Abstract
Serous ovarian cancer is one of the most lethal gynecological malignancies. Progress on effective diagnostics and therapeutics for this disease are hampered by ambiguity as to the cellular origins of this histotype of ovarian cancer, as well as limited suitable animal models to analyze early stages of disease. In this report, we will review current animal models with respect to the two proposed progenitor cells for serous ovarian cancer, the ovarian surface epithelium and the fallopian tube epithelium.
Keywords: serous ovarian cancer, fallopian tube epithelium, ovarian surface epithelium, ovulation, animal model
Introduction
Treatment of ovarian cancer presents several unique problems, including identification of the correct cell of origin for the disease. It is becoming widely accepted that either the ovarian surface epithelium (OSE) or the epithelium of the distal fallopian tube (FTE) may serve as a progenitor for high-grade serous cancer, the most common and deadly form of ovarian cancer. In order to study the initiation and progression of this disease, many animal models have been developed to recapitulate the course of neoplastic transformation; however, many of these models have focused only on analysis of cancer arising from the OSE. The goal of this review is to analyze how current animal models provide insights into development of serous cancer from the distal fallopian tube as well as the OSE.
Ovarian cancer
While advances have been made in prevention, diagnosis, and treatment of many types of cancers affecting women, including breast and cervical cancer, ovarian cancer remains the most deadly gynecological malignancy and the fifth leading cause of cancer death in women (1). Slow progress in reducing mortality rates for this disease is attributed in part to a lack of detection methods, as the ovaries are situated deep within the pelvic cavity, limiting screening tests. As a result, the disease often goes undiagnosed until late stages, limiting treatment options and providing fewer experimental resources for analysis of early stage disease. While tumors can arise from other cell types of the ovary, epithelial cell cancers, or carcinomas, account for ~90% of ovarian cancers, and are the only form of ovarian cancer in which the progenitor cell is in question (2).
Further complicating diagnoses and treatment of ovarian cancer are the existence of four distinct histotypes of ovarian cancer, each with a separate morphology, set of common molecular abnormalities, and clinical progression. The clear cell and mucinous histotypes are found at very low frequencies, and little is known about the progenior cells for these types of ovarian cancer. Endometrioid carcinomas account for approximately 10% of ovarian carcinomas, with histology that resembles glandular structures normally found in the uterus (3). Endometrioid carcinoma risk is correlated with endometriosis, and the most common molecular abnormalities detected are mutations in genes forβ-catenin, a member of the Wnt signaling pathway, and PTEN, a phosphatase involved in downregulating the PI3-kinase/Akt pathway (4). The most common histotype of ovarian cancer is the serous histotype, occurring with a frequency of approximately 70% of ovarian epithelial cancers, and as such will be discussed further in this review (4).
Serous tumors resemble the FTE, but molecular abnormalities and the tissue of origin associated with this type of tumor vary depending on the grade of carcinoma. By far the most common type of ovarian carcinoma is high-grade serous carcinoma (HGSC), which is very aggressive, with high proliferation rates. This may be the result of frequently observed mutations in p53, occurring in 50–80% of high-grade serous cancers, as well as amplification of the HER-2/neu oncogene, which is observed in 10–20% of cases (5). There are at least two potential sites of origin for HGSC, including the OSE or OSE-lined epithelial inclusion cysts and the FTE (6–8).
Ovulation and the ovarian surface epithelium
The OSE has long been postulated as a source of serous ovarian cancer. The OSE is a single layer of squamous-to-cuboidal epithelium surrounding the outer surface of the ovary (9). Following release of the oocyte during ovulation, the OSE proliferate and migrate to cover the wound in the ovarian surface (10). To facilitate this process, the cells are responsive to hormonal signals, proteases, extracellular matrix components, and inflammatory molecules. This has led to the development of hypotheses linking ovulation to development of ovarian cancer.
While the precise etiology of ovarian cancer is unknown, there is a strong correlation between an increased number of lifetime ovulations and increased risk of ovarian cancer (11). There are at least three distinct components of ovulation that likely contribute to this risk: proliferation of OSE following ovulation, proliferation in response to the gonadotropins, and release of inflammatory cytokines during ovulation. If serous cancer originates in the FTE, it is likely that components of ovulation also impact these cells.
The tear-and-repair hypothesis was proposed by Fathalla in 1971, and suggests that incessant ovulation is a risk factor for ovarian cancer due to constant remodeling of the ovarian surface, which allows opportunities for spontaneous DNA damage as the cells rapidly proliferate (12). Increased proliferation of OSE following ovulation has been validated in many animal systems (10, 13). The OSE express receptors for the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and are thought to respond to gonadotropin stimulation by an increase in proliferation and inhibition of apoptosis (14–16). The majority of women are diagnosed with ovarian cancer following menopause, when depletion of follicles and the lack of negative feedback by estrogen leads to tonically high levels of circulating FSH and LH (17). Ovulation is also thought to release inflammatory cytokines, which generate oxidative stress (18). In addition to oxidative stress generated by prostaglandins, bradykinins, and leukotrienes, macrophage infiltration into the periovulatory site releases additional sources of reactive oxygen (19). Oxidative stress associated with ovulation has been shown to induce DNA damage in OSE, contributing to neoplastic transformation of these cells (20). Due to the close proximity of the FTE to the ovary, many ovulatory factors that affect the OSE may additionally impact the fallopian tube. While these effects have been well characterized in the OSE, future studies should examine how ovulation impacts the fallopian tube.
The combination of proliferation following ovulation, stimulation by the gonadotropins, and DNA damage induced by inflammatory molecules likely contributes to development of ovarian cancer over a lifetime of repeated monthly ovulations. Repeated cycles of ovulation lead to development of epithelial inclusion cysts, which are thought to be a precursor lesion for serous ovarian cancer originating from the OSE (7, 21, 22). The cells lining inclusion cysts are exposed to an environment rich with concentrated hormones and growth factors, which may contribute to progression to serous adenocarcinomas (22). A similar mechanism may explain how serous cancer originating in the fallopian tube is often detected in the ovary, as FTE might be sloughed from the distal fimbriae, fall inside or become trapped in the ovarian cortex after ovulation, and are subsequently exposed to hormones and growth factors.
The fallopian tube epithelium and hereditary risk of ovarian cancer
While the OSE has long been accepted as a site of origin for serous ovarian cancer, several studies have suggested that the distal fimbriae of the fallopian tube may be an alternative site of origin (8, 23–25). The FTE lies in close proximity and is contiguous with the OSE, although the two types of epithelium have different molecular characteristics (11). Both types of cells express certain epithelial markers, such as cytokeratins, but the FTE expresses E-cadherin while the OSE does not, although E-cadherin is often expressed in inclusion cysts (26). Additionally, the FTE express tubal-specific markers such as oviductin, Pax8, and markers of ciliated cells (27). Interestingly, advanced HGSC demonstrates expression of some tubal-specific proteins, such as CA-125, Pax8, and E-cadherin, which are not normally expressed in the OSE (28, 29).
