Abstract
Dry eye disease is a multifactorial disorder of the tears and ocular surface characterized by symptoms of dryness and irritation. Although the pathogenesis of dry eye disease is not fully understood, it is recognized that inflammation has a prominent role in the development and propagation of this debilitating condition. Factors that adversely affect tear film stability and osmolarity can induce ocular surface damage and initiate an inflammatory cascade that generates innate and adaptive immune responses. These immunoinflammatory responses lead to further ocular surface damage and the development of a self-perpetuating inflammatory cycle. Herein, we review the fundamental links between inflammation and dry eye disease and discuss the clinical implications of inflammation in disease management.
INTRODUCTION
Dry eye disease (DED), also known as keratoconjunctivitis sicca, is a multifactorial disorder of the tears and ocular surface.1 Common symptoms of DED include dryness, irritation, foreign body sensation, light sensitivity, and itching. It is estimated that almost 5 million Americans 50 years and older have DED, and millions more experience episodic symptoms of dry eye2; of these, approximately two-thirds are women.3– 4 The prevalence of DED rises dramatically with increasing age, and as older populations grow, so too will the burden of DED-associated morbidity.5 Dry eye disease can hinder the performance of activities of daily living, and DED is associated with an overall decrease in quality of life.6 Patients with DED are significantly more likely than the general population to experience symptoms of anxiety and depression.7 Risk factors for the development of DED include advanced age, female sex, hormonal imbalance, autoimmune disease, abnormal corneal innervation, vitamin deficiency, environmental stress, contact lens use, infection, medication use, and ophthalmic surgery.1 The pathogenesis of DED is not fully understood; however, it is recognized that inflammation has a prominent role in the development and amplification of the signs and symptoms of DED.
IMMUNOPATHOGENESIS OF DRY EYE
Immunoinflammatory Pathways
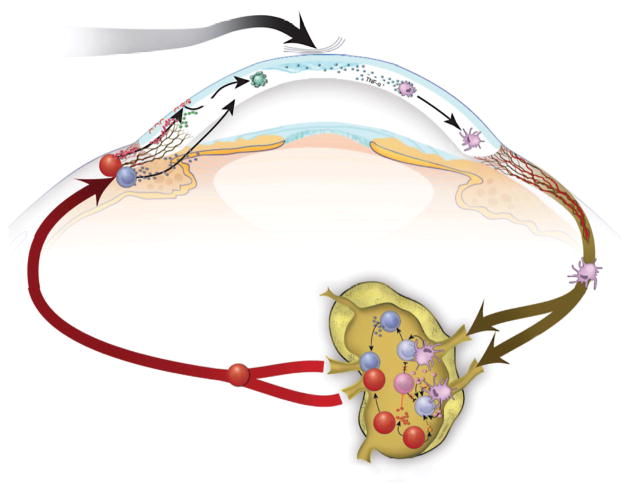
The ocular surface system consists of the cornea, conjunctiva, lacrimal glands, meibomian glands, nasolacrimal duct, and their associated tear and connective tissue matrices, as well as the eyelids and eyelashes, all integrated by continuous epithelia and interconnected nervous, endocrine, immune, and vascular systems.8 Factors that disturb the delicate homeostatic balance of the ocular surface system can adversely affect tear film stability and osmolarity, resulting in osmotic, mechanical, and inflammatory damage.9 Exposure of ocular surface epithelial cells to elevated tear osmolarity activates stress-associated mitogen-activated protein kinases, such as c-Jun N-terminal kinase, extracellular signal–related kinase, and p38.10– 12 Mitogen-activated protein kinase signaling pathways stimulate the transcription factors nuclear factor κB and activator protein 1, thereby initiating the production of proinflammatory cytokines, chemokines, and matrix metalloproteinases (MMPs).12 These inflammatory mediators promote the activation (maturation) of immature antigen-presenting cells (APCs) and induce their migration to draining lymphoid tissues (Figure 1). The APCs are responsible for priming naive T cells in the lymphoid compartment, leading to the expansion of autoreactive CD4+ helper T cell (TH) subtype 1 and TH17 cell subsets. 13– 14 T cells subsequently infiltrate the ocular surface, where they secrete additional proinflammatory cytokines. Helper T cell subtype 1–secreted interferon (IFN) γ upregulates the production of chemokines, chemokine receptors, and cell adhesion molecules (CAMs) that facilitate the ingress of pathogenic immune cells, including TH17 cells that secrete interleukin (IL) 17, which further promotes epithelial damage by stimulating the production of proinflammatory cytokines and MMPs. Regardless of the origin, a self-perpetuating cycle of inflammation develops that is central to the pathogenesis of DED.
Figure 1.
Immunoinflammatory pathways. Desiccating stress induces tear hyperosmolarity, activating intracellular signaling pathways that initiate the production of proinflammatory cytokines (eg, interleukin [IL] 1, tumor necrosis factor [TNF], and IL-6). This proinflammatory milieu facilitates the activation and maturation of immature antigen-presenting cells (iAPC). Mature APCs (mAPC) migrate through the afferent lymphatics to draining lymph nodes, where they induce effector helper T cell 1 (TH1) and TH17 cells that subsequently migrate through efferent blood vessels to the ocular surface. The TH17 cells antagonize regulatory T cell (Treg) functions and lead to further expansion of T effectors in the draining lymph nodes. Effector TH1-secreted interferon (IFN) γ and TH17-secreted IL-17 exert their pathogenic effects by promoting the production of proinflammatory cytokines, chemokines, matrix metalloproteinases (eg, MMP-3 and MMP-9), cell adhesion molecules (CAM), and prolymphangiogenic molecules (vascular endothelial growth factor [VEGF] D and VEGF-C) that facilitate the infiltration of pathogenic immune cells, leading to further damage of the ocular surface. IL-17R indicates IL-17 receptor; TGF, transforming growth factor.
