Abstract
Purpose
Development of chemoresistance, poor prognosis, and metastasis often renders the current treatments for colorectal cancer (CRC) ineffective. Whether ursolic acid (UA), a component of numerous medicinal plants, either alone or in combination with capecitabine, can inhibit the growth and metastasis of human CRC was investigated.
Experimental design
The effect of UA on proliferation of colorectal cancer cell lines was examined by mitochondrial dye-uptake assay, apoptosis by esterase staining, NF-κB activation by DNA binding assay and protein expression by western blot. The effect of UA on the growth and chemosensitization was also examined in orthotopically-implanted CRC in nude mice.
Results
We found that UA inhibited the proliferation of different colon cancer cell lines. This is correlated with inhibition of constitutive NF-κB activation and downregulation of cell survival (Bcl-xL, Bcl-2, cFLIP, survivin), proliferative (Cyclin D1), and metastatic (MMP-9, VEGF, ICAM-1) proteins. When examined in an orthotopic nude-mice model, UA significantly inhibited tumor volume, ascites formation and distant organ metastasis, and this effect was enhanced with capecitabine. Immunohistochemistry of tumor tissue indicated that UA downregulated biomarkers of proliferation (Ki-67) and microvessel density (CD31). This effect was accompanied by suppression of NF-κB, STAT3, and β-catenin. In addition, UA suppressed EGFR, and induced p53, and p21 expression. We also observed bioavailability of UA in the serum and tissue of animals.
Conclusion
Overall our results demonstrate that UA can inhibit the growth and metastasis of CRC and further enhance the therapeutic effects of capecitabine through suppression of multiple biomarkers linked to inflammation, proliferation, invasion, angiogenesis, and metastasis.
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer death among men ages 40 to 79 years (1), with an annual global mortality of greater than 639,000 persons. In the year 2010 in the United States alone, 72,090 men (9% of all cancers) and 70,480 women (10% of the all cancer) will be diagnosed with CRC. Deaths from CRC are expected to be 51,370 in 2010, up from 49,920 in 2009. Chemotherapy, radiotherapy, and surgery are the basic treatment methods used against CRC. However, long-term use of chemotherapy can make patients feel very sick and can also make their condition worse. Tumors also develop resistance to the chemotherapy over time.
Accumulating evidence suggests that the development and progression of many malignancies, including colorectal cancer, are associated with constitutive activation of multiple signaling pathways that promote proliferation, inhibit apoptosis and induce metastasis (2). NF-κB and STAT3 transcription factors, which primarily regulate inflammation, play a crucial role in regulating several pathways that affect tumor cell survival, angiogenesis, motility and invasiveness (3). Epidermal growth factor receptor (EGFR), one of the family of four erbB receptors, is found to be overexpressed in a variety of human tumors, including colorectal cancers (4). Aberrant activation of EGFR and the EGF signal pathway is associated with neoplastic cell proliferation, migration, stromal invasion, resistance to apoptosis and angiogenesis (5). Thus agents that can control these multiple signaling pathway have potential for use against CRC.
Although natural remedies have been claimed to have potential for the prevention or treatment of CRC, neither an active component nor a mechanism has been well characterized. Ursolic acid (UA), a pentacyclic triterpenoid, as a common active compound of numerous medicinal plants including holy basil (6) has been shown to exhibit anticancer potential in vitro and in vivo. It suppressed proliferation and induced apoptosis in cells of various cancers, including breast cancer (7), colon cancer (8), non-small cell lung cancer (9), cervical cancer (10), multiple myeloma (11), pancreatic cancer (12), melanoma (13), and prostate cancer (14). In animal models, UA inhibited tumorigenesis (15-17), suppressed tumor invasion (18), and inhibited metastasis of esophageal carcinoma (19). How UA acts as an anticancer agent is not yet clear. However, several reports have suggested that it inhibits DNA replication (20), induces Ca2+ release (21), activates caspases (13, 22) and JNK (14), downregulates antiapoptotic genes (23, 24), inhibits COX-2 and iNOS (25, 26), suppresses MMP-9 (18), and inhibits protein tyrosine kinase (27), STAT3 (11) and NF-κB activation (24). All these reports suggest that UA has potential against CRC.
In the current report, we investigated whether UA inhibits the growth and metastasis of human colon cancer in vitro and in an orthotopic mouse model of CRC. We found that UA significantly inhibited the growth and metastasis of CRC and further enhanced the effect of capecitabine through downregulation of multiple cell signaling pathways.
Materials and Methods
Materials
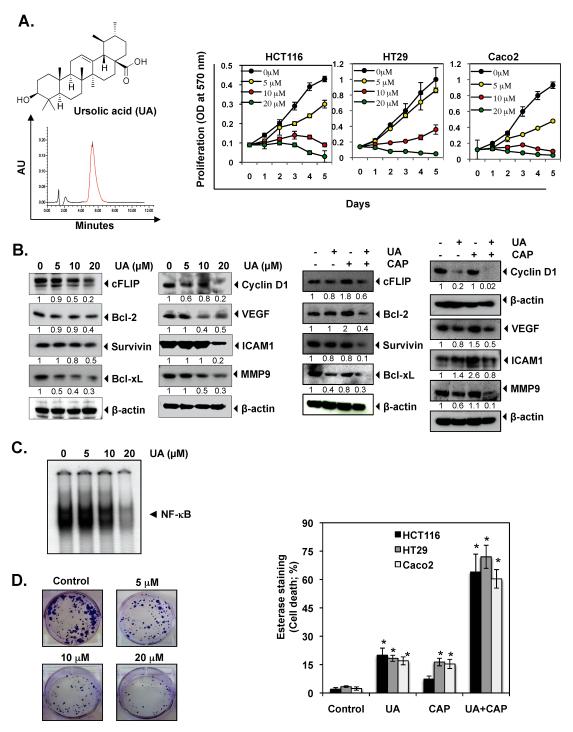
Ursolic acid (Fig. 1A) was kindly supplied by King Sing Guan (Haikou, China), and its purity was ascertained by HPLC (Fig 1A, left panel). Polyclonal antibodies against p65, ICAM-1, cyclin D1, MMP-9, survivin, and cIAP-1 and monoclonal antibodies against VEGF, c-myc, Bcl-2, and Bcl-xL were obtained from Santa Cruz Biotechnology. The liquid 3,3-diaminobenzidine + substrate chromogen system–horseradish peroxidase used for immunohistochemistry was obtained from DakoCytomation. Penicillin, streptomycin, DMEM/F12 medium, and fetal bovine serum were obtained from Invitrogen. All other chemicals were obtained from Sigma unless otherwise stated.
Figure 1.
