiovascular disease. ‘U’ and ‘M’ indicate unmethylated and methylated promoters, respectively. Lanes 1, 3 and 5, unmethylated/unmethylated status; Lanes 2 and 4, methylated/unmethylated status and L, Ladder.
Statistical analysis. Statistical analyses were performed using the SPSS 15.0 software. P values for statistical comparison across methylation status was obtained from tests for categorical and continuous variables by chi-square, Fisher's exact test, ANOVA or t-test and Mann-Whitney and Kruskal-Wallis tests for non-parametric. OR and 95% CI were computed with the use of binary logistic regression analyses. Adjusted OR were stratified by age, sex, LLD usage, ethnicity, history of CVD, hypertension and diabetes mellitus. Probability value below 0.05 was determined as being statistically significant.
Results
Multivariate linear regression analysis of demog-raphic characteristics of all subjects showed that sex, LLD usage, ethnicity, history of CVD, hypertension and diabetes mellitus, serum levels of total triglycerides, total cholesterol, HDL-C and LDL-C were statistically different in CVD patients and healthy controls (Table 1). Since in this study only about 50% of all CVD patients took the LLD, in comparison with control group, their serum total triglycerides, total cholesterol and LDL-C levels were significantly higher and HDL-C levels were significantly lower in CVD patients (P = 0.0001).
We examined the promoter methylation status of the NPC1 gene on the DNA samples of 50 CVD patients and 50 healthy subjects. As shown in Table 3, the frequency of semi-methylated promoter (methylated/ unmethylated, methylated/unmethylated status) in CVD patients is much higher than that in controls, showing a statistically significant difference between the two groups (OR = 6.521, 95% CI = 2.211-19.215, P = 0.008). Additionally, the prevalence of the methylated allele was elevated in CVD than healthy subjects, demonstrating the impact of NPC1 promoter methylation as a risk factor for susceptibility to CVD (OR = 2.011, 95% CI = 1.116-3.594, P = 0.028). However, a completely methylated promoter (methylated/methylated status) was not identified in any individual in either of the two groups tested. The association remained significant after adjustment for age, sex, LLD usage, ethnicity, history of CVD, hypertension and diabetes mellitus. Table 4 shows demographic characteristics of subjects based on the methylation status of NPC1 promoter. Our data show that LLD usage, serum levels of total triglycerides, total cholesterol, HDL-C and LDL-C varies according to the methylation status of NPC1 gene, so that subjects with a completely unmethylated promoter for the NPC1 gene possess lower levels of total triglycerides, total cholesterol and LDL-C and higher levels of HDL-C than individuals with a methylated allele for the NPC1 gene.
Table 3.
Promoter methylation frequency of Niemann-pick type C1 gene in cardiovascular disease patients (CVD) and healthy individuals
| Methylation Status | CVD n (%) | Control n (%) | Adjusted OR* | Adjusted P value |
|---|---|---|---|---|
| UU | 5 (10.0) | 21 (42.0) | - | Ref. |
| MU | 45 (90.0) | 29 (58.0) | 6.521 (2.211-19.215) | 0.008 |
| MM | 0 (0.0) | 0 (0.0) | - | - |
| Allele | ||||
| U | 55 (55.0) | 71 (71.0) | - | Ref. |
| M | 45 (45.0) | 29 (29.0) | 2.011(1.116-3.594) | 0.028 |
UU, fully unmethylated promoter; MU, promoter with methylated/unmethylated status or semi-methylated promoter; MM, fully methylated promoter. *Adjustments were stratified by age, sex, lipid-lowering drugs, ethnicity, history of CVD, hypertension and diabetes mellitus
Table 4.
Demographic characteristics of subjects according to methylation status of Niemann-pick type C1 (NPC1) gene
| Parameters |
NPC1 methylation status
|
|||
|---|---|---|---|---|
| UU | MU | P | ||
| Age (years) | 63.83 | 58.54 | 0.0670 | |
| Men n (%) | 11 (42.3) | 37 (50.0) | 0.4990 | |
| Use of LLD n (%) | 1 (3.8) | 18 (24.3) | 0.0180 | |
| Ethnicity | ||||
| Fars Balouch |
19 (73.1) 7 (26.9) |
64 (86.5) 10 (13.5) |
0.1170 | |
| Current smoking n (%) | 3 (11.5) | 20 (27.0) | 0.1970 | |
| History of CVD n (%) | 4 (15.4) | 20 (27.0) | 0.4810 | |
| History of hypertension n (%) | 1 (3.8) | 7 (9.5) | 0.3480 | |
| History of diabetes n (%) | 15 (57.7) | 32 (43.2) | 0.4310 | |
| Triglyceride level (mg/dl) | 134.3 ± 47.24 | 183.9 ± 38.50 | 0.0001 | |
| Cholesterol level (mg/dl) | 178.8 ± 38.51 | 207.8 ± 34.22 | 0.0010 | |
| LDL-cholesterol (mg/dl) | 114.6 ± 33.32 | 129.1 ± 31.22 | 0.0570 | |
| HDL-cholestrol (mg/dl) | 48.1 ± 9.33 | 40.1 ± 8.28 | 0.0001 | |
Clinical characteristics of age, LDL-C, HDL-C, total cholesterol and triglyceride values are given as mean ± SD and other values as number and percent of individuals. LLD, Lipid-lowering drugs; UU, fully unmethylated promoter; MU, promoter with methylated/unmethylated status or semi-methylated promoter
Discussion
Our findings revealed that the frequency of semi-methylated promoter (methylated/unmethylated status) is higher in CVD patients than that in controls, showing the influence of NPC1 methylated promoter as a risk factor for susceptibility to CVD.
