Abstract
Objective
To evaluate the efficacy of topoisomerase I inhibitor, topotecan, in patients with recurrent BRCA+ versus BRCA− ovarian, fallopian tube, and primary peritoneal carcinomas.
Methods
A single-institution retrospective analysis of platinum-resistant patients characterized for the presence or absence of known deleterious BRCA mutations. Patients received topotecan at a dose and schedule determined by their treating physician (five day or weekly). Response rate and progression-free survival (PFS) were assessed.
Results
A total of 50 patients (9 BRCA+, 41 BRCA−) were treated with topotecan. Both groups were well balanced in terms of age, stage, grade, and number of prior therapies. All patients had high-grade serous carcinoma. The clinical benefit rate in BRCA+ and BRCA− patients was 0% and 26.8% (6 PRs, 6 SDs), respectively (p = 0.18). Median PFS in BRCA+ and BRCA− pts was 1.7 months (95%CI: 1.0 – 2.8mos) and 2.5 months (95%CI: 1.9 - 2.8 mos), respectively (p = 0.057). Median time to best response was 1.9 months, and median response duration 2.6 months.
Conclusions
This analysis in a heavily pretreated cohort of patients fails to support the superiority of topotecan in BRCA+ platinum-resistant ovarian, fallopian tube, and primary peritoneal cancers. Further study of this class of agents, specifically in less heavily-pretreated patients, may still be warranted.
Keywords: Ovarian cancer, BRCA, topoisomerase I inhibitors, topotecan
INTRODUCTION
Ovarian cancer (OC) is the leading cause of death from gynecologic malignancies in the United States and is the fourth most common cause of cancer death in women.[1] An estimated 21,000 cases are diagnosed in the United States each year, resulting in 15,000 deaths. It is now recognized that approximately 10% of these cases, and 16-21% of the high-grade serous subtype, are due to a hereditary susceptibility, of which BRCA1 and BRCA2 mutations account for the majority.[2, 3] Female BRCA1 and BRCA2 carriers have a 36-60% and 16-27% lifetime risk of developing OC, respectively.[4, 5] A risk-reducing bilateral salpingo-oophorectomy can significantly reduce, although not entirely eliminate, these risks.[6, 7]
Numerous retrospective and case-controlled studies have reported that patients with BRCA-associated (BRCA+) OC have better overall survival than patients with sporadic (BRCA−) OC.[8, 9] Preclinical data suggest that BRCA+ OCs are more sensitive to platinum-based chemotherapy.[10] BRCA+ OCs are now understood to have impaired homologous recombination, the DNA repair pathway responsible for the high-fidelity resolution of platinum-induced double-stranded DNA breaks.[11] One case-control analysis reported higher complete response rates to first, second, and third-line platinum-based chemotherapy in BRCA+ compared with BRCA− OC.[12] Another case series suggested that BRCA+ OC patients have improved overall survival even when accounting for platinum-sensitivity in a multivariate analysis.[13] These data suggest that both the chemosensitivity and underlying biology of BRCA+ OC may be more favorable then BRCA− OC. Despite these observations, nearly all patients with recurrent BRCA+ OC will eventually develop platinum resistance and as a result, ultimately die of their disease.
Topotecan is an accepted treatment for platinum-resistant disease, with a historic response rate of 10-15% in several large phase II and phase III studies.[14-16] Topotecan inhibits topoisomerase I leading to stalled replication forks, double-stranded DNA breaks, and irreversible DNA cross-linking.[17, 18] The similarity in DNA-damage caused by topoisomerase I inhibitors and platinums raises the possibility that BRCA+ OCs may also have increased sensitivity to topotecan. To our knowledge, the activity of topotecan in BRCA+ versus BRCA− OC has never been reported.
We therefore conducted a retrospective analysis of our institutional experience with topotecan in patients characterized for germline BRCA mutations. Our objectives were to determine the response rate and progression free survival of topotecan in patients with BRCA+ versus BRCA− OC.