UA inhibits the growth and proliferation of colorectal cancer (CRC) and enhances the apoptotic effects of capecitabine. (A) Chemical structure and HPLC profile of UA (left panel). HCT116, HT29, and Caco2 cells were pretreated with the indicated concentrations of UA for 1, 2, 3, 4, and 5 days. Cell viability was then analyzed by the MTT method as described under Materials and Methods (right panel). (B) HCT116 cells were pretreated with indicated concentration of UA for 24 h. Whole-cell extracts were prepared and subjected to western blotting (Left panel). HCT116 cells were pretreated with 20 μM of UA for 8 h and then exposed to capecitabine (20 μM) for 24 h. Whole-cell extracts were prepared and subjected to western blotting (Right panel). (C) HCT116 cells were treated with the indicated concentrations of UA for 8 h, and then nuclear extracts were prepared and assayed for NF-κB activation by EMSA. (D) HCT116 cells were treated with indicated concentration of UA for 12 h. The cells were then reseeded in 6 well plates and allowed to form colonies for 14 days, after which they were stained with crystal violet, as described in Materials and Methods (left panel). HCT116, HT29, and Caco2 cells were pretreated with 20 μM of UA for 8 h. The media were removed, and the cells were then exposed capecitabine for 24 h. Cell viability was then analyzed by the Live/Dead assay (right panel). * represents significant (Bonferroni Adj (for 3 comparisons) p < 0.05) over vehicle control within each cell line.
Cell lines
The human colon cancer cell lines HCT116, HT29, and Caco2 were obtained from American Type Culture Collection. HCT116 cells were cultured in DMEM, and HT29 and Caco2 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin.
Proliferation assay
The effect of UA on cell proliferation was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) uptake method as described previously (28). The absorbance was measured at 570 nm using an MRX Revelation 96-well multiscanner (Dynex Technologies).
Clonogenic assay
HCT116 cells (3,000 per well) were seeded in 12-well plates, incubated for 12 hours, and treated with different concentration of UA for 12 hours. The cells were then reseeded in six-well plates and allowed to form colonies for 14 days. Colonies were stained with 0.3% crystal violet solution for 20 minutes. The excess crystal violet solution was washed with distilled water to visualize the clonogenic potential of the cells.
Apoptosis assay
To determine whether UA can potentiate the apoptotic effects of capecitabine in colon cancer cells, we used a Live/Dead assay kit (Molecular Probes). In brief, cells (5,000 per well) were incubated in chamber slides and co-incubated with UA and capecitabine for 24 h. Cells were then stained with assay reagents for 30 min at ambient temperature. Cell viability was determined under a fluorescence microscope by counting live (green) and dead (red) cells.
Animals
Male athymic nu/nu mice (4 weeks old) were obtained from the breeding section of the Department of Experimental Radiation Oncology at The University of Texas MD Anderson Cancer Center. Three mice per cage were housed in standard acrylic glass mouse cages in a room maintained at constant temperature and humidity with a 12-hour light:dark cycle; mice were fed regular sterilized chow diet with water ad libitum. Our experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at MD Anderson Cancer Center.
Orthotopic implantation of HCT116 cells
HCT116 cells were stably transfected with luciferase as previously described (28). Luciferase-transfected HCT116 cells were harvested from subconfluent cultures after a brief exposure to 0.25% trypsin and 0.2% EDTA. Trypsinization was stopped with medium containing 10% FBS. The cells were washed once in serum-free medium and resuspended in PBS. Only suspensions consisting of single cells, with >90% viability, were used for the injections. Mice were anesthetized with ketamine-xylazine solution, a small left abdominal flank incision was made, and HCT116 cells (1.5 × 106) in 50 μL of PBS were injected into the cecum using a 30-gauge needle and a calibrated push button-controlled dispensing device (Hamilton Syringe Company). To prevent leakage, a cotton swab was held cautiously for 1 min over the site of injection. The abdominal wound was closed in one layer with wound clips (Braintree Scientific, Inc).
Experimental protocol
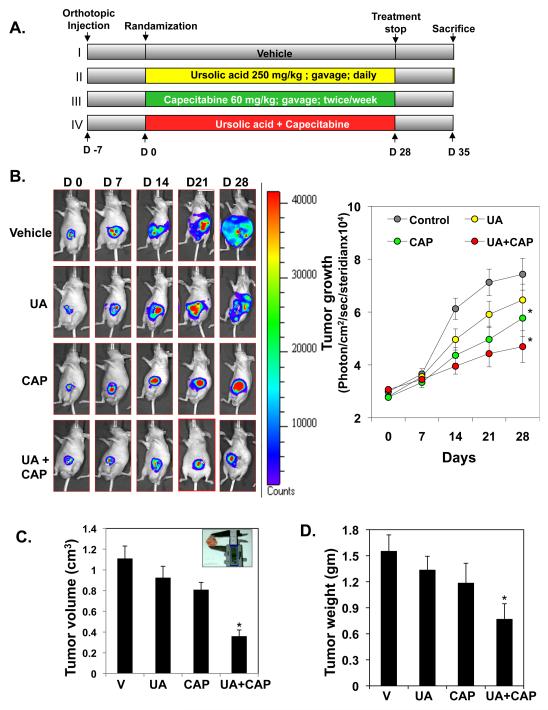
One week after implantation, mice were randomly assigned to the following treatment groups (six mice per group): (i) corn oil (vehicle) (100 μL daily); (ii) UA alone (250 mg/kg once daily, orally), (iii) capecitabine alone (60 mg/kg, twice weekly by gavage), and (iv) UA (250 mg/kg, once daily, orally) and capecitabine (60 mg/kg, twice weekly by gavage). In combination group, UA were given 6-8 hours before capecitabine. Tumor volumes were monitored weekly by the bioluminescence imaging system (IVIS) 200 and Living Image software (Caliper Life Sciences). Before imaging, animals were anesthetized in an acrylic chamber with 2.5% isofluorane/air mixture and injected intraperitoneally with D-luciferin potassium salt in PBS at a dose of 150 mg/kg body weight. After 10 min of incubation with luciferin, mice were placed in a right lateral decubitus position, and a digital gray-scale animal image was acquired, followed by acquisition and overlay of a pseudocolor image representing the spatial distribution of detected photons emerging from active luciferase within the animal. Signal intensity was quantified as the sum of all detected photons within the region of interest per second per steradian. Mice were imaged on days 0, 7, 14, 21, and 28 of treatment. Therapy was continued for 4 weeks, and the mice were sacrificed 1 week later. Primary tumors in the cecum were excised, and the final tumor volume was measured as V = 2/3 πr3, where r is the mean of the three dimensions (length, width, and depth). The number of metastasis colonies was counted in the liver, intestines, lungs, rectum, and spleen. Each tumor excised from mice was divided into three parts. The first part of the tumor tissue was formalin-fixed, second part fixed in OCT and third part was snap frozen in liquid nitrogen and stored at −80°C. Hematoxylin and eosin staining confirmed the presence of tumors in each cecum.