Epitheliopathy
Epitheliopathy is one of the most easily recognizable clinical features of DED. Staining the ocular surface with diagnostic dyes, such as fluorescein, rose bengal, and lissamine green, provides a practical method for evaluating ocular surface integrity. Dry eye disease increases epithelial cell density and thickness, decreases epithelial cell size, and increases epithelial cell turnover.15– 16 Inflammation of the ocular surface is intimately linked to this epithelial dysfunction. The proinflammatory cytokines IL-1 and IFN-γ cause squamous metaplasia of ocular surface epithelial cells, and IFN-γ decreases goblet cell differentiation.17– 18 Apoptosis of ocular surface cells in DED can be induced by intrinsic (stress-associated mitogen-activated protein kinase) and extrinsic (tumor necrosis factor [TNF] and Fas/Fas ligand) pathways.19– 20 The MMPs (eg, MMP-9) are produced in response to desiccating stress and promote corneal extracellular matrix degradation and epithelial cell loss.21 Helper T cell subtype 17–secreted IL-17 was recently shown to disrupt corneal epithelial barrier function.14
Lymphangiogenesis
Traditionally, angiogenesis has been thought to involve lymphangiogenesis and hemangiogenesis, producing afferent lymphatic vessels and efferent blood vessels, respectively. Dry eye disease involves a unique form of pathologic angiogenesis that produces lymphangiogenesis without associated hemangiogenesis, and this is observed in experimental and clinical settings. The presence of lymphatic endothelial marker 1–staining monocytic cells in the conjunctiva has been described and linked to the growth of lymphatic vessels into the cornea.22
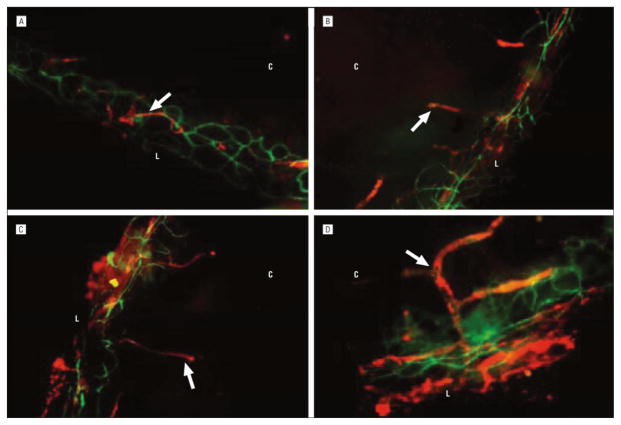
Immunohistochemical analysis of dry eye corneas identified the infiltration of lymphatic endothelial marker 1–expressing macrophages and lymphatic vessels (Figure 2).23 Dry eye induction increases the expression of factors that promote lymphangiogenesis, including vascular endothelial growth factor (VEGF) C and VEGF-D and associated receptors VEGFR-2 and VEGFR-3. Vascular endothelial growth factor A is also upregulated, contributing to lymphangiogenesis through the recruitment of VEGF-C–and VEGF-D–producing macrophages. In addition, recent data suggest that TH17-secreted IL-17 upregulates expression of VEGF-C and VEGF-D and promotes corneal lymphangiogenesis in DED.24 Pathogenic immune cells are present in the regional lymph nodes of DED-induced mice.13 The identification of newly formed lymphatic vessels in the cornea provides a potential route by which antigens and APCs can travel from the ocular surface to these draining lymph nodes. Accordingly, blockage of lymphangiogenesis may prove to be an effective treatment for DED.25
Figure 2.
Analysis of corneal lymphangiogenesis in normal eye (A) and in dry eye on day 6 (B), day 10 (C), and day 14 (D) (original magnification ×100). The lymphatic vessels (arrows) increased in area and caliber and progressed toward the center of the cornea with disease progression. The lymphatic vessels were unaccompanied by blood vessels (CD31 high and lymphatic endothelial marker 1 negative). L indicates limbus; C, center of the cornea. Adapted from Goyal et al.
Neuropathy
The corneal epithelium has approximately 7000 nerve endings per square millimeter, making the cornea one of the most densely innervated tissues in the human body.26 The nervous system is an important component of the ocular surface system, and optimal functioning of the corneal nerves is essential for the maintenance of a healthy ocular surface. In healthy corneas, nerve endings are located between epithelial wing cell layers, where they are protected from external stimuli.27 Decreased density and altered morphologic structure of the subbasal nerves have been reported in DED-induced corneas.28– 33 These abnormalities are generally found to correlate with DED severity. Elevated tear osmolarity induces inflammatory-mediated epitheliopathy that results in the exposure of corneal nerves to mechanical and inflammatory insults. Inflammatory cytokines in turn increase the synthesis of neurotrophic factors that stimulate nerve growth, potentially explaining the altered nerve morphologic structure (nerve sprouts, tortuosity, and thinning) commonly observed in DED.34 Corneal nerve abnormalities lead to further ocular surface damage and help perpetuate the vicious inflammatory cycle of DED.35
FUNDAMENTAL LINKS BETWEEN INFLAMMATION AND DRY EYE
MOLECULAR MEDIATORS
Cytokines
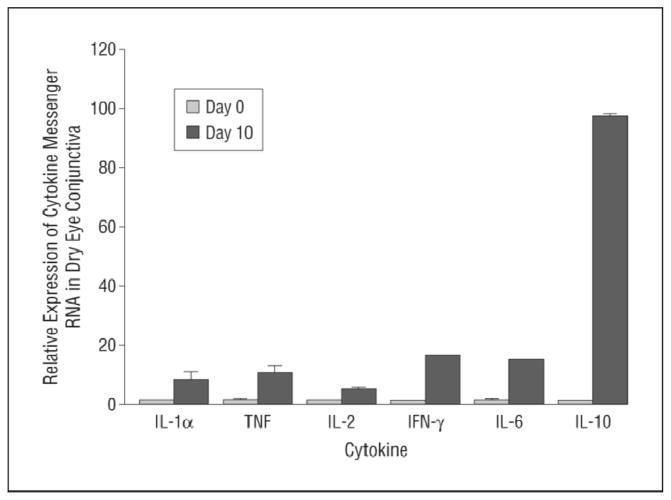
Cytokines are signaling molecules that mediate intercellular communication. The production of proinflammatory cytokines is upregulated by osmotic, inflammatory, and mechanical damage. The term interleukin alludes to intercellular communication between leukocytes; however, many different cell types are capable of producing and responding to cytokines. For example, virtually all nucleated cells, including epithelial cells of the ocular surface, are capable of producing IL-1, IL-6, and TNF.36 Clinical studies37– 42 consistently report elevated levels of these cytokines in the tears of patients with DED. Similar trends are noted in the conjunctival epithelium, which contains elevated levels of IL-1, IL-6, TNF, and transforming growth factor β1.41,43 Several additional cytokines have been isolated from the ocular surface of patients with DED, including IL-2, IL-4, IL-5, IL-10, and IFN-γ.41 These clinical findings have been corroborated by studies44– 46 involving experimental models of DED (Figure 3).
Figure 3.
Real-time polymerase chain reaction results showing increased relative expression of various cytokine transcripts in dry eye conjunctiva (day 10) compared with normal conjunctiva. Data are presented as the mean (SE) (error bars) (n = 18 for interleukin [IL] 1α and tumor necrosis factor [TNF] and n = 6 for the remaining cytokines). IFN-γ indicates interferon γ. Adapted from Rashid et al.
