Abstract
Pericardial effusion is an independent predictor of mortality in patients with pulmonary arterial hypertension (PAH). However, the management and outcomes of patients with pulmonary hypertension and pericardial effusion are not well described.
A retrospective observational study was conducted at Baylor College of Medicine and The Methodist Hospital by screening all patients admitted between June 1, 2005 and June 1, 2010 with the ICD-9 codes for pulmonary hypertension and pericardial effusion. 138 patients with pericardial effusion were identified, and 103 patients were excluded if they had valvular heart disease, recent surgery or end stage renal disease. Thirty-five patients with PH diagnosed by a historical right heart catheterization or echocardiography and with documented pericardial effusion were included in this analysis. Demographic, hemodynamic, laboratory and survival data was collected.
The mean age was 49.5±36 years (mean ± SD), 31 of 35 patients were females (93%) and pulmonary artery systolic pressure was 77±19 mm Hg. Mean follow-up period was 20.5±12.9 months. Fifteen patients had PAH associated with connective tissue disease (50%). Majority of the patients (87%) with pericardial effusion were managed conservatively. Four patients (13%) who were hemodynamically unstable underwent pericardial window placement. One of them was started on epoprostenol and two patients had the doses of PAH-specific medications uptitrated. Three of four pericardial window patients survived to the conclusion of the follow-up period. The overall survival in our cohort was 60% with three patients lost to follow-up.
Connective tissue disease-associated PAH and female gender were predominant in our cohort of patients with pericardial effusion. Seventy-five percent of patients who were treated with pericardial window for hemodynamically unstable pericardial effusion survived till the end of the study period. Pericardial window may be a therapeutic option in unstable PH patients with pericardial effusion. Further studies are needed to determine the optimal treatment strategy for such patients.
Keywords: pulmonary arterial hypertension, pericardial effusion, right heart failure, echocardiogram
Background
Pericardial effusion in patients with pulmonary arterial hypertension (PAH) has been identified as an independent mortality risk factor with a hazard ratio of 1.35(2). PAH due to connective tissue disease such as scleroderma with pericardial effusion may confer additional risk(2, 3, 4) Data from the REVEAL registry suggest the prevalence of pericardial effusion among WHO diagnostic class 1 PAH patients may be as high as 25%(2) Pericardiocentesis or surgical pericardial window have been used for refractory, recurrent, or hemodynamically unstable pericardial effusions due to a wide range of etiologies(1). A case series of six PAH patients with pericardial tamponade revealed 50% mortality over one year period if effusion was not drained and of two patients who received intervention for effusion with pericardial window (PW) placement(5), one patient expired. The management of such patients, particularly in the setting of impending or active hemodynamic instability, has not been well described in the literature. We present our single center experience of 35 patients with pulmonary hypertension and comorbid pericardial effusion, management of pericardial effusion, and long-term outcome.
Methods and Materials
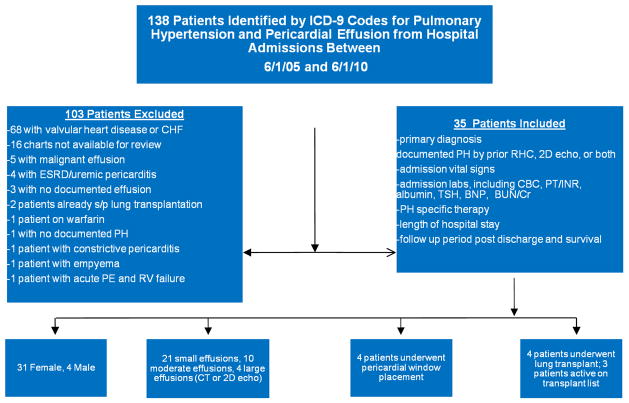
This was a retrospective observational study conducted through reviewing the medical records of The Methodist Hospital in Houston, TX. Institutional review board approval for the study was granted through Baylor College of Medicine and the Methodist Hospital, and following approval, the medical records of the Methodist Hospital were queried. The hospital charts of all patients admitted to the Methodist hospital between June 1, 2005 and June 1, 2010 were searched using the ICD-9 codes for “pulmonary hypertension” and “pericardial effusion.” Patient charts found to contain the relevant ICD-9 codes were then screened, and those patient encounters with a documented history of WHO diagnostics class 1, 3, 4, or 5 pulmonary hypertension by historical right heart catheterization, 2D echocardiogram, or both, as well as pericardial effusion demonstrated by 2D echocardiogram or computed tomography were identified. One hundred and thirty eight patients with pericardial effusion were identified, and 103 patients who had valvular heart disease, recent cardiothoracic surgery, end stage renal disease or other reasons were excluded (Figure 1). Thirty-five patients were identified who met the inclusion criteria were identified.