NF-κB activation in colon cancer cells
To assess NF-κB activation, we isolated nuclei from the colon cancer cell lines and performed the electrophoretic mobility shift assay (EMSA) essentially as previously described (29).
Immunolocalization of NF-κB p65, β-catenin, VEGF and MMP-9 in tumor samples
The nuclear localization of p65 and the expressions of MMP-9, and VEGF were examined using an immunohistochemical method described previously (28). Pictures were captured with a Photometrics CoolSNAP CF color camera (Nikon) and MetaMorph version 4.6.5 software (Universal Imaging).
Ki-67 immunohistochemistry
Frozen sections (5 μm) were stained with anti-Ki-67 (rabbit monoclonal clone SP6, NeoMarkers) antibody as previously described (28). Results for Ki-67+cells are shown as 40× magnification. A total of ten 40× fields were examined from three tumors of each of the treatment groups.
Microvessel density
Frozen sections (5 μm) were fixed in cold acetone and stained with rat anti-mouse CD31 monoclonal antibody (PharMingen) as previously described (28). The CD31 stained slides were observed under Leica DM4000B fluorescence microscope (Leica Microsystems, Inc.) equipped with a SPOT-RTKE digital camera, and the images were acquired and stored using SPOT advanced software (Diagnostic Instruments).
Protein extraction and western blot analysis
Colorectal tumor tissues (75–100 mg) from control and experimental mice were minced and incubated on ice for 30 min in 0.5 mL of ice-cold whole-cell lysis buffer. The minced tissue was homogenized using a Dounce homogenizer and centrifuged at 16,000g at 4 °C for 10 min. The proteins were then fractionated by SDS-PAGE, electrotransferred to nitrocellulose membranes, blotted with each antibody, and detected by enhanced chemiluminescence (Amersham Pharmacia Biotech).
High-performance liquid chromatography analysis
The extraction of UA was performed as described by Chen et al., (30). Frozen tissue (100 mg) was thawed, 400 μL of ethanol was added, and the mixture was homogenized for 30 sec. Next 500 μL of water was added, and the solution was homogenized for 15 sec. Then, 500 μL of hexane was added, and the solution was again homogenized for 15 sec. The samples were centrifuged at 5,000 rpm for 5 min at 4°C. The organic layer was evaporated to dryness under purified air at 40 °C. The pellets were reconstituted with mobile phase and estimated by HPLC (Waters Inc.). A silica-based C18 column and a mobile phase that was the mixture of acetonitrile:methanol:acetic acid (10%) in a ratio of 45:45:10 were used for separation. Analyses were conducted at a flow rate of 0.4 ml/min with the detector operating at a wavelength of 291 nm. Tissues from vehicle-treated mice were set as controls that reflect the base line.
Combination effects of UA and Capecitabine
Assessment of synergistic drug combination treatments between UA and capecitabine were evaluated using MTT assays on HCT116 cells. A total of 4.0 × 103 cells were plated in triplets and treated with UA alone (0.25xIC50, 0.5xIC50, 1xIC50,2xIC50,4xIC50), Capecitabine alone (0.25xIC50, 0.5xIC50, 1xIC50, 2xIC50, 4xIC50), and UA in combination with capecitabine at fixed ratio (Supplementary Table 1). After incubation, 5.0 mg/ml MTT reagent was added into each well, incubated for 2 h in the dark at 37°C and then MTT lysis buffer was added as described previously (28). The absorbance was measured at 570 nm using an MRX Revelation 96-well multiscanner (Dynex Technologies).
Statistical analysis
For the effect of UA on cell proliferation over time, we modeled the percentage of viable cells using linear mixed effects models with fixed effects as dose, day of measurement, and their interaction and (replicated) cell growth as random effects. In cases where the fixed effects were statistically significant, pair wise comparisons between doses (within each time point) were conducted by comparing the least square means. Multiple comparison adjustments were made using the Bonferroni’s method. Similarly, we examined the effect of UA and CAP on tumor growth of nude mice over time using a linear mixed models with fixed effects as UA or CAP, day of measurement, and interactions between treatment and day (other 2-way and 3-way interactions were not significant), along with random effect of mice nested within treatment group. Repeated measurements over time were modeled using an (first order) autoregressive covariance structure.
To model the effects of UA and CAP on apoptosis, metastatic score and tumor weight of nude mice, we first modeled the outcomes using linear models with fixed effects as UA and CAP, and their interaction. Pairwise comparisons were conducted as above with multiple comparison adjustments made using the Bonferroni’s method. When multiple cell lines were tested, we examined each cell line separately.
For all linear models, we examined the normality assumption of the residuals using Q-Q plots and used transformation to approximate normality where this assumption was violated (e.g. for tumor weight we used square-root transformation). We computed the incidence rate of ascite with exact 95% confidence interval. The differences in ascite incidence rate among treatment groups were compared using Fisher’s exact test.
Assessment of the type of drug interactions was done using Chou and Talalay’s method. Assuming that a dose-effect curve follows Chou and Talalay’s median effect equation, where d is the dose of a drug, Dm is the median effective dose of the drug, and m is a slope parameter. The above equation can be rewritten as . We regress on log d to get a marginal dose-effect curve. Then the dose level to achieve certain effect can be estimated for each of the three regimens (drug 1 alone, drug 2 alone, and the combination of drugs 1 and 2 with constant relative potency between them). Suppose that combination dose (d1,d2) elicits the same effect y as drug 1 alone at dose Dy,1, and drug 2 alone at dose Dy,2, then the interaction index (II) as defined by the Loewe additivity model [31], , which measures the magnitude of drug interaction. The value of II is estimated by the above equation and the corresponding 95% confidence interval is computed using the delta method [32] to calculate the variance of II. The value of II < 1, II =1, and II >1 correspond to the drug interactions’ being synergistic, additive, and antagonistic, respectively. Statistical software SAS 9.1.3 (SAS, Cary, NC) and S-Plus 8.0 (TIBCO Software Inc., Palto Alto, CA) were used for all the analyses.
Results
Our goal in the current study was to determine whether UA can affect the growth and metastasis of CRC and enhance the effect of capecitabine, which is used routinely to treat CRC, and if so, to determine the mechanism by which UA manifests its effects in vitro and in vivo against human CRC. We used three different well-characterized human colon cancer cell lines (HCT116, HT29, and Caco2) that exhibit distinct characteristics. To facilitate the monitoring of tumor growth in animals, HCT116 was stably transfected with the luciferase reporter gene and used in an orthotopic transplant model in mice.