NPC1, a crucial participant in the cellular lipid trafficking, plays a protective role against cholesterol intracellular accumulation [24]. The efflux process for the elimination of lipids from arterial wall cells and macrophages in the peripheral tissues is critical for the maintenance of lipid homeostasis and protection against atherosclerosis [25]. This process is principally carried out by the HDL in a process known as reverse cholesterol transport. Reverse cholesterol transport is a key process through which peripheral cells unload their unwanted, surplus cholesterol by returning it to the liver for eventual disposal.
ABCA1 mediates the efflux of free cholesterol or unesterified cholesterol/phospholipid complexes from peripheral cells, such as macrophage-derived foam cells, to apolipoprotein A-I and is responsible for nascent HDL particle formation [4, 16]. ABCA1 belongs to a superfamily of ATP-binding cassette transporters and is a potent cardioprotective factor. ABCA1 is expressed ubiquitously, but in highest concentrations in the liver, brain, adrenal glands, and macrophage foam cells. In NPC1-deficient cells, despite of increased cell cholesterol content, ABCA1 protein level is reduced and ABCA1-mediated cholesterol efflux is impaired [26]. Therefore, HDL-C levels are extremely low which can accelerate the risk for CVD [27], type 2 diabetes and metabolic syndrome [26]. In agreement with these proofs, our findings showed that individuals with a methylated allele for NPC1 gene had lower levels of serum HDL-C, probably due to impaired function of NPC1 in these subjects (Table 4).
From the other point of view, impairment of ABCA1-mediated cholesterol efflux in the NPC1-deficient cells causes peripheral macrophages to be filled with cholesterol [28]. Accumulation of LDL such as oxidized LDL and recruitment of monocytes in the arterial subendothelial spaces are early events in atherosclerosis. Macrophages, which are derived from monocytes in these areas, take up oxidized LDL through the scavenger receptor pathways and finally become foam cells. As sterol uptake mediated by scavenger receptors is not under negative feedback control, scavenger receptor-mediated events may really contribute to excessive cholesterol accumulation in macrophages leading to foam cell formation [29, 30].
Other important abnormal metabolic event in NPC1 deficient macrophages is formation of cholesterol crystals. This abnormality results from excessive uptake of cholesteryl ester lipid droplets and accumulation of unesterified cholesterol by the macrophages [31]. These cholesterol crystals may be outside or inside cells, appearing within cellular vacuoles or lysosomes. Cholesterol crystals acts as an endogenous danger signal that initiates local inflammatory response [32]. Local inflammation is critical in formation and expansion of the necrotic core that can cause plaque rupture and/or erosion by expanding sharp-tipped cholesterol crystals that leads to initiation of a systemic inflammation. A systemic process can influence the local milieu by activating the macrophages to become foam cells and phagocytotic [33]. In addition, crystals in the foam cells induce apoptosis, leading to further attraction of macrophages that forms an extracellular lipid pool and vulnerable plaque within the arterial wall [33].
Dysregulation of cholesterol homeostasis is also thought to be another irregularity in NPC1 deficient macrophages [34]. The ER serves as a cholesterol sensor, allowing the cell to regulate cholesterol synthesis and uptake via the sterol regulatory element-binding protein pathway [35]. In NPC cells, LDL-C transports to the cell surface and ER is also greatly delayed, and the rising cholesterol level in the cell is not sensed by the ER, thus homeostatic mechanisms are not initiated and it fails to downregulate cholesterol synthesis. Therefore, the late endosome/E continues to accumulate cholesterol and become highly enlarged leading to the formation of lipid droplet rich foam cells [26, 34]. In accord with these facts, our results demonstrated that subjects with unmethylated/ unmethylated status for the NPC1 methylation elevated levels of HDL-C and lower levels of total triglycerides, total cholesterol and LDL-C, suggesting the protective effect of NPC1 against rising of serum lipids and thus susceptibility to CVD (Table 4).