Figure 1.
Schematic of study sample with exclusions from analysis.
Included patients were then divided according to the size of the pericardial effusion, and the presence of pericardial tamponade physiology. The baseline patient demographics that were recorded included age, follow up time from admission, pulmonary hypertension specific medications being administered on admission or added during the admission, baseline laboratory values including creatinine, prothrombin time/INR, B-type natriuretic peptide levels, and whether the patient underwent solid organ transplantation during the observation period.
Statistical Analysis
Hemodynamic parameters obtained for each pericardial effusion size group, small, moderate, large, and tamponade, mean pulmonary artery pressure (by right heart catheterization), mean right atrial pressure, pulmonary artery systolic pressure (by 2D echocardiography), and mean arterial pressure (by blood pressure cuff) were compared using ANOVA analysis, as well as baseline laboratory values for BNP, creatinine, INR, and platelet count. Logistic regression for multivariate analysis with death as an endpoint was performed for multiple covariates including age, gender (male or female), pulmonary artery systolic pressure (as estimated by 2D echocardiogram), mean right atrial pressure, mean arterial blood pressure (measured non-invasively by blood pressure cuff), B-type natriuretic peptide level on admission, INR, creatinine, and the size of pericardial effusion (small, moderate, large, or tamponade physiology). Kaplan-Meier survival curves were constructed for all patients with PH and pericardial effusion, those patients with pericardial tamponade who underwent pericardial window placement, those patients managed conservatively (medical management only), and those patients who underwent transplantation during the follow up period. All statistical calculations were done using SAS software (SAS Institute, Cary, NC).
Results
Demographics characteristics
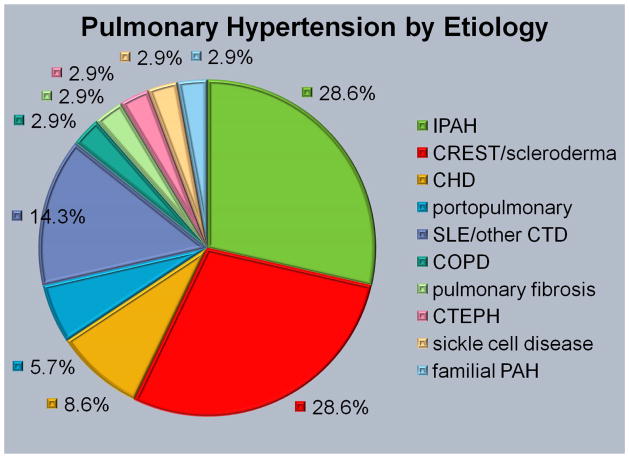
The mean age of the cohort was 46.1±15.8 years, 31 females and 4 males, and with a mean follow up time from hospitalization of 22.5±15.1 months (Table 1). Ten patients (28.6%) carried a diagnosis of idiopathic pulmonary arterial hypertension (IPAH), ten patients had CREST/scleroderma-related pulmonary hypertension (28.6%), 5 patients had a diagnosis of systemic lupus erythematosus or other connective tissue disease-associated pulmonary hypertension (14.3%), 3 patients had a history of congenital heart disease-related pulmonary hypertension (8.6%), 2 patients had portopulmonary hypertension (5.7%), and five patients had miscellaneous etiologies (2.9% each) (Figure 2). All but one patient (34 of 35, 97.1%) were on pulmonary hypertension specific therapy at the time of hospitalization, although the patient not on PH-specific therapy had not been evaluated by the pulmonary hypertension service during their admission. The four patients who developed pericardial tamponade comprised the major represented etiologies in our cohort: 2 patients with IPAH and 2 with scleroderma/CREST-associated PH.
Table 1.