UA inhibits the proliferation of CRC cells in vitro
We first determined whether UA inhibits the proliferation of human CRC cells. We used one cell line with mutant K-ras (HCT116) and two cell lines with wild-type K-ras (HT29 and Caco2). We found that UA significantly (p<0.001) inhibited cell proliferation in all three-cell lines in a dose- and time-dependent manner. HT29 and Caco2 cells were found to be the more sensitive than HCT116 cells as indicated by their IC50 value (HT29 – 8.7 μM, Caco2 – 6.2 μM and HCT116 – 9.8 μM) (Fig. 1A, right panel).
UA alone and in combination with capecitabine downregulates the expression of proteins linked to survival, proliferation, and metastasis in CRC cells
We also examined whether UA alone or in combination with capecitabine can downregulate the expression of proteins associated with survival, proliferation, invasion, and metastasis. Our results showed that UA inhibited the expression of CRC survival (c-FLIP, Bcl-2, survivin, Bcl-xL), proliferation (cyclin D1), and metastasis (MMP-9, VEGF, ICAM-1) proteins in dose dependent manner (Fig. 1B, left panel). When UA treated in combination with capecitabine the effect was prominent (Fig. 1B, right panel). However, induction of cFLIP, Bcl-2, VEGF and ICAM-1 was observed by capecitabine alone treatment.
UA inhibits the constitutive action of NF-κB in CRC cells
Because NF-κB activation has been closely associated with proliferation of tumor cells (33) and CRC is known to express constitutively active NF-κB, we investigated whether UA inhibits this transcription factor in colon cancer cells. Therefore, we performed the DNA-binding assay to determine whether UA inhibits constitutive NF-κB activation. We found that UA inhibited NF-κB activation in a dose-dependent manner (Fig. 1C).
UA suppresses colony formation
Next, we determined whether UA could affect the long-term colony formation assay. We found that UA treatment significantly suppressed the colony-forming ability of HCT116 tumor cells in dose dependent manner (Fig 1D, left panel).
UA sensitizes CRC cells to apoptosis by capecitabine
We next investigated whether UA can enhance the effect of capecitabine-induced apoptosis in all three-cell lines. For this, we performed the Live/Dead apoptosis assay. Our linear models showed that there was significant interaction between the two drugs for all three-cell lines. The UA significantly enhance capecitabine-induced apoptosis on all three-cell lines (p-value for interaction term <0.001) (Fig. 1D, right panel). These observations together suggested that UA has potential in the treatment of CRC. Therefore we designed in vivo studies.
UA synergistically enhances capecitabine-induced cytotoxicity
Based on MTT assay Chou and Talalay’s median effect results, it was found that UA was able to reduce the viability of HCT116 cells to a higher extent when used in combination with capecitabine. Drug interaction index (II) analysis indicates synergistic effects for all combinations where UA and capecitabine were used equal to or higher than IC50 concentration. However, additive effects were observed where UA and capecitabine were used less than IC50 concentration (Supplementary Table 1).
UA inhibits the growth of human CRC in an orthotopic nude mouse model
On the basis of these results, we decided to determine whether UA affects the growth of CRC, alone and in combination with capecitabine. For this, we used orthotopically implanted human HCT116 cancer cells in nude mice. The experimental protocol is depicted in Figure 2A. Luciferase-transfected HCT116 cells were implanted in the colorectal part of nude mice. After 1 week, on the basis of the initial IVIS image, we randomly assigned the mice to four groups (six mice per group) and began the treatment as per the experimental protocol. The treatment was continued for 4 weeks, and the mice were sacrificed 6 weeks after tumor cell injection. The IVIS imaging was performed weekly after tumor implantation (Fig. 2B, left panel). Fig. 2B, right panel shows the bioluminescence imaging of tumor volume over time. Results showed a gradual increase in tumor volume in the control group (Fig. 2B, right panel) compared with the remaining groups. Overall, there was significant day and treatment-by-day effect (Type 3 test p<0.0001). However, the differences in IVIS imaging were not significant on day 0 and day 7. On day 14, 21, and 28, the differences between the control and the CAP group and between the control and UA + CAP group were significant. Fig. 2C showed the effect of capecitabine and UA on tumor volume measured on the last day of the experiment at autopsy. Capecitabine significantly reduced tumor volume (p=0.0335). There was a trend that UA also reduced tumor volume, but the effect was not statistically significant (p=0.11). Tumor volume of the mice treated with UA plus capecitabine was significantly smaller than that of the vehicle control group. The linear model showed that CAP significantly decreases tumor weight (p=0.021). The effect of UA is not statistically significant (p=0.12), possibly due to small sample size. Compared to the vehicle control, tumor weight of the combination treatment was significantly smaller (Bonferroni adjusted (for 3 comparisons) p=0.027) (Fig. 2D).
Figure 2.
UA enhances the ability of capecitabine to inhibit the growth of orthotopically implanted CRC tumors in nude mice. (A) Schematic representation of the experimental protocol described in Materials and Methods (n = 6). (B) Bioluminescence imaging of orthotopically implanted CRC in live, anesthetized mice (left panel) every week, showing measurements (photons/sec) of tumor volume at various time points using live bioluminescence imaging (BLI) at the indicated times (right panel) * indicates significant over control at each time points (Bonferroni Adj (for a total of 15 comparisons) p<0.05). (C) Tumor volumes measured on the last day of the experiment at autopsy using Vernier calipers and calculated using the formula V = 2/3 πr3 (n = 6). * represents significant (Bonferroni Adj (for 3 comparisons) p < 0.05) over vehicle control. (D) Average tumor weight of each group weighed on the last day of the experiment. * represents significant (Bonferroni Adj (for 3 comparisons) p < 0.05) over vehicle control.
UA inhibits distant organ metastasis and ascites
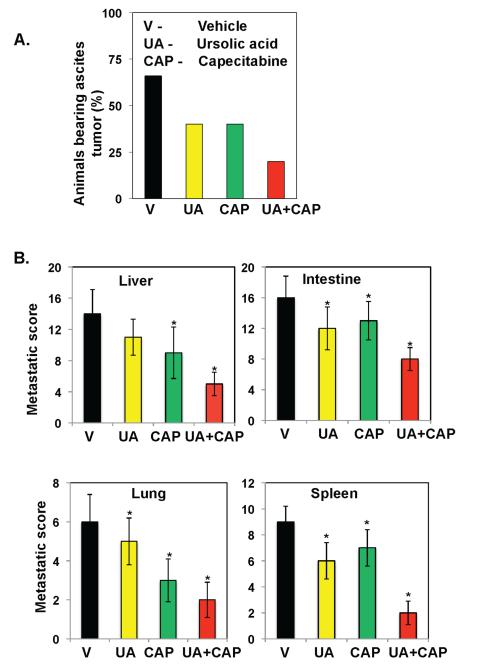
We also evaluated whether UA alone or in combination with capecitabine can inhibit ascites formation and distant organ metastasis in nude mice. We monitored the animals every day for ascites tumors by observing the increase in belly size. Vehicle-treated mice developed ascites tumors 27 days after tumor cell implantation, and the presence of ascites tumor was confirmed at the time of death. There were no statistically significant differences in ascite incidence rate among treatment groups (Fisher’s exact test p=0.57) due to small sample size (Fig. 3A). On day 42, mice were sacrificed and examined for the presence of metastases. The examination showed that colon cancer metastases developed in the liver, intestines, lungs, and spleen of vehicle-treated mice. UA alone inhibited metastasis to some extent, as did capecitabine in most organs; however, when they were used in combination, the inhibition of metastasis was maximal in these mice (Fig. 3B).
