Abstract
Objective
To determine the response of bone marrow progenitor cells from patients with myelodysplastic syndromes (MDS) to culture in physiologic oxygen tension.
Methods
Methylcellulose progenitor assays using both unfractionated bone marrow mononuclear cells (MNCs) and purified CD34+ progenitors were performed in atmospheric oxygen (18.6% O2) or one of two levels of hypoxia (1% and 3% O2). Assays were performed using normal donor marrow, MDS patient marrow, acute myelogenous leukemia marrow or peripheral blood blasts, chronic phase chronic myelogenous leukemia (CML) marrow MNCs, and blast crisis CML peripheral blood.
Results
The majority of MDS samples showed decreased colony-forming units (CFU) in 18.6% O2 compared to normal controls, as expected. However, in either 1% or 3% O2, 9 of 13 MDS samples demonstrated augmentation of CFUs beyond that observed in normal controls, with 6 of 13 demonstrating a greater than ninefold augmentation. This effect is cell autonomous, as it persisted after purification of CD34+ progenitor cells. Additionally, the augmented response to physiologic oxygen tension is specific to MDS, as it was not observed in either acute or chronic myelogenous leukemia samples.
Conclusion
These results suggest that the reported decrease in MDS CFUs reflects greater sensitivity of MDS progenitors or their progeny to the nonphysiologic oxygen tensions routinely used in vitro, rather than a true decrease in progenitor frequency. Importantly, these experiments for the first time describe an experimental system that can be used to study the growth of primary cells from patients with MDS.
Myelodysplastic syndromes (MDS) are a diverse group of hematopoietic disorders characterized by persistent cytopenias and a variable rate of progression to acute myelogenous leukemia (AML) [1,2]. Historically, these disorders were recognized as anemias that were “refractory” to treatment [3–6] or as “preleukemia” in patients who subsequently developed AML [7–13]. While the French-American-British classification system for MDS recognized a distinction between AML and MDS as early as 1982 [14], the concept of preleukemia remains [15–19]. Recent work has detailed the biologic distinctions between MDS and de novo AML [20], but the historical focus on leukemic biology continues to influence studies of these poorly understood disorders [21–23]. Although a number of hypotheses based upon the preleukemia paradigm have been explored [24–27], an understanding of the underlying pathophysiology remains elusive [28].
The absence of a coherent mechanistic explanation for these disorders has prompted us to reexamine the assumptions behind current models of MDS [21–23]. The marrow of MDS patients consistently demonstrates decreased hematopoietic progenitor colonies [29–34] as well as enhanced levels of apoptosis [35–47]. These two findings are felt to explain the paradox of a hypercellular bone marrow with profound peripheral cytopenias observed in MDS patients [48] and suggests that a propensity to apoptosis is responsible for the initiation of these disorders. However, a predisposition to apoptosis is seemingly incompatible with the self-renewal of MDS-initiating cells implied by the persistence of these diseases over months to years.
Accumulating evidence for a low oxygen tension in the-hematopoietic stem cell (HSC) niche and a rising gradient of oxygen tension during subsequent differentiation [49–57], as well as reports of mitochondrial dysfunction and oxidative damage in MDS marrows [58–66], led us to hypothesize that MDS results from a lesion that causes apoptosis only at oxygen tensions higher than that of the HSC niche. Such a lesion would permit unlimited self-renewal of MDS-initiating cells, provided these cells stayed at an oxygen tension comparable to that of the HSC niche. Consistent with our hypothesis, we demonstrate here that MDS progenitor cells cultured in both 3% O2 and 1% O2 demonstrate an augmentation of colony-forming unit (CFU) yield far in excess of what has previously been described in normal progenitors [67–76]. This degree of augmentation with physiologic oxygen was not observed in either acute or chronic myelogenous leukemias, but was maintained following the removal of CD34− accessory cells. These results demonstrate that MDS progenitor cells or their progeny have a unique sensitivity to increased oxygen tension and provide an improved methodology for the study of MDS cells.
Materials and methods
Cell isolation
Mononuclear cells (MNCs) from normal volunteer donors and patients with MDS, AML, and chronic myelogenous leukemia (CML) were obtained from the Stem Cell and Leukemia Core Facility at the Abramson Cancer Center of the University of Pennsylvania Center. Samples were collected from bone marrow aspirations (MDS, CML chronic phase, AML, and normal) and from peripheral blood or pheresis packs (CML blast crisis and AML) after informed consent in accordance with institutional guidelines. Following Ficoll-gradient centrifugation, MNCs were frozen as viable cells in fetal bovine serum (FBS) with 10% dimethyl sulfoxide then stored in liquid nitrogen.
Cell culture
Methylcellulose plates were cultured for 14 days in one of three experimental conditions. All three conditions used fully humidified atmospheres at 37°C (6.2% H2O) supplemented to 5% CO2. Standard conditions using room air provided 18.6% O2/70.2% N2. Culture under reduced oxygen tensions was achieved by purging a HERAcell 150 3-gas incubator (Thermo Electron, Asheville, NC) with purified N2 to maintain either 3% O2/85.8% N2 or 1% O2/87.8% N2. All MDS and normal control experiments were performed with two dilutions of cells split into either triplicate plates in two conditions (standard vs 3%) or duplicate plates in three conditions (standard vs 3% vs 1%). All AML and CML experiments were performed in standard vs 3% O2 conditions. AML MNC experiments were performed with single dilutions and either duplicate or triplicate plates, while the CML and CD34 selected AML experiments were performed with two dilutions and triplicate plates.
Normal/MDS/CML MNC plating
Cryopreserved MNCs from normal donors, MDS patients, and CML patients were thawed and washed in phosphate-buffered saline with 10% FBS, 0.5 mM ethylenediamine tetraacetic acid (Invitrogen, Carlsbad, CA, USA), and 8 µg/mL DNase I (Roche Applied Science, Indianapolis, IN, USA). Following the enumeration of viable cells, two dilutions of MNCs were prepared in MethoCult (StemCell Technologies, Vancouver, BC, Canada) methylcellulose media. Cells were diluted into 8 mL media to allow preparation of six 1-mL replicate plates from the same cell dilution. All MDS and normal samples, as well as the CML blast crisis sample, were plated in MethoCult H4434 media containing 30% FBS, 50 ng/mL stem cell factor, 10 ng/mL granulocyte-macrophage colony stimulating factor, 10 ng/mL interleukin (IL)-3, and 3 U/mL erythropoietin. The CML chronic phase sample was plated in MethoCult H4230 media containing 30% FBS without cytokines, which had been supplemented with 10 ng/mL IL-3. This change was made with the CML chronic phase sample only because culture in complete H4434 medium resulted in >500 colonies per plate in all conditions. All normal MNC experiments were performed at dilutions of 2 × 103/mL and 1 × 104/mL. Except as noted, all MDS and CML MNC experiments were performed at dilutions of 1 × 104/mL and 5 × 104/mL.
AML MNC plating
Cryopreserved MNCs from AML patient bone marrow, peripheral blood, or pheresis packs were thawed as above and then cultured in EGM-2 media (Cambrex Bio Science, Walkersville, MD, USA) as described previously [77]. This step was done for AML samples and not other samples because of a high rate of spontaneous cell death over the first 48 hours after thawing of cryopreserved AML samples. Following 48 hours of culture, the remaining viable cells were enumerated and single dilutions of viable cells (either 5000/mL or 8000/mL) were prepared and plated in H4434.
CD34+ progenitor plating
Selection of CD34+ cells was performed with either cryopreserved MNCs prepared as already described (patient samples) or freshly isolated MNCs (normal donors). Two rounds of positive selection were performed over MS columns using the CD34 Progenitor Cell Isolation Kit according to manufacturer’s instructions (Miltenyi Biotec, Auburn, CA, USA). Following the enumeration of viable cells, replicate plates were prepared as described above in H4434 with dilutions of 100/mL and 500/mL for normal CD34+ cells, 100/mL, and 1000/mL for CML CD34+ cells, 500/mL and 5000/mL for AML CD34+ cells, and 500/mL and 2500/mL for MDS CD34+ cells. Purity of CD34+ cells was confirmed by flow cytometry.