The OSE and FTE share a common embryologic precursor in the coelomic epithelium, which gives rise to the epithelia lining the Mullerian structures, including the fallopian tube, endometrium, and endocervix, as well as the OSE (9). The OSE retains mesothelial characteristics that indicate it may not be terminally differentiated (11). Recent hypotheses have suggested that due to the undifferentiated nature of the OSE as well as its similarity to the FTE and their common embryologic origin, the OSE and FTE are contiguous and represent such similar tissues that it may not be necessary to delineate from which cell type serous ovarian cancer arises (29). Alternatively, there are at least two scenarios for initiation of serous cancer: the OSE terminally differentiates to resemble the FTE, or the cancer originates in the fallopian tube and metastasizes to the ovary. In approximately 50% of women with serous cancer who underwent removal and analysis of the fallopian tubes, early serous carcinoma was detected in the fallopian tube (24). Often the dominant ovarian mass and the early lesions detected in the fallopian tube exhibit the same mutations in p53, indicating that the two types of cancer share a common source (25).
While the majority of HGSC occurs spontaneously, approximately 10% of cases are associated with a familial history of ovarian cancer, due to inherited mutations in BRCA1 and BRCA2 (30). Preneoplastic lesions of the fallopian tube in BRCA women have molecular alterations similar to those found in overt serous ovarian cancer, including activation of the PI3-kinase pathway, increase in p53 expression, and decrease of p21 expression (31, 32). Women with BRCA mutations are more likely to exhibit the “p53 signature” and are also more likely to exhibit primary fallopian tube carcinomas, which histologically resemble serous carcinomas (8, 33). The “p53 signature” refers to foci of p53 expression in the fallopian tube that occur without accompanying changes in cell morphology or increased proliferation (8). However, this phenomenon has also been observed to a lesser degree in the OSE, and may reflect the fact that in the human, the OSE and FTE are contiguous, with an increased incidence of the “p53 signature” observed as the cells become more differentiated toward the proximal fallopian tube (29).
Inactivation of DNA damage sensing and repair mechanisms seems to play a key role in development of serous cancer originating in the FTE, as women with Li-Fraumeni syndrome, resulting from a germline mutation in p53, are at higher risk for exhibiting the “p53 signature” while also exhibiting increased levels of DNA damage in the FTE (34). Despite a high incidence of expression of the “p53 signature”, women with Li-Fraumeni syndrome are not at a higher risk for serous cancer, indicating that disruption of BRCA1, and not necessarily p53, is critical for cancer initiation. In support of this hypothesis, when p53 knockout mice were superovulated with exogenous gonadotropins, no tumors or neoplastic lesions were observed (35).
There is a clear role for genetic predisposition and disruption in DNA damage repair genes in development of serous carcinoma originating in the fallopian tube, but it is likely that ovulation also impacts the fallopian tube, perhaps leading to neoplastic changes in the FTE. The controversy surrounding the source of HGSC, as well as the mechanisms leading to its initiation and progression point to a need for animal models that can determine how HGSC originates in the FTE or the OSE. These systems can also be used to model spontaneous changes in epithelia, such as might be observed following ovulation, or to determine the contributions of specific genetic changes to development of HGSC. This review highlights animal models of spontaneous ovarian cancer, HGSC induced by chemical and hormonal carcinogens, and genetic models of serous cancer, including promoter-driven expression of transgenes as well as conditional expression of genes regulated by intrabursal injection of adenovirus expressing Cre recombinase into floxed mouse models. In particular, analysis of each system with regard to defining a cell of origin for serous cancer will be highlighted.
Animal models that develop spontaneous ovarian carcinoma
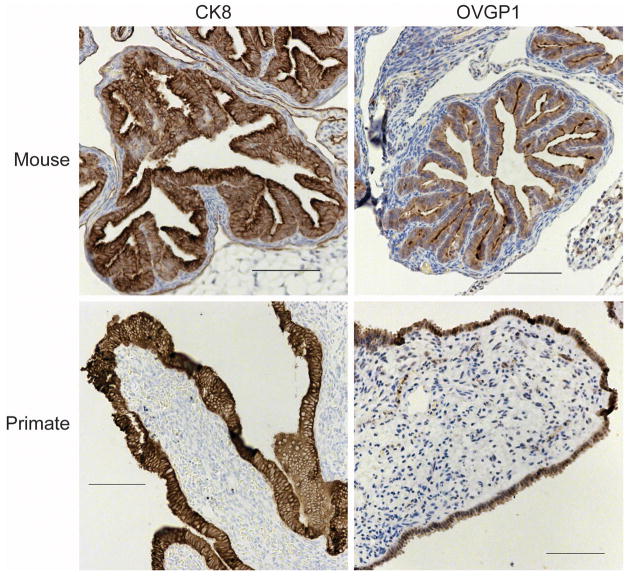
To closely mimic the progression of ovarian cancer seen in humans, investigators have used a non-human primate model, specifically the macaque. Features of the macaque reproductive system closely mimic that of the human, with similar anatomy, length of menstrual cycles, and development of menopause as the animal ages (36). Importantly, unlike other animal models used to study ovarian cancer such as the rodent or the laying hen, the non-human primate exhibits fallopian tubes similar to those of humans, with the epithelial cells on the outer surface of the tissue in contact with the peritoneal surface and contiguous with the OSE (Figure 1). This may facilitate better analysis of disease initiation as well as analysis of the effects of ovulation on the fallopian tube.
Figure 1.
Comparison of the location of FTE cells in primates and rodents. Distal fallopian tube fimbriae (primates) or oviduct (mouse) were analyzed by immunohistochemistry for an epithelial protein (CK8) or a tubal specific protein (OVGP1). In primates, FTE are on the outer surface of the stromal component of the tissue, and in mice, the FTE line the lumen of the oviductal tube.
The progression of ovarian carcinomas in macaques is largely similar to that observed in humans. Non-human primates develop spontaneous ovarian cancer in an age-dependent manner, similar to the development of ovarian cancer in humans following menopause (37). Both humans and non-human primates develop ovarian cancers at low rates, with human females having an approximately 2% risk of developing ovarian carcinomas, and non-human primates having about a 0.4% risk (37–39). However, unlike in humans, where the vast majority of ovarian cancers are epithelial, the most common types of ovarian cancer in non-human primates are granulosa cell tumors or sex-cord stromal tumors (36, 40). Carcinomas account for about 23% of the ovarian cancers detected in non-human primates, and these show remarkable similarity to those observed in humans, as all four histotypes have been observed and show similar patterns of progression and metastasis (36). However, no fallopian tube cancers have been observed in the macaque, although hyperplasia and nuclear atypia have been observed (36).
The non-human primate model has been used primarily to study prevention of spontaneous ovarian cancer using hormone-based treatments, which address the onset of ovarian cancer during menopause, as well as the influence of FSH, LH, estradiol, and progesterone on the reproductive tissues. Treatment of macaques with oral contraceptives, or with the progestin-only component of oral contraceptives increased apoptosis in the OSE (41, 42). This prevention strategy addresses the clearing of OSE spontaneously damaged during ovulation and the subsequent rapid proliferation following extrusion of the oocyte. However, because non-human primates develop spontaneous ovarian cancer at such low rates over a long time course, these studies cannot directly address prevention of ovarian cancer, as no animals developed tumors during the course of the study. Additionally, these studies were performed prior to studies proposing the fallopian tube as a source of serous cancer, and future analysis will be important to evaluate if oral contraceptives could potentially reduce initiation of tubal lesions following ovulation through apoptosis.