Figure 3.
UA potentiates capecitabine to inhibit ascites and distant organ metastasis in orthotopic CRC in nude mice. (A) UA combined with capecitabine inhibited the development of ascites in CRC. The number of mice with ascites was counted on the day of experiment termination, and the percentages of mice with ascites tumors were plotted. (B) Combined UA and capecitabine inhibited metastasis to the liver, intestines, lungs, and spleen. Mice were sacrificed, and abdomens were opened surgically. The number of metastatic foci in each organ was then counted. * represents significant (Bonferroni Adj (for 3 comparisons) p < 0.05) over vehicle control (V, vehicle; UA, ursolic acid; CAP, capecitabine).
UA is bioavailable in serum and CRC tumor tissue
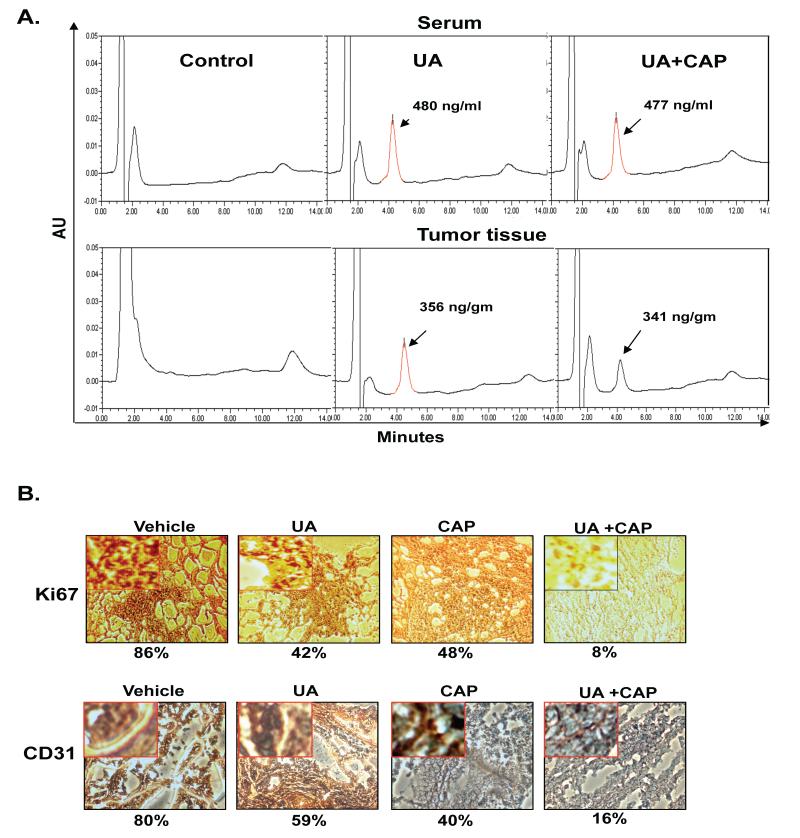
Because UA inhibited tumorigenesis in nude mice, we therefore investigated whether UA is bioavailable in the serum and the CRC tumor of the mice. HPLC results showed that a significant amount of UA was available in the serum of the mice treated with UA alone (480 ng/ml) or with UA plus capecitabine (477 ng/ml). The presence of capecitabine did not affect the level of UA concentrations in serum. UA was also found in CRC tumors (Fig. 4A). Although the amount of UA in CRC tumor was lesser than that in the serum of mice but it was comparable. Purified UA was used as a control.
Figure 4.
(A) UA is bioavailable in the serum of nude mice and in CRC tumors. Animals were fed with UA 4 h before being sacrificed. Mice were anesthetized and blood was collected by cardiac puncture, and then the mice were sacrificed humanely. HPLC analysis was performed to determine UA bioavailability in the serum and CRC tumors. (B) Immunohistochemical analysis of the proliferation marker Ki-67 and CD31 microvessel density in tumor tissue. Percentages indicate positive staining for the given biomarker.
UA inhibits CD31 and Ki-67 expression
The Ki-67-positive index is used as a marker for cell proliferation, and the CD31 index is a marker for microvessel density. We examined whether UA and capecitabine can modulate these biomarkers. We found that both UA alone and capecitabine alone downregulated the expression of Ki-67 in CRC tissue and the combination of the two was more effective (Fig. 4B). Similarly, we found that both agents alone reduced CD31 expression, compared with the control group, and the combined UA plus capecitabine was the most effective (Fig. 4B). The differences in expression of Ki-67 and CD31 between each of the three active treatments and the vehicle control were statistically significant (Bonferroni adjusted p-value <0.007).
UA inhibits NF-κB in CRC tumors
NF-κB is known to be overexpressed in CRC tumors (34). Therefore, we determined whether UA could inhibit activated NF-κB in the CRC tumors. We found that UA inhibited activated NF-κB in these tissues and that combined UA plus capecitabine was more effective (Fig. 5A). The differences in expression of NF-κ between each of the three active treatments and the vehicle control were statistically significant (Bonferroni adjusted (for all 6 pair-wise comparisons) p-value <0.001).
Figure 5.