MATERIALS AND METHODS
Patients
Institutional review board approval was obtained for this retrospective analysis. Eligible patients were seen at Memorial Sloan-Kettering Cancer Center (MSKCC) between January 1st, 1995 and February 1st, 2011. Patients were required to have recurrent ovarian, fallopian tube, or primary peritoneal cancer and have had BRCA mutation testing on one of two IRB approved research studies. Histologic confirmation of the original primary was required. All patients were platinum resistant according to GOG criteria (platinum-free interval with evidence of progressive disease of < 6 months from last platinum). All patients received topotecan. Patients may have received daily topotecan (Days 1-5, 21-day cycle) or weekly topotecan (Days 1, 8, and 15, 28-day cycle). Patients who received topotecan with an addition cytotoxic, biologic, or hormonal therapy were excluded from analysis.
Tumor Response and Progression Assessment
Responses were determined by clinician adjudicated CT, MRI, or PET scans. The retrospective nature of this cohort precluded use of the Response Evaluation Criteria for Solid Tumors (RECIST) to formally assess these scans. Progression was based on Rustin criteria (CA 125 ≥ 2x the nadir value)[19], radiologic progression or clinical progression (including death).
Statistical Design
This was a single institutional retrospective analysis with the primary objective of determining the clinical benefit (complete response + partial response + stable disease) rate of topotecan in BRCA+ and BRCA− patients. The associations between clinical factors and BRCA status/clinical response were tested by either using the Wilcoxon-rank sum test for the continuous variables or the Fisher-exact test for categorical variables. The PFS/OS time is calculated from the therapy starting date to the progression date/the death date/last follow-up date. Univariate PFS analysis/OS analyses were performed stratifying for BRCA status. The PFS/OS rate is estimated using Kaplan-Meier method. P-values are obtained by Wilcoxon-Gehan test.
RESULTS
A total of 50 patients were analyzed. Among this group, 9 patients were BRCA+ and 41 patients were BRCA−. Baseline demographic data and treatments received are listed in Table 1. There were no major differences between the two cohorts in the distribution of age, histology, grade, pathologic stage, or number of prior therapies. This was a heavily pre-treated cohort with a median of 5 prior therapies (range: 2-9).
Table 1.
Baseline Demographics
| Variables | All | BRCA (−) | BRCA (+) | p-values* |
|---|---|---|---|---|
| Whole Cohort | 50 | 41 | 9 | |
|
| ||||
| Age at Diagnosis | ||||
| Median (Mean) | 60(60.04) | 62(60.76) | 60(56.78) | 0.215 |
| Range | 40-78 | 40-78 | 43-66 | |
|
| ||||
| Diagnosis | ||||
| Ovarian Ca | 46(92%) | 37(90.2%) | 9(100%) | 1.000 |
| Fallopian Tube Ca | 3(6%) | 3(7.3%) | 0(0%) | |
| Peritoneum Ca NOS | 1(2%) | 1(2.4%) | 0(0%) | |
|
| ||||
| Pathologic Stage (4 missing) | ||||
| II | 2(4.3%) | 1(2.7%) | 1(11.1%) | 0.333 |
| III | 40(87%) | 33(89.2%) | 7(77.8%) | |
| IV | 4(8.7%) | 3(8.1%) | 1(11.1%) | |
|
| ||||
| Grade (1 missing) | ||||
| G1 | 0(0%) | 0(0%) | 0(0%) | 1.000 |
| G2 | 3(6.1%) | 3(7.5%) | 0(0%) | |
| G3 | 46(93.9%) | 37(92.5%) | 9(100%) | |
|
| ||||
| Number of Previous Therapies (line) | ||||
| Median (Mean) | 5(4.92) | 5(4.76) | 5(5.67) | 0.223 |
| Range | 2-9 | 2-9 | 2-9 | |
|
| ||||
| Rx | ||||
| 5-Day Topotecan | 8(16%) | 7(17.1%) | 1(11.1%) | 1.000 |
| Weekly Topotecan | 42(84%) | 34(82.9%) | 8(88.9%) | |
p-values for “Age at Diagnosis” and “Number of Previous Therapies“ were obtained by Wilcoxon-Rank sum test; other p-values were obtained by Fisher-Exact test.