The laying hen offers insights into the role of incessant ovulation in the development of spontaneous ovarian cancer, but has some dissimilarity to the human anatomy. The hen maintains an ovulatory cycle lasting 24–26 hours, and the ovulated egg passes through the oviduct, which is roughly comparable to the human fallopian tube, although the oviduct functions as an endocrine organ to complete the development of the egg shell prior to laying (43). The hen does not have a luteal phase and some have postulated that the lack of circulating progesterone is responsible for the increased risk of cancer, not incessant ovulation (44). The hen also does not menstruate and some have speculated that retrograde menstruation is the mechanical force allowing for peritoneal dissemination of uterine cells or FTE (28). Although hens can lay for up to 8–9 years, most animals used in agriculture are culled after about 2 years due to the high incidence of ovarian and oviductal tumors (45). Over the course of a 4 year study, 32% of birds developed ovarian tumors, and 8% of birds developed oviductal tumors (45). The most common histotypes of ovarian carcinoma found in laying hens are the endometrioid and serous types, which are found at approximately similar rates, in contrast to the majority of human ovarian cancers, which are of the serous histotype (46). Therefore, the laying hen is particularly well suited to study whether the cell type of initiation directly influences the histotype of ovarian cancer.
Due to the high incidence of spontaneous serous cancer formation in laying hens, many studies have analyzed the molecular changes occurring in these types of tumors in comparison to their human counterparts. Mutations in p53 are found in greater than 50% of human serous ovarian cancers, and a recent study demonstrated that 48% of chicken ovarian carcinomas also contained mutations in p53 (47). Overexpression of HER-2/neu was increased in ovarian carcinomas from hens, and in humans, an increase in HER-2/neu expression is commonly associated with progression of primary fallopian tube carcinoma as well as HGSC (47–49).
In addition to providing valuable information about the effects of incessant ovulation on initiation and progression of serous ovarian cancer, the laying hen can also be used to study the fallopian tube (oviduct) as a progenitor for serous cancer. Since hens develop spontaneous oviductal tumors, gene expression profiling has been completed to compare genetic changes in ovarian tumors to serous cancer from humans (50). Trevino et al. found that hen ovarian tumors when compared to normal ovaries contained upregulated transcripts of genes found in normal hen oviducts, such as Pax2 and ovalbumin (50). However, the tumor samples analyzed were not divided by histotype or stage and transcripts were not compared to those from normal oviducts, so it remains ambiguous whether ovarian tumors in the hen originate in the oviduct, or if tumors originating in the OSE terminally differentiate to resemble the oviduct.
Very few spontaneous ovarian or fallopian tube cancers have been noted in laboratory rodents. Overall, rats and mice develop ovarian carcinomas at a low rate of approximately 2% of all animals, which is not that dissimilar from human women or non-human primates (51, 52). A spontaneous carcinoma arising in a Lewis rat was well characterized as being of the endometrioid histotype, and expression patterns of genes such as β-catenin, HER-2/neu, and CA125 were similar to that of humans (53).
Of the animal models that develop spontaneous ovarian carcinomas, primarily the laying hen and the non-human primate, both models provide insight into the role of the three hypotheses linking ovulation to serous ovarian cancer originating from the OSE. In examining the fallopian tube as a site of origin for serous cancer, neither the hen nor the non-human primate has been examined with regard to mutations in BRCA1, which seem to be a predisposing factor for initiation of serous cancer from the fallopian tube in women. However, it is likely that ovulation impacts the fallopian tube as well as the OSE, and the non-human primate is better poised to analyze the influx of hormones and inflammatory factors acting on this tissue as the hen oviduct is significantly anatomically different from that of the human. Alternatively, laboratory rodents exhibit anatomy more similar to the human and non-human primate, with the rodent oviduct comparable to the human fallopian tube (54, 55). Importantly, rodents demonstrate similar ovulatory cycles to the human; the existence of a luteal phase, although shorter than that of the human, allows for examination of progesterone signaling (56). Unlike the hen, the rodent oviduct retains the same cell types and expression patterns of molecular markers of the human fallopian tube and performs the same biological functions (27).
Ovarian carcinoma induced by carcinogens
While rats and mice have very low rates of spontaneous ovarian carcinoma, these animals have been used extensively in studies of carcinoma induced by carcinogens, as well as in genetically engineered models of ovarian cancer. In order to study early events in ovarian cancer formation, several studies have treated rodents with chemical carcinogens. Broadly, these carcinogens include molecules that interfere with normal biological processes in cells of the ovary, such as hormones or hormone antagonists, or molecules that function as toxins or DNA damaging agents (57). While treatment of rodents with hormones such as estradiol or testosterone does not induce ovarian tumors, many studies have noted preneoplastic changes in the OSE following administration of hormone. Estrogen has been shown to stimulate proliferation of the OSE, but it is more likely that hormones play a role in progression of ovarian cancer rather than initiation (58). As the FTE expresses receptors for estradiol, FSH, and LH, it is likely that if hormones play a role in progression of ovarian cancer they would also impact those originating in the fallopian tube (59, 60).
Studies utilizing DNA damaging agents, such as N-methyl-N′-nitrosourea (MNU) or 7,12-dimethylbenz[a]anthracene (DMBA) attempt to mimic the types of random DNA damage that may occur following repeated iterations of the tear-and-repair process. One caveat is the fact the types of carcinogens that have been used to model ovarian cancer are not chemicals to which humans are normally exposed, but these studies can be used to provide information about the impact of random DNA damage on ovarian cancer initiation.
To date, the most effective route of administration of DMBA or MNU to initiate ovarian tumors is by implanting a sterile suture coated with the carcinogen directly in contact with the ovarian surface (61–63). When sutures impregnated with DMBA or MNU were sewn into the ovaries of rats, all members of the treatment group developed ovarian adenomas or adenocarcinomas, indicating the tumors likely arose from the OSE, the cell type in closest contact to the suture (61). Although the histotype of the adenocarcinomas was not extensively noted, some serous lesions were observed following treatment with carcinogens (61). Interestingly, because of placement of the suture within the bursal space encompassing the ovary, Tunca et al. also noted uterine hyperplasia and neoplastic lesions. This indicates that the uterus, and likely the oviduct, was affected by treatment with DMBA or MNU, as the oviduct is contiguous with the uterus in rodents (61).
Additional studies using DMBA-coated sutures implanted into rat ovaries confirmed formation of adenocarcinomas appearing to originate from the OSE (63). However, this study noted that mostly undifferentiated carcinomas resulted from treatment with DMBA, with the exception of one papillary serous cystadenocarcinoma that was observed (63). To further analyze the impact of ovulation on carcinogen-induced ovarian cancer formation, Stewart et al. combined superovulation studies with implantation of DMBA-coated sutures into the ovaries of rats (62). Superovulation was induced by multiple rounds of stimulation with FSH and LH to promote proliferation of the OSE. Superovulation increased tumor complexity, with more tumors resembling the serous histotype of ovarian cancer as compared to animals receiving DMBA treatment without gonadotropin stimulation (62). These serous lesions were analyzed for molecular alterations and compared to those commonly identified in human ovarian carcinomas, and mutations in p53 as well as activating mutations in K-ras were found (62). This study indicates that the additional stimulation by the gonadotropins may impact progression of ovarian cancer, likely due to activation of proliferative signaling pathways in cells already spontaneously damaged by DMBA. The change in p53 as a significant factor influencing cancer formation is also common to serous cancer originating from the FTE, potentially suggesting that ovulation may modulate neoplastic changes induced by spontaneous DNA damage in the microenvironment, including the fallopian tube.
