Abstract
Objective
Nonalcoholic fatty liver disease (NAFLD) is associated with abnormalities in basal glucose and free fatty acid (FFA) metabolism, multi-organ insulin resistance, and alterations in lipoprotein kinetics. These metabolic outcomes can be evaluated in vivo by using stable isotopically-labeled tracer methods. An understanding of the reproducibility of these measures is necessary to ensure adequate statistical power in studies designed to evaluate metabolic function in subjects with NAFLD.
Methods
We determined the degree of intra-individual variability of skeletal muscle, adipose tissue, and hepatic insulin sensitivity and basal plasma glucose, FFA, and very-low-density lipoprotein (VLDL) triglyceride (TG) and apolipoprotein B-100 (apoB-100) kinetics in 8 obese subjects with NAFLD (age: 44±3 yr; body mass index: 38.2±1.7 kg/m2; intrahepatic triglyceride content: 24.5±3.9%), by using the hyperinsulinemic-euglycemic clamp technique and stable isotope-labeled tracer methods and mathematical modeling on two separate occasions ~2 months apart.
Results
The intra-individual variability (coefficient of variation) ranged from 6% for basal glucose production to 21% for insulin-stimulated glucose disposal (% increase from basal). We estimated that a 25% difference in any outcome measure can be detected with a sample size of ≤8 subjects for paired studies and ≤15 subjects per group for unpaired studies, assuming an α value of 0.05 and a β value of 0.20 (i.e., 80% power).
Conclusion
These results demonstrate that only a small number of subjects are needed to detect clinically relevant effects in insulin sensitivity and hepatic lipoprotein metabolism in obese subjects with NAFLD, and will be useful to determine appropriate sample size for future metabolic studies.
Keywords: NALFD, insulin resistance, tracers, VLDL kinetics, repeatability, reliability
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) comprises a spectrum of liver abnormalities1 characterized by the excessive accumulation of triglyceride (TG), with or without inflammation and fibrosis.2 The presence of NAFLD is strongly associated with the presence of metabolic abnormalities that are risk factors for coronary heart disease, particularly alterations in glucose and fatty acid kinetics, insulin action, and hepatic lipoprotein metabolism.3–5 Therefore, assessment of these metabolic functions is an important outcome measure in NAFLD research.
Substrate metabolism and multi-organ insulin sensitivity can be reliably evaluated in vivo in human subjects. A two-stage hyperinsulinemic-euglycemic clamp procedure combined with stable isotope-labeled tracer infusion can be used to evaluate insulin sensitivity in skeletal muscle, adipose tissue, and liver.6 Stable isotope-labeled tracer techniques can also be used to assess basal glucose, free fatty acid (FFA), very-low-density lipoprotein (VLDL) triglyceride (TG) and VLDL-apolipoprotein B-100 (apoB-100) metabolism.7,8 These types of studies are usually conducted in small numbers of subjects because they are expensive, require sophisticated analytical techniques, and are burdensome for study participants.9–14 Therefore, an understanding of the reproducibility of these metabolic outcome measures is necessary to be able to design studies that contain the appropriate number of subjects needed to detect an effect of an intervention or differences between study groups. This issue might be particularly important in obese subjects with NAFLD, due to fluctuations in liver biochemistries,15,16 which could represent an underlying variability in metabolic function.
The purpose of the present study was to determine the reproducibility and intra-individual variability of skeletal muscle, adipose tissue, and hepatic insulin sensitivity and basal glucose, FFA, VLDL-TG, and VLDL-apoB-100 kinetics in obese subjects with NAFLD. Stable isotope tracer infusion studies were performed at baseline and repeated about two months later. This information was used to provide estimates of minimum differences needed to detect a statistically significant effect on selected metabolic outcomes for any given sample size for both paired (longitudinal) and unpaired (cross-sectional) study designs.
MATERIALS AND METHODS
Subjects
Eight obese subjects with NAFLD (intrahepatic triglyceride [IHTG] content >10%) participated in this study (44 ± 3 years old, 6 women and 2 men; Table 1). All subjects completed a comprehensive medical evaluation, which included a 2-h oral glucose tolerance test. Body fat mass and fat-free mass were determined by using dual-energy x-ray absorptiometry (Delphi-W densitometer, Hologic, Waltham, MA), and IHTG content was measured by using proton magnetic resonance spectroscopy (Siemens, Erlanger, Germany) as previously described.17 Potential participants who smoked cigarettes, consumed ≥20 g/d of alcohol, had severe hypertriglyceridemia (>300 mg/dl), diabetes or IHTG content ≤10% were excluded. All subjects had been weight-stable (≤2% change in weight) and sedentary (exercise <1 h/wk) for at least 3 months before enrollment. Subjects provided their written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine.
TABLE 1.