Efficacy
Response data by BRCA status and treatment received are depicted in Table 2. The clinical benefit rate (complete response + partial response + stable disease) observed in BRCA+ and BRCA− patients was 0% and 26.8%, respectively (p=0.18). Among the BRCA− patients, 6 patients had partial responses, and 6 had stable disease. The median time to best response in this group was 1.9 months (range: 0.7 – 4.4 mos), and median response duration 2.6 months (range: 1.1 - 8.3 mos).
Table 2.
Treatment Outcomes
| Variable | Best Response |
|||
|---|---|---|---|---|
| Pt# | PR/SD | No-response | p-value* | |
| All | 50 | 11 | 39 | |
|
| ||||
| BRCA | ||||
| (−) | 41 | 11(26.8%) | 30(73.2%) | 0.177 |
| (+) | 9 | 0(0%) | 9(100%) | |
| Rx | ||||
| 5-Day Topotecan | 8 | 4(50%) | 4(50%) | |
| Weekly Topotecan | 42 | 7(16.7%) | 35(83.3%) | |
p-value was obtained by the Fisher-Exact test.
Progression-Free and Overall Survival
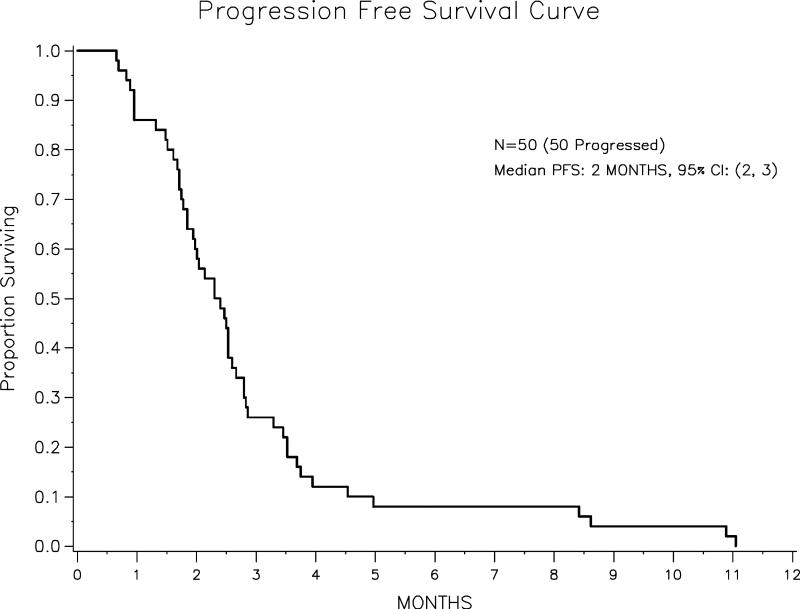
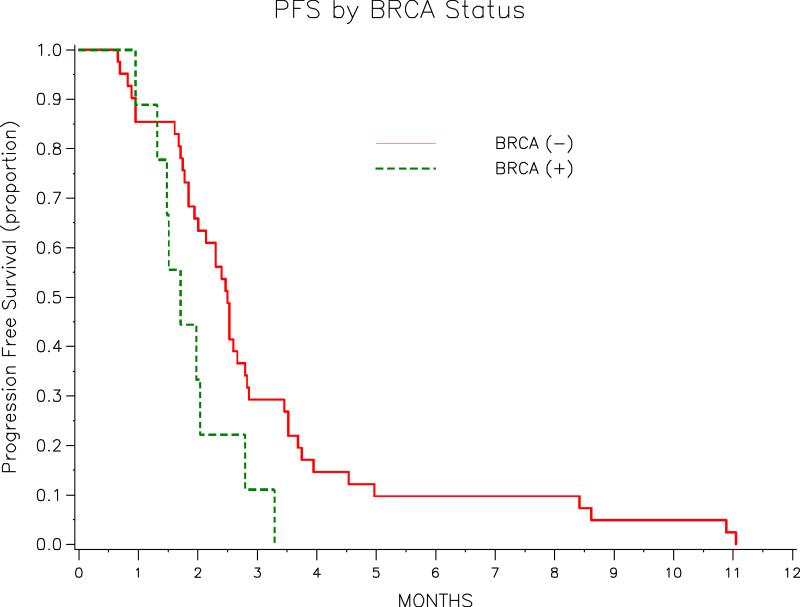
The median PFS time for the entire 50 patient cohort was 2.4 months (range: 0.66– 11.05 mos). The median PFS in the BRCA+ and BRCA− groups was 1.7 months (95% CI: 0.95-2.80) and 2.5 months (95% CI: 1.9-2.8), respectively (p = 0.057). The univariate PFS analysis results are listed in Table 3. Of the 50 patients, 39 had died at the time of the analysis. The median OS for the cohort overall was 13.5 months (range: 7.3 – 18.6 mos). The OS of each group by BRCA status are listed in Table 4. The median duration of follow-up for the 11 survivors was 7.0 months. The Kaplan-Meier PFS curves for the entire cohort and stratified by BRCA status are show in Figure 1 and Figure 2, respectively. Among responders the median time to best response was 1.9 months (range: 0.7 – 4.4 mos), and the median response duration 2.6 months (range: 1.1 – 8.3 mos).