Patient Characteristics
| Patient | Sex | Age | PH Specific Diagnosis | Follow-Up (Months) | Outcome at Study End (6/1/10) | PH Therapy on Admission |
|---|---|---|---|---|---|---|
| 1 | F | 75 | CREST (limited scleroderma) | 40 | Alive | PDE5-I; ETRA started |
| 2 | F | 62 | idiopathic PAH | 12 | Deceased; 1/7/08 | PDE5-I; epoprostenol started |
| 3 | F | 46 | chronic thromboembolic PH | 14 | Alive | ETRA |
| 4 | F | 22 | systemic lupus erythematosus | 36 | Alive | None |
| 5 | F | 74 | COPD | 30 | Alive | ETRA |
| 6 | F | 63 | CREST (limited scleroderma) | 48 | Alive | None |
| 7 | F | 57 | portopulmonary hypertension | 25 | Alive | ETRA |
| 8 | F | 47 | scleroderma and interstitial lung disease | 13 | Alive | ETRA; inhaled iloprost |
| 9 | F | 44 | mixed connective tissue disease | 8 | Deceased; 7/22/09 | epoprostenol |
| 10 | F | 27 | sickle cell disease | 6 | Deceased; 5/22/09 | None |
| 11 | F | 63 | scleroderma and interstitial lung disease | 26 | Alive | ETRA |
| 12 | F | 47 | scleroderma | 16 | Unknown; lost to f/u (last admit 4/2010) | None |
| 13 | F | 41 | CREST (limited scleroderma) | 26 | Alive; active on transplant list | ETRA, epo started |
| 14 | F | 61 | Idiopathic PAH | 42 | Alive; s/p BLTx 2/08 | None |
| 15 | M | 36 | CHD with ASD | 32 | Unknown; lost to f/u (last contact 2/10) | epoprostenol started |
| 16 | F | 37 | Idiopathic PAH | 6 | Deceased; 4/17/07 | ETRA; epoprostenol |
| 17 | F | 32 | Idiopathic PAH | 28 | Deceased; 6/4/09 | None |
| 18 | F | 73 | Idiopathic PAH and questionable CTD | 1 | Deceased; 1/1/09 | PDE5-I; IV treprostinil |
| 19 | F | 31 | idiopathic PAH | 18 | Alive; s/p OHT/DLT 9/13/09 | ETRA; epoprostenol |
| 20 | F | 23 | systemic lupus erythematosus | 22 | Unknown; last patient contact 2/10 | PDE5-I |
| 21 | F | 44 | Scleroderma | 12 | Deceased; 11/30/07 | PDE5-I; ETRA; epoprostenol |
| 22 | M | 43 | portopulmonary hypertension | 50 | Alive; s/p OLT 2/14/07 | None |
| 23 | F | 67 | NSIP/pulm fibrosis | 1 | Deceased; 8/27/08 | PDE5-I; ETRA |
| 24 | F | 40 | systemic lupus erythematosus | 26 | Alive; s/p OHT/DLT 11/09 | epoprostenol |
| 25 | F | 28 | idiopathic PAH | 16 | Alive; on list for OHT/DLT | None |
| 26 | F | 24 | idiopathic PAH | 49 | Alive | PDE5-I |
| 27 | F | 24 | idiopathic PAH | 34 | Alive; on transplant list | PDE5-I; epoprostenol |
| 28 | F | 47 | Scleroderma | 44 | Alive; s/p OHT/DLT 7/10 | PDE5-I started |
| 29 | F | 65 | Scleroderma | 24 | Deceased; 1/5/08 | PDE5-I; ETRA; inhaled iloprost |
| 30 | F | 49 | Scleroderma | 9 | Alive | None |
| 31 | M | 50 | familial PAH | 16 | Alive | PDE5-I; ETRA; epoprostenol |
| 32 | M | 41 | CHD with VSD | 1 | Deceased 8/28/07 | PDE5-I; IV treprostinil |
| 33 | F | 24 | CHD with ASD; Crouzon’s syndrome | 6 | Alive | ETRA |
| 34 | F | 57 | idiopathic PAH | 4 | Deceased (before 6/10); lost to f/u ’06 | PDE5-I; ETRA; IV treprostinil |
| 35 | F | 48 | Wegener’s granulomatosis; PAH | 45 | Alive | ETRA; epoprostenol |
| Total | 4 M, 31 F | 21 Alive; 11 Deceased; 3 Lost to F/U | ||||
| Mean | 46.1 | 22.5 | 5 TX | |||
| SD | 15.8 | 15.1 |
Figure 2.