Subject characteristics at baseline (Study 1) and approximately 2 months later (Study 2)
| Study 1 Mean ± SEM (range) |
Study 2 Mean ± SEM |
|
|---|---|---|
| Body weight (kg) | 111 ± 5 (85–129) | 112 ± 5 |
| Body mass index (kg/m2) | 38.2 ± 1.7 (32–45) | 38.5 ± 1.7 |
| Body fat (% of body weight) | 41 ± 2 (32–49) | 41 ± 2 |
| Fat-free mass (kg) | 59.0 ± 2.2 (51–68) | 58.8 ± 2.1 |
| Intrahepatic triglyceride content (%) | 24.5 ± 3.9 (11–43) | 24.4 ± 4.1 |
Experimental protocol
Each subject underwent a 9.5-h, two-stage hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotope tracer infusions, to evaluate adipose tissue, skeletal muscle, and hepatic insulin sensitivity, and a 12-h isotope infusion study in conjunction with mathematical modeling to determine VLDL-TG and VLDL-apoB-100 kinetics approximately 1 week apart. Both studies were repeated approximately 2 months later. Subjects were instructed to maintain their usual dietary and physical activity habits during this period.
Hyperinsulinemic-euglycemic clamp procedure
Subjects were admitted to the Washington University School of Medicine Clinical Research Unit in the evening before the study and consumed a standard meal (~10 kcal/kg of fat-free mass) at 1900 h and a 250-kcal liquid snack (Ensure™, Ross Laboratories, Columbus, OH) at 2000 h. At 0500 h the following morning, one catheter was inserted into a forearm vein to infuse stable isotopically labeled tracers (Cambridge Isotope Laboratories, Andover, MA), dextrose and insulin, and a second catheter was inserted into a radial artery in the contralateral hand to obtain blood samples. At 0600 h, a primed continuous infusion of [6,6-2H2]glucose (priming dose: 22.5 µmol/kg; infusion rate, 0.25 µmol/kg·min), dissolved in 0.9% NaCl solution, was started and maintained for 5.5 hours, until the end of stage 1 of the euglycemic-hyperinsulinemic clamp procedure. At 0800 h, a continuous infusion of [2,2-2H2]palmitate (infusion rate: 0.035 µmol/kg·min), bound to human albumin, was started and maintained for 3.5 hours (through the end of stage 1 of the euglycemic-hyperinsulinemic clamp procedure). At 0930 h (3.5 hours after starting the infusion of glucose tracer), a 2-stage euglycemic-hyperinsulinemic clamp procedure was started and continued for 6 hours. During stage 1 of the clamp procedure (3.5 to 5.5 hours), insulin was infused at a rate of 20 mU/m2 body surface area (BSA)·min (initiated with a priming dose of 80 mU/m2 BSA·min for 5 minutes and then 40 mU/m2 BSA·min for 5 minutes). During stage 2 of the clamp procedure (5.5 to 9.5 hours), insulin was infused at a rate of 50 mU/m2 BSA·min (initiated with a priming dose of 200 mU/m2 BSA·min for 5 minutes and then 100 mU/m2 BSA·min for 5 minutes). Euglycemia was maintained at a blood glucose concentration of approximately 5.6 mmol/L (100 mg/dl) throughout stages 1 and 2 by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose at variable rates. The infusion rates of [6,6-2H2]glucose and [2,2-2H2]palmitate were reduced by 50% during stage 1, and [6,6-2H2]glucose infusion was reduced by 75% during stage 2 of the clamp procedure to account for changes in hepatic glucose production and adipose tissue lipolytic rates.
Blood samples were obtained immediately before starting the tracer infusion and every 10 min during the final 30 min of the basal period and stages 1 and 2 of the clamp procedure. To determine plasma glucose concentration, blood was collected in tubes containing heparin; plasma was separated by centrifugation and analyzed immediately. All other blood samples were collected in chilled tubes containing sodium EDTA. Samples were placed immediately in an ice bath, and plasma was separated by centrifugation within 30 min of collection.
VLDL kinetics study
Approximately 1 week after the clamp protocol, subjects were readmitted to the Clinical Research Unit and consumed the same evening meal and snack as in the evening before the clamp procedure. At 0600 h the following morning, VLDL kinetics were determined as previously described.10 A bolus of [1,1,2,3,3-2H5]glycerol (75 µmol/kg) was administered, and constant infusions of [2,2-2H2]palmitate (0.03 µmol/kg·min) and [5,5,5-2H3]leucine (0.12 µmol/kg·min; priming dose: 7.2 µmol/kg) were started and maintained for 12 h. Blood samples were obtained immediately before starting the tracer infusion and at 5, 15, 30, 60, 90, and 120 min and then every hour for 10 h. Aliquots of plasma were kept in the refrigerator to isolate VLDL,18,19 and the remaining plasma samples were stored at −80°C until additional analyses were performed.