Table 3.
Univariate PFS Analysis
| Variable | Pt# | Median PFS Time (Months; 95%CI) | p-value* |
|---|---|---|---|
| All | 50 | 2.4 (1.8-2.6) | |
|
| |||
| BRCA | |||
| (−) | 41 | 2.5 (1.9-2.8) | 0.057 |
| (+) | 9 | 1.7 (1.0-2.8) | |
p-value was obtained by Wilcoxon-Gehan test.
Table 4.
Univariate OS Analysis
| Variable | Pt# | Death # | Median OS Time (Months; 95%CI) | p-value* |
|---|---|---|---|---|
| All | 50 | 39 | 13.5 (7.3-18.6) | |
|
| ||||
| BRCA | ||||
| (−) | 41 | 33 | 13.5 (7.1-18.3) | 0.371 |
| (+) | 9 | 6 | 18.6 (0.95-Not Estimable) | |
p-value was obtained by Wicoxon-Gehan test.
Figure 1.
Figure 2.
DISCUSSION
This retrospective analysis did not demonstrate superior activity of the topoisomerase I inhibitor, topotecan, in BRCA+ compared with BRCA− OC. In fact, BRCA+ patients appeared to fair worse both in terms of clinical benefit rate and progression-free survival, although neither difference was statistically significant. This outcome may reflect the limited number of BRCA+ patients included in this analysis. It is also possible that the heavily pretreated nature of this cohort obscured differences that would be more apparent in a healthier group of patients. The median PFS for the entire cohort was only 2.4 months, somewhat shorter then would be expected from historical controls. Sehouli et al [15] recently reported a median PFS of 3.7 in 194 platinum-resistant OC patients treated prospectively with one of two topotecan schedules. Those patients had a median of 2 prior therapies, and upfront platinum-refractory disease was excluded. By comparison, the median number of prior therapies in this group was 5, with some patients receiving as many as 9 prior treatments. Unfortunately, germline BRCA status of the patients treated in these prospective studies is not available.