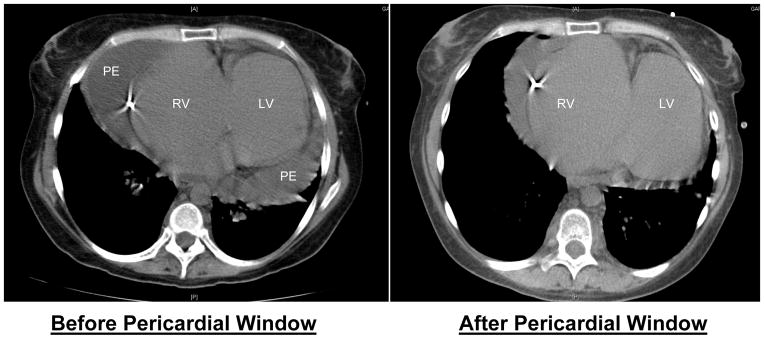
Computed tomography of chest in patients with cardiac tamponade. Patient presented with decompensated right heart failure and hypotension. The patient successfully underwent subxiphoid pericardial window for refractory pericardial effusion and unstable hemodynamics. LV, left ventricle; RV, right ventricle; PE, pericardial effusion.
Pericardial Effusion Size
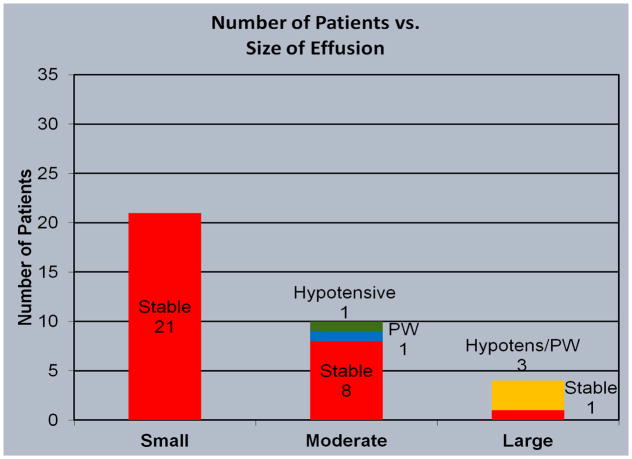
The included patients were also stratified by the qualitative size of the pericardial effusion determined by echocardiography or computed tomography into small, moderate, large, or any size with evidence of pericardial tamponade evidenced by diastolic collapse of the right atrium and/or right ventricle (Figure 3). Twenty-one patients (60%) were found to have a small pericardial effusion, 9 patients with a moderate-sized pericardial effusion (25.7%), one with a large pericardial effusion but without tamponade (2.8%), and four patients with pericardial tamponade (11.5%) of which three had large pericardial effusions and one a moderate-sized effusion. Pericardial windows were done for all four patients found to have pericardial tamponade (Figure 4).
Figure 3.
Etiology of pulmonary hypertension in the studied population. CHD, congenital heart disease; CTEPH, chronic thromboembolic pulmonary hypertension; iPAH, idiopathic pulmonary arterial hypertension.
Figure 4.
Number of patients with small, moderate and large pericardial effusions.
Hemodynamics analysis
Hemodynamic parameters were analyzed within individual subgroups of pericardial effusion sizes. Using ANOVA methodology, there were no significant differences in the mean pulmonary artery pressure measured by right heart catheterization (p = 0.636), pulmonary artery systolic pressure by echocardiography (p = 0.636), mean right atrial pressure measured by RHC (p = 0.467), or mean arterial pressure by non-invasive blood pressure monitoring (p = 0.867) at the time of admission of any of the pericardial effusion subgroups.
Laboratory Parameters
Laboratory parameters for the pericardial effusion subgroups were likewise compared using ANOVA analysis (Table 2). There were no significant differences in the B-type natriuretic peptide (P = 0.773), INR (P = 0.847), platelets (P = 0.813), creatinine (P = 0.926) for any of the pericardial effusion subgroups.
Table 2.
Laboratory values for the studied population
|
INR IU |
Platelets 1000/μL |
Creatinine mg/dL |
|
|---|---|---|---|
| Small (N=21) | 1.8±1.5 | 243.0±145.4 | 1.2±1.0 |
| Moderate (N=9) | 2.0±1.5 | 198.9±64.7 | 1.0±0.3 |
| Large (N=1) | ND | 177.0 | 0.7 |
| Tamponade (N=4) | 1.8±1.0 | 231.5±102.5 | 1.1±0.5 |
| Mean, All Patients (N=35) | 1.9±1.4 | 228.5±122.1 | 1.1±0.8 |
Outcome Analysis
Logistic regression with death as an endpoint was performed for multiple variables. There were no significant associations found between an increased risk of death and patient age (OR 1.04, 95% CI 0.93–1.16, P = 0.531), patient gender (male vs. female, OR 0.28, 95% CI 0.01–14.76, P = 0.526), mean PASP, mean RAP, MAP, BNP, creatinine, INR, size of pericardial effusion (OR 5.77, 95% CI 0.22–156.62, P = 0.294), or the placement of a pericardial window (OR 0.01, 95% CI <0.001–50.31, P = 0.296).