Sample analyses
Substrate and hormone concentrations
Plasma glucose concentration was determined on an automated glucose analyzer (YSI 2300 STAT plus, Yellow Spring Instrument Co., Yellow Springs, OH). Plasma insulin concentration was measured by a radioimmunoassay specific for insulin (Linco Research, St. Louis, MO). Plasma FFA concentrations were quantified by gas chromatography (HP 5890 Series II GC, Hewlett-Packard, Palo Alto, CA) after adding heptadecanoic acid to plasma as an internal standard.20 Total plasma TG and VLDL-TG concentrations were determined with a colorimetric enzymatic kit (Sigma Chemicals, St. Louis, MO) and VLDL-apoB-100 concentration with a turbidimetric immunoassay (Wako Pure Chemical Industries, Osaka, Japan).18
Substrate enrichments
The tracer-to-tracee ratios (TTRs) of plasma glycerol, glucose, palmitate and leucine, the TTRs of glycerol and palmitate in VLDL-TG, and the TTR of leucine in VLDL-apoB-100 were determined by using gas chromatography-mass spectrometry (Agilent Technologies/HP 6890 Series GC System – 5973 Mass Selective Detector, Hewlett-Packard, Palo Alto, CA), as described previously.18–22 The heptafluorobutyryl derivative was formed for the analysis of plasma glucose and glycerol in plasma and VLDL-TG, the tertiary-butyldimethylsilyl derivative was prepared for plasma leucine, and the N-heptafluorobutyryl n-propyl ester derivative was used for leucine in VLDL-apoB-100. Plasma free palmitate and palmitate in VLDL-TG were analyzed as methyl esters.
Calculations
Insulin sensitivity
The two insulin infusion rates during the clamp procedure were chosen to evaluate adipose tissue insulin sensitivity (low-dose insulin infusion to submaximally suppress lipolysis of adipose tissue TG), hepatic insulin sensitivity (low-dose insulin infusion to submaximally suppress hepatic glucose production), and skeletal muscle insulin sensitivity (high-dose insulin infusion to adequately stimulate muscle glucose uptake).6 Isotopic steady-state conditions were achieved during the final 30 min of the basal period and stages 1 and 2 of the clamp procedure. Endogenous glucose rate of appearance (Ra) in plasma was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR during the last 30 min of the basal period and stages 1 and 2 of the clamp procedure. Glucose rate of disappearance (Rd) was calculated as the sum of endogenous glucose Ra and the infusion rate of exogenous glucose. Palmitate Ra was calculated by dividing the palmitate tracer infusion rate by the average plasma palmitate TTR obtained during the final 30 min of the basal period and stage 1 of the clamp procedure. The degree of insulin-induced suppression of glucose Ra (stage 1 vs basal state) was used as an index of hepatic insulin sensitivity, the degree of insulin-induced suppression of palmitate Ra (stage 1 vs basal state) was used as an index of adipose tissue insulin sensitivity, and the degree of insulin-induced stimulation of glucose Rd (stage 2 vs basal state) was used as an index of skeletal muscle insulin sensitivity.6
VLDL kinetics
The fractional turnover rate (FTR) of VLDL-TG was determined by fitting the TTR time-courses of free glycerol in plasma and glycerol in VLDL-TG to a compartmental model.22 We have previously shown that plasma concentrations of VLDL-TG and VLDL-apoB-100 remain constant during the time-frame of our experiments.18,23 The rate of VLDL-TG secretion, which represents the amount of VLDL-TG secreted by the liver per unit of plasma, was calculated by multiplying the FTR by the steady-state plasma concentration of VLDL-TG. The plasma clearance rate of VLDL-TG, which is an index of the removal efficiency of VLDL-TG from plasma, was calculated as FTR times volume of distribution; VLDL-TG volume of distribution was assumed to equal plasma volume (calculated as 55 ml per kg of fat-free mass) because VLDL particles are essentially confined into the plasma compartment.24
The relative contribution of systemic plasma fatty acids (i.e., FFA from the systemic circulation that are taken up by the liver and directly incorporated into VLDL-TG) to total VLDL-TG production was determined by the degree of isotopic dilution upon fitting the palmitate TTR in plasma and VLDL-TG to a compartmental model,18,19,22 and was used to calculate the relative contribution of nonsystemic fatty acids by subtraction. Nonsystemic fatty acids are derived from pools of fatty acids that are not labeled with 2H2-palmitate during the infusion period, including fatty acids released from pre-existing, slowly turning over lipid stores in the liver and tissues draining directly into the portal vein, fatty acids resulting from lipolysis of plasma lipoproteins that are taken up by the liver, and fatty acids derived from hepatic de novo lipogenesis.25
The FTR of VLDL-apoB-100 was calculated by fitting the TTR time-courses of free leucine in plasma and leucine in VLDL-apoB-100 to a compartmental model.18,19 Hepatic secretion and plasma clearance rates of VLDL-apoB-100 were calculated based on plasma VLDL-apoB-100 concentration and VLDL-apoB-100 FTR as described above for VLDL-TG. The kinetic parameters of VLDL-apoB-100 are indices of the secretion and plasma clearance rates of VLDL particles, because each VLDL particle contains a single molecule of apoB-100.26
Statistical analysis and reproducibility statistics
All data sets were normally distributed according to the Kolmogorov-Smirnov criteria. Results from the two study days were compared with Student’s paired, two-tailed t-test. Summary data are presented as means ± SEMs. A P-value ≤ 0.05 was considered statistically significant.