Despite the small sample size of this cohort, which precludes any definitive conclusions, these data suggest that it is unlikely that heavily pretreated BRCA+ platinum-resistant OCs have substantially improved sensitivity to topotecan. The mechanisms by which BRCA+ and BRCA− OCs develop resistance to DNA-damaging agents such as platinums are not well understood. Preclinical data suggest that increased BRCA1 expression is correlated inversely with platinum sensitivity and that antisense inhibition of BRCA1 reverses platinum-refractoriness.[10] Other data suggest that BRCA-associated OCs may regain functional BRCA1/2 proteins through a secondary somatic mutation following platinum exposure and that this second mutation confers drug resistance.[20] Similar findings have been observed following treatment of BRCA-deficient cell lines with PARP inhibitors, a novel class of anti-neoplastics that specifically exploit tumor deficiency in the homologous recombination DNA-repair pathway.[21] Other data from OC cell lines and mouse models suggest that expression of the drug efflux transporter ABCG2 markedly reduces the protein levels of Top1, the target of topoisomerase inhibitors, and that this results in resistance to the combination of topotecan and olaparib (a PARP inhibitor).[22] Other more traditional mechanisms of acquired drug resistance, such as drug efflux through cellular pumps or secondary non-mutation in non-BRCA genes, also likely play important roles in resistance to DNA-damaging agents.
In light of this preclinical data, the disappointing outcomes of the topotecan in BRCA+ OCs reported here suggest that the mechanisms which confer these tumors with resistance to platinums may also confer resistance to other drug classes that lead to similar double-stranded DNA-breaks. Despite this finding, these two groups of patients appeared to have similar overall survival. It remains unknown whether BRCA+ OC patients who were less heavily pretreated would benefit disproportionally from treatment with topotecan. . This hypothesis generating exercise suggests that therapies designed to exploit the DNA repair deficiencies of BRCA+ OCs may best used in less heavily pretreated patients whose tumors have not developed multiple resistant mechanisms.
Footnotes
Conflicts of Interest: NK has received consulting fees and has been an expert witness for Pfizer. No other authors have conflicts to report.
REFERENCES
- [1].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. l2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- [2].Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. l2005;104:2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- [3].Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–10. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365–72. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- [5].Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–89. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. l2002;346:1609–15. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- [7].Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. l2002;346:1616–22. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- [8].Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol. l2008;26:20–5. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- [9].Ben David Y, Chetrit A, Hirsh-Yechezkel G, et al. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol. 2002;20:463–6. doi: 10.1200/JCO.2002.20.2.463. [DOI] [PubMed] [Google Scholar]
- [10].Husain A, He G, Venkatraman ES, Spriggs DR. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II) Cancer Res. 1998;58:1120–3. [PubMed] [Google Scholar]
- [11].Farmer H, McCabe N, Lord CJ. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. l2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- [12].Tan DS, Rothermundt C, Thomas K, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26:5530–6. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- [13].Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127–32. doi: 10.1093/annonc/mdq577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. l2001;19:3312–22. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- [15].Herzog TJ, Sill MW, Walker JL, et al. A phase II study of two topotecan regimens evaluated in recurrent platinum-sensitive ovarian, fallopian tube or primary peritoneal cancer: A Gynecologic Oncology Group Study (GOG 146Q) Gynecol Oncol. 2011;120:454–8. doi: 10.1016/j.ygyno.2010.11.008. [DOI] [PubMed] [Google Scholar]
- [16].Sehouli J, Stengel D, Harter P, et al. Topotecan Weekly Versus Conventional 5-Day Schedule in Patients With Platinum-Resistant Ovarian Cancer: a randomized multicenter phase II trial of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol. 2011;29:242–8. doi: 10.1200/JCO.2009.27.8911. [DOI] [PubMed] [Google Scholar]
- [17].Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. l2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- [18].Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. l2010;17:421–33. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rustin GJ, Quinn M, Thigpen T, et al. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. l2004;96:487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- [20].Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. l2008;451:1116–20. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–5. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- [22].Zander SA, Kersbergen A, van der Burg E, et al. Sensitivity and acquired resistance of BRCA1;p53-deficient mouse mammary tumors to the topoisomerase I inhibitor topotecan. Cancer Res. 2010;70:1700–10. doi: 10.1158/0008-5472.CAN-09-3367. [DOI] [PubMed] [Google Scholar]