Chemokines
Chemokines are cytokines that regulate the chemotaxis, or directed migration, of immune cells. The chemokine IL-8 (CXCL8) is consistently identified in the tears and conjunctiva of patients with DED.40– 43 Interleukin 8 can be produced by any cell with a toll-like receptor (eg, epithelial cells and macrophages), and it is a neutrophil chemoattractant involved in the innate immune response. The closely related chemokines CXCL9, CXCL10, and CXCL11 are elevated in the tear film and ocular surface of patients with DED.42,47– 49 These latter chemokines are produced in response to IFN-γ and function as T-cell chemoattractants on binding to the chemokine (CXCR3 motif) receptor. Animal models of DED provide further evidence of chemokine activity. The chemokine (CC motif) ligands CCL3 (macrophage inflammatory protein 1α) and CCL4 (macrophage inflammatory protein 1β) are upregulated in DED.48– 49 These chemokines are produced by macrophages, recruit inflammatory cells (such as neutrophils), and upregulate the production of proinflammatory cytokines. Another potent T-cell chemoattractant, CCL5 (RANTES), is upregulated in DED.40,49 The chemokine receptors CCR1, CCR2, CCR5, and CXCR3 have been implicated in the pathogenesis of DED and represent promising targets for immunomodulation.50– 52
Matrix Metalloproteinases
Matrix metalloproteinases are endopeptidases involved in tissue remodeling. Corneal epithelial cells produce MMP-1, MMP-3, MMP-9, and MMP-13 in response to hyperosmolar stress.53 Experimental dry eye increases MMP-1, MMP-3, MMP-9, and MMP-10 levels in the tears and corneal epithelium of mice.21 Elevated levels of pro–MMP-9 and increased activity of MMP-9 have been identified in the tears of patients with dry eye.38,54– 55 Knockout of MMP-9 tempers the severity of experimental dry eye, implicating MMP-9 in the pathogenesis of DED.56 Not only is MMP-9 produced by granulocytes, but it is also involved in the activation of latent IL-1.38,57 The MMPs are thought to modulate the severity of DED by promoting corneal extracellular matrix degradation and epithelial cell loss.16,21 Doxycycline, a tetracycline derivative, ameliorates DED by inhibiting the activity of MMPs, primarily MMP-9, promoting ocular surface integrity. 16,58
Major Histocompatibility Complex Class II and Costimulatory Molecules
The expression of various cell-associated immunomodulatory molecules is increased in DED. The ocular surface of patients with dry eye contains elevated levels of HLA type DR (HLADR), CD40, CD154 (CD40L), CD80, CD86, Fas, and Fas ligand.59– 65 HLADR is a major histocompatibility complex (MHC) class II cell surface receptor involved in antigen presentation. CD40, CD154, CD80, and CD86 are costimulatory molecules involved in APC–T-cell interactions. Increased expression of these molecules suggests that antigen (presumably autoantigen) presentation occurs efficiently in DED. Fas is a death receptor that induces apoptosis on binding to Fas ligand. The presence of these molecules in the conjunctiva and lacrimal glands of patients with dry eye is indicative of cellular infiltration, as these molecules are responsible for regulating the activity of immune cells.
Adhesion Molecules
Cell adhesion molecules are cell surface proteins that facilitate cellular migration by binding to extracellular matrix components. Cell adhesion molecules promote the infiltration of immune cells into the ocular surface of patients with dry eye. Elevated levels of intercellular adhesion molecule 1 and vascular CAM-1 have been identified in the conjunctiva and lacrimal glands of patients with dry eye.66– 68 Intercellular adhesion molecule 1 binds to lymphocyte function–associated antigen 1. Vascular CAM-1 is expressed by the vascular endothelium and binds to immune cell–expressed very late antigen 4, also known as integrin α4β1. Treatment with monoclonal antibodies against murine intercellular adhesion molecule 1 and lymphocyte function–associated antigen 1 resulted in decreased ocular surface inflammatory infiltrates in experimental DED.66 Topical inhibition of very late antigen 4 decreases dry eye severity and suppresses inflammation in a murine model of DED.69
CELLULAR MEDIATORS
Antigen-Presenting Cells
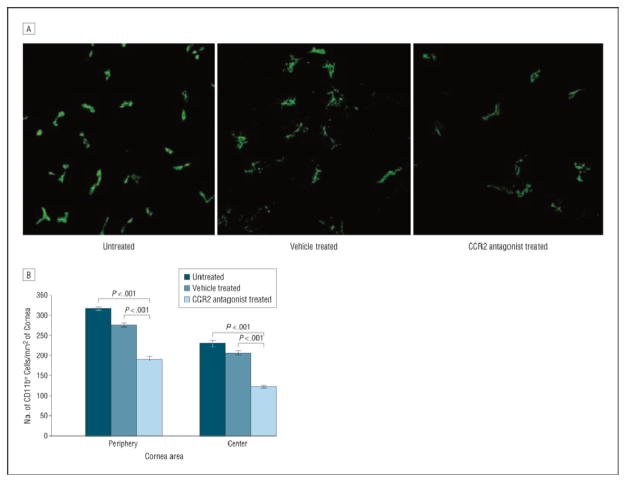
Antigen-presenting cells are sentinel cells of the immune system that respond to danger signals (eg, microbial pathogens) by internalizing, processing, and presenting antigens.70 The phenotype, or state of maturation, of an APC determines its function. Immature APCs express low levels of MHC class II and costimulatory molecules (eg, CD80 [B7.1]); although immature APCs are proficient at capturing antigens, they are inefficient at presenting antigens and promoting T-cell activation. Inflammatory microenvionments can induce APC maturation via increased expression of MHC class II and costimulatory molecules, rendering APCs efficient at priming T cells.71 Antigen-presenting cells of the ocular surface include monocytes and macrophages, dendritic cells (DCs), and Langerhans cells (LCs). The LCs are the only cells in the corneal epithelium that constitutively express MHC class II.72 The peripheral corneal epithelium contains MHC class II–positive and MHC class II–negative LCs, while the central cornea contains only MHC class II–negative, costimulatory molecule–negative LCs; however, LCs of the central cornea are capable of expressing MHC class II and costimulatory molecules following inflammatory stimuli.73 The anterior corneal stroma contains differentiated and undifferentiated resident monocytic cell–derived DCs.73– 74 Recently, the presence of a unique population of (non-LC) langerin-positive DCs was reported in the corneal stroma.75 In contrast, MHC class II–positive DCs are abundantly present in the conjunctiva. In vivo microscopy reveals that DED dramatically increases the presence of DCs in the central corneal epithelium of patients.76 Similarly, in DED-induced murine corneas, there is evidence of an influx of CD11b+ APCs (Figure 4). The APC-mediated priming of TH1 and TH17 cells against autoantigens has been proposed as a potential source of autoimmunity in DED.77
Figure 4.