Evaluation of colonies
Following 14 days of incubation, plates were scored using an inverted microscope on the basis of cell number and colony morphology. Groups of 40 or more cells were scored as a colony. Granulocyte, monocyte, and granulocyte-monocyte (CFU-GM) colonies were each scored as one myeloid colony. Erythroid colonies, either single or clustered, were each scored as one erythroid colony. Replicates with between 10 and 150 total colonies per 1-mL plate were deemed valid for enumeration of colony number, and each lineage with at least 10 colonies per 1 mL plate was likewise deemed valid. Note in Table 2 that erythroid colonies were nonevaluable in several samples, as there were > 150 myeloid colonies with < 10 erythroid colonies. Error bars represent the standard error of the measurement.
Table 2.
MDS samples: Demographics, clinical characteristics, absolute CFUs, and fold-augmentation in 3% O2
| Absolute CFU/104 MNC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | E | Fold change* | |||||||||||||
| Sample | Patient | Sex | Age (y) | Diagnosis | WBC (103/µL) |
Hb (g/dL) |
Plt (103/µL) |
Blasts (%) | IPSS | 18.6% | 3% | 18.6% | 3% | GM | E |
| 202 | R0283 | M | 63 | RAEB-2 | 1.7 | 9.1 | 22 | 19 | INT-2 | 37.3 | >150 | <2 | <2 | 14.19† | NE |
| 218‡ | R0296 | F | 59 | RAEB-2 | 7.1 | 9.4 | 45 | 16 | High | 21.8 | 29.2 | <2 | <2 | 1.34 | NE |
| 234§ | R0306 | M | 60 | RCMD | 2.7 | 7.1 | 46 | 2 | INT-1 | 3.5 | 59.3 | <2 | 2.3 | 16.79 | NE |
| 246‡ | R0296 | F | 59 | RAEB-2 | 18.4 | 9.2 | 5 | 14 | High | 45.8 | 60.2 | <2 | <2 | 1.31 | NE |
| 253§ | R0306 | M | 60 | RCMD | 2.3 | 9.6 | 34 | 2 | INT-1 | 7.2 | 65.0 | <2 | <2 | 9.03 | NE |
| 260 | R0321 | M | 78 | RCMD | 1.9 | 6.9 | 189 | <5 | INT-1 | 7.0 | 17.7 | 16.4 | 25.8 | 2.34 | 1.45 |
| 298 | R0350 | M | 63 | RCMD | 8.8 | 13.9 | 52 | 4 | Low | 21.7 | 52.7 | 6.7 | 18.0 | 2.66 | 1.75 |
| 306 | R0356 | M | 77 | RCMD | 3.7 | 8.9 | 185 | 1 | Low | 21.3 | 45.3 | <2 | 29.3 | 2.13 | > 14.67 |
| 308 | R0342 | M | 73 | RAEB-2 | 1.9 | 11.1 | 56 | 18 | INT-2 | 2.5 | 3.8 | 5.3 | 5.7 | 1.50 | 1.09 |
| 313 | R0361 | M | 65 | RAEB-1 | 12.7 | 13 | 34 | 8 | INT-1 | 10.2 | 16.5 | 27.0 | 21.0 | 1.63 | 0.78 |
| 470 | R0398 | M | 70 | RAEB-1 | 2.3 | 10.8 | 7 | 5 | INT-2 | 13.5 | 350 | 2.3 | 2.3 | 25.89 | 1.00 |
| 496 | R0496 | M | 45 | RA | 2.8 | 11.8 | 90 | 0 | INT-2 | 7.5 | 9.5 | 7.1 | 10.1 | 1.26 | 1.42 |
| 584 | R0556 | M | 62 | RCMD | 8.1 | 9.4 | 93 | 3 | Low | 7.9 | 75.8 | <2 | 16.7 | 9.60 | > 8.33 |
CFU = colony-forming units; E = erythroid colonies; F = female; GM = combined myeloid (granulocyte, monocyte, and granulocyte-monocyte) colonies; Hb = hemoglobin; INT = intermediate; IPSS = International Prognostic System Score; MNC = mononuclear cells; NE = not evaluable; Plt = platelet count; R XXXX5 patient registry number XXXX; M = male; RAEB = refractory anemia with excess blasts; RA = refractory anemia; RCMD = refractory cytopenia with multilineage dysplasia; WBC = white blood cell count.
Italics indicates CFU fold-changes greater than that observed in normal controls but less than eightfold, while bold indicates greater than eightfold CFU augmentation.
Fold-change based on CD34+ cell results.
Sample 246 was obtained 50 days after sample 218, both from patient R0296.
Sample 253 was obtained 50 days after sample 234, both from patient R0306.
Results
Characteristics of normal marrow donors and MDS patients
Methylcellulose CFU assays were performed using MNCs obtained from volunteer donors and MDS patients seen at the Abramson Cancer Center of the University of Pennsylvania. Normal controls ranged from 29 to 59 years old (Table 1) and all reported themselves to be in good health; no clinical testing was performed on the controls. Patient samples had a diagnosis of MDS as defined by the Hematopathology Department at the University of Pennsylvania. For each sample, the subtype of MDS according to World Health Organization criteria [78], International Prognostic System Score [79], marrow blast count, blood counts, and age at the time of sample acquisition, as well as the patient’s sex and registry identification, are presented in Table 2. Note that although the majority were classified as refractory cytopenia with multilineage dysplasia, several subtypes of MDS are represented. With one exception, all subjects were male. Two pairs of samples, 234/253 and 218/246, were acquired prior to and following 6 weeks of oral valproic acid on University of Pennsylvania Cancer Center study 02702.
Table 1.
Normal controls: Demographics, absolute CFUs, and fold-augmentation in 3% O2
| Absolute CFU/104 MNC | ||||||||
|---|---|---|---|---|---|---|---|---|
| 18.6% O2 | 3% O2 | Fold change | ||||||
| Sample | Sex | Age (y) | GM | E | GM | E | GM | E |
| NBM10 | F | 29 | 55.6 | 57.0 | 70.8 | 72 | 1.27 | 1.26 |
| NBM11 | F | 35 | 32.0 | 70.0 | 54.2 | 65.8 | 1.63 | 1.02 |
| NBM12 | M | 46 | 106.7 | 100.0 | 243.3 | 130 | 2.28 | 1.30 |
| NBM13 | F | 55 | 39.2 | 33.0 | 52.0 | 37.2 | 1.40 | 1.15 |
| NBM14 | M | 29 | 10.7 | 17.7 | 22.7 | 18.7 | 2.13 | 1.06 |
| NBM19 | F | 53 | 44.5 | 27.0 | 54.3 | 21.3 | 1.22 | 0.79 |
| NBM22 | M | 59 | 15.0 | 30.7 | 25.0 | 29.3 | 1.67 | 0.96 |
CFU = colony-forming unit; F = female; GM = combined myeloid (granulocyte, monocyte, and granulocyte-monocyte) colonies; E = erythroid colonies; M = male; MNC, mononuclear cells; NBM = normal bone marrow sample.