To combine spontaneous DNA damage induced by carcinogens with an altered genetic background, such as might be found in women with a genetic predisposition to serous ovarian cancer, DMBA-coated sutures were implanted into the ovaries of mice heterozygous for a point mutation in p53 (64). Compared to their wild-type littermates, a higher percentage of DMBA implanted p53Ala135Val/wt mice developed ovarian tumors, some of which were adenocarcinomas possibly originating from the OSE, although the p53 mutation would be likely to impact the FTE as well (64). This study provides an interesting comparison to studies of the “p53 signature”, which is described as an overexpression of p53 (8, 65). Point mutations in p53 often lead to stabilization and ovexpression of the protein, and if the “p53 signature” is the result of a point mutation(s) similar to that described in the study by Wang et al., it is possible that at least a subset of the adenocarcinomas observed may have originated from the oviduct (8, 64–66).
Conditional inactivation of transgenes using Cre-loxP
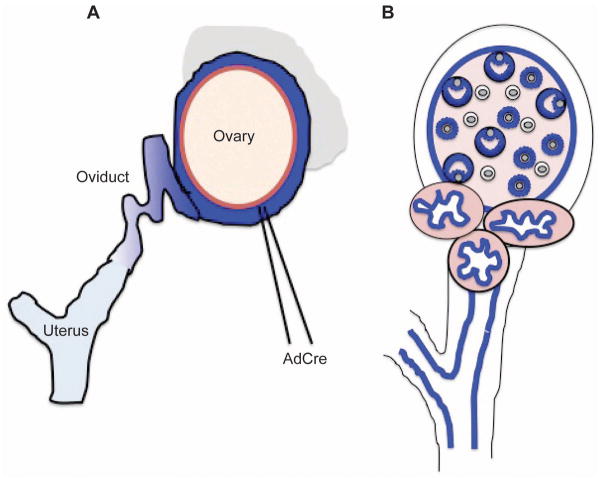
Often, constitutive expression of genes involved in cancer initiation result in embryonic lethality, such as the case for BRCA1 (67). One strategy that researchers have used to induce conditional inactivation is intrabursal injection of adenoviruses expressing Cre recombinase (AdCre) to inactivate floxed alleles of specific genes. This system requires creation of a transgenic mouse expressing a constitutive or tissue-specific promoter driving expression of a particular gene flanked by loxP sites, which act as a substrate for the Cre recombinase enzyme (68). To inactivate the gene in the OSE, AdCre is injected into the intrabursal space, often near the uterotubular junction; however, these injections can be leaky and lead to inactivation of floxed alleles in the oviduct and uterus, and potentially other tissues (Figure 2A) (68, 69).
Figure 2.
Targeting of reproductive epithelium by intrabursal AdCre injection or MISIIR promoter. A) Adenovirus expressing Cre recombinase is injected into the intrabursal space. Blue represents Cre expression, with darker shades of blue demonstrating higher expression levels than lighter shades of blue. It is likely that intrabursal injection of AdCre leads to varying degrees of recombination in the OSE, FTE, and possibly the endometrium. B) MISIIR promoter targets transgene expression to the Müllerian epithelium, shown in blue. Affected tissues include the granulosa cells, the OSE, the FTE, and the endometrium. A detailed analysis of MISIIR promoter strength in these various cellular compartments has not yet been completed.
Floxed alleles of the tumor suppressors p53, BRCA1, and retinoblastoma (Rb) have been conditionally inactivated singly or in various combinations by intrabursal injection of AdCre. Mutations in p53 are found in more than 50% of ovarian cancers, and disruption of BRCA1 is often associated with mutations in p53, particularly in women with a familial history of breast and ovarian cancer (70). Additionally, Rb has been shown to modulate BRCA1 by direct binding to BRCA1 as well as by transcriptional modification of BRCA1 (71). Disruption of these tumor suppressors have been shown to correlate specifically with HGSC originating from the OSE and the FTE (3).
Following injection of AdCre to inactivate BRCA1, researchers observed no overt tumors but found preneoplastic lesions, including OSE hyperplasia and epithelial inclusion cysts (72). Quinn et al. found a very low rate of tumor formation for inactivation of p53 alone, while Clark-Knowles et al. found that inactivation of p53 resulted in tumors in 100% of animals, although these were not of epithelial origin (71, 73). Co-inactivation of p53 with BRCA1, but not Rb, dramatically accelerated tumor growth, although the tumors formed were leiomyosarcomas, which did not exhibit characteristics indicative of epithelial-derived tumors and were thought to potentially arise from the bursal membrane (71, 73).
In contrast to these findings, Flesken-Nikitin et al. found that conditional inactivation of p53 and Rb resulted in tumor formation with a very high penetrance, and that 39% of these tumors were well differentiated and of the serous histotype, although few tumors were observed when either p53 or Rb was inactivated singly (74). Notably, this study introduced the AdCre injection following ovulation to induce proliferation of the OSE, which may impact infectivity and expression levels of the Cre recombinase enzyme, as well as allowing for the impact of ovulatory hormones on cancer initiation.
An alternative strategy to disrupt p53 and Rb expression used conditional expression of the SV40 T antigen (SV40 TAg) by intrabursal injection of AdCre (75). Following conditional expression of SV40 TAg, poorly differentiated epithelial tumors were observed, with involvement of the oviduct, uterus, and other tissues. Tumors found in the oviduct resembled those found in the ovary and retained SV40 TAg expression, though it was not determined whether the oviductal tumors metastasized from the ovary or originated in the oviduct (75). In this study, AdCre injection clearly affected cells of the oviduct as well as the OSE, hampering identification of the source of the oviductal tumors.
Promoter-driven expression of transgenes
Conditional expression of floxed genes by intrabursal injection of AdCre allows for expression of the genes in specific tissues at specific times. An alternative strategy for conditional expression of genes involved in serous ovarian cancer is by use of a tissue-specific promoter. OSE-specific promoters have been proposed, including ovarian-specific promoter 1 (OSP1), and the promoters for secretory leukoproteinase inhibitor (SLP1) and epithelium-specific ets transcription factor (ESE1) (76). However, the OSP1, ESE1, and SLP1 promoters have largely been tested only in cell lines, or demonstrate promiscuous localization in mouse tissues (68, 76).
To date, two mouse models have been published which permit analysis of gene expression in the FTE without simultaneous expression in the OSE. While a transgenic mouse expressing Cre recombinase under the FoxJ1 promoter demonstrates recombination within ciliated cells of the oviduct, recombination is also observed within bronchial cells (77). This model has not yet been crossed to mice carrying floxed alleles of genes relevant for serous cancer initiation, so it is unknown if gene inactivation specifically within the ciliated cells of the oviduct will lead to cancer formation. Miyoshi et al. generated a transgenic mouse line using the oviductal glycoprotein (OVGP1) promoter driving SV40 expression, resulting in formation of large tumors in the oviduct and uterus, but not the ovary (78). Although SV40 TAg expression was detected by RT-PCR in the ovaries of adult transgenic mice, no tumors were observed in the ovary, possibly indicating that cancer cells originating in the oviduct or uterus may have metastasized to the ovary. Interestingly, this study observed that tumor formation in the oviduct and uterus was highly dependent on estradiol, likely as a result of estradiol regulation of OVGP1 expression (59). Unfortunately, due to tumor formation, female animals expressing the transgene exhibit severely limited fertility, which may hamper future studies using this model to study the impact of ovulation on serous cancer development.