Enumeration of corneal CD11b+ monocytes. A, Representative confocal images of the center of whole-mount corneas showing CD11b+ cells (green) in untreated and vehicle-treated eyes and in eyes treated with a topical chemokine receptor 2 (CCR2) antagonist. B, Treatment with topical CCR2 antagonist significantly decreased the number of CD11b+ cells in the periphery and the center of corneas with dry eye compared with the untreated and vehicle-treated groups. Bars represent the mean values; limit lines, SEMs. Adapted from Goyal et al.
Effector T Cells
T-cell infiltration of the ocular surface is a pervasive finding in DED (Figure 5). CD4+ T cells, including IFN-γ–secreting TH1 cells and IL-17–secreting TH17 cells, are thought to be the primary effector T cells of DED.13– 14 Although the relative contributions of TH1 and TH17 cells remain unclear, recent findings suggest that TH17 cells have a prominent role in the pathogenesis of DED.14,77– 79 This is an important finding, particularly given that TH1 and TH17 cells differentiate via divergent pathways. Elevated expression of IL-6 has long been recognized in DED; however, the role of IL-6 in the pathogenesis of DED has been largely unknown. It is understood that IL-6 and transforming growth factor β promote the differentiation of TH17 cells, while transforming growth factor β suppresses TH1-mediated responses.78 The attenuating effects of CD4+CD25+Foxp3+ regulatory T cells (Tregs) have been described in models of ocular surface inflammation, but the inability of Tregs to suppress DED has been incompletely explained. It was recently demonstrated that the TH17, but not TH1, cell subset is resistant and functionally antagonistic to Treg-mediated suppression in DED, and in vivo blockade of IL-17 significantly decreases DED severity.78
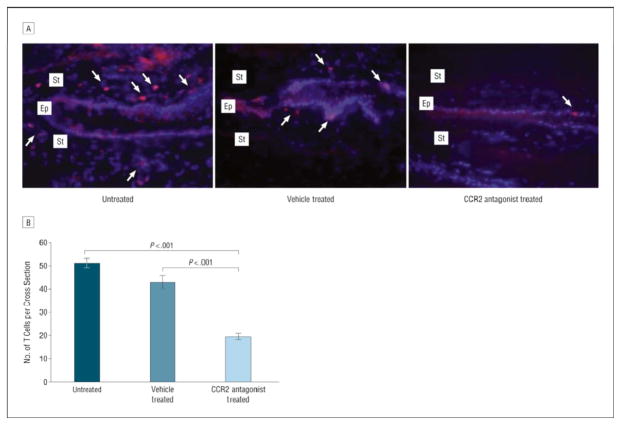
Figure 5.
Enumeration of conjunctival T cells. A, Representative images of conjunctival cross sections immunostained for CD3 (red) and nucleus (blue) of untreated and vehicle-treated eyes and of eyes treated with a topical chemokine receptor 2 (CCR2) antagonist (T cells are marked by arrows). Ep indicates epithelial layer; St, stromal layer. B, Treatment with topical CCR2 antagonist significantly decreased the number of conjunctival T cells compared with the untreated and vehicle-treated groups. Bars represent the mean values; limit lines, SEMs. Adapted from Goyal et al.
Regulatory T Cells
Regulatory T cells are a distinct family of T cells involved in the suppression of immune responses. Treg abnormalities in systemic autoimmune diseases associated with DED, such as Sjögren syndrome, systemic lupus erythematosus, and rheumatoid arthritis, have long been recognized.80 Treg abnormalities were recently implicated in the development of experimental DED. Nude mice that lack CD4+CD25+Foxp3+ Tregs were adoptively transferred with DED-primed T cells and subsequently developed Sjögren syndrome–like DED.81 Tregs have been shown to suppress DED-associated ocular surface inflammation.82 The resistance of effector T cells, particularly TH17 cells, to Treg-mediated suppression has been identified as an important factor in the pathogenesis of DED.78
Natural Killer Cells
Natural killer (NK) cells are large granular lymphocytes capable of secreting proinflammatory cytokines and killing infected or transformed cells. Although NK cells, T cells, and B cells are derived from common bone marrow–derived lymphoid progenitors, they differ significantly in phenotype and function. Natural killer cells have been implicated in the pathogenesis of various autoimmune diseases; however, little is known about the function of NK cells in DED. Investigations of NK cell activity and frequency in Sjögren syndrome have yielded varying results.83– 87 Natural killer cells do not seem to infiltrate the conjunctival epithelium of patients with dry eye.88 However, IFN-γ–secreting NK cells located in draining lymph nodes have been implicated in the early development of experimental DED.89
CLINICAL IMPLICATIONS OF INFLAMMATION IN DRY EYE
INFLAMMATION AS A MEASURE OF CLINICAL DISEASE
Numerous tests and guidelines are available to help direct the clinical management of DED.90 Unfortunately, decision making is often complicated by a lack of concordance among the signs and symptoms of DED.91 Some of the techniques being used to investigate inflammation and dry eye in the experimental setting may one day be available in the clinical setting to help overcome this incongruity. As previously described, many markers of inflammation can be identified in the tears and conjunctiva of patients with DED. Some of these markers, including IL-6 and HLADR, correlate with clinical measures of disease severity and treatment efficacy.92– 93 In vivo confocal microscopy is being used to examine the effects of ocular surface inflammation on immune cell infiltration and on morphologic structure of epithelial cells, subbasal nerves, and meibomian glands.35,76,94– 95 As experimental techniques that evaluate ocular surface inflammation are further refined, they may become valuable tools in the physician’s options. Diagnostic methods that combine conventional tests with experimental measures of inflammation (eg, the scraping cytology score system96) have been proposed, potentially bridging the gap between bench and bedside.
ANTI-INFLAMMATORY TREATMENT
Cyclosporine A
Cyclosporine A exerts immunosuppressive activity through several pathways. It forms a complex with cyclophilin that inhibits the calcineurin phosphatase pathway responsible for the transcription of T-cell–activating cytokines (such as IL-2).97 Cyclosporine A binds cyclophilin D, inhibiting the activity of the mitochondrial permeability transition pore and subsequent cytochrome c–mediated apoptosis. The immunomodulatory activity of cyclosporine A is used in the treatment of immune-based disorders, such as transplant rejection, psoriasis, ulcerative colitis, rheumatoid arthritis, and DED. Topical administration of cyclosporine A has been shown to increase tear fluid secretion, possibly by promoting the local release of parasympathetic nervous system–associated neurotransmitters.98 The beneficial effects of cyclosporine A treatment in DED are well established; however, it is clear that many patients with DED do not show a consistent therapeutic response to topical cyclosporine A. The cumulative findings of several clinical trials indicate that long-term treatment with cyclosporine A, 0.05%, ophthalmic emulsion can yield positive results with regard to objective and subjective findings, including corneal surface staining, Schirmer test with anesthesia, blurred vision, and frequency of artificial tear application.99– 100 In addition, topical cyclosporine A treatment may be associated with a significant improvement in many of the cellular and molecular markers of disease severity.92– 93,101 Increased frequency of topical cyclosporine A administration may be of benefit to patients refractory to the standard dosing regimen.102 Although higher dosing frequencies may increase treatment efficacy, some patients experience bothersome adverse effects (eg, burning or irritation) that impair medication tolerability.