Culture in 3% O2 enhances MDS CFUs out of proportion to normal CFUs
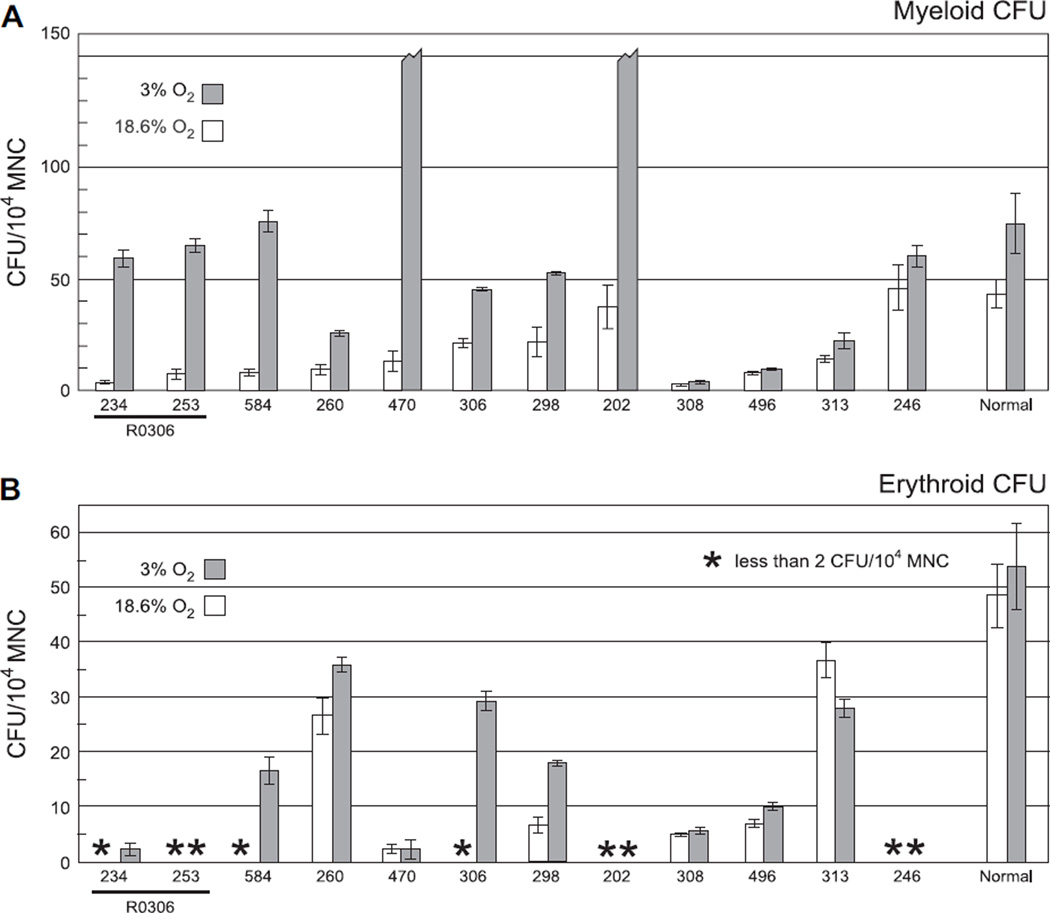
In order to determine if oxygen tension would affect colony-forming activity, we performed colony-forming assays in both standard conditions (18.6% O2) and in 3% O2. Cells were mixed in complete methylcellulose media containing 30% FBS, stem cell factor, granulocyte-macrophage colony-stimulating factor, IL-3, and erythropoietin in sufficient volume for six replicate plates of each cell dilution. Alternate replicate plates were then cultured for 14 days in either 18.6% O2 or 3% O2. Culture of normal MNCs in 18.6% O2 produced myeloid and erythroid CFUs of 10.7 to 106.7 and 17.7 to 100.0 per 104 MNCs, respectively (Table 1). Mean myeloid and erythroid CFUs were 42.8 and 49.9 per 104 MNCs, respectively (Fig. 1, white bars on far right). Growth of normal MNCs in 3% O2 resulted in a modest increase in colony-forming activity as reported previously [71–76]. This augmentation ranged from 1.2- to 2.3-fold and 0.8-to 1.3-fold for myeloid and erythroid CFUs, respectively (Table 1). Consistent with published results [33], both myeloid and erythroid CFUs from MDS MNCs grown in 18.6% O2 were lower than controls, with means ranging from 2.6 to 45.8 and < 2 to 36.7 per 104 MNCs, respectively (Table 2 and Fig. 1, white bars). In distinct contrast to the response of normal controls, 8 of 12 MDS samples demonstrated greater augmentation than observed in normal samples (Fig. 1, gray bars; Table 2, italics) and 6 of 12 samples demonstrated greater than eightfold augmentation of at least one lineage (Table 2, bold). There was no significant correlation between cytogenetics and response to culture in 3% O2 (data not shown). These results demonstrate that culture of MDS cells in 3% O2 produces significantly more CFUs than culture in 18.6% O2.
Figure 1.
Culture in 3% O2 enhances colony-forming unit (CFU) yield from myelodysplastic syndrome (MDS) mononuclear cells (MNCs). Myeloid (A) and erythroid (B) CFUs from MNCs of normal volunteer donors (“Normal” on far right) and MDS patients (indicated by unique sample numbers) were counted following 14 days of culture in either standard conditions using 18.6% O2 (white bars) or in 3% O2 (gray bars). Results are normalized to CFUs per 104 MNCs. Broken bars represent samples with > 150 colonies per 1-mL plate. Samples 234 and 253 were collected from the same patient, R0306, prior to and following 6 weeks of treatment with the histone deacetylase inhibitor valproic acid.
Culture in 1% O2 does not further enhance MDS CFU growth
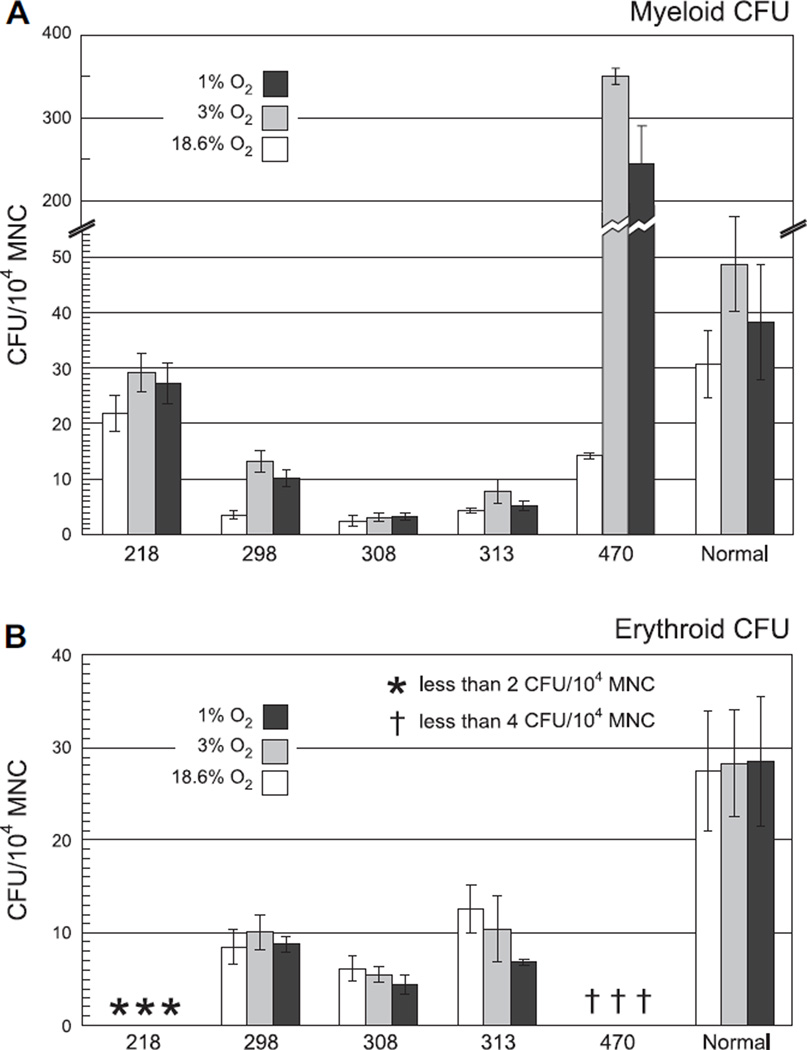
While 3% O2 is close to the 2.3% O2 observed in whole murine bone marrow [80], the HSC niche may experience an oxygen tension significantly lower than this [55]. It is possible that culture at a lower oxygen tension would allow further augmentation of MDS progenitor growth. It is also possible that samples such as 308, which demonstrated low CFUs even with culture in 3% O2, are as sensitive to culture in 3% O2 as other MDS samples are to culture in 18.6% O2. In order to examine these possibilities, additional experiments were performed in 1% O2 as well as the conditions described above. Culture in 1% O2 did not enhance either myeloid or erythroid CFUs from MDS samples compared to culture in 3% O2 (Fig. 2). Culture in 1% O2 trended toward a decrease compared with culture in 3% O2 (compare dark gray bars to light gray bars), but samples that demonstrated a response to culture in 3% O2 (e.g., 470) also demonstrated increased CFUs with culture in 1% O2 when compared to culture in 18.6% O2. Overall, we do not see significant changes in the augmentation of MDS CFUs with a further decrease in oxygen tension below 3% O2.