Reproducibility statistics (i.e., change in the mean, total error, typical error) and sample size estimations were computed according to Hopkins.27 The change in the mean represents the average difference between the paired individual values obtained in the two studies; it consists of a random change component and a systematic (non-random) change component. The 95% confidence interval (CI) of the change in the mean was calculated to assess the absence (when the 95% CI spans zero) or presence (when the 95% CI does not include zero) of a systematic change. The total error of measurement, which reflects total measurement variability, was assessed by the intra-individual coefficient of variation (CV), calculated as the individual SD for the values obtained on the two different study days divided by the mean of the two values, and expressed as percent. We have previously evaluated the analytical variability of substrate concentration and enrichment measurements and found that almost all (>92%) of the total measurement variability is of biological origin.18 The typical error of measurement, which reflects within-subject variability and includes all sources of error, was calculated as the SD of the change in the mean divided by the square root of 2, and expressed as percent; the typical error was used to estimate the required sample size as a function of the minimum detectable difference for key outcome variables, assuming α = 0.05 and β = 0.20 (i.e., 80% power), for both paired and unpaired experimental designs.
RESULTS
Subject characteristics and basal metabolic profile on the two study days
Body weight and body mass index, body fat, fat-free mass and IHTG content were not different between the two study days (Table 1). Basal plasma glucose (5.5 ± 0.2 and 5.7 ± 0.3 mmol/l, P = 0.272), insulin (25 ± 2 and 24 ± 3 mU/l, P = 0.601), and FFA (0.58 ± 0.03 and 0.55 ± 0.05 mmol/l, P = 0.450) concentrations were not different during the first and second clamp procedure. Plasma total TG (1.93 ± 0.20 and 2.00 ± 0.24 mmol/l, P = 0.619), VLDL-TG (0.78 ± 0.10 and 0.76 ± 0.09 mmol/l, P = 0.629), and VLDL-apoB-100 (111 ± 16 and 106 ± 14 nmol/l, P = 0.555) concentrations were not different during the first and second VLDL kinetics study.
Reproducibility of basal substrate kinetics and insulin sensitivity
No significant differences were detected between the first and second studies in mean basal glucose and palmitate Ra, stage 1 insulin-mediated suppression of glucose Ra and palmitate Ra and stage 2 stimulation of glucose Rd, VLDL-TG and VLDL-apoB-100 secretion and clearance rates, or the relative contribution of nonsystemic fatty acids to VLDL-TG production (Table 2). In all cases, the 95% CI for the change in the mean included zero, indicating the absence of systematic changes in metabolic outcome measures; intra-individual CVs ranged from 6% to 21% for all metabolic variables (Table 2).
TABLE 2.
Reproducibility of basal substrate kinetics and multi-organ insulin sensitivity assessed at baseline (Study 1) and approximately 2 months later (Study 2)
| Study 1 | Study 2 | Change in the mean | Intra-individual | |||
|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Average | (95% CI) | CV, % (range) | ||
| Glucose Ra | (mmol/min) | 0.92 ± 0.04 | 0.93 ± 0.05 | 0.01 | (−0.07, 0.10) | 6.2 (2–14) |
| Palmitate Ra | (µmol/min) | 159 ± 11 | 150 ± 10 | −9 | (−32, 13) | 8.3 (2–26) |
| Insulin-mediated suppression of glucose Ra | (%) | 69 ± 6 | 66 ± 4 | −3 | (−14, 8) | 10.1 (2–19) |
| Insulin-mediated suppression of palmitate Ra | (%) | 63 ± 3 | 56 ± 5 | −7 | (−20, 5) | 15.5 (3–39) |
| Insulin-mediated stimulation of glucose Rd | (%) | 123 ± 20 | 145 ± 29 | 21 | (−12, 54) | 21.0 (15–28) |
| VLDL-TG secretion rate | (µmol/L·min) | 6.2 ± 0.6 | 6.8 ± 0.7 | 0.6 | (−1.2, 2.5) | 15.6 (2–39) |
| Contribution of nonsystemic plasma fatty acids to total VLDL-TG production | (%) | 57 ± 6 | 54 ± 6 | −3 | (−11, 5) | 11.4 (2–33) |
| VLDL-TG plasma clearance rate | (mL/min) | 32 ± 6 | 38 ± 7 | 6 | (−3, 14) | 13.5 (0–40) |
| VLDL-apoB-100 secretion rate | (nmol/L·min) | 0.46 ± 0.08 | 0.47 ± 0.06 | 0.01 | (−0.07, 0.08) | 8.8 (2–18) |
| VLDL-apoB-100 plasma clearance rate | (mL/min) | 16 ± 2 | 17 ± 2 | 1 | (−1, 3) | 11.1 (2–29) |
SEM, standard error of the mean; CI, confidence interval; CV, coefficient of variation; Ra, rate of appearance; Rd, rate of disappearance; VLDL, very low-density lipoprotein; TG, triglyceride; apoB-100, apolipoprotein B-100.