Corticosteroids
Corticosteroids are steroid hormones that can be used to suppress inflammation. Corticosteroids bind to glucocorticoid receptors and inhibit the expression of proinflammatory molecules, promote the expression of anti-inflammatory molecules, and stimulate the apoptosis of lymphocytes.103– 109 Experimental studies108– 110 have demonstrated the efficacy of corticosteroids in the treatment of DED at the cellular, molecular, and clinical levels. Clinical trials have demonstrated the efficacy of topical corticosteroid treatment at diminishing symptom severity and minimizing ocular surface staining.111– 113 Systemic corticosteroid administration may also be effective in the treatment of severe acute dry eye refractive to more traditional treatment modalities.114 Unfortunately, long-term topical or systemic corticosteroid use is associated with deleterious adverse effects, such as ocular hypertension, cataracts, and opportunistic infections. Repetitive short-term pulsatile administration of topical corticosteroids is a promising method of harnessing their beneficial effects, while minimizing the risk of adverse events.115
Tetracycline Derivatives
Tetracycline derivatives are unique in that they possess antibacterial and anti-inflammatory properties. They exert antibacterial activity by reversibly binding to the bacterial ribosome and inhibiting the production of proteins. Tetracycline derivatives have been noted to possess immunomodulatory properties at submicrobial doses. Experimental investigations have demonstrated that the tetracycline derivative doxycycline can inhibit c-Jun N-terminal kinase and extracellular signal–related kinase mitogen-activated protein kinase signaling in epithelial cells of the ocular surface exposed to hyperosmolar stress, downregulating the expression of CXCL8 and proinflammatory cytokines IL-1β and TNF.103 Doxycycline inhibits the activity of MMPs (eg, MMP-9) and supports ocular surface integrity.58,109 In addition, the tetracycline-derivative minocycline inhibits the expression of cell-associated proinflammatory molecules, including MHC class II.116 A novel topically applied liposome-bound form of tetracycline has shown some promise in the treatment of experimental DED.117 The anti-inflammatory benefits of orally administered tetracycline derivatives have been used in the treatment of chronic immunomediated diseases, including dry eye secondary to ocular rosacea and blepharitis.118– 122 Despite extensive evidence from experimental trials indicating the potential benefits of administration of tetracycline derivatives in the treatment of DED, there is limited clinical evidence of their efficacy.
Essential Fatty Acids
Essential fatty acids (EFAs) are biologically necessary fatty acids (FAs) that must be ingested because they cannot be synthesized de novo by the human body. Humans require 2 EFAs for optimal health, -3 (α-linolenic acid) and -6 (linoleic acid) FAs. Essential fatty acids are the precursors of eicosanoids (prostaglandins, prostacyclins, thromboxanes, and leukotrienes) that modulate immune responses. Omega-3 FAs are generally classified as anti-inflammatory, while -6 FAs are generally proinflammatory.123 Omega-3 FAs block the production of proinflammatory eicosanoids (prostaglandin E2 and leukotriene B4) and cytokines (IL-1 and TNF). 124– 125 The anti-inflammatory effects of -3 FAs have been used in the treatment of immunomediated disorders, including Sjögren syndrome. Investigations on the use of EFAs in the treatment of DED have produced conflicting results; however, most of the available evidence suggests that administration of EFAs, particularly -3 FAs, can lessen DED severity.126– 131 Several clinical trials have investigated the systemic administration of various EFAs and demonstrated beneficial effects with regard to the signs and symptoms of DED.126– 129 Topical administration of -3 FAs significantly decreased ocular surface staining, cytokine expression, and immune cell infiltration in an experimental model of murine dry eye.45 Topical administration of resolvin E1, an -3 FA derivative, increased tear production, helped maintain ocular surface integrity, decreased cyclooxygenase 2 expression, and decreased immune cell infiltration in experimental dry eye.132 Available data suggest that EFAs can ameliorate DED; however, more evidence is needed to identify the most efficacious forms and doses of EFAs.
Novel Therapeutics
The past several years have yielded a veritable explosion of new information about the immunopathogenesis of DED. The successful application of cyclosporine in the clinical management of DED has implicated the inflammatory mediators of DED as promising targets for therapeutic intervention. A thorough review of the therapeutic agents being investigated is beyond the scope of this article. Our laboratory has been involved in the evaluation of anti-inflammatory agents using a short-term experimental model of murine dry eye. Positive results have been reported using various therapeutic agents that target inflammatory mediators, including CCR2, very late antigen 4, and IL-17, to name a few.52,69,78 Other laboratories have reported positive results using medications that inhibit inflammation. Needless to say, interest in evaluating potential therapeutic agents for DED has increased exponentially.
In conclusion, the evidence implicating inflammation in the pathogenesis of DED has opened new avenues for the treatment of this complex disorder. The successful application of anti-inflammatory medications in the treatment of DED provides hope for the millions of individuals who daily experience this deleterious condition. We anticipate that the advent of novel immunomodulatory agents will herald a new era of DED treatment.
Acknowledgments
Funding/Support: This work was supported in part by the Sjögren’s Syndrome Foundation (Dr. Chauhan) and by grant EY20889 from the National Institutes of Health (Dr. Dana).