Figure 2.
Culture in 1% O2 does not further enhance colony-forming unit (CFU) yield from myelodysplastic syndromes (MDS) mononuclear cells (MNCs). Myeloid (A) and erythroid (B) CFUs from bone marrow MNCs of normal volunteer donors (“Normal” on far right) and MDS patients (indicated by unique sample numbers) were counted following 14 days of culture in standard conditions using 18.6% O2 (white bars), in 3% O2 (light gray bars), or in 1% O2 (dark gray bars). Results are normalized to CFUs per 104 MNCs. Sample 470 was plated at dilutions of 1 × 103/mL and 2.5 × 104/mL.
Effect of 3% O2 is cell-intrinsic
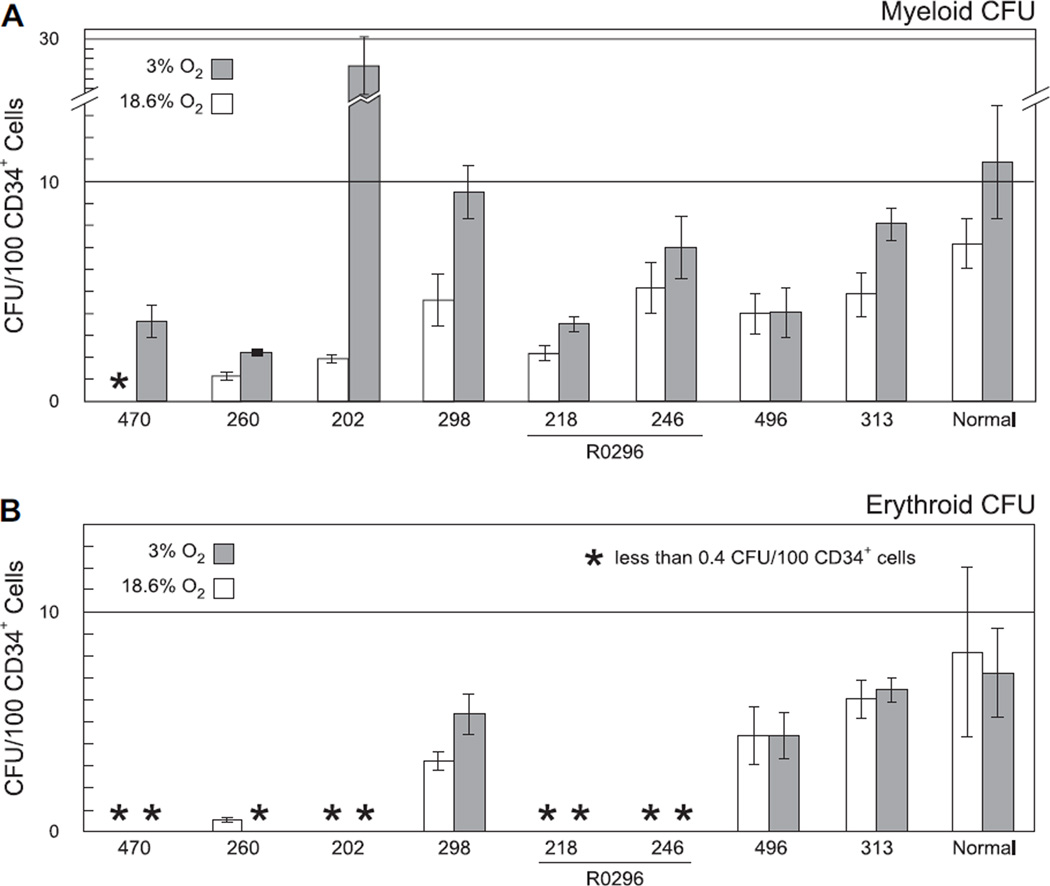
It is possible that the observed increase in MDS CFUs is not intrinsic to MDS progenitors, but instead results from “by-stander” effects, such as increased cytokine production by accessory cells in response to hypoxia. The relative roles of cell-intrinsic and cell-extrinsic factors were assessed by culturing purified CD34+ progenitor cells from MDS patient samples. Methylcellulose plates were generated as described above using CD34+ cells of 98–99% purity (data not shown) from a subset of samples with sufficient numbers of banked MNCs and placed in either 18.6% O2 or 3% O2. Culture of normal CD34+ cells in 18.6% O2 yielded mean myeloid and erythroid CFUs of 7.2 and 8.2 per 100 CD34+ cells, respectively (Fig. 3A and B, white bars on far right). Culture of purified CD34+ cells from MDS samples resulted in recapitulation of the response of myeloid CFUs following culture of MNCs in 3% O2, with continued augmentation of myeloid CFUs (Fig. 3A, samples 470, 260, 202, and 298) or lack of augmentation of any CFUs (Fig. 3, samples 218, 246, 496, and 313), respectively. Only one sample with increased erythroid CFUs following culture of MNCs in 3% O2 (Fig. 1B, sample 298) had sufficient cells available for purification of CD34+ cells, rendering conclusions regarding the contribution of accessory cells to the erythroid response suspect. Overall, these CD34+ progenitor experiments demonstrate that augmentation of myeloid CFUs with culture in 3% O2 is cell-intrinsic.
Figure 3.
Effect of culture in 3% O2 is maintained in purified CD34+ progenitor cells. Myeloid (A) and erythroid (B) colony-forming units (CFU) from CD34+ cells of normal volunteer donors (“Normal” on far right) and myelodysplastic syndrome (MDS) patients (numbered bars) were counted following 14 days of culture in either standard conditions using 18.6% O2 (white bars) or in 3% O2 (gray bars). Results are normalized to CFUs per 100 CD34+ cells. Samples 218 and 246 were collected from the same patient, R0296, prior to and following six weeks of treatment with the histone deacetylase inhibitor valproic acid.
Culture in 3% O2 does not alter differentiation in methylcellulose
The augmented CFU yield observed when MDS progenitors are grown in 3% O2 may reflect alterations in the ability of these cells to differentiate in 3% O2. In order to examine this possibility, the morphology of cells derived from colonies grown in both 18.6% O2 and 3% O2 was examined. While cells from both conditions demonstrated an abnormal morphology consistent with their derivation from patients with MDS, no significant morphologic differences were observed between cells cultured at different oxygen tensions (data not shown). This result suggests that the response to culture in 3% O2 oxygen does not reflect changes in myeloid differentiation.