Sample size estimations for minimum detectable differences
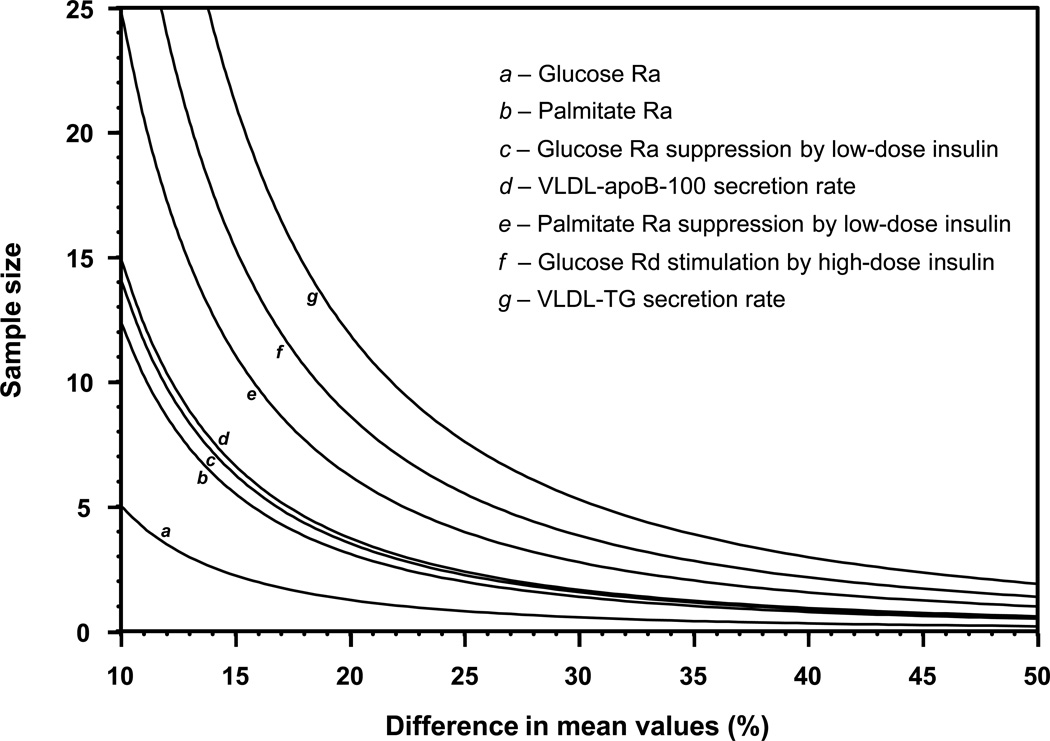
An estimate of the minimum number of subjects needed to detect significant changes in key outcome variables in a paired study design, assuming α = 0.05 and β = 0.20 (power = 80%), was used to construct a nomogram (Figure 1). Total sample size (experimental plus control subjects) required for a cross-sectional study design with two groups can be derived by multiplying the number of subjects required for the paired design by 4. The sample size per group needed to detect significant differences between two groups (containing an equal number of subjects/group) in a cross-sectional study design can be calculated by multiplying the number of subjects required for the paired study design by 2. Sample size requirements for selected differences in mean values, for a paired study design and for an unpaired study design with two groups, are given in Table 3.
Figure 1.
Minimum number of subjects needed to detect significant differences in basal plasma glucose, palmitate and VLDL kinetics, and hepatic (glucose Ra suppression by low-dose insulin), adipose tissue (palmitate Ra suppression by low-dose insulin), and skeletal muscle (glucose Rd stimulation by high-dose insulin) insulin sensitivity assuming α = 0.05 and β = 0.20 (power = 80%) in a paired study design. The sample size/group needed to detect significant differences between two groups in a cross-sectional study design can be calculated by multiplying the number of subjects required for the paired study design by 2. Ra, rate of appearance; Rd, rate of disappearance; VLDL, very low density lipoprotein; TG, triglyceride; apoB-100, apolipoprotein B-100.