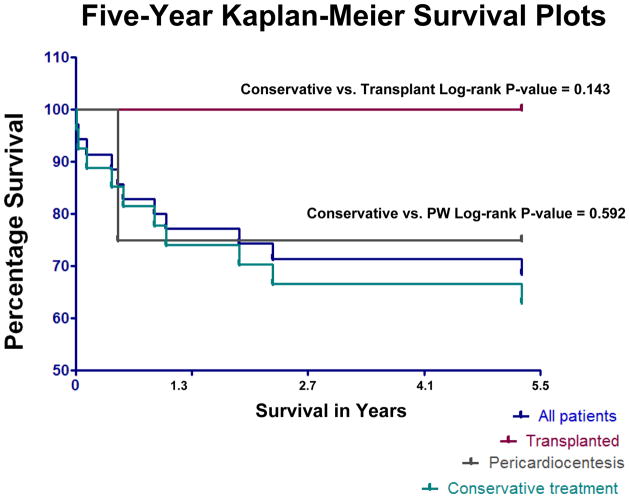
At the conclusion of the observation period, 21 patients of 35 (60%) were alive, with 12 patients deceased (34.3%), and two patients lost to follow up (5.7%) (mean follow-up period 22.5±15.1 months). Four patients ultimately underwent solid organ transplantation (11.4%), three heart/double lung transplantations and one orthotopic liver transplantation, and all four survived to the conclusion of the observation period (mean follow-up post transplant 21.3±15.5 months). Of the four patients who had a pericardial window placed for tamponade physiology, three survived to the end of the observation period (75%), and one expired (Figure 5). Two patients who had pericardial window placed for hemodynamically significant effusions were active on the list for heart/double lung transplantation (mean follow-up 29.5±4.9 months). None of the patients who ultimately went on to solid organ transplantation during the observation period had a pericardial window placed.
Figure 5.
Kaplan-Meier plot of mortality in PH patients in different treatment groups. PW, pericardial window. Patients who were transplanted did not have a significant survival advantage compared to the other subgroups, although this study may be underpowered to detect this difference.
Discussion
Evidence that the presence of pericardial effusion is an independent mortality risk factor in the setting of PAH(2) presents a challenge to clinicians in the management of this subset of the pulmonary hypertension cohort. In our patient population, we did not demonstrate a correlation between mortality and the size of pericardial effusion or tamponade, nor between multiple covariates including hemodynamics and demographic characteristics and increased risk of patient death. These findings may be reflective of small sample size, although suggest that the size of pericardial effusion is less important from a standpoint of prognosis than is the presence or absence of effusion. In the special case of pericardial tamponade complicating pericardial effusion, we did not show any mortality or survival benefit between the non-operative pharmacologic management of PH patients with pericardial effusion and the surgical creation of a pericardial window.
The accurate assessment of the mortality benefit in this subgroup of patients is difficult retrospectively for at least two reasons. First, the standard of care for treatment of patients with pericardial tamponade and impending hemodynamic collapse has been pericardial drainage(1,3,4,6) either percutaneously by pericardiocentesis or by surgical decompression. Previously reported data by Hemnes et al.(6) suggested that the one year mortality of PH patients with hemodynamically significant pericardial effusion in the absence of intervention may be 50%, and that the perioperative mortality associated with operative intervention in their patient population was also 50% (one of two patients died). The mechanisms responsible for this high mortality post-drainage of effusion in the PH cohort are not well understood. It is also unknown whether the high mortality rates associated with pericardial effusion in pulmonary hypertension are directly related to the effusion, or are a surrogate for deteriorating hemodynamics or another, as of yet, undiscovered risk factor. Second, in our cohort, additional interventions were performed at the time of pericardial window placement including the initiation or titration of PH specific medications perioperatively. The effect of optimization of PH-specific pharmacotherapy in combination with pericardial window placement cannot be defined with these data.
The significant limitation present in the available data is limited sample size, which may affect determinations of statistically significant differences between subgroups, as well as the retrospective nature of the study design. In addition, of the patients who survived to undergo solid organ transplantation, none had pericardial tamponade, although two of the four patients with tamponade survived to the conclusion of the observation period and were active on the list for solid organ transplantation.