Footnotes
Financial Disclosure: None reported.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 4.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127(6):763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286(17):2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 6.Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36(1):1–7. doi: 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 8.Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48(10):4391–4398. doi: 10.1167/iovs.07-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):179–193. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- 10.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31(5):186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 11.De Paiva CS, Pangelinan SB, Chang E, et al. Essential role for c-Jun N-terminal kinase 2 in corneal epithelial response to desiccating stress. Arch Ophthalmol. 2009;127(12):1625–1631. doi: 10.1001/archophthalmol.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82(4):588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50(8):3802–3807. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2(3):243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabiani C, Barabino S, Rashid S, Dana MR. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp Eye Res. 2009;89(2):166–171. doi: 10.1016/j.exer.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beardsley RM, De Paiva CS, Power DF, Pflugfelder SC. Desiccating stress decreases apical corneal epithelial cell size—modulation by the metalloproteinase inhibitor doxycycline. Cornea. 2008;27(8):935–940. doi: 10.1097/ICO.0b013e3181757997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YT, Nikulina K, Lazarev S, et al. Interleukin-1 as a phenotypic immunomodulator in keratinizing squamous metaplasia of the ocular surface in Sjögren’s syndrome. Am J Pathol. 2010;177(3):1333–1343. doi: 10.2353/ajpath.2010.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye–induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Invest Ophthalmol Vis Sci. 2007;48(6):2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 19.Luo L, Li DQ, Pflugfelder SC. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea. 2007;26(4):452–460. doi: 10.1097/ICO.0b013e318030d259. [DOI] [PubMed] [Google Scholar]
- 20.Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44(1):124–129. doi: 10.1167/iovs.02-0581. [DOI] [PubMed] [Google Scholar]
- 21.Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47(8):3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Cursiefen C, Barabino S, Zhang Q, Dana MR. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest Ophthalmol Vis Sci. 2005;46(12):4536–4540. doi: 10.1167/iovs.05-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal S, Chauhan SK, El Annan J, Nallasamy N, Zhang Q, Dana R. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010;128(7):819–824. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan SK, Jin Y, Goyal S, et al. A novel prolymphangiogenic function for Th17/IL17 [published online September 8, 2011] Blood. doi: 10.1182/blood-2011-01-332049. [DOI] [Google Scholar]
- 25.Skobe M, Dana R. Blocking the path of lymphatic vessels. Nat Med. 2009;15(9):993–994. doi: 10.1038/nm0909-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 27.Müller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37(4):476–488. [PubMed] [Google Scholar]
- 28.Tuominen IS, Konttinen YT, Vesaluoma MH, Moilanen JA, Helintö M, Tervo TM. Corneal innervation and morphology in primary Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44(6):2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 29.Benítez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45(9):3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 30.Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48(1):173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 31.Hoşal BM, Ornek N, Zileliolu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond) 2005;19(12):1276–1279. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 32.Erdélyi B, Kraak R, Zhivov A, Guthoff R, Németh J. In vivo confocal laser scanning microscopy of the cornea in dry eye. Graefes Arch Clin Exp Ophthalmol. 2007;245(1):39–44. doi: 10.1007/s00417-006-0375-6. [DOI] [PubMed] [Google Scholar]
- 33.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48(5):2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 34.Lee HK, Ryu IH, Seo KY, Hong S, Kim HC, Kim EK. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology. 2006;113(2):198–205. doi: 10.1016/j.ophtha.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Dastjerdi MH, Dana R. Corneal nerve alterations in dry eye–associated ocular surface disease. Int Ophthalmol Clin. 2009;49(1):11–20. doi: 10.1097/IIO.0b013e31819242c9. [DOI] [PubMed] [Google Scholar]
- 36.Cannon JG. Inflammatory cytokines in nonpathological states. News Physiol Sci. 2000:15298–303. doi: 10.1152/physiologyonline.2000.15.6.298. [DOI] [PubMed] [Google Scholar]
- 37.Tishler M, Yaron I, Geyer O, Shirazi I, Naftaliev E, Yaron M. Elevated tear interleukin-6 levels in patients with Sjögren syndrome. Ophthalmology. 1998;105(12):2327–2329. doi: 10.1016/S0161-6420(98)91236-2. [DOI] [PubMed] [Google Scholar]
- 38.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283–2292. [PubMed] [Google Scholar]
- 39.Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-α levels in tears of patients with dry eye syndrome. Cornea. 2007;26(4):431–437. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]
- 40.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205. e1. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 42.Enríquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010:16862–873. [PMC free article] [PubMed] [Google Scholar]
- 43.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19(3):201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 44.Corrales RM, Villarreal A, Farley W, Stern ME, Li DQ, Pflugfelder SC. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26(5):579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- 45.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 46.Zhu L, Shen J, Zhang C, et al. Inflammatory cytokine expression on the ocular surface in the Botulium toxin B induced murine dry eye model. Mol Vis. 2009:15250–258. [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51(2):643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48(6):2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 49.El Annan J, Goyal S, Zhang Q, Freeman GJ, Sharpe AH, Dana R. Regulation of T-cell chemotaxis by programmed death-ligand 1 (PD-L1) in dry eye–associated corneal inflammation. Invest Ophthalmol Vis Sci. 2010;51(7):3418–3423. doi: 10.1167/iovs.09-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Törnwall J, Lane TE, Fox RI, Fox HS. T cell attractant chemokine expression initiates lacrimal gland destruction in nonobese diabetic mice. Lab Invest. 1999;79(12):1719–1726. [PubMed] [Google Scholar]
- 51.Gulati A, Sacchetti M, Bonini S, Dana R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch Ophthalmol. 2006;124(5):710–716. doi: 10.1001/archopht.124.5.710. [DOI] [PubMed] [Google Scholar]
- 52.Goyal S, Chauhan SK, Zhang Q, Dana R. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch Ophthalmol. 2009;127(7):882–887. doi: 10.1001/archophthalmol.2009.125. [DOI] [PubMed] [Google Scholar]
- 53.Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45(12):4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 54.Chotikavanich S, de Paiva CS, Li Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acera A, Rocha G, Vecino E, Lema I, Durán JA. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008;40(6):315–321. doi: 10.1159/000150445. [DOI] [PubMed] [Google Scholar]