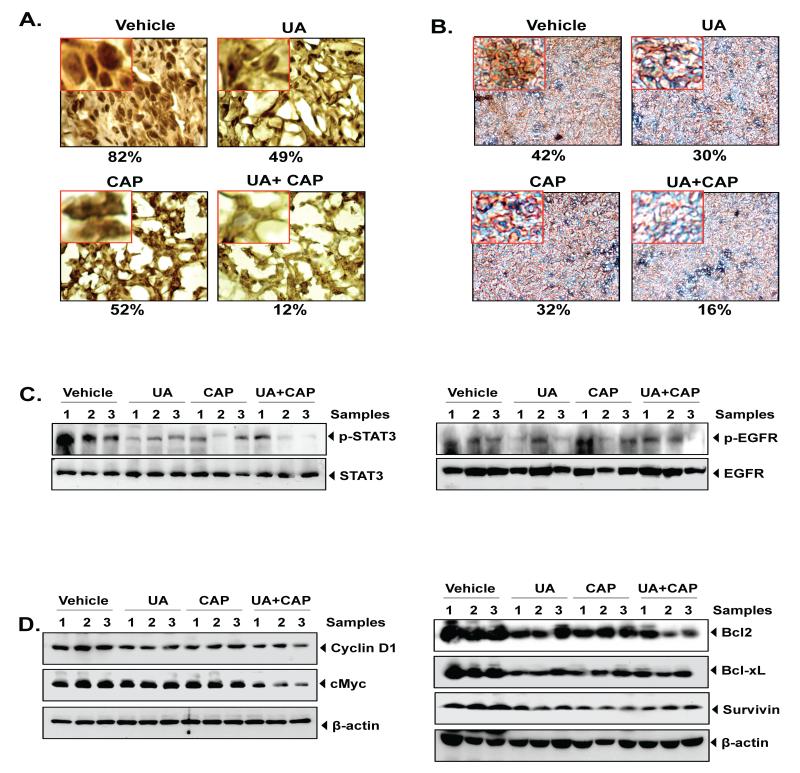
UA enhances the ability of capecitabine to inhibit the expression of NF-κB and genes linked to proliferation, invasion, angiogenesis, and metastasis of CRC tumors. Immunohistochemical analysis of (A) nuclear p65 and (B) β-catenin in tumor tissues. Percentages indicate positive staining for the given biomarker. (C) Western blot analysis of phospho-STAT3 (left panel) and EGFR phosphorylation (right panel). (D) Western blot analysis of proteins involves in proliferation (Cyclin D1, c-myc), cell survival (Bcl-2, Bcl-xL and survivin). Three samples were taken from each group.
UA inhibits β-catenin expression in CRC tumors
Overexpression of nuclear β-catenin in colorectal cancer is known and found to strongly associated with metastasis (35). Therefore, we investigated to determine whether UA inhibits the expression of β-catenin in tumors. We observed that treatment of UA resulted in suppression of β-catenin. The combination of UA and CAP treatment results in greater inhibition of β-catenin than each agent alone Bonferroni adjusted (for all 6 pair-wise comparisons) p-value <0.01) (Fig. 5B).
UA suppressed the activation of transcription factor STAT3
Next we examined whether UA modulates the activation of STAT3 overexpression because STAT3 is found to constitutively active (36) in CRC tumors. We found that UA and capecitabine alone moderately suppressed the activated STAT3 by inhibiting phosphorylation at Tyr705, however UA in combination with capecitabine markedly inhibited its phosphorylation (Fig 5C, left panel). Thus, inhibition of this transcription factor by UA could be associated with enhancement of capecitabine-induced CRC tumor regression.
UA suppressed the activation of epidermal growth factor receptor (EGFR)
Most of CRC is characterized with overexpression of EGFR and predicted with high risk of metastasis and recurrence (37). Targeting EGFR seems to be a promising approach for the CRC treatment. In present experiment, moderate inhibition of EGFR activation was observed by either UA alone or capecitabine alone or by combination (Fig 5C, right panel).
UA inhibits the expression of various biomarkers
We also examined the expression of various biomarkers in CRC tumor samples by western blot (Fig. 5D). The results showed that UA alone or capecitabine suppressed the expression of cyclin D1, c-myc (involved in proliferation), Bcl-2, Bcl-xL, and survivin (involved in cell survival). UA in combination with capecitabine inhibited proteins like cyclin D1, c-myc, and Bcl-2 more profoundly than either drug alone (Fig. 5D). However, inhibition of survivin and Bcl-xL was not prominent in combination compared to either UA alone or capecitabine alone.
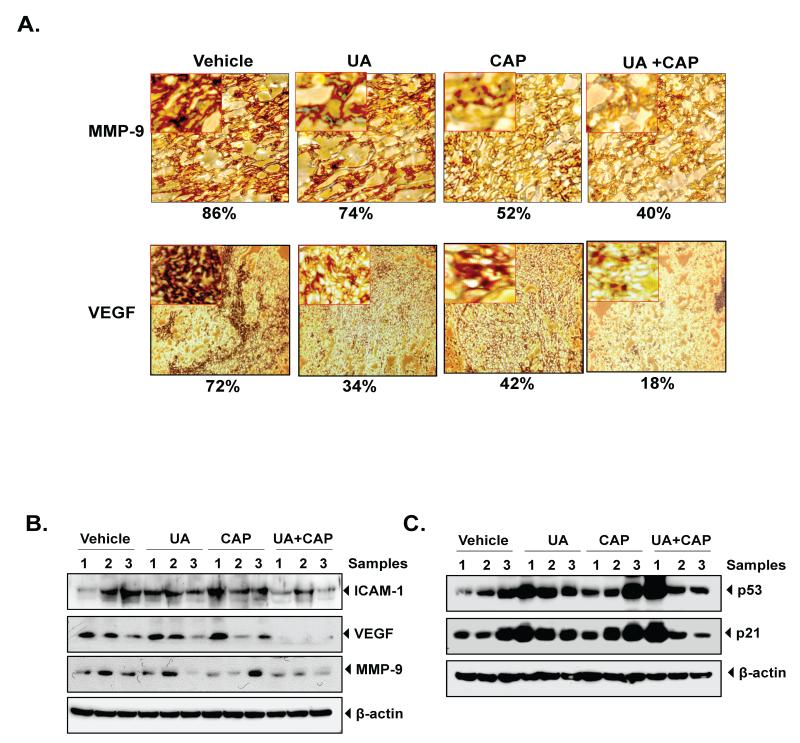
Immunohistochemical analysis showed reductions in the expression of MMP-9 and VEGF (involved in invasion and metastasis) in tumors from the UA alone group (Bonferroni adjusted p-value =0.053) and those from the capecitabine-alone group (Bonferroni adjusted p-value <0.001) compared with the control group (Fig. 6A). Results also indicated that the combination of UA and capecitabine was more effective than treatment with either UA alone (Bonferroni adjusted (for all 6 pair-wise comparisons) p-value <0.001) or CAP alone (Bonferroni adjusted p-value =0.053). Western blotting results also confirmed that expression of proteins involved in invasion and metastasis like MMP-9, VEGF and ICAM-1 decreased by either drug compared with the control treatment. However, in combination with capecitabine, decrease of MMP-9, VEGF and ICAM-1 expression by UA was more prominent compared with individual in CRC tumor tissues (Fig 6B).
Figure 6.
(A) UA enhances the effect of capecitabine against the expression of MMP-9, and VEGF in CRC samples analyzed by immunohistochemistry. Samples from three animals in each group were analyzed, and representative data are shown. Percentages indicate positive staining for the given biomarker. (B) Western blot analysis of proteins involves in invasion, angiogenesis and (ICAM-1, VEGF and MMP-9). (C) UA induced the expression of tumor suppressor p53 and p21. Tissue extracts were subjected to western blotting using respective antibodies. Samples from three animals in each group were analyzed, and representative data are shown.