As previously discussed, there was no statistically significant difference between conservative management and surgical drainage of pericardial effusion in the PH cohort, nor was there a statistically significant difference for pericardial window placement vs. transplantation, although there may have been a trend towards a survival benefit of transplantation.
The central question to be addressed in future investigations is what, if anything, can be done to mitigate the dismal mortality statistics for this subset of pulmonary hypertension patients. Lung transplantation has been shown to improve survival in pulmonary hypertension patients with NYHA class III to IV symptoms and deteriorating clinical status despite medical therapy (5,7,8). Nevertheless, there has been criticism of the Lung Allocation Score(9) (LAS) used by United Network for Organ Sharing (UNOS) for prioritizing organ transplant recipients in placing pulmonary hypertension patients at a relative disadvantage compared with other advanced lung disease categories.
The current LAS algorithm does not take into account the presence of pericardial effusion as a harbinger of poor prognosis in pulmonary hypertension. Further studies on patient outcomes would be helpful in clarifying the possibility of adding pericardial effusion to the LAS as a surrogate for mortality risk in pulmonary hypertension patients.
More data are needed to define the role of medical and/or surgical interventions in the management of pulmonary hypertension patients with pericardial effusion, although lung transplantation remains an option in end-stage patients.
Conclusions
Patients with pulmonary hypertension complicated by pericardial effusion currently carry a poor prognosis, and little data exists to support management options in this complex clinical scenario. The standard of care for hemodynamically unstable pericardial effusion has historically been catheter or surgical decompression, although it is unclear from the available information whether this approach is beneficial in the setting of pulmonary hypertension-associated effusions. The pathophysiology of hemodynamic decompensation in pulmonary hypertension patients after pericardiocentesis or pericardial window placement is not well understood. In addition, the size of pericardial effusion may be less important in this patient population than the presence of effusion. Further studies with larger patient cohorts will be needed to clarify the proper evaluation and risk stratification of pulmonary hypertension patients with pericardial effusion, and which interventions, if any, can be used to alter their clinical outcomes.
Acknowledgments
This study was supported by the National Institute of Health grant K23HL-093214 to ZS. Author appreciates the editorial help of Ms Janice Brister in the preparation of this manuscript.
References
- 1.Becit N, Unlü Y, Ceviz M, et al. Subxiphoid pericardiostomy in the management of pericardial effusions: case series analysis of 368 patients. Heart. 2005 Jun;91(6):785–90. doi: 10.1136/hrt.2004.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010 Jul 13;122(2):164–72. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 3.Campo A, Mathai SC, Le Pavec J, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010 Jul 15;182(2):252–60. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunne JV, Chou JP, Viswanathan M, et al. Cardiac tamponade and large pericardial effusions in systemic sclerosis: A report of four cases and a review of the literature. Clin Rheumatol. 2011 Mar;30(3):433–8. doi: 10.1007/s10067-010-1667-0. [DOI] [PubMed] [Google Scholar]
- 5.de Perrot M, Granton JT, McRae K, Pierre AF, Singer LG, Waddell TK, Keshavjee S. Outcome of patients with pulmonary arterial hypertension referred for lung transplantation: a 14-year single-center experience. J Thorac Cardiovasc Surg. 2012 Apr;143(4):910–8. doi: 10.1016/j.jtcvs.2011.08.055. Epub 2012 Feb 4. [DOI] [PubMed] [Google Scholar]
- 6.Hemnes AR, Gaine SP, Wiener CM. Poor outcomes associated with drainage of pericardial effusions in patients with pulmonary arterial hypertension. South Med J. 2008 May;101(5):490–4. doi: 10.1097/SMJ.0b013e31816c0169. [DOI] [PubMed] [Google Scholar]
- 7.Lordan JL, Corris PA. Pulmonary arterial hypertension and lung transplantation. Expert Rev Respir Med. 2011 Jun;5(3):441–54. doi: 10.1586/ers.11.21. Review. [DOI] [PubMed] [Google Scholar]
- 8.Mendeloff EN, Meyers BF, Sundt TM, Guthrie TJ, Sweet SC, de la Morena M, Shapiro S, Balzer DT, Trulock EP, Lynch JP, Pasque MK, Cooper JD, Huddleston CB, Patterson GA. Lung transplantation for pulmonary vascular disease. Ann Thorac Surg. 2002 Jan;73(1):209–17. doi: 10.1016/s0003-4975(01)03082-x. discussion 217–9. [DOI] [PubMed] [Google Scholar]
- 9.http://www.unos.org/docs/lung_allocation_score.pdf