- 56.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166(1):61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001;85(2):147–153. doi: 10.1136/bjo.85.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Brignole F, Pisella PJ, Goldschild M, De Saint Jean M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000;41(6):1356–1363. [PubMed] [Google Scholar]
- 60.Baudouin C, Brignole F, Pisella PJ, De Saint Jean M, Goguel A. Flow cytometric analysis of the inflammatory marker HLA DR in dry eye syndrome: results from 12 months of randomized treatment with topical cyclosporin A. Adv Exp Med Biol. 2002;506(pt B):761–769. doi: 10.1007/978-1-4615-0717-8_107. [DOI] [PubMed] [Google Scholar]
- 61.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43(8):2609–2614. [PubMed] [Google Scholar]
- 62.Ogawa Y, Kuwana M, Yamazaki K, et al. Periductal area as the primary site for T-cell activation in lacrimal gland chronic graft-versus-host disease. Invest Ophthalmol Vis Sci. 2003;44(5):1888–1896. doi: 10.1167/iovs.02-0699. [DOI] [PubMed] [Google Scholar]
- 63.Tsubota K, Fujihara T, Saito K, Takeuchi T. Conjunctival epithelium expression of HLA-DR in dry eye patients. Ophthalmologica. 1999;213(1):16–19. doi: 10.1159/000027387. [DOI] [PubMed] [Google Scholar]
- 64.Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjögren’s syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol Vis Sci. 1994;35(9):3493–3504. [PubMed] [Google Scholar]
- 65.Tsubota K, Fujita H, Tsuzaka K, Takeuchi T. Quantitative analysis of lacrimal gland function, apoptotic figures, Fas and Fas ligand expression of lacrimal glands in dry eye patients. Exp Eye Res. 2003;76(2):233–240. doi: 10.1016/s0014-4835(02)00279-8. [DOI] [PubMed] [Google Scholar]
- 66.Gao J, Morgan G, Tieu D, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjögrens syndrome-like MRL/lpr mice. Exp Eye Res. 2004;78(4):823–835. doi: 10.1016/j.exer.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 67.Aronni S, Cortes M, Sacchetti M, et al. Upregulation of ICAM-1 expression in the conjunctiva of patients with chronic graft-versus-host disease. Eur J Ophthalmol. 2006;16(1):17–23. doi: 10.1177/112067210601600104. [DOI] [PubMed] [Google Scholar]
- 68.Saito I, Terauchi K, Shimuta M, et al. Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren’s syndrome. J Clin Lab Anal. 1993;7(3):180–187. doi: 10.1002/jcla.1860070309. [DOI] [PubMed] [Google Scholar]
- 69.Ecoiffier T, El Annan J, Rashid S, Schaumberg D, Dana R. Modulation of integrin α4β1 (VLA-4) in dry eye disease. Arch Ophthalmol. 2008;126(12):1695–1699. doi: 10.1001/archopht.126.12.1695. [DOI] [PubMed] [Google Scholar]
- 70.Sallusto F, Lanzavecchia A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 2002;4(suppl 3):S127–S132. doi: 10.1186/ar567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamrah P, Liu Y, Zhang Q, Dana MR. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol. 2003;121(8):1132–1140. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 72.Klareskog L, Forsum U, Tjernlund UM, Rask L, Peterson PA. Expression of Ia antigen-like molecules on cells in the corneal epithelium. Invest Ophthalmol Vis Sci. 1979;18(3):310–313. [PubMed] [Google Scholar]
- 73.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44(2):581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 74.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43(7):2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 75.Hattori T, Chauhan SK, Lee H, et al. Characterization of Langerin-expressing dendritic cell subsetsin the normal cornea. Invest Ophthalmol Vis Sci. 2011;52(7):4598–4604. doi: 10.1167/iovs.10-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin H, Li W, Dong N, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci. 2010;51(1):122–128. doi: 10.1167/iovs.09-3629. [DOI] [PubMed] [Google Scholar]
- 77.Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci. 2010;51(6):3083–3091. doi: 10.1167/iovs.09-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182(3):1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol. 2009;2(4):375–376. doi: 10.1038/mi.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bernard F, Romano A, Granel B. Regulatory T cells and systemic autoimmune diseases: systemic lupus erythematosus, rheumatoid arthritis, primary Sjögren’s syndrome. Rev Med Interne. 2010;31(2):116–127. doi: 10.1016/j.revmed.2009.03.364. [DOI] [PubMed] [Google Scholar]
- 81.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren’s syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176(7):3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 82.Siemasko KF, Gao J, Calder VL, et al. In vitro expanded CD4+CD25+Foxp3+ regulatory T cells maintain a normal phenotype and suppress immune-mediated ocular surface inflammation. Invest Ophthalmol Vis Sci. 2008;49(12):5434–5440. doi: 10.1167/iovs.08-2075. [DOI] [PubMed] [Google Scholar]
- 83.Pedersen BK, Oxholm P. Impaired release of natural killer cytotoxic factor in patients with primary Sjögren’s syndrome. Clin Exp Immunol. 1988;72(2):299–302. [PMC free article] [PubMed] [Google Scholar]
- 84.Struyf NJ, Snoeck HW, Bridts CH, De Clerck LS, Stevens WJ. Natural killer cell activity in Sjögren’s syndrome and systemic lupus erythematosus: stimulation with interferons and interleukin-2 and correlation with immune complexes. Ann Rheum Dis. 1990;49(9):690–693. doi: 10.1136/ard.49.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Izumi Y, Ida H, Huang M, et al. Characterization of peripheral natural killer cells in primary Sjögren’s syndrome: impaired NK cell activity and low NK cell number. J Lab Clin Med. 2006;147(5):242–249. doi: 10.1016/j.lab.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Szodoray P, Gal I, Barath S, et al. Immunological alterations in newly diagnosed primary Sjögren’s syndrome characterized by skewed peripheral T-cell subsets and inflammatory cytokines. Scand J Rheumatol. 2008;37(3):205–212. doi: 10.1080/03009740801910361. [DOI] [PubMed] [Google Scholar]
- 87.Szodoray P, Papp G, Horvath IF, et al. Cells with regulatory function of the innate and adaptive immune system in primary Sjögren’s syndrome. Clin Exp Immunol. 2009;157(3):343–349. doi: 10.1111/j.1365-2249.2009.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barabino S, Montaldo E, Solignani F, Valente C, Mingari MC, Rolando M. Immune response in the conjunctival epithelium of patients with dry eye. Exp Eye Res. 2010;91(4):524–529. doi: 10.1016/j.exer.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon-γ-secreting NK cells promote induction of dry eye disease. J Leukoc Biol. 2011;89(6):965–972. doi: 10.1189/jlb.1110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 91.Nichols KK. Patient-reported symptoms in dry dye disease. Ocul Surf. 2006;4(3):137–145. doi: 10.1016/s1542-0124(12)70040-x. [DOI] [PubMed] [Google Scholar]
- 92.Turner K, Pflugfelder SC, Ji Z, Feuer WJ, Stern M, Reis BL. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000;19(4):492–496. doi: 10.1097/00003226-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 93.Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001;42(1):90–95. [PubMed] [Google Scholar]
- 94.Kojima T, Matsumoto Y, Dogru M, Tsubota K. The application of in vivo laser scanning confocal microscopy as a tool of conjunctival in vivo cytology in the diagnosis of dry eye ocular surface disease. Mol Vis. 2010:162457–2464. [PMC free article] [PubMed] [Google Scholar]