TABLE 3.
Sample size needed in paired and unpaired studies to detect a given relative difference within or between groups (α = 0.05 and 80% power)
| Paired design Within-group difference |
Unpaired design Between-group difference |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15% | 20% | 25% | 30% | 35% | 15% | 20% | 25% | 30% | 35% | |
| Total number of subjects required | Number of subjects required per group | |||||||||
| Glucose Ra | 2 | 1 | 1 | 1 | 1 | 4 | 3 | 2 | 1 | 1 |
| Palmitate Ra | 6 | 3 | 2 | 1 | 1 | 11 | 6 | 4 | 3 | 2 |
| Insulin-mediated suppression of glucose Ra | 6 | 4 | 2 | 2 | 1 | 13 | 7 | 5 | 3 | 2 |
| Insulin-mediated suppression of palmitate Ra | 11 | 6 | 4 | 3 | 2 | 22 | 12 | 8 | 6 | 4 |
| Insulin-mediated stimulation of glucose Rd | 15 | 9 | 6 | 4 | 3 | 31 | 17 | 11 | 8 | 6 |
| VLDL-TG secretion rate | 21 | 12 | 8 | 5 | 4 | 42 | 24 | 15 | 11 | 8 |
| Contribution of nonsystemic plasma fatty acids to total VLDL-TG production | 5 | 3 | 2 | 1 | 1 | 10 | 6 | 4 | 3 | 2 |
| VLDL-TG plasma clearance rate | 15 | 8 | 5 | 4 | 3 | 30 | 17 | 11 | 7 | 5 |
| VLDL-apoB-100 secretion rate | 7 | 4 | 2 | 2 | 1 | 13 | 7 | 5 | 3 | 2 |
| VLDL-apoB-100 plasma clearance rate | 5 | 3 | 2 | 1 | 1 | 9 | 5 | 3 | 2 | 2 |
Ra, rate of appearance; Rd, rate of disappearance; VLDL, very low-density lipoprotein; TG, triglyceride; apoB-100, apolipoprotein B-100.
DISCUSSION
In this study, we examined the reproducibility of evaluating basal substrate kinetics and organ-specific insulin sensitivity in obese subjects with NAFLD. The variability in our outcome measures includes both technical (variability in performance of the clamp procedure and the isotope infusions and sample analyses) and physiological (variability in metabolic function) factors. Our results indicate that metabolic function in obese subjects is stable and the intra-individual variability (i.e., CV) in these metabolic outcome measures ranges from 6% to 21%. In general, the variability was less for straightforward outcome measures that involved a constant infusion of tracer and calculations based on standard isotope tracer dilution principles (e.g., basal glucose and palmitate Ra) than for more complex measures that involved manipulating the physiological environment (e.g., organ insulin sensitivity) or based on bolus tracer injection with mathematical modeling (e.g., VLDL-TG kinetics). Nevertheless, for all metabolic variables examined, we found that a 25% difference in any outcome measure can be detected with a sample size of ≤8 subjects for paired studies and ≤15 subjects per group for unpaired studies, assuming an α value of 0.05 and a β value of 0.20 (i.e., 80% power). However, even though significant differences in some metabolic outcome variables might be detected with only few subjects, both within and between groups, such small studies (e.g., fewer than 5 subjects) would not likely generate convincing conclusions.
Our results demonstrate that multi-organ insulin sensitivity is stable over 2 months in obese persons with NAFLD. Furthermore, we found that the intra-individual variability of insulin action in liver (suppression of glucose Ra) and adipose tissue (suppression of palmitate Ra) (CVs of 10–15%) was smaller than the variability of insulin action in skeletal muscle (stimulation of glucose Rd) (CV of 21%), possibly because the dose of infused insulin resulted in near maximum suppression of glucose production and lipolytic rate, but was not adequate to maximally stimulate muscle glucose uptake.28 Our estimates compare favorably with those from previous studies which only evaluated the variability in whole-body insulin sensitivity, determined as the glucose infusion rate needed to maintain euglycemia during insulin infusion without isotope tracers, and reported CVs between 3% and 35%.29–36 The results from our study demonstrate that only a small number of subjects are needed to detect clinically relevant improvements in insulin sensitivity. Moderate (~10%) diet-induced weight loss and thiazolidinedione therapy increase skeletal muscle insulin sensitivity (i.e., insulin-stimulated increase in glucose disposal) by 25–75%,12,13,37–39 hepatic insulin sensitivity (i.e., insulin-mediated suppression in glucose production) by 40–125%,37,40,41 and adipose insulin sensitivity (i.e., insulin-mediated suppression of lipolysis) by 30–45%.42,43 Our data suggest that a 25% improvement or a between-group difference in skeletal muscle insulin sensitivity can be detected in obese subjects with NAFLD with only 6 subjects in a paired design and 11 subjects per group in a two-arm unpaired study at the 5% level of significance with 80% power; fewer subjects would be needed to detect a 25% improvement or a between-group difference in hepatic and adipose tissue insulin sensitivity.