One limitation of the current study is a relatively small sample size. Larger studies with different ethnicities are required to confirm our findings.
To the best of our knowledge, no report has been published yet regarding the status of NPC1 promoter methylation in CVD. Overall, in the present study, we found that the frequency of NPC1 promoter methylation was much higher in CVD patients than that in healthy subjects, suggesting that the NPC1 promoter methylation is a probable mechanism that accounts for reduced/impaired NPC1 expression and may thus contribute to progression of CVD which remains to be cleared.
ACKNOWLEDGEMENTS
This work was financially assisted by a dissertation grant (M.Sc. thesis of MA) from Zahedan University of Medical Sciences. The authors are grateful to all individuals who freely participated in the research.
References
- 1.Windler E, Schoffauer M, Zyriax BC. The significance of low HDL-cholesterol levels in an ageing society at increased risk for cardiovascular disease. Diab Vasc Dis Res. 2007 Jun;4(2):136–42. doi: 10.3132/dvdr.2007.032. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2009;4(2):113–9. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laitinen DL, Manthena S. Impact of change in high-density lipoprotein cholesterol from baseline on risk for major cardiovascular events. Adv Ther. 2010 Apr;27(4):233–44. doi: 10.1007/s12325-010-0019-4. [DOI] [PubMed] [Google Scholar]
- 4.Mahdy Ali K, Wonnerth A, Huber K, Wojta J. Cardiovascular disease risk reduction by raising HDL cholesterol-current therapies and future opportunities. Br J Pharmacol. 2012 Nov;167(6):1177–94. doi: 10.1111/j.1476-5381.2012.02081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burillo E, Andres EM, Mateo-Gallego R, Fiddyment S, Jarauta E, Cenarro A, et al. High-density lipoprotein cholesterol increase and non-cardiovascular mortality: a meta-analysis. Heart. 2010 Sep;96(17):1345–51. doi: 10.1136/hrt.2010.195396. [DOI] [PubMed] [Google Scholar]
- 6.Liu JP, Tang Y, Zhou S, Toh BH, McLean C, Li H. Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol Cell Neurosci. 2010 Jan;43(1):33–42. doi: 10.1016/j.mcn.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Scolari F, Ravani P. Atheroembolic renal disease. Lancet. 2010 May;375(9726):1650–60. doi: 10.1016/S0140-6736(09)62073-0. [DOI] [PubMed] [Google Scholar]
- 8.Peake KB, Vance JE. Defective cholesterol trafficking in Niemann-Pick C-deficient cells. FEBS Lett. 2010 Jul;584(13):2731–9. doi: 10.1016/j.febslet.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 9.Lecerf JM, de Lorgeril M. Dietary cholesterol: from physiology to cardiovascular risk. Br J Nutr. 2011 Jul;106(1):6–14. doi: 10.1017/S0007114511000237. [DOI] [PubMed] [Google Scholar]
- 10.Bi X, Liao G. Cholesterol in Niemann-Pick Type C disease. Subcell Biochem. 2010;51:319–35. doi: 10.1007/978-90-481-8622-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karten B, Peake KB, Vance JE. Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim Biophys Acta. 2009 Jul;1791(7):659–70. doi: 10.1016/j.bbalip.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Tomkin GH. Targets for intervention in dyslipidemia in diabetes. Diabetes Care. 2008 Feb;31(Suppl 2):S241–8. doi: 10.2337/dc08-s260. [DOI] [PubMed] [Google Scholar]
- 13.Ramjee V, Sperling LS, Jacobson TA. Non-high-density lipoprotein cholesterol versus apolipoprotein B in cardiovascular risk stratification: do the math. J Am Coll Cardiol. 2011 Jul;58(5):457–63. doi: 10.1016/j.jacc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Ye D, Lammers B, Zhao Y, Meurs I, Van Berkel TJ, Van Eck M. ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis. Curr Drug Targets. 2011 May;12(5):647–60. doi: 10.2174/138945011795378522. [DOI] [PubMed] [Google Scholar]
- 15.Toth PP. Should we target HDL cholesterol level in lowering cardiovascular risk? Pol Arch Med Wewn. 2009 Oct;119(10):667–72. [PubMed] [Google Scholar]
- 16.Iatan I, Alrasadi K, Ruel I, Alwaili K, Genest J. Effect of ABCA1 mutations on risk for myocardial infarction. Curr Atheroscler Rep. 2008 Oct;10(5):413–26. doi: 10.1007/s11883-008-0064-5. [DOI] [PubMed] [Google Scholar]