Several mouse models have been generated using the Müllerian inhibiting substance type II receptor (MISIIR or Amhr2) promoter, which drives expression of transgenes in the OSE, granulosa cells, oviduct, and uterine epithelium (Figure 2B) (68). When SV40 TAg was expressed under the MISIIR promoter, tumors were observed in approximately 50% of the animals, and these tumors were primarily poorly differentiated carcinomas (79). This is very similar to the types of tumors observed following intrabursal injection of AdCre into mice carrying floxed alleles of SV40 TAg (75); however, tumors from the animals expressing SV40 TAg driven by the MISIIR promoter also exhibited areas of cyst formation and papillary structures coincident with the OSE, suggesting a serous histotype for at least a subset of tumors. These tumors follow a pattern of dissemination, metastasis, and formation of ascites that mimics the course of human disease (79). Despite expression of MISIIR in the oviductal epithelium, no SV40 TAg expression was detected and no tumors were observed originating from the oviduct in the initial studies (79). Different results were obtained in this study as compared to the Miyoshi et al. study utilizing the OVGP1 promoter to drive expression of SV40 in the oviduct, likely due to differences in the strength of the OVGP1 and MISIIR promoters in the oviduct.
Since original publication of this mouse model, transgenic lines expressing varying levels of SV40 TAg driven by MISIIR have been developed. Most notably, Quinn et al. have recently shown that one line of mice exhibits expression of SV40 TAg only in the oviduct and not in the OSE; however, these animals do not develop tumors (80). This is in accordance with a recent study showing that SV40 expression drives immortalization of human FTE cells, but is not sufficient for transformation of these cells (81). As disruption of p53 and Rb mediated by SV40 TAg was not sufficient to drive neoplastic transformation, these studies point to the necessity for additional genetic disruptions in the fallopian tube. This study questions whether the “p53 signature” may represent a true preneoplastic lesion in the fallopian tube, as inactivation of p53 is not sufficient to drive transformation; however, as the “p53 signature” is more commonly observed in BRCA mutant women, it may serve as a preneoplastic lesion when BRCA1 is also mutated or inactivated.
An interesting study from Chodankar et al. generated mice with conditional inactivation of BRCA1 by crossing animals expressing Cre recombinase driven by the FSH receptor (FSHR) promoter to animals expressing floxed alleles of BRCA1, with the intention of targeting BRCA1 in granulosa cells (82). Following inactivation of BRCA1 by this method, benign serous cystadenomas were observed in the ovary and uterus but not specifically mentioned in the oviduct (82). The cysts exhibited epithelial morphology and lacked expression of Mullerian inhibiting substance (MIS), indicating that they did not originate from granulosa cells. The authors postulate that in this system, inactivation of BRCA1 in the granulosa cells disrupts release of a signal interpreted by the OSE, leading to cystadenoma formation (82). Alternatively, it is possible that BRCA1 is also inactivated in tissues such as the OSE and potentially the FTE, as these tissues have been shown to express FSHR (14, 83). However, expression of MIS might be expected in these cystadenomas, as several studies have indicated that the OSE and oviduct express MIS as well as MISIIR (84, 85). Formation of preneoplastic lesions, specifically areas of OSE hyperplasia and inclusion cysts, following BRCA1 inactivation in the OSE and potentially FTE is consistent with AdCre-mediated inactivation of BRCA1. (72).
Two studies have examined the role of disruption of the PI-3 kinase signaling pathway in serous ovarian cancer. Using the MISIIR promoter to drive overexpression of the catalytic p110αsubunit of PI-3 kinase, Liang et al. observed hyperplasia of the OSE, consistent with a role for this signaling pathway in proliferation of epithelial cells (86). No tumors were observed, but this may correspond to an early event in OSE transformation, which upon additional genetic insult may lead to tumorigenicity.
Mullany et al. examined alteration of the PI 3-kinase signaling pathway by a different strategy using inactivation of PTEN phosphatase. PTEN is a negative regulator of Akt, a kinase downstream of PI 3-kinase that is often altered in human cancers (87, 88). Constitutive activation of PI 3-kinase signaling was combined with expression of an activating mutation in K-Ras to generate low grade serous papillary adenocarcinoma (87). In this study, mice expressing floxed PTEN alleles and floxed KrasG12D alleles were crossed to mice expressing Cre recombinase under the MISIIR promoter. Serous papillary adenocarcinomas formed in 100% of the animals, and when the investigators compared transcripts from their murine tumor samples to human tumor samples, they observed many genes in the K-ras pathway that were similarly regulated. Additionally, overexpression of p53 was found in OSE isolated from transgenic ovaries, and this was found to be downstream of disruption of PTEN. This study further demonstrates that in OSE, inactivation of PTEN and overexpression of K-rasG12D drives serous ovarian carcinoma formation; however, it does not rule out the possibility that loss of PTEN in the tubal epithelium leads to an increase in p53 expression, which may result in carcinoma originating in the FTE, which also expresses MISIIR.
A previous study used AdCre to express the same activating mutation in K-ras in combination with inactivation of PTEN, but with strikingly different results (89). Following intrabursal injection, Dinulescu et al. observed endometriosis with peritoneal spread when K-rasG12D was expressed, and observed endometrioid carcinoma when K-rasG12D was co-expressed with inactivation of PTEN. The investigators concluded that at least some endometrioid carcinoma lesions directly arose from the OSE, as ovaries subjected to AdCre injection that were isolated and re-implanted under the bursas of mice wild type for K-ras and PTEN exhibited the same endometrioid carcinomas as previously observed.
Comparison of these two studies demonstrates that the method in which transgenes are expressed in murine models of ovarian cancer can make a significant difference in the histotype of tumor observed. A closer investigation into viral leakage and the strength of the MISIIR promoter in the various reproductive tissues is necessary to clarify the origin of tumors in many transgenic animal models. Additionally, when a tissue-specific promoter drives the transgenes, the alleles are inactivated prior to puberty, limiting the contribution of ovarian steroid hormones and gonadotropins to tumor initiation and progression. In contrast, intrabursal injection of AdCre to inactivate the alleles is performed in adult animals, where hormones may have already impacted the OSE and FTE. Moreover, AdCre expression is transient, usually lasting about 21 days, which may or may not be sufficient expression time to establish lasting neoplastic changes in the cells (74, 89).
Conclusion
To enable earlier diagnosis of ovarian cancer, identification of the source of the tumors is necessary. While the OSE has long been accepted as the progenitor cell for ovarian carcinomas, it is likely that at least a subset of these cancers may originate in the fallopian tube and metastasize to the ovary, where the cancer is detected. Due to these ambiguities, researchers are faced with difficulties in using human tissue to study the course of this disease. To this end, many animal models have been developed to study spontaneous transformation leading to ovarian carcinomas, as well as targeted disruption of genes found to be involved in the progression of human disease. Many of these studies provide useful information about the tubal epithelium as well as the OSE as a source of disease. Future animal models are likely to specifically target the FTE to determine how ovulation and inherited risk factors, such as BRCA1 mutations, impact serous cancer development.