We found that the intra-individual variability of basal VLDL-TG and VLDL-apoB-100 kinetics in our obese subjects with NAFLD ranged from 9% to 15%, which is similar to values reported in studies conducted in healthy lean men18 and premenopausal women.44,45 Therefore, even though our subjects had increased IHTG content, which is associated with an increase in VLDL-TG secretion rate,4,10,46 the reproducibility in VLDL kinetics was not different than that observed in subjects who have a normal amount of IHTG. Our estimates demonstrate that few subjects are needed to detect the clinically relevant effects of moderate (~10%) diet-induced weight loss or treatment with niacin, fenofibrate, or atorvastatin, which cause a 35–65% change in VLDL-TG secretion and clearance rates and a 25–60% change in VLDL-apoB-100 secretion and clearance rates.14,19,47–49
In conclusion, our data demonstrate that longitudinal and cross-sectional differences in basal substrate kinetics and organ-specific insulin sensitivity, assessed by using sophisticated in vivo metabolic research techniques, can be detected with acceptable statistical power in small numbers of obese subjects with NAFLD. This information will be useful in designing studies that investigate metabolic function in subjects with NAFLD. In addition, these data illustrate the potential efficiency in conducting studies in small numbers of subjects to evaluate the metabolic effects of novel interventions, before embarking on large multicenter studies to detect effects on clinical outcomes.
ACKNOWLEDGEMENTS
This study was supported by National Institutes of Health grants DK 37948, DK 56341 (Nutrition Obesity Research Center), RR024992 (Clinical and Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource).
The authors thank Freida Custodio and Jennifer Shew for technical assistance, the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
Footnotes
CONFLICT OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 4.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magkos F, Mittendorfer B. Stable isotope-labeled tracers for the investigation of fatty acid and triglyceride metabolism in humans in vivo. Clin Lipidol. 2009;4:215–230. doi: 10.2217/clp.09.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnett JR, Barrett PH. Apolipoprotein B metabolism: tracer kinetics, models, and metabolic studies. Crit Rev Clin Lab Sci. 2002;39:89–137. doi: 10.1080/10408360208951113. [DOI] [PubMed] [Google Scholar]
- 9.Kotronen A, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia. 2008;51:130–138. doi: 10.1007/s00125-007-0867-x. [DOI] [PubMed] [Google Scholar]
- 10.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbrini E, deHaseth D, Deivanayagam S, Mohammed BS, Vitola BE, Klein S. Alterations in fatty acid kinetics in obese adolescents with increased intrahepatic triglyceride content. Obesity (Silver Spring) 2009;17:25–29. doi: 10.1038/oby.2008.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitola BE, Deivanayagam S, Stein RI, Mohammed BS, Magkos F, Kirk EP, et al. Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity (Silver Spring) 2009;17:1744–1748. doi: 10.1038/oby.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, et al. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:2727–2735. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 16.Lazo M, Selvin E, Clark JM. Brief communication: clinical implications of short-term variability in liver function test results. Ann Intern Med. 2008;148:348–352. doi: 10.7326/0003-4819-148-5-200803040-00005. [Erratum in: Ann Intern Med. 2009 Apr 2007;2150(2007):2504]. [DOI] [PubMed] [Google Scholar]
- 17.Frimel TN, Deivanayagam S, Bashir A, O'Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 18.Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res. 2007;48:1204–1211. doi: 10.1194/jlr.D600048-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–E556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 20.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 21.Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 22.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233. [PubMed] [Google Scholar]
- 23.Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr. 2003;77:573–579. doi: 10.1093/ajcn/77.3.573. [DOI] [PubMed] [Google Scholar]
- 24.Magkos F, Wright DC, Patterson BW, Mohammed BS, Mittendorfer B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab. 2006;290:E355–E362. doi: 10.1152/ajpendo.00259.2005. [DOI] [PubMed] [Google Scholar]
- 25.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Elovson J, Chatterton JE, Bell GT, Schumaker VN, Reuben MA, Puppione DL, et al. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res. 1988;29:1461–1473. [PubMed] [Google Scholar]