Myeloid leukemia cells do not recapitulate response of MDS cells to culture in 3% O2
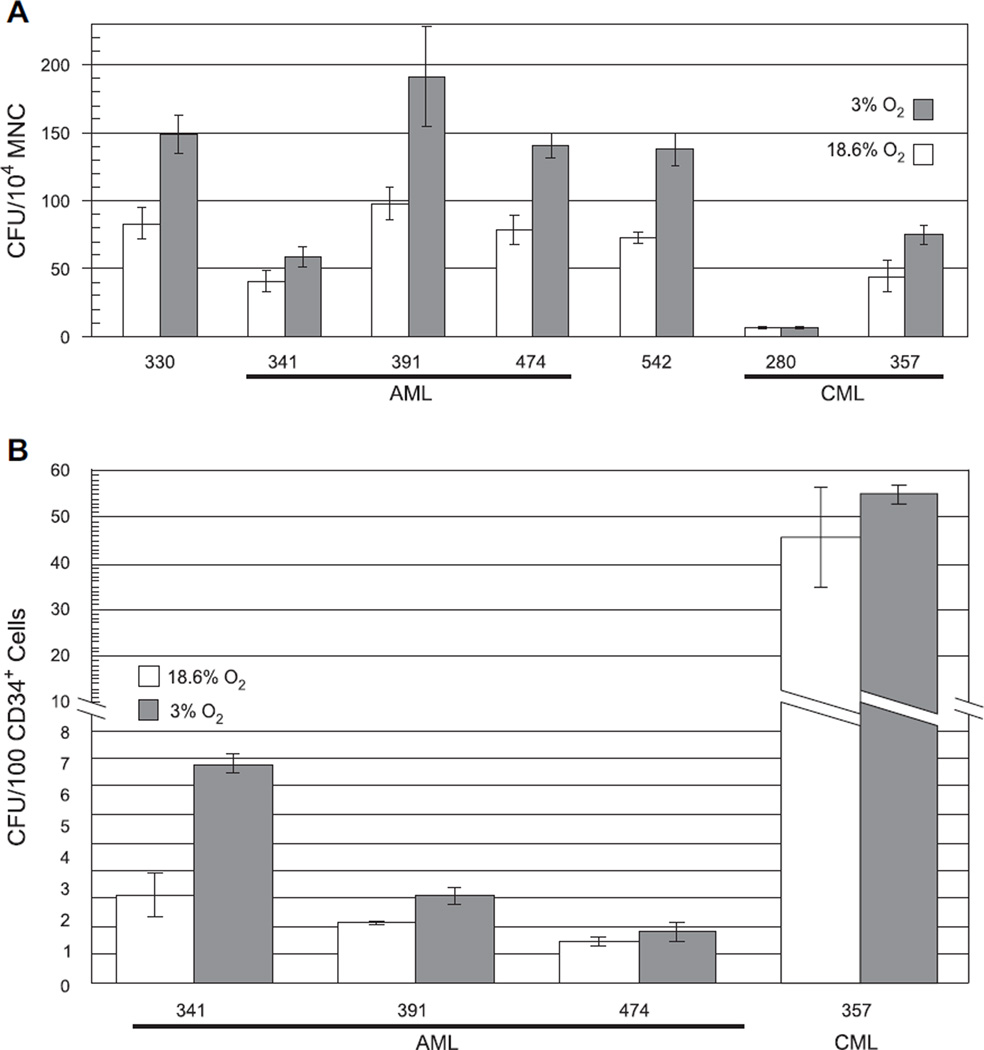
The striking response of MDS progenitors to culture in 3% O2 may reflect a generalized sensitivity of abnormal hematopoietic cells to the elevated oxygen tensions routinely used for in vitro culture. Additional experiments with MNCs from two CML and five AML patients, as well as CD34+ cells from a subset of these patients, were performed to address this possibility. For each leukemia sample the subtype according to World Health Organization criteria [78], sample blast count, blood counts, and age at the time of sample acquisition, as well as the patient’s sex and registry identification, are presented in Table 3. Culture of these leukemic samples in 3% O2 produced augmentation of CFUs from both total MNCs (Table 3 and Fig. 4A) and CD34+ cells (Fig. 4B), which was more consistent with the response of normal controls (Table 1) [71–74] than of the responding MDS samples (Table 2 and Figs. 1 and 3). Thus, a marked response to culture in 3% O2 is specific to MDS marrow cells and is not indicative of leukemic transformation.
Table 3.
Leukemia samples: Demographics, clinical characteristics, absolute CFUs, and fold-augmentation in 3% O2
| Absolute CFU/104 MNC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Patient | Sex | Age (y) | Diagnosis | WBC (103/µL) |
Hb (g/dL) |
Plt (103/µL) |
Blasts (%) | 18.6% O2 | 3% O2 | Fold change |
| 330 | R0375 | F | 25 | Acute myelomonocytic leukemia | 250.2 | 6.3 | 27 | 67 | 83.1 | 148.8 | 1.79 |
| 341 | R0385 | F | 52 | Acute monocytic leukemia | 191 | 7 | 45 | 62 | 40.6 | 58.8 | 1.45 |
| 391 | R0423 | M | 55 | AML with 11q23 abnormalities | 193.8 | 6.3 | 107 | 66 | 98.0 | 191.3 | 1.95 |
| 474 | R0481 | F | 70 | AML without maturation | 24.1 | 8.3 | 62 | 84 | 78.7 | 140.7 | 1.79 |
| 542 | R0532 | M | 42 | AML with maturation | 24.3 | 12.9 | 68 | 80 | 72.7 | 138.0 | 1.90 |
| 280 | R0337 | F | 45 | CML in blast crisis | 7.7 | 8.1 | 42 | 25 | 7.0 | 6.5 | 0.93 |
| 357 | R0394 | F | 56 | CML in chronic phase | 31.5 | 12.4 | 647 | 0 | 44.3 | 75.0 | 1.69 |
AML = acute myelogenous leukemia; CFU = colony-forming units; CML = chronic myelogenous leukemia; F = female; Hb = hemoglobin; M = male; MNC = mononuclear cells; Plt = platelet count; R XXXX = patient registry number XXXX; WBC = white blood cell count.
Figure 4.
Culture in 3% O2 provides minimal enhancement of colony-forming unit (CFU) yield from leukemia samples. (A) Graph of total CFUs obtained from mononuclear cells (MNCs) of five acute myelogenous leukemia (AML) patients and two chronic myelogenous leukemia (CML) patients following 14 days of culture in either standard conditions using 18.6% O2 (white bars) or in 3% O2 (gray bars). Results are normalized to CFUs per 104 MNCs. (B) Graph of total CFUs obtained from CD34+ cells of three AML patients and one CML patient following 14 days of culture in either standard conditions using 18.6% O2 (white bars) or in 3% O2 (gray bars). Results are normalized to CFUs per 100 CD34+ cells.
Discussion
We have demonstrated that bone marrow progenitor cells from patients with MDS, or the progeny of those progenitor cells, are abnormally sensitive to the elevated oxygen tension used in routine cell culture. We have additionally demonstrated that this sensitivity is alleviated by culture in oxygen tensions closer to those found in normal bone marrow. The response of MDS myeloid progenitors to reduced oxygen tension far exceeds that observed in normal progenitors, either in our hands (compare Table 1 and Table 2) or in numerous reports from the literature in which fold changes of CFU-GM ranging from 1.5-fold [72–74] to two-fold [71] were seen. In a few MDS samples an effect can be seen in the erythroid lineage, but the most pronounced effect was in the myeloid lineage. In fact, the decrease in erythroid CFU augmentation of sample 298 with culture in 3% O2 following purification of CD34+ progenitors may indicate that the response of MDS erythroid progenitors to culture in 3% O2 results from cell-extrinsic effects. Cell-extrinsic effects of lowered oxygen tension on the growth of erythroid colonies have been reported previously [69]. In that report, this effect was eliminated with removal of either macrophages or T cells. However, the clear maintenance of hypoxic augmentation in myeloid CFUs following selection of CD34+ progenitor cells demonstrates that this response is cell autonomous. Although the physiologic explanation for the dramatic effect of hypoxia has not been determined, these results for the first time describe an experimental system which can be used to study the growth of primary cells from patients with MDS.