Acknowledgments
This work was supported by the Ovarian Cancer Research Fund Liz Tilberis (L/T/UIC/01.20211) and NIH CA139492.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Auersperg N, Maines-Bandiera SL, Dyck HG. Ovarian carcinogenesis and the biology of ovarian surface epithelium. J Cell Physiol. 1997;173:261–265. doi: 10.1002/(SICI)1097-4652(199711)173:2<261::AID-JCP32>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2):S19–32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 4.Gilks CB. Molecular abnormalities in ovarian cancer subtypes other than high-grade serous carcinoma. J Oncol. 2010;2010:740968. doi: 10.1155/2010/740968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auersperg N, Ota T, Mitchell GW. Early events in ovarian epithelial carcinogenesis: progress and problems in experimental approaches. Int J Gynecol Cancer. 2002;12:691–703. doi: 10.1046/j.1525-1438.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- 7.Scully RE. Early de novo ovarian cancer and cancer developing in benign ovarian lesions. Int J Gynaecol Obstet. 1995;49(Suppl):S9–15. doi: 10.1016/0020-7292(95)02404-z. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 9.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 10.Burdette JE, Kurley SJ, Kilen SM, Mayo KE, Woodruff TK. Gonadotropin-induced superovulation drives ovarian surface epithelia proliferation in CD1 mice. Endocrinology. 2006;147:2338–2345. doi: 10.1210/en.2005-1629. [DOI] [PubMed] [Google Scholar]
- 11.Auersperg N, Woo MM, Gilks CB. The origin of ovarian carcinomas: a developmental view. Gynecol Oncol. 2008;110:452–454. doi: 10.1016/j.ygyno.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 13.Wright JW, Pejovic T, Fanton J, Stouffer RL. Induction of proliferation in the primate ovarian surface epithelium in vivo. Hum Reprod. 2008;23:129–138. doi: 10.1093/humrep/dem347. [DOI] [PubMed] [Google Scholar]
- 14.Parrott JA, Doraiswamy V, Kim G, Mosher R, Skinner MK. Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol Cell Endocrinol. 2001;172:213–222. doi: 10.1016/s0303-7207(00)00340-3. [DOI] [PubMed] [Google Scholar]
- 15.Konishi I, Kuroda H, Mandai M. Review: gonadotropins and development of ovarian cancer. Oncology. 1999;57(Suppl 2):45–48. doi: 10.1159/000055274. [DOI] [PubMed] [Google Scholar]
- 16.Edmondson RJ, Monaghan JM, Davies BR. Gonadotropins mediate DNA synthesis and protection from spontaneous cell death in human ovarian surface epithelium. Int J Gynecol Cancer. 2006;16:171–177. doi: 10.1111/j.1525-1438.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarti S, Collins WP, Forecast JD, Newton JR, Oram DH, Studd JW. Hormonal profiles after the menopause. Br Med J. 1976;2:784–787. doi: 10.1136/bmj.2.6039.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch WJ. Ovulatory factor in ovarian carcinogenesis. Adv Exp Med Biol. 2008;622:119–128. doi: 10.1007/978-0-387-68969-2_10. [DOI] [PubMed] [Google Scholar]
- 20.Murdoch WJ, Townsend RS, McDonnel AC. Ovulation-induced DNA damage in ovarian surface epithelial cells of ewes: prospective regulatory mechanisms of repair/survival and apoptosis. Biol Reprod. 2001;65:1417–1424. doi: 10.1095/biolreprod65.5.1417. [DOI] [PubMed] [Google Scholar]
- 21.Burdette JE, Oliver RM, Ulyanov V, Kilen SM, Mayo KE, Woodruff TK. Ovarian epithelial inclusion cysts in chronically superovulated CD1 and Smad2 dominant-negative mice. Endocrinology. 2007;148:3595–3604. doi: 10.1210/en.2007-0030. [DOI] [PubMed] [Google Scholar]
- 22.Ghahremani M, Foghi A, Dorrington JH. Etiology of ovarian cancer: a proposed mechanism. Med Hypotheses. 1999;52:23–26. doi: 10.1054/mehy.1997.0620. [DOI] [PubMed] [Google Scholar]
- 23.Roh MH, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma and the dominant ovarian mass: clues to serous tumororigin? Am J Surg Pathol. 2009;33:376–383. doi: 10.1097/PAS.0b013e3181868904. [DOI] [PubMed] [Google Scholar]
- 24.Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, Lee Y, Crum CP. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 25.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 26.Sundfeldt K, Piontkewitz Y, Ivarsson K, Nilsson O, Hellberg P, Brannstrom M, Janson PO, Enerback S, Hedin L. E-cadherin expression in human epithelial ovarian cancer and normal ovary. Int J Cancer. 1997;74:275–280. doi: 10.1002/(sici)1097-0215(19970620)74:3<275::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA, Drapkin R. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auersperg N. The origin of ovarian carcinomas: a unifying hypothesis. Int J Gynecol Pathol. 2011;30:12–21. doi: 10.1097/PGP.0b013e3181f45f3e. [DOI] [PubMed] [Google Scholar]
- 30.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Press JZ, De Luca A, Boyd N, Young S, Troussard A, Ridge Y, Kaurah P, Kalloger SE, Blood KA, Smith M, Spellman PT, Wang Y, Miller DM, Horsman D, Faham M, Gilks CB, Gray J, Huntsman DG. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Press JZ, Wurz K, Norquist BM, Lee MK, Pennil C, Garcia R, Welcsh P, Goff BA, Swisher EM. Identification of a Preneoplastic Gene Expression Profile in Tubal Epithelium of BRCA1 Mutation Carriers. Neoplasia. 2010;12:993–1002. doi: 10.1593/neo.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cass I, Holschneider C, Datta N, Barbuto D, Walts AE, Karlan BY. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstet Gynecol. 2005;106:1327–1334. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- 34.Xian W, Miron A, Roh M, Semmel DR, Yassin Y, Garber J, Oliva E, Goodman A, Mehra K, Berkowitz RS, Crum CP, Quade BJ. The Li-Fraumeni syndrome (LFS): a model for the initiation of p53 signatures in the distal Fallopian tube. J Pathol. 2010;220:17–23. doi: 10.1002/path.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander BM, Van Kirk EA, Naughton LM, Murdoch WJ. Ovarian morphometrics in TP53-deficient mice. Anat Rec (Hoboken) 2007;290:59–64. doi: 10.1002/ar.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper TK, Gabrielson KL. Spontaneous lesions in the reproductive tract and mammary gland of female non-human primates. Birth Defects Res B Dev Reprod Toxicol. 2007;80:149–170. doi: 10.1002/bdrb.20105. [DOI] [PubMed] [Google Scholar]