UA upregulates tumor suppressor proteins
The activation of oncogenes and inactivation of tumor suppressor genes have been implicated in the development of many human and animal malignancies. Therefore, we determined whether UA modulated p53. We found that p53 and its downstream target p21 was upregulated by either UA or capecitabine treatment. Combination of UA and capecitabine enhance the level of p53 and p21 (Fig 6C).
Discussion
Although capecitabine as a chemotherapeutic drug is frequently used in patients with stage III CRC after surgery to remove their tumors, this agent has limited in both efficacy and has side effects (38). Therefore, in this study, we investigated whether ursolic acid derived from various fruits and vegetables has potential either alone or in combination with capecitabine in the treatment of CRC. Whether examined in cell culture or in animal model, we found that UA alone inhibited the growth of CRC and enhanced the activity of capecitabine. When examined for the mechanism, we found that UA inhibited several transcription factors such as NF-κB, STAT3, and β-catenin activation and various biomarkers linked to survival, proliferation, and metastasis. We also observed that UA potentiated capecitabine-induced cell death.
This is the first report to show that UA can suppress CRC growth and enhances the effect of capecitabine in an orthotopic mouse model. Whether UA affects the concentration of capecitabine or its analog is not known at present. However, UA is known to inhibit cytochrome P450 1A2 activities in human liver microsomes (39). Although one study reported that UA enhanced the post-irradiation responses to gamma radiation and decreased undesirable radiation damage to hematopoietic tissue after radiotherapy (9), this study did not describe the mechanism of action. In our study, the inhibition of tumor growth by UA alone or in combination with capecitabine appeared to be due to suppression of multiple biomarkers. UA inhibited proliferative proteins cyclin D1 and Ki-67, indicating one of the probable mechanisms of the anti-tumorigenic potential of UA. EGFR overexpression has also been shown in some of the CRC tumor cells (40), which was downregulated by UA in present study. Previously Shan et al (41) showed that UA suppressed the phosphorylation of EGFR, ERK1/2, p38 MAPK, and JNK in colorectal cancer cells, which was correlated with its growth inhibitory effect.
We also found that UA alone can inhibit CRC metastasis to different organs and also suppress CRC-induced ascites. The inhibition of metastasis could be due to the suppression of MMP-9 and VEGF. UA indeed decreased angiogenesis, as indicated by inhibition of CD31, a marker for microvessel density, and VEGF. It has been reported earlier that UA inhibited tumor-associated capillary formation by reducing VEGF, and proinflammatory cytokines in C57BL/6 mice induced by highly metastatic B16F-10 melanoma cells (42). UA can also downregulate MMP-9, which is in agreement with previous reports (18). Inhibition of metastasis by UA may also be due to CXCR4, a chemokine receptor closely linked to metastasis and which has been reported to be downregulated by UA (43).
Our finding that UA downregulated expressions of MMP-9 and ICAM-1, which are involved in tumor invasion, were significantly suppressed in tumor tissues from UA-treated mice. It has been shown that MMP-9 and ICAM-1 are overexpressed in CRC patients (44, 45), and it has also been observed that negative MMP-9 expression levels correlate with longer survival time in CRC patients (44). Finally, our results showed that, in CRC, UA inhibited several antiapoptotic proteins regulated by NF-κB, including survivin, Bcl-2, Bcl-xL and cIAP-1. These results suggest that UA enhances the apoptotic effect of capecitabine by inhibiting antiapoptotic proteins in CRC cells.
Overall, our results suggest that UA has significant potential for the treatment of CRC and it can further enhance the effects of capecitabine by inhibiting NF-κB and associated biomarkers that are involved in proliferation, angiogenesis, invasion, and metastasis. On the basis of these results, further studies are required in patients to explore the potential of UA as an anticancer agent.
Supplementary Material
Translational Relevance.
Despite advances in earlier detection and therapy, colorectal cancer (CRC) is the leading cause of death from gastrointestinal malignancy. Existing chemotherapeutic regimens are associated with lack of efficacy and yet are highly toxic. Long-term use of chemotherapeutic drugs also resulted into development of tumor resistance overtime. Thus the agents, which can overcome the resistance and can enhance the effect of chemotherapeutic drugs, are urgently needed. Through in vitro and in vivo experiments, we demonstrate for the first time that ursolic acid (UA), an active compound present in numerous medicinal plants including holy basil, can potentiate the effect of capecitabine, a standard drug, against colorectal cancer. Our preclinical findings show that UA is effective in inhibition of tumor growth and metastasis and that this effect is further enhanced when combined with capecitabine. This antitumor effect of UA was mediated through multiple pathways linked to inflammation, proliferation, invasion, angiogenesis, and metastasis.
Acknowledgements
We thank Michael Worley and the Department of Scientific Publications for carefully editing the manuscript and Dr. Juri G. Gelovani, Department of Experimental Diagnostic Imaging, for providing bioluminescence imaging system (IVIS) 200 facility. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from Center for Targeted Therapy of MD Anderson Cancer Center. Veera Baladandayuthapani’s and Caimiao Wei’s work is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Prakobwong S, Gupta SC, Kim JH, Sung B, Pinlaor P, Hiraku Y, et al. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis. 2011;32:1372–80. doi: 10.1093/carcin/bgr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 4.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 5.Dancey JE, Freidlin B. Targeting epidermal growth factor receptor--are we missing the mark? Lancet. 2003;362:62–4. doi: 10.1016/S0140-6736(03)13810-X. [DOI] [PubMed] [Google Scholar]
- 6.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 7.Es-saady D, Simon A, Ollier M, Maurizis JC, Chulia AJ, Delage C. Inhibitory effect of ursolic acid on B16 proliferation through cell cycle arrest. Cancer Lett. 1996;106:193–7. doi: 10.1016/0304-3835(96)04312-1. [DOI] [PubMed] [Google Scholar]
- 8.Andersson D, Liu JJ, Nilsson A, Duan RD. Ursolic acid inhibits proliferation and stimulates apoptosis in HT29 cells following activation of alkaline sphingomyelinase. Anticancer Res. 2003;23:3317–22. [PubMed] [Google Scholar]
- 9.Hsu HY, Yang JJ, Lin CC. Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett. 1997;111:7–13. doi: 10.1016/s0304-3835(96)04481-3. [DOI] [PubMed] [Google Scholar]