There are several practical aspects of our data that bear comment. First, the methodology we have described is quite robust. For example, results shown in Figures 1 and 3 are independent experiments from cryopreserved specimens. Additionally, samples drawn from the same patient 7 weeks later continue to show either the absence (Table 2, compare 218 to 246) or presence (Table 2, compare 234 to 253) of a response to culture in 3% O2. Thus, this assay should be amenable to use in laboratories that do not have access to fresh bone marrow aspirates from MDS patients. Second, we note that the increase in CFUs is primarily in the myeloid lineage and less so in the erythroid lineage. The explanation for this result is unclear, but it is an important question for future investigations. The difference in lineage response may be dependent on the subtype of MDS, the presence of different accessory cell populations, or intrinsic differences in myeloid and erythroid responses to the underlying abnormalities responsible for these disorders. Third, the robust response to decreased oxygen tension we report appears to be specific to MDS and is not seen in myeloid leukemias. Although studies in aplastic anemia and folate deficiency would be interesting, samples of viable bone marrow MNCs from such patients are not available to us for such studies. Finally, a subset of samples show low overall progenitor frequency in all oxygen tensions tested. These samples were not distinguished by diagnosis, age, cytogenetics, or patient treatment. Of note, all samples described here were processed using standard Ficoll density centrifugation and plated in methylcellulose in room air rather than a controlled oxygen atmosphere. Each sample was therefore exposed to an extended period of hyperoxia both prior to and following cryopreservation. Alternatively, there may be variability in the ability of MDS cells to recover from the effects of cryopreservation and thawing. A larger study comparing fresh to frozen samples could address these issues. Assessment of the relevance of exposure to high oxygen tension during sample acquisition will require direct comparative studies.
Oxygen tensions vary widely in normal human tissues, from a maximum of 13% in arterial blood, through 9% in spleen and 5% in mixed venous blood, down to 3% in myocardium, liver, and brain [81]. In situ measurements of murine bone marrow, which avoid the technical limitations inherent to marrow aspiration such as contamination by sinusoidal blood [82] demonstrate an oxygen tension of 2.3% [80]. There is growing evidence that the unique environment provided by the HSC niche includes an oxygen tension even lower than that of the marrow as a whole [49–57]. Our results challenge a model of MDS in which these disorders are the result of decreased numbers of progenitor cells [30–34]. This model is based on the observation that CFUs from these patients are decreased upon culture in 18.6% O2 [29–34]. In most of our MDS samples CFUs increase from low to normal by shifting to culture in physiologic oxygen tension. This suggests that the number of progenitor cells in these patients is in fact normal, but that the maturing progeny of the progenitor cells cannot mature efficiently when exposed to more than 3% O2, resulting in their elimination through apoptosis. Our results suggest that oxygen tension may act as a context-specific trigger of apoptosis in hematopoietic progenitor cells, or their progeny, in MDS patients. MDS-specific HSCs carrying this defect would be especially well protected from a trigger based on increased oxygen tension by virtue of the deeper hypoxia of the HSC niche. If this model is correct, it is conceivable that such an abnormal HSC could maintain the unlimited self-renewal potential of normal HSCs so long as it remained in the HSC niche.
In summary, we have demonstrated that MDS progenitor cells are exquisitely sensitive to oxygen tensions higher than those found in the bone marrow, and propose that this sensitivity offers a window to better illuminate the pathophysiology underlying these puzzling disorders. These results also have practical implications, as studies of MDS have been difficult due to the inability to consistently grow cells from these patients using standard in vitro techniques. Expansion of primary cells from patients with MDS can be markedly improved by reducing the oxygen tension to which these cells are exposed.
Acknowledgments
We would like to thank the patients and staff of the Hematologic Malignancies Unit at the Hospital of the University of Pennsylvania, without whom these studies would not be possible. We would also like to thank Dr. M. Celeste Simon and Dr. Brian D. Keith for assistance with hypoxia experiments and the members of the Carroll lab for advice and assistance. Finally, we would like to thank Dr. Elizabeth Hexner, Dr. Sasha Perl, and Beth A. Burke for critical reading of the manuscript. J.E.T. is supported by grant K08-HL73977 from the National Heart, Lung and Blood Institute. M.C. is a recipient of the Leukemia and Lymphoma Society of America Clinical Scholar Award. P.V.S. is supported by grant T32-DK07780 from the National Institute of Diabetes & Digestive & Kidney Diseases. This work was supported by a Leukemia and Lymphoma Society of America grant.
References
- 1.Vergilio JA, Bagg A. Myelodysplastic syndromes. Contemporary biologic concepts and emerging diagnostic approaches. Am J Clin Pathol. 2003;119(suppl):S58–S77. doi: 10.1309/T8DF-XEXM-YLHW-51DG. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg EH. Strategies for biology- and molecular-based treatment of myelodysplastic syndromes. Curr Drug Targets. 2005;6:713–725. doi: 10.2174/1389450054863707. [DOI] [PubMed] [Google Scholar]
- 3.Rhoads C, Barker W. Refractory anemia: analysis of 100 cases. JAMA. 1938;110:794–796. [Google Scholar]
- 4.Bomford P, Rhoads C. Refractory anemia: I. Clinical and pathological aspects. Q J Med. 1941;10:175. [Google Scholar]
- 5.Björkman SE. Chronic refractory anemia with sideroblastic bone marrow: a study of four cases. Blood. 1956;11:250–259. [PubMed] [Google Scholar]
- 6.Vilter RW, Jarrold T, Will JJ, Mueller JF, Friedman BI, Hawkins VR. Refractory anemia with hyperplastic bone marrow. Blood. 1960;15:1–29. [PubMed] [Google Scholar]
- 7.Hamilton-Paterson JL. Pre leukaemic anaemia. Acta Haematol. 1949;2:309–316. doi: 10.1159/000203474. [DOI] [PubMed] [Google Scholar]
- 8.Williams MJ. Myeloblastic leukemia preceded by prolonged hematologic disorder. Blood. 1955;10:502–509. [PubMed] [Google Scholar]
- 9.Rowley JD, Blaisdell RK, Jacobson LO, Mikuta J, Byrne R, Shepley L. Chromosome studies in preleukemia: I. Aneuploidy of group C chromosomes in three patients. Blood. 1966;27:782–799. [PubMed] [Google Scholar]
- 10.Kumar S, Bhargava M. Pre-leukaemic acute myelogenous leukaemia. Acta Haematol. 1970;43:21–30. doi: 10.1159/000208710. [DOI] [PubMed] [Google Scholar]
- 11.Saarni MI, Linman JW. Preleukemia. The hematologic syndrome preceding acute leukemia. Am J Med. 1973;55:38–48. doi: 10.1016/0002-9343(73)90148-4. [DOI] [PubMed] [Google Scholar]
- 12.Fisher WB, Armentrout SA, Weisman R, Jr, Graham RC., Jr “Preleukemia.” A myelodysplastic syndrome often terminating in acute leukemia. Arch Intern Med. 1973;132:226–232. doi: 10.1001/archinte.132.2.226. [DOI] [PubMed] [Google Scholar]