- 37.Marr-Belvin AK, Bailey CC, Knight HL, Klumpp SA, Westmoreland SV, Miller AD. Ovarian pathology in rhesus macaques: a 12-year retrospective. J Med Primatol. 2010;39:170–176. doi: 10.1111/j.1600-0684.2010.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore CM, Hubbard GB, Leland MM, Dunn BG, Best RG. Spontaneous ovarian tumors in twelve baboons: a review ofovarian neoplasms in non-human primates. J Med Primatol. 2003;32:48–56. doi: 10.1034/j.1600-0684.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 39.Swisher E. Ovarian cancer associated with inherited mutations in BRCA1 or BRCA2. Curr Womens Health Rep. 2003;3:27–32. [PubMed] [Google Scholar]
- 40.Cline JM, Wood CE, Vidal JD, Tarara RP, Buse E, Weinbauer GF, de Rijk EP, van Esch E. Selected Background Findings and Interpretation of Common Lesions in the Female Reproductive System in Macaques. Toxicol Pathol. 2008;36:142s–163s. doi: 10.1177/0192623308327117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez GC, Nagarsheth NP, Lee KL, Bentley RC, Walmer DK, Cline M, Whitaker RS, Isner P, Berchuck A, Dodge RK, Hughes CL. Progestin-induced apoptosis in the Macaque ovarian epithelium: differential regulation of transforming growth factor-beta. J Natl Cancer Inst. 2002;94:50–60. doi: 10.1093/jnci/94.1.50. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez GC, Walmer DK, Cline M, Krigman H, Lessey BA, Whitaker RS, Dodge R, Hughes CL. Effect of progestin on the ovarian epithelium of macaques: cancer prevention through apoptosis? J Soc Gynecol Investig. 1998;5:271–276. doi: 10.1016/s1071-5576(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 43.Lu KH, Yates MS, Mok SC. The monkey, the hen, and the mouse: models to advance ovarian cancer chemoprevention. Cancer Prev Res (Phila Pa) 2009;2:773–775. doi: 10.1158/1940-6207.CAPR-09-0156. [DOI] [PubMed] [Google Scholar]
- 44.Barnes MN, Berry WD, Straughn JM, Kirby TO, Leath CA, Huh WK, Grizzle WE, Partridge EE. A pilot study of ovarian cancer chemoprevention using medroxyprogesterone acetate in an avian model of spontaneous ovarian carcinogenesis. Gynecol Oncol. 2002;87:57–63. doi: 10.1006/gyno.2002.6806. [DOI] [PubMed] [Google Scholar]
- 45.Fredrickson TN. Ovarian tumors of the hen. Environ Health Perspect. 1987;73:35–51. doi: 10.1289/ehp.877335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barua A, Bitterman P, Abramowicz JS, Dirks AL, Bahr JM, Hales DB, Bradaric MJ, Edassery SL, Rotmensch J, Luborsky JL. Histopathology of ovarian tumors in laying hens: a preclinical model of human ovarian cancer. Int J Gynecol Cancer. 2009;19:531–539. doi: 10.1111/IGC.0b013e3181a41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hakim AA, Barry CP, Barnes HJ, Anderson KE, Petitte J, Whitaker R, Lancaster JM, Wenham RM, Carver DK, Turbov J, Berchuck A, Kopelovich L, Rodriguez GC. Ovarian adenocarcinomas in the laying hen and women share similar alterations in p53, ras, and HER-2/neu. Cancer Prev Res (Phila) 2009;2:114–121. doi: 10.1158/1940-6207.CAPR-08-0065. [DOI] [PubMed] [Google Scholar]
- 48.Nofech-Mozes S, Khalifa MA, Ismiil N, Saad RS, Hanna WM, Covens A, Ghorab Z. Immunophenotyping of serous carcinoma of the female genital tract. Mod Pathol. 2008;21:1147–1155. doi: 10.1038/modpathol.2008.108. [DOI] [PubMed] [Google Scholar]
- 49.Nowee ME, Dorsman JC, Piek JM, Kosma VM, Hamalainen K, Verheijen RH, van Diest PJ. HER-2/neu and p27Kip1 in progression of Fallopian tube carcinoma: an immunohistochemical and array comparative genomic hybridization study. Histopathology. 2007;51:666–673. doi: 10.1111/j.1365-2559.2007.02850.x. [DOI] [PubMed] [Google Scholar]
- 50.Trevino LS, Giles JR, Wang W, Urick ME, Johnson PA. Gene Expression Profiling Reveals Differentially Expressed Genes in Ovarian Cancer of the Hen: Support for Oviductal Origin? Hormones and Cancer. 2010;1:177–186. doi: 10.1007/s12672-010-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenacre CB. Spontaneous tumors of small mammals. Vet Clin North Am Exot Anim Pract. 2004;7:627–651. vi. doi: 10.1016/j.cvex.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Gregson RL, Lewis DJ, Abbott DP. Spontaneous ovarian neoplasms of the laboratory rat. Vet Pathol. 1984;21:292–299. doi: 10.1177/030098588402100305. [DOI] [PubMed] [Google Scholar]
- 53.Sharrow AC, Ronnett BM, Thoburn CJ, Barber JP, Giuntoli RL, 2nd, Armstrong DK, Jones RJ, Hess AD. Identification and characterization of a spontaneous ovarian carcinoma in Lewis rats. J Ovarian Res. 2010;3:9. doi: 10.1186/1757-2215-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komatsu M, Fujita H. Electron-microscopic studies on the development and aging of the oviduct epithelium of mice. Anat Embryol (Berl) 1978;152:243–259. doi: 10.1007/BF00350523. [DOI] [PubMed] [Google Scholar]
- 55.Critoph FN, Dennis KJ. The cellular composition of the human oviduct epithelium. Br J Obstet Gynaecol. 1977;84:219–221. doi: 10.1111/j.1471-0528.1977.tb12559.x. [DOI] [PubMed] [Google Scholar]
- 56.Baird DT, Baker TG, McNatty KP, Neal P. Relationship between the secretion of the corpus luteum and the length of the follicular phase of the ovarian cycle. J Reprod Fertil. 1975;45:611–619. doi: 10.1530/jrf.0.0450611. [DOI] [PubMed] [Google Scholar]
- 57.Maronpot RR. Ovarian toxicity and carcinogenicity in eight recent National Toxicology Program studies. Environ Health Perspect. 1987;73:125–130. doi: 10.1289/ehp.8773125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai W, Oliveros-Saunders B, Wang Q, Acevedo-Duncan ME, Nicosia SV. Estrogen stimulation of ovarian surface epithelial cell proliferation. In Vitro Cell Dev Biol Anim. 2000;36:657–666. doi: 10.1290/1071-2690(2000)036<0657:ESOOSE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Umezu T, Hanazono M, Aizawa S, Tomooka Y. Characterization of newly established clonal oviductal cell lines and differential hormonal regulation of gene expression. In Vitro Cell Dev Biol Anim. 2003;39:146–156. doi: 10.1007/s11626-003-0009-9. [DOI] [PubMed] [Google Scholar]
- 60.Ziecik AJ, Kaczmarek MM, Blitek A, Kowalczyk AE, Li X, Rahman NA. Novel biological and possible applicable roles of LH/hCG receptor. Mol Cell Endocrinol. 2007;269:51–60. doi: 10.1016/j.mce.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Tunca JC, Erturk E, Bryan GT. Chemical induction of ovarian tumors in rats. Gynecol Oncol. 1985;21:54–64. doi: 10.1016/0090-8258(85)90232-x. [DOI] [PubMed] [Google Scholar]