- 10.Yim EK, Lee MJ, Lee KH, Um SJ, Park JS. Antiproliferative and antiviral mechanisms of ursolic acid and dexamethasone in cervical carcinoma cell lines. Int J Gynecol Cancer. 2006;16:2023–31. doi: 10.1111/j.1525-1438.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 11.Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–55. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 12.Chadalapaka G, Jutooru I, McAlees A, Stefanac T, Safe S. Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg Med Chem Lett. 2008;18:2633–9. doi: 10.1016/j.bmcl.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmand PO, Duval R, Delage C, Simon A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int J Cancer. 2005;114:1–11. doi: 10.1002/ijc.20588. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Kong C, Zeng Y, Wang L, Li Z, Wang H, et al. Ursolic acid induces PC-3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro. Mol Carcinog. 2010;49:374–85. doi: 10.1002/mc.20610. [DOI] [PubMed] [Google Scholar]
- 15.Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104:43–8. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- 16.Gayathri R, Priya DK, Gunassekaran GR, Sakthisekaran D. Ursolic acid attenuates oxidative stress-mediated hepatocellular carcinoma induction by diethylnitrosamine in male Wistar rats. Asian Pac J Cancer Prev. 2009;10:933–8. [PubMed] [Google Scholar]
- 17.Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, et al. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–8. [PubMed] [Google Scholar]
- 18.Cha HJ, Park MT, Chung HY, Kim ND, Sato H, Seiki M, et al. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene. 1998;16:771–8. doi: 10.1038/sj.onc.1201587. [DOI] [PubMed] [Google Scholar]
- 19.Yamai H, Sawada N, Yoshida T, Seike J, Takizawa H, Kenzaki K, et al. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int J Cancer. 2009;125:952–60. doi: 10.1002/ijc.24433. [DOI] [PubMed] [Google Scholar]
- 20.Kim DK, Baek JH, Kang CM, Yoo MA, Sung JW, Chung HY, et al. Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication. Int J Cancer. 2000;87:629–36. [PubMed] [Google Scholar]
- 21.Baek JH, Lee YS, Kang CM, Kim JA, Kwon KS, Son HC, et al. Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells. Int J Cancer. 1997;73:725–8. doi: 10.1002/(sici)1097-0215(19971127)73:5<725::aid-ijc19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Choi YH, Baek JH, Yoo MA, Chung HY, Kim ND, Kim KW. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int J Oncol. 2000;17:565–71. [PubMed] [Google Scholar]
- 23.Kassi E, Sourlingas TG, Spiliotaki M, Papoutsi Z, Pratsinis H, Aligiannis N, et al. Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Invest. 2009;27:723–33. doi: 10.1080/07357900802672712. [DOI] [PubMed] [Google Scholar]
- 24.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–83. [PubMed] [Google Scholar]
- 25.Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 2000;60:2399–404. [PubMed] [Google Scholar]
- 26.Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998;58:717–23. [PubMed] [Google Scholar]
- 27.Hollosy F, Meszaros G, Bokonyi G, Idei M, Seprödi A, Szende B, et al. Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumor cells. Anticancer Res. 2000;20:4563–70. [PubMed] [Google Scholar]
- 28.Kunnumakkara AB, Diagaradjane P, Anand P, Harikumar KB, Deorukhkar A, Gelovani J, et al. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int J Cancer. 2009;125:2187–97. doi: 10.1002/ijc.24593. [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi MM, LaPushin R, Aggarwal BB. Tumor necrosis factor and lymphotoxin. Qualitative and quantitative differences in the mediation of early and late cellular response. J Biol Chem. 1994;269:14575–83. [PubMed] [Google Scholar]
- 30.Chen Q, Luo S, Zhang Y, Chen Z. Development of a liquid chromatography-mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: application to the pharmacokinetic and tissue distribution study. Anal Bioanal Chem. 2011;399:2877–84. doi: 10.1007/s00216-011-4651-x. [DOI] [PubMed] [Google Scholar]
- 31.Lee JJ, Kong M, Ayers GD, Lotan R. Interaction index and different methods for determining drug interaction in combination therapy. J Biopharm Stat. 2007;17:461–80. doi: 10.1080/10543400701199593. [DOI] [PubMed] [Google Scholar]
- 32.Oehlert GW. A Note on the Delta Method. Ame Stat. 1992;46:27–9. [Google Scholar]
- 33.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30:1615–30. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, et al. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–9. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Cheng H, Liu Y, Wang L, Yu W, Zhang G, et al. Prognostic value of nuclear beta-catenin overexpression at invasive front in colorectal cancer for synchronous liver metastasis. Ann Surg Oncol. 2011;18:1553–9. doi: 10.1245/s10434-010-1519-9. [DOI] [PubMed] [Google Scholar]
- 36.Ma XT, Wang S, Ye YJ, Du RY, Cui ZR, Somsouk M. Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma. World J Gastroenterol. 2004;10:1569–73. doi: 10.3748/wjg.v10.i11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 38.Disel U, Gurkut O, Abali H, Kaleağasi H, Mertsoylu H, Ozyilkan O, et al. Unilateral hand-foot syndrome: an extraordinary side effect of capecitabine. Cutan Ocul Toxicol. 2010;29:140–2. doi: 10.3109/15569521003699585. [DOI] [PubMed] [Google Scholar]
- 39.Kim KA, Lee JS, Park HJ, Kim JW, Kim CJ, Shim IS, et al. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004;74:2769–79. doi: 10.1016/j.lfs.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Guo GF, Cai YC, Zhang B, Xu RH, Qiu HJ, Xia LP, et al. Overexpression of SGLT1 and EGFR in colorectal cancer showing a correlation with the prognosis. Med Oncol. 2011;28(Suppl 1):S197–203. doi: 10.1007/s12032-010-9696-8. [DOI] [PubMed] [Google Scholar]
- 41.Shan JZ, Xuan YY, Zheng S, Dong Q, Zhang SZ. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J Zhejiang Univ Sci B. 2009;10:668–74. doi: 10.1631/jzus.B0920149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanjoormana M, Kuttan G. Antiangiogenic activity of ursolic acid. Integr Cancer Ther. 2010;9:224–35. doi: 10.1177/1534735410367647. [DOI] [PubMed] [Google Scholar]
- 43.Shanmugam MK, Manu KA, Ong TH, Ramachandran L, Surana R, Bist P, et al. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int J Cancer. 2011;129:1552–63. doi: 10.1002/ijc.26120. [DOI] [PubMed] [Google Scholar]
- 44.Bendardaf R, Buhmeida A, Hilska M, Laato M, Syrjänen S, Syrjänen K, et al. MMP-9 (gelatinase B) expression is associated with disease-free survival and disease-specific survival in colorectal cancer patients. Cancer Invest. 2010;28:38–43. doi: 10.3109/07357900802672761. [DOI] [PubMed] [Google Scholar]
- 45.Maurer CA, Friess H, Kretschmann B, Wildi S, Müller C, Graber H, et al. Over-expression of ICAM-1, VCAM-1 and ELAM-1 might influence tumor progression in colorectal cancer. Int J Cancer. 1998;79:76–81. doi: 10.1002/(sici)1097-0215(19980220)79:1<76::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.