- 95.Villani E, Beretta S, De Capitani M, Galimberti D, Viola F, Ratiglia R. In vivo confocal microscopy of meibomian glands in Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2011;52(2):933–939. doi: 10.1167/iovs.10-5995. [DOI] [PubMed] [Google Scholar]
- 96.Versura P, Profazio V, Fresina M, Campos EC. A novel scraping cytology score system (SCSS) grades inflammation in dry eye patients. Curr Eye Res. 2009;34(5):340–346. doi: 10.1080/02713680902816290. [DOI] [PubMed] [Google Scholar]
- 97.Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2–3):119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida A, Fujihara T, Nakata K. Cyclosporin A increases tear fluid secretion via release of sensory neurotransmitters and muscarinic pathway in mice. Exp Eye Res. 1999;68(5):541–546. doi: 10.1006/exer.1998.0619. [DOI] [PubMed] [Google Scholar]
- 99.Stevenson D, Tauber J, Reis BL. Cyclosporin A Phase 2 Study Group. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Ophthalmology. 2000;107(5):967–974. doi: 10.1016/s0161-6420(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 100.Sall K, Stevenson OD, Mundorf TK, Reis BL. CsA Phase 3 Study Group. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000;107(4):631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 101.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330–337. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 102.Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28(10):1091–1096. doi: 10.1097/ICO.0b013e3181a16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Solomon A, Rosenblatt M, Li D, et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Am J Ophthalmol. 2000;130(5):688. doi: 10.1016/s0002-9394(00)00755-8. [DOI] [PubMed] [Google Scholar]
- 104.Hashimoto S, Gon Y, Matsumoto K, Takeshita I, Maruoka S, Horie T. Inhalant corticosteroids inhibit hyperosmolarity-induced, and cooling and rewarming-induced interleukin-8 and RANTES production by human bronchial epithelial cells. Am J Respir Crit Care Med. 2000;162(3 pt 1):1075–1080. doi: 10.1164/ajrccm.162.3.9911099. [DOI] [PubMed] [Google Scholar]
- 105.Liden J, Rafter I, Truss M, Gustafsson JA, Okret S. Glucocorticoid effects on NF-kappaB binding in the transcription of the ICAM-1 gene. Biochem Biophys Res Commun. 2000;273(3):1008–1014. doi: 10.1006/bbrc.2000.3079. [DOI] [PubMed] [Google Scholar]
- 106.Aksoy MO, Li X, Borenstein M, Yi Y, Kelsen SG. Effects of topical corticosteroids on inflammatory mediator-induced eicosanoid release by human airway epithelial cells. J Allergy Clin Immunol. 1999;103(6):1081–1091. doi: 10.1016/s0091-6749(99)70183-1. [DOI] [PubMed] [Google Scholar]
- 107.Brunner T, Arnold D, Wasem C, Herren S, Frutschi C. Regulation of cell death and survival in intestinal intraepithelial lymphocytes. Cell Death Differ. 2001;8(7):706–714. doi: 10.1038/sj.cdd.4400854. [DOI] [PubMed] [Google Scholar]
- 108.Dursun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132(1):8–13. doi: 10.1016/s0002-9394(01)00913-8. [DOI] [PubMed] [Google Scholar]
- 109.De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47(7):2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 110.Lekhanont K, Leyngold IM, Suwan-Apichon O, Rangsin R, Chuck RS. Comparison of topical dry eye medications for the treatment of keratoconjunctivitis sicca in a botulinum toxin B–induced mouse model. Cornea. 2007;26(1):84–89. doi: 10.1097/01.ico.0000240079.24583.a1. [DOI] [PubMed] [Google Scholar]
- 111.Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology. 1999;106(4):811–816. doi: 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- 112.Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003;136(4):593–602. doi: 10.1016/s0002-9394(03)00326-x. [DOI] [PubMed] [Google Scholar]
- 113.Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138(3):444–457. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 114.Cordero-Coma M, Anzaar F, Sobrin L, Foster CS. Systemic immunomodulatory therapy in severe dry eye secondary to inflammation. Ocul Immunol Inflamm. 2007;15(2):99–104. doi: 10.1080/09273940701299354. [DOI] [PubMed] [Google Scholar]
- 115.Hong S, Kim T, Chung SH, Kim EK, Seo KY. Recurrence after topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren’s syndrome. J Ocul Pharmacol Ther. 2007;23(1):78–82. doi: 10.1089/jop.2006.0091. [DOI] [PubMed] [Google Scholar]
- 116.Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)α/βII. J Biol Chem. 2007;282(20):15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- 117.Shafaa MW, El Shazly LH, El Shazly AH, El gohary AA, El hossary GG. Efficacy of topically applied liposome-bound tetracycline in the treatment of dry eye model. Vet Ophthalmol. 2011;14(1):18–25. doi: 10.1111/j.1463-5224.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- 118.Voils SA, Evans ME, Lane MT, Schosser RH, Rapp RP. Use of macrolides and tetracyclines for chronic inflammatory diseases. Ann Pharmacother. 2005;39(1):86–94. doi: 10.1345/aph.1E282. [DOI] [PubMed] [Google Scholar]
- 119.Stone DU, Chodosh J. Oral tetracyclines for ocular rosacea: an evidence-based review of the literature. Cornea. 2004;23(1):106–109. doi: 10.1097/00003226-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 120.Gilbard JP. Dry eye, blepharitis and chronic eye irritation: divide and conquer. J Ophthalmic Nurs Technol. 1999;18(3):109–115. [PubMed] [Google Scholar]
- 121.Bartholomew RS, Reid BJ, Cheesbrough MJ, Macdonald M, Galloway NR. Oxytetracycline in the treatment of ocular rosacea: a double-blind trial. Br J Ophthalmol. 1982;66(6):386–388. doi: 10.1136/bjo.66.6.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seal DV, Wright P, Ficker L, Hagan K, Troski M, Menday P. Placebo controlled trial of fusidic acid gel and oxytetracycline for recurrent blepharitis and rosacea. Br J Ophthalmol. 1995;79(1):42–45. doi: 10.1136/bjo.79.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocul Surf. 2010;8(1):18–28. doi: 10.1016/s1542-0124(12)70214-8. [DOI] [PubMed] [Google Scholar]
- 124.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(1 suppl):343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 125.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 126.Miljanović B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82(4):887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barabino S, Rolando M, Camicione P, et al. Systemic linoleic and γ-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22(2):97–101. doi: 10.1097/00003226-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 128.Aragona P, Bucolo C, Spinella R, Giuffrida S, Ferreri G. Systemic omega-6 essential fatty acid treatment and pge1 tear content in Sjögren’s syndrome patients. Invest Ophthalmol Vis Sci. 2005;46(12):4474–4479. doi: 10.1167/iovs.04-1394. [DOI] [PubMed] [Google Scholar]
- 129.Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30(3):308–314. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 130.Oxholm P, Manthorpe R, Prause JU, Horrobin D. Patients with primary Sjögren’s syndrome treated for two months with evening primrose oil. Scand J Rheumatol. 1986;15(2):103–108. doi: 10.3109/03009748609102073. [DOI] [PubMed] [Google Scholar]
- 131.Theander E, Horrobin DF, Jacobsson LT, Manthorpe R. Gammalinolenic acid treatment of fatigue associated with primary Sjögren’s syndrome. Scand J Rheumatol. 2002;31(2):72–79. doi: 10.1080/03009740252937577. [DOI] [PubMed] [Google Scholar]
- 132.Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26(5):431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]