- 13.Linman JW, Bagby C., Jr The preleukemic syndrome: clinical and laboratory features, natural course, and management. Nouv Rev Fr Hematol Blood Cells. 1976;17:11–31. doi: 10.1007/978-3-642-66312-3_2. [DOI] [PubMed] [Google Scholar]
- 14.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 15.Killmann SA. Preleukemia: does it exist? Nouv Rev Fr Hematol Blood Cells. 1976;17:81–105. doi: 10.1007/978-3-642-66312-3_6. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg PL. The smoldering myeloid leukemic states: clinical and biologic features. Blood. 1983;61:1035–1044. [PubMed] [Google Scholar]
- 17.Bagby GC., Jr The concept of preleukemia: clinical and laboratory studies. Crit Rev Oncol Hematol. 1986;4:203–220. doi: 10.1016/s1040-8428(86)80012-9. [DOI] [PubMed] [Google Scholar]
- 18.Yang SS, Chau T, Dai MS, Lin SH. Steroid-induced tumor lysis syndrome in a patient with preleukemia. Clin Nephrol. 2003;59:201–205. doi: 10.5414/cnp59201. [DOI] [PubMed] [Google Scholar]
- 19.Heiss S, Erdel M, Gunsilius E, Nachbaur D, Tzankov A. Myelodysplastic/ myeloproliferative disease with erythropoietic hyperplasia (erythroid preleukemia) and the unique translocation (8;9)(p23;p24): first description of a case. Hum Pathol. 2005;36:1148–1151. doi: 10.1016/j.humpath.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Albitar M, Manshouri T, Shen Y, et al. Myelodysplastic syndrome is not merely “preleukemia. Blood. 2002;100:791–798. doi: 10.1182/blood.v100.3.791. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto K, Hirano N, Toyoshima H, et al. Mutations of the p53 gene in myelodysplastic syndrome (MDS) and MDS-derived leukemia. Blood. 1993;81:3022–3026. [PubMed] [Google Scholar]
- 22.Stephenson J, Mufti GJ, Yoshida Y. Myelodysplastic syndromes: from morphology to molecular biology. Part II. The molecular genetics of myelodysplasia. Int J Hematol. 1993;57:99–112. [PubMed] [Google Scholar]
- 23.Shih LY, Huang CF, Wang PN, et al. Acquisition of FLT3 or N-ras mutations is frequently associated with progression of myelodysplastic syndrome to acute myeloid leukemia. Leukemia. 2004;18:466–475. doi: 10.1038/sj.leu.2403274. [DOI] [PubMed] [Google Scholar]
- 24.Allampallam K, Dutt D, Nair C, et al. The clinical and biologic significance of abnormal lipid profiles in patients with myelodysplastic syndromes. J Hematother Stem Cell Res. 2000;9:247–255. doi: 10.1089/152581600319469. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld C, List A. Ahypothesis for the pathogenesis of myelodysplastic syndromes: implications for new therapies. Leukemia. 2000;14:2–8. doi: 10.1038/sj.leu.2401618. [DOI] [PubMed] [Google Scholar]
- 26.Wolfler A, Erkeland SJ, Bodner C, et al. A functional single-nucleotide polymorphism of the G-CSF receptor gene predisposes individuals to high-risk myelodysplastic syndrome. Blood. 2005;105:3731–3736. doi: 10.1182/blood-2004-06-2094. [DOI] [PubMed] [Google Scholar]
- 27.Ly H, Calado RT, Allard P, et al. Functional characterization of telomerase RNA variants found in patients with hematologic disorders. Blood. 2005;105:2332. doi: 10.1182/blood-2004-09-3659. [DOI] [PubMed] [Google Scholar]
- 28.Catenacci DV, Schiller GJ. Myelodysplasic syndromes: a comprehensive review. Blood Rev. 2005;19:301–319. doi: 10.1016/j.blre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg PL, Mara B. The preleukemic syndrome: correlation of in vitro parameters of granulopoiesis with clinical features. Am J Med. 1979;66:951–958. doi: 10.1016/0002-9343(79)90450-9. [DOI] [PubMed] [Google Scholar]
- 30.Milner GR, Testa NG, Geary CG, et al. Bone marrow culture studies in refractory cytopenia and ‘smouldering leukaemia’. Br J Haematol. 1977;35:251–261. doi: 10.1111/j.1365-2141.1977.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 31.Chui DH, Clarke BJ. Abnormal erythroid progenitor cells in human preleukemia. Blood. 1982;60:362–367. [PubMed] [Google Scholar]
- 32.Ruutu T, Partanen S, Lintula R, Teerenhovi L, Knuutila S. Erythroid and granulocyte-macrophage colony formation in myelodysplastic syndromes. Scand J Haematol. 1984;32:395–402. doi: 10.1111/j.1600-0609.1984.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 33.Dan K, An E, Futaki M, et al. Megakaryocyte, erythroid and granulocyte-macrophage colony formation in myelodysplastic syndromes. Acta Haematol. 1993;89:113–118. doi: 10.1159/000204502. [DOI] [PubMed] [Google Scholar]
- 34.Juvonen E, Partanen S, Knuutila S, Ruutu T. Megakaryocyte colony formation by bone marrow progenitors in myelodysplastic syndromes. Br J Haematol. 1986;63:331–334. doi: 10.1111/j.1365-2141.1986.tb05556.x. [DOI] [PubMed] [Google Scholar]
- 35.Clark D, Lampert I. Apoptosis is a common histopathological finding in myelodysplasia: the correlate of ineffective hematopoiesis. Leuk Lymphoma. 1990;2:415–418. doi: 10.3109/10428199009069295. [DOI] [PubMed] [Google Scholar]
- 36.Raza A, Gezer S, Mundle S, et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes. Blood. 1995;86:268–276. [PubMed] [Google Scholar]
- 37.Hellström-Lindberg E, Kanter-Lewensohn L, Öst Å. Morphological changes and apoptosis in bone marrow from patients with myelodysplastic syndromes treated with granulocyte-CSF and erythropoietin. Leuk Res. 1997;21:415–425. doi: 10.1016/s0145-2126(96)00110-5. [DOI] [PubMed] [Google Scholar]
- 38.Tsoplou P, Kouraklis-Symeonidis A, Thanopoulou E, Zikos P, Orphanos V, Zoumbos NC. Apoptosis in patients with myelodysplastic syndromes: differential involvement of marrow cells in ‘good’ versus ‘poor’ prognosis patients and correlation with apoptosis-related genes. Leukemia. 1999;13:1554–1563. doi: 10.1038/sj.leu.2401538. [DOI] [PubMed] [Google Scholar]
- 39.Washington LT, Jilani I, Estey E, Albitar M. Less apoptosis in patients with 5q-syndrome than in patients with refractory anemia. Leuk Res. 2002;26:899–902. doi: 10.1016/s0145-2126(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 40.Bogdanovic A, Jankovic G, Colovic M, Trpinac D, Bumbasirevic V. Apoptosis in bone marrow of myelodysplastic syndrome patients. Blood. 1996;87:3064. [PubMed] [Google Scholar]
- 41.Shetty V, Mundle S, Alvi S, et al. Measurement of apoptosis, proliferation and three cytokines in 46 patients with myelodysplastic syndromes. Leuk Res. 1996;20:891–900. doi: 10.1016/s0145-2126(96)00008-2. [DOI] [PubMed] [Google Scholar]
- 42.Jaju RJ, Boultwood J, Oliver FJ, et al. Molecular cytogenetic delineation of the critical deleted region in the 5q-syndrome. Genes Chromosomes Cancer. 1998;22:251–256. doi: 10.1002/(sici)1098-2264(199807)22:3<251::aid-gcc11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 43.Bincoletto C, Saad ST, Soares da Silva E, Queiroz ML. Autonomous proliferation and bcl-2 expression involving haematopoietic cells in patients with myelodysplastic syndrome. Br J Cancer. 1998;78:621–624. doi: 10.1038/bjc.1998.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker JE, Fishlock KL, Mijovic A, Czepulkowski B, Pagliuca A, Mufti GJ. ‘Low-risk’ myelodysplastic syndrome is associated with excessive apoptosis and an increased ratio of pro- versus anti-apoptotic bcl-2-related proteins. Br J Haematol. 1998;103:1075–1082. doi: 10.1046/j.1365-2141.1998.01114.x. [DOI] [PubMed] [Google Scholar]
- 45.Parker JE, Mufti GJ, Rasool F, Mijovic A, Devereux S, Pagliuca A. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood. 2000;96:3932–3938. [PubMed] [Google Scholar]