- 62.Stewart SL, Querec TD, Ochman AR, Gruver BN, Bao R, Babb JS, Wong TS, Koutroukides T, Pinnola AD, Klein-Szanto A, Hamilton TC, Patriotis C. Characterization of a carcinogenesis rat model of ovarian preneoplasia and neoplasia. Cancer Res. 2004;64:8177–8183. doi: 10.1158/0008-5472.CAN-04-1702. [DOI] [PubMed] [Google Scholar]
- 63.Crist KA, Zhang Z, You M, Gunning WT, Conran PB, Steele VE, Lubet RA. Characterization of rat ovarian adenocarcinomas developed in response to direct instillation of 7,12-dimethylbenz[a]anthracene (DMBA) coated suture. Carcinogenesis. 2005;26:951–957. doi: 10.1093/carcin/bgi039. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Zhang Z, Lu Y, Yao R, Jia D, Wen W, LaRegina M, Crist K, Lubet R, You M. Enhanced susceptibility to chemical induction of ovarian tumors in mice with a germ line p53 mutation. Mol Cancer Res. 2008;6:99–109. doi: 10.1158/1541-7786.MCR-07-0216. [DOI] [PubMed] [Google Scholar]
- 65.Jarboe EA, Pizer ES, Miron A, Monte N, Mutter GL, Crum CP. Evidence for a latent precursor (p53 signature) that may precede serous endometrial intraepithelial carcinoma. Mod Pathol. 2009;22:345–350. doi: 10.1038/modpathol.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corney DC, Flesken-Nikitin A, Choi J, Nikitin AY. Role of p53 and Rb in ovarian cancer. Adv Exp Med Biol. 2008;622:99–117. doi: 10.1007/978-0-387-68969-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gowen LC, Johnson BL, Latour AM, Sulik KK, Koller BH. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat Genet. 1996;12:191–194. doi: 10.1038/ng0296-191. [DOI] [PubMed] [Google Scholar]
- 68.Garson K, Shaw TJ, Clark KV, Yao DS, Vanderhyden BC. Models of ovarian cancer--are we there yet? Mol Cell Endocrinol. 2005;239:15–26. doi: 10.1016/j.mce.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 69.Boyd J. Mouse models of gynecologic pathology. N Engl J Med. 2005;352:2240–2242. doi: 10.1056/NEJMcibr051024. [DOI] [PubMed] [Google Scholar]
- 70.Buller RE, Lallas TA, Shahin MS, Sood AK, Hatterman-Zogg M, Anderson B, Sorosky JI, Kirby PA. The p53 mutational spectrum associated with BRCA1 mutant ovarian cancer. Clin Cancer Res. 2001;7:831–838. [PubMed] [Google Scholar]
- 71.Clark-Knowles KV, Senterman MK, Collins O, Vanderhyden BC. Conditional inactivation of Brca1, p53 and Rb in mouse ovaries results in the development of leiomyosarcomas. PLoS One. 2009;4:e8534. doi: 10.1371/journal.pone.0008534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark-Knowles KV, Garson K, Jonkers J, Vanderhyden BC. Conditional inactivation of Brca1 in the mouse ovarian surface epithelium results in an increase in preneoplastic changes. Exp Cell Res. 2007;313:133–145. doi: 10.1016/j.yexcr.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 73.Quinn BA, Brake T, Hua X, Baxter-Jones K, Litwin S, Ellenson LH, Connolly DC. Induction of ovarian leiomyosarcomas in mice by conditional inactivation of Brca1 and p53. PLoS One. 2009;4:e8404. doi: 10.1371/journal.pone.0008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003;63:3459–3463. [PubMed] [Google Scholar]
- 75.Laviolette LA, Garson K, Macdonald EA, Senterman MK, Courville K, Crane CA, Vanderhyden BC. 17beta-estradiol accelerates tumor onset and decreases survival in a transgenic mouse model of ovarian cancer. Endocrinology. 2010;151:929–938. doi: 10.1210/en.2009-0602. [DOI] [PubMed] [Google Scholar]
- 76.Tanyi JL, Lapushin R, Eder A, Auersperg N, Tabassam FH, Roth JA, Gu J, Fang B, Mills GB, Wolf J. Identification of tissue-and cancer-selective promoters for the introduction of genes into human ovarian cancer cells. Gynecol Oncol. 2002;85:451–458. doi: 10.1006/gyno.2002.6644. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Huang G, Shornick LP, Roswit WT, Shipley JM, Brody SL, Holtzman MJ. A transgenic FOXJ1-Cre system for gene inactivation in ciliated epithelial cells. Am J Respir Cell Mol Biol. 2007;36:515–519. doi: 10.1165/rcmb.2006-0475RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyoshi I, Takahashi K, Kon Y, Okamura T, Mototani Y, Araki Y, Kasai N. Mouse transgenic for murine oviduct-specific glycoprotein promoter-driven Simian Virus 40 large T-antigen: tumor formation and its hormonal regulation. Mol Reprod Dev. 2002;63:168–176. doi: 10.1002/mrd.10175. [DOI] [PubMed] [Google Scholar]
- 79.Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIRpromoter develop epithelial ovarian cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 80.Quinn BA, Xiao F, Bickel L, Martin L, Hua X, Klein-Szanto A, Connolly DC. Development of a syngeneic mouse model of epithelial ovarian cancer. J Ovarian Res. 2010;3:24. doi: 10.1186/1757-2215-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A. 2011;108:7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chodankar R, Kwang S, Sangiorgi F, Hong H, Yen HY, Deng C, Pike MC, Shuler CF, Maxson R, Dubeau L. Cell-nonautonomous induction of ovarian and uterine serous cystadenomas in mice lacking a functional Brca1 in ovarian granulosa cells. Curr Biol. 2005;15:561–565. doi: 10.1016/j.cub.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 83.Zheng W, Magid MS, Kramer EE, Chen YT. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am J Pathol. 1996;148:47–53. [PMC free article] [PubMed] [Google Scholar]
- 84.Mishina Y, Whitworth DJ, Racine C, Behringer RR. High specificity of Mullerian-inhibiting substance signaling in vivo. Endocrinology. 1999;140:2084–2088. doi: 10.1210/endo.140.5.6705. [DOI] [PubMed] [Google Scholar]
- 85.Bristol-Gould SK, Hutten CG, Sturgis C, Kilen SM, Mayo KE, Woodruff TK. The development of a mouse model of ovarian endosalpingiosis. Endocrinology. 2005;146:5228–5236. doi: 10.1210/en.2005-0697. [DOI] [PubMed] [Google Scholar]
- 86.Liang S, Yang N, Pan Y, Deng S, Lin X, Yang X, Katsaros D, Roby KF, Hamilton TC, Connolly DC, Coukos G, Zhang L. Expression of activated PIK3CA in ovarian surface epithelium results in hyperplasia but not tumor formation. PLoS One. 2009;4:e4295. doi: 10.1371/journal.pone.0004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mullany LK, Fan HY, Liu Z, White LD, Marshall A, Gunaratne P, Anderson ML, Creighton CJ, Xin L, Deavers M, Wong KK, Richards JS. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene. 2011 doi: 10.1038/onc.2011.70. Epub ahead of print March 21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 89.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]