- 17.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003 Jun;326(7404) doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Despres JP, Lemieux I, Dagenais GR, Cantin B, Lamarche B. HDL-cholesterol as a marker of coronary heart disease risk: the Quebec cardiovascular study. Atherosclerosis. 2000 Dec;153(2):263–72. doi: 10.1016/s0021-9150(00)00603-1. [DOI] [PubMed] [Google Scholar]
- 19.Koga Y, Pelizzola M, Cheng E, Krauthammer M, Sznol M, Ariyan S, et al. Genome-wide screen of promoter methylation identifies novel markers in melanoma. Genome Res. 2009 Aug;19(8):1462–70. doi: 10.1101/gr.091447.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, et al. Ambulatory blood pressure An independent predictor of prognosis in essential hypertension. Hypertension. 1994 Dec;24(6):793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PWF, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino Sr RB. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Int Med. 2007;167(10):1068–74. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 22.Hashemi M, Moazeni-Roodi AK, Fazaeli A, Sandoughi M, Bardestani GR, Kordi-Tamandani DM, et al. Lack of association between paraoxonase-1 Q192R poly-morphism and rheumatoid arthritis in southeast Iran. Genet Mol Res. 2010 Feb;9(1):333–9. doi: 10.4238/vol9-1gmr728. [DOI] [PubMed] [Google Scholar]
- 23.Hashemi M, Rezaei H, Eskandari-Nasab E, Kaykhaei MA, Taheri M. Association of promoter methylation and 32-bp deletion of the PTEN gene with susceptibility to metabolic syndrome. Mol Med Report. 2012 Nov;7:342–6. doi: 10.3892/mmr.2012.1174. [DOI] [PubMed] [Google Scholar]
- 24.Scott C, Ioannou YA. The NPC1 protein: structure implies function. Biochim Biophys Acta. 2004 Oct;1685(1-3):8–13. doi: 10.1016/j.bbalip.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Reiss AB, Anwar F, Chan ES, Anwar K. Disruption of cholesterol efflux by coxib medications and inflammatory processes: link to increased cardio-vascular risk. J Investig Med. 2009 Aug;57(6):695–702. doi: 10.2310/JIM.0b013e31819ec3c7. [DOI] [PubMed] [Google Scholar]
- 26.Boadu E, Francis GA. The role of vesicular transport in ABCA1-dependent lipid efflux and its connection with NPC pathways. J Mol Med (Berl) 2006 Apr;84(4):266–75. doi: 10.1007/s00109-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 27.Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006 Nov;99(10):1031–43. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 28.Cao XL, Yin RX, Wu DF, Miao L, Aung LH, Hu XJ, et al. Genetic variant of V825I in the ATP-binding cassette transporter A1 gene and serum lipid levels in the Guangxi Bai Ku Yao and Han populations. Lipids Health Dis. 2011 Jan;10:14. doi: 10.1186/1476-511X-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie C, Richardson JA, Turley SD, Dietschy JM. Cholesterol substrate pools and steroid hormone levels are normal in the face of mutational inactivation of NPC1 protein. J Lipid Res. 2006 May;47(5):953–63. doi: 10.1194/jlr.M500534-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JR, Coleman T, Langmade SJ, Scherrer DE, Lane L, Lanier MH, et al. Niemann-Pick C1 protects against atherosclerosis in mice via regulation of macrophage intracellular cholesterol trafficking. J Clin Invest. 2008 Jun;118(6):2281–90. doi: 10.1172/JCI32561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruth HS. Lipoprotein cholesterol and atherosclerosis. Curr Mol Med. 2001 Dec;1(6):633–53. doi: 10.2174/1566524013363212. [DOI] [PubMed] [Google Scholar]
- 32.Dunne A. Inflammasome activation: from inflammatory disease to infection. Biochem Soc Trans. 2011 Apr;39(2):669–73. doi: 10.1042/BST0390669. [DOI] [PubMed] [Google Scholar]
- 33.Abela GS. Cholesterol crystals piercing the arterial plaque and intima trigger local and systemic inflammation. J Clin Lipidol. 2010 May-Jun;4(3):156–64. doi: 10.1016/j.jacl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Ory DS. The niemann-pick disease genes; regulators of cellular cholesterol homeostasis. Trends Cardiovasc Med. 2004 Feb;14(2):66–72. doi: 10.1016/j.tcm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Blanchette-Mackie EJ. Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim Biophys Acta. 2000 Jun 26;1486(1):171–83. doi: 10.1016/s1388-1981(00)00055-x. [DOI] [PubMed] [Google Scholar]