- 46.Matthes TW, Meyer G, Samii K, Beris P. Increased apoptosis in acquired sideroblastic anaemia. Br J Haematol. 2000;111:843–852. [PubMed] [Google Scholar]
- 47.Span LF, Vierwinden G, Pennings AH, Boezeman JB, Raymakers RA, de Witte T. Programmed cell death is an intrinsic feature of MDS progenitors, predominantly found in the cluster-forming cells. Exp Hematol. 2005;33:435–442. doi: 10.1016/j.exphem.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Parker JE, Mufti GJ. Excessive apoptosis in low risk myelodysplastic syndromes (MDS) Leuk Lymphoma. 2000;40:1–24. doi: 10.3109/10428190009054877. [DOI] [PubMed] [Google Scholar]
- 49.Allalunis MJ, Turner AR, Partington JP, Urtasun RC. Effect of misonidazole therapy on human granulopoietic stem cells. Cancer Treat Rep. 1980;64:1097–1102. [PubMed] [Google Scholar]
- 50.Turner AR, Allalunis MJ, Urtasun RC, Pedersen JE, Meeker BE. Cytotoxic and radiosensitizing effects of misonidazole on hematopoiesis in normal and tumor-bearing mice. Int J Radiat Oncol Biol Phys. 1980;6:1157–1162. doi: 10.1016/0360-3016(80)90168-6. [DOI] [PubMed] [Google Scholar]
- 51.Allalunis MJ, Chapman JD, Turner AR. Identification of a hypoxic population of bone marrow cells. Int J Radiat Oncol Biol Phys. 1983;9:227–232. doi: 10.1016/0360-3016(83)90104-9. [DOI] [PubMed] [Google Scholar]
- 52.Allalunis-Turner J, Chapman JD. The in vitro sensitivities to radiation and misonidazole of mouse bone marrow cells derived from different microenvironments. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:415–422. doi: 10.1080/09553008514552641. [DOI] [PubMed] [Google Scholar]
- 53.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J. 2001;81:685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. I. Krogh’s model. Biophys J. 2001;81:675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Down JD, Parmar K, Clyne J, Mauch PM, Sackstein R. Defining the bone marrow stem cell niche according to a Hoechst dye diffusion gradient and hypoxia [abstract] Blood. 2004;104(11) 192a Abstract 666. [Google Scholar]
- 56.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 57.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 58.Aoki Y. Multiple enzymatic defects in mitochondria in hematological cells of patients with primary sideroblastic anemia. J Clin Invest. 1980;66:43–49. doi: 10.1172/JCI109833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowen D, Peddie C. Mitochondrial oxygen consumption and ineffective haematopoiesis in patients with myelodysplastic syndromes. Br J Haematol. 2002;118:345–346. doi: 10.1046/j.1365-2141.2002.03576_2.x. [DOI] [PubMed] [Google Scholar]
- 60.Broker S, Meunier B, Rich P, Gattermann N, Hofhaus G. MtDNA mutations associated with sideroblastic anaemia cause a defect of mitochondrial cytochrome c oxidase. Eur J Biochem. 1998;258:132–138. doi: 10.1046/j.1432-1327.1998.2580132.x. [DOI] [PubMed] [Google Scholar]
- 61.Gattermann N, Retzlaff S, Wang YL, et al. A heteroplasmic point mutation of mitochondrial tRNALeu(CUN) in non-lymphoid haemopoietic cell lineages from a patient with acquired idiopathic sideroblastic anaemia. Br J Haematol. 1996;93:845–855. doi: 10.1046/j.1365-2141.1996.d01-1724.x. [DOI] [PubMed] [Google Scholar]
- 62.Gattermann N, Retzlaff S, Wang YL, et al. Heteroplasmic point mutations of mitochondrial DNA affecting subunit I of cytochrome c oxidase in two patients with acquired idiopathic sideroblastic anemia. Blood. 1997;90:4961–4972. [PubMed] [Google Scholar]
- 63.Gattermann N, Wulfert M, Junge B, Germing U, Haas R, Hofhaus G. Ineffective hematopoiesis linked with a mitochondrial tRNA mutation (G3242A) in a patient with myelodysplastic syndrome. Blood. 2004;103:1499–1502. doi: 10.1182/blood-2003-07-2446. [DOI] [PubMed] [Google Scholar]
- 64.Peddie CM, Wolf CR, McLellan LI, Collins AR, Bowen DT. Oxidative DNA damage in CD34+ myelodysplastic cells is associated with intracellular redox changes and elevated plasma tumour necrosis factor-alpha concentration. Br J Haematol. 1997;99:625–631. doi: 10.1046/j.1365-2141.1997.4373247.x. [DOI] [PubMed] [Google Scholar]
- 65.Reddy PL, Shetty VT, Dutt D, et al. Increased incidence of mitochondrial cytochrome c-oxidase gene mutations in patients with myelodysplastic syndromes. Br J Haematol. 2002;116:564–575. doi: 10.1046/j.0007-1048.2001.03323.x. [DOI] [PubMed] [Google Scholar]
- 66.Shin MG, Kajigaya S, Levin BC, Young NS. Mitochondrial DNA mutations in patients with myelodysplastic syndromes. Blood. 2003;101:3118–3125. doi: 10.1182/blood-2002-06-1825. [DOI] [PubMed] [Google Scholar]
- 67.Rich IN, Kubanek B. The effect of reduced oxygen tension on colony formation of erythropoietic cells in vitro. Br J Haematol. 1982;52:579–588. doi: 10.1111/j.1365-2141.1982.tb03934.x. [DOI] [PubMed] [Google Scholar]
- 68.Lu L, Broxmeyer HE. Comparative influences of phytohemagglutinin-stimulated leukocyte conditioned medium, hemin, prostaglandin E, and low oxygen tension on colony formation by erythroid progenitor cells in normal human bone marrow. Exp Hematol. 1985;13:989–993. [PubMed] [Google Scholar]
- 69.Pennathur-Das R, Levitt L. Augmentation of in vitro human marrow erythropoiesis under physiological oxygen tensions is mediated by monocytes and T lymphocytes. Blood. 1987;69:899–907. [PubMed] [Google Scholar]
- 70.Broxmeyer HE, Cooper S, Lu L, Miller ME, Langefeld CD, Ralph P. Enhanced stimulation of human bone marrow macrophage colony formation in vitro by recombinant human macrophage colony-stimulating factor in agarose medium and at low oxygen tension. Blood. 1990;76:323–329. [PubMed] [Google Scholar]
- 71.Bradley TR, Hodgson GS, Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978;97:517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- 72.Maeda H, Hotta T, Yamada H. Enhanced colony formation of human hemopoietic stem cells in reduced oxygen tension. Exp Hematol. 1986;14:930–934. [PubMed] [Google Scholar]
- 73.Katahira J, Mizoguchi H. Improvement of culture conditions for human megakaryocytic and pluripotent progenitor cells by low oxygen tension. Int J Cell Cloning. 1987;5:412–420. doi: 10.1002/stem.5530050506. [DOI] [PubMed] [Google Scholar]
- 74.Ishikawa Y, Ito T. Kinetics of hemopoietic stem cells in a hypoxic culture. Eur J Haematol. 1988;40:126–129. doi: 10.1111/j.1600-0609.1988.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 75.Broxmeyer HE, Cooper S, Gabig T. The effects of oxidizing species derived from molecular oxygen on the proliferation in vitro of human granulocyte-macrophage progenitor cells. Ann N Y Acad Sci. 1989;554:177–184. doi: 10.1111/j.1749-6632.1989.tb22419.x. [DOI] [PubMed] [Google Scholar]
- 76.Cipolleschi MG, D’Ippolito G, Bernabei PA, et al. Severe hypoxia enhances the formation of erythroid bursts from human cord blood cells and the maintenance of BFU-E in vitro. Exp Hematol. 1997;25:1187–1194. [PubMed] [Google Scholar]
- 77.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–4268. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 79.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 80.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 81.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 82.Grant WC, Root WS. The relation of O2 in bone marrow blood to posthemorrhagic erythropoiesis. Am J Physiol. 1947;150:618–627. doi: 10.1152/ajplegacy.1947.150.4.618. [DOI] [PubMed] [Google Scholar]