- 27.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition. 2010;26:686–693. doi: 10.1016/j.nut.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 30.Fossum E, Hoieggen A, Moan A, Nordby G, Kjeldsen SE. Insulin sensitivity relates to other cardiovascular risk factors in young men: validation of some modifications of the hyperinsulinaemic, isoglycaemic glucose clamp technique. Blood Press Suppl. 1997;2:113–119. [PubMed] [Google Scholar]
- 31.Mather KJ, Hunt AE, Steinberg HO, Paradisi G, Hook G, Katz A, et al. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab. 2001;86:5457–5464. doi: 10.1210/jcem.86.11.7880. [DOI] [PubMed] [Google Scholar]
- 32.Soop M, Nygren J, Brismar K, Thorell A, Ljungqvist O. The hyperinsulinaemic-euglycaemic glucose clamp: reproducibility and metabolic effects of prolonged insulin infusion in healthy subjects. Clin Sci (Lond) 2000;98:367–374. doi: 10.1042/cs0980367. [DOI] [PubMed] [Google Scholar]
- 33.Becker RH, Frick AD, Teichert L, Nosek L, Heinemann L, Heise T, et al. Fluctuation and reproducibility of exposure and effect of insulin glargine in healthy subjects. Diabetes Obes Metab. 2008;10:1105–1113. doi: 10.1111/j.1463-1326.2008.00874.x. [DOI] [PubMed] [Google Scholar]
- 34.Morris AD, Ueda S, Petrie JR, Connell JM, Elliott HL, Donnelly R. The euglycaemic hyperinsulinaemic clamp: an evaluation of current methodology. Clin Exp Pharmacol Physiol. 1997;24:513–518. doi: 10.1111/j.1440-1681.1997.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 35.Bokemark L, Froden A, Attvall S, Wikstrand J, Fagerberg B. The euglycemic hyperinsulinemic clamp examination: variability and reproducibility. Scand J Clin Lab Invest. 2000;60:27–36. doi: 10.1080/00365510050185010. [DOI] [PubMed] [Google Scholar]
- 36.Hedman A, Berglund L, Essen-Gustavsson B, Reneland R, Lithell H. Relationships between muscle morphology and insulin sensitivity are improved after adjustment for intra-individual variability in 70-year-old men. Acta Physiol Scand. 2000;169:125–132. doi: 10.1046/j.1365-201x.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Tonelli J, Kishore P, Owen R, Goodman E, Scherer PE, et al. Insulin-sensitizing effects of thiazolidinediones are not linked to adiponectin receptor expression in human fat or muscle. Am J Physiol Endocrinol Metab. 2007;292:E1301–E1307. doi: 10.1152/ajpendo.00312.2006. [DOI] [PubMed] [Google Scholar]
- 38.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 39.Schrauwen-Hinderling VB, Mensink M, Hesselink MK, Sels JP, Kooi ME, Schrauwen P. The insulin-sensitizing effect of rosiglitazone in type 2 diabetes mellitus patients does not require improved in vivo muscle mitochondrial function. J Clin Endocrinol Metab. 2008;93:2917–2921. doi: 10.1210/jc.2008-0267. [DOI] [PubMed] [Google Scholar]
- 40.Haus JM, Solomon TP, Marchetti CM, Edmison JM, Gonzalez F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab. 2010;95:323–327. doi: 10.1210/jc.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juurinen L, Kotronen A, Graner M, Yki-Jarvinen H. Rosiglitazone reduces liver fat and insulin requirements and improves hepatic insulin sensitivity and glycemic control in patients with type 2 diabetes requiring high insulin doses. J Clin Endocrinol Metab. 2008;93:118–124. doi: 10.1210/jc.2007-1825. [DOI] [PubMed] [Google Scholar]
- 42.Racette SB, Davis AO, McGill JB, Klein S. Thiazolidinediones enhance insulin-mediated suppression of fatty acid flux in type 2 diabetes mellitus. Metabolism. 2002;51:169–174. doi: 10.1053/meta.2002.29981. [DOI] [PubMed] [Google Scholar]
- 43.Shadid S, Jensen MD. Pioglitazone increases non-esterified fatty acid clearance in upper body obesity. Diabetologia. 2006;49:149–157. doi: 10.1007/s00125-005-0051-0. [DOI] [PubMed] [Google Scholar]
- 44.Magkos F, Patterson BW, Mittendorfer B. No effect of menstrual cycle phase on basal very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Am J Physiol Endocrinol Metab. 2006;291:E1243–E1249. doi: 10.1152/ajpendo.00246.2006. [DOI] [PubMed] [Google Scholar]
- 45.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325:1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 46.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 47.Bilz S, Wagner S, Schmitz M, Bedynek A, Keller U, Demant T. Effects of atorvastatin versus fenofibrate on apoB-100 and apoA-I kinetics in mixed hyperlipidemia. J Lipid Res. 2004;45:174–185. doi: 10.1194/jlr.M300309-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Watts GF, Barrett PH, Ji J, Serone AP, Chan DC, Croft KD, et al. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 2003;52:803–811. doi: 10.2337/diabetes.52.3.803. [DOI] [PubMed] [Google Scholar]
- 49.Ginsberg HN, Le NA, Gibson JC. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects. Effect of weight loss. J Clin Invest. 1985;75:614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]