Abstract
Background/Aim
Limited clinical data exist to guide practice patterns and evidence-based use of inotropes and vasopressors following CABG.
Methods
Contemporary Analysis of Perioperative Cardiovascular Surgical Care (CAPS-Care) collected detailed perioperative data from 2,390 CABG patients between 2004 and 2005 at 55 US hospitals. High-risk elective or urgent CABG patients were eligible for inclusion. We stratified participating hospitals into high, medium, and low tertiles of inotrope use. Hospital-level outcomes were compared before and after risk adjustment for baseline characteristics.
Results
Hospital-level risk-adjusted rates of any inotrope/vasopressor use varied from 100–35%. Hospitals in the highest tertile of use had more patients with mitral regurgitation compared to medium or low use hospitals (P<0.001), more previous cardiovascular interventions (P=0.002), longer cardiopulmonary bypass (P<0.001), longer cross-clamp times (P<0.001), and required more transfusions (P=0.001). Despite these differences, unadjusted outcomes were similar between high-, medium-, and low-use hospitals for operative mortality (4.5% vs. 5.3% vs. 5.2%; P=0.702), 30-day mortality (4.1% vs. 4.6% vs. 5.0%; P=0.690), postoperative renal failure (7.2% vs. 9.2% vs. 6.6%; P=0.142), atrial fibrillation (23.0% vs. 27.2% vs. 25.6%; P=0.106), and acute limb ischemia (0.6% vs. 0.5% vs. 0.5%; P=0.945). These similar outcomes persisted after risk adjustment: adjusted OR=0.97 (95% CI [0.94, 1.00], P=0.086) for operative mortality and adjusted OR=1.00 (95% CI [0.96, 1.04], P=0.974) for postoperative renal failure.
Conclusion
While considerable variability is present among hospitals in inotrope use following CABG, observational comparison of outcomes did not distinguish a superior pattern; thus, randomized prospective data are needed to better guide clinical practice.
Outcomes following coronary artery bypass grafting (CABG) continue to improve, despite the fact that the recipient patient population is older and has more comorbidities, compared to previous years. Advances in perioperative processes of care continue to play an important role in these improvements.1, 2 However, while many perioperative processes of care have been defined and linked to improvements in outcomes, data is lacking with regards to inotrope and vasopressor use for treatment of low cardiac output syndrome (LCOS) in the early postoperative period following CABG.
Several clinical studies have examined the factors associated with the development of LCOS and the subsequent need for inotropic support.3–5 Commonly defined as low cardiac output (confidence interval [CI] <2.5) with evidence of end-organ dysfunction such as low urine output (<0.5mL/kg/hr), LCOS is associated with increased morbidity and mortality following CABG6 and is known to increase length of stay, resource use, and overall costs.5 The multitude of positive vasoactive agents enlisted alone or in combination for the management of LCOS include phosphodiesterase inhibitors (amrinone, milrinone), antidiuretic hormone analogues (vasopressin), pure alpha adrenergic agonists (phenylephrine), and both natural (epinephrine, dopamine) and synthetic (dobutamine, dopexamine) catecholamines.7
While individual physiological effects have been outlined, insufficient clinical data exist to guide general practice patterns for use of these agents following CABG. Clinicians generally balance perceived physiologic benefits against perceived risks, including induced arrhythmias or decreased peripheral, renal, and bypass graft perfusion.8 While some clinicians/institutions routinely administer inotropic agents to all patients undergoing CABG, others administer these agents selectively to patients with either authentic or anticipated LCOS. Without evidence-based guidance, variation by clinician and institution is not only expected, but has been demonstrated in large multicenter registries.7, 9, 10 This study seeks to address the present gap in knowledge regarding optimal pattern of application of inotropic agents during the critical recovery period following CABG.
MATERIALS AND METHODS
Data Sources
Clinical data from the Society of Thoracic Surgeons National Cardiac Database (STS NCD) were collected using methods described fully elsewhere.11 Briefly, the STS NCD was established in 1987 as a multicenter repository for quality improvement and clinical research. The STS NCD collects data from approximately 90% of all US hospitals with cardiothoracic surgical programs and contains detailed data on patient demographics, clinical profile, and short-term outcomes for over 3.5 million procedures. Sites voluntarily submit data to the data coordinating center (Duke Clinical Research Institute, Durham, NC) on a quarterly basis and sites receive site-specific feedback. Data definitions are standardized and accuracy of individual data elements has been validated in regional analyses with an agreement rate >95%.11 Overall, completeness of procedure reporting and mortality event reporting in patients age ≥65 has been validated against national Medicare claims files.1, 12 The STS also conducts annual on-site data audits on randomly selected database participants.
We performed a retrospective chart abstraction using a detailed data collection form to augment the STS NCD with perioperative care data. No patients were contacted as part of this study. STS NCD participants were invited to join this study entitled, the Contemporary Analysis of Perioperative Cardiovascular Surgical Care (CAPS-Care). We considered only those sites performing at least 50 high-risk CABG operations annually in order to ensure adequate sample size. From among those that expressed interest, 50 database participants were selected based on prior experience submitting high-quality data. Of the 50 database participants selected, 48 obtained appropriate institutional review board approval and proceeded with data collection—the 48 STS NCD database participants collect data for 55 total hospitals. Sites were trained to abstract charts using the CAPS-Care case report form. These procedures are described in more detail elsewhere.9
The data collected included preoperative clinical variables, intraoperative care, postoperative pharmacologic care, postoperative management, and postoperative events. The missing data rate was low, with <0.1% missing for any inotrope/vasopressor use. CAPS-Care participating hospitals were generally similar compared with other STS NCD hospitals in baseline clinical and institutional characteristics except, by design, CAPS-Care hospitals were overall higher volume. CAPS-Care data were entered into a computerized database and linked to the STS NCD data with the same unique record identification. The STS NCD already contained baseline demographic variables, clinical and operative variables, and major adverse events during hospitalization. American Hospital Association files were linked to supplement the dataset with hospital characteristics of interest including bed size, teaching status, geographic region, intensivist staffing, and other factors.
Patient Population
Fifty patients who underwent high-risk CABG and met selection criteria were randomly selected from each of the 48 participating CAPS-Care sites with institutional review board approval. Patients who underwent elective or urgent CABG from January 2004 to June 2005 were screened. To be eligible for inclusion, patients either had to have a preoperative ejection fraction <40% or be ≥65 years with either diabetes mellitus, or estimated glomerular filtration rate <60 mL/min per 1.73 m2. Patients were excluded from selection if they were ≤18 years or if they had emergent/salvage operation or preoperative cardiogenic shock.
Outcome Measures and Statistical Analysis
Our study focused on patients receiving any inotrope/vasopressor postoperatively up to 12 hours after skin closure, including any agents initiated preoperatively or intraoperatively. Positive vasoactive therapies were any inotrope or vasopressor, including dobutamine, dopamine, milrinone, epinephrine, norepinephrine, phenylephrine, and vasopressin. Hospital level of any vasoactive therapies used among all patients within each hospital was calculated and presented as a percentage. Hospitals were categorized to three groups with high, medium, low used of any vasoactive therapies based on tertile of percent of inotrope/vasopressor use. Patient baseline and operative characteristics are summarized among three groups.
Categorical variables are presented as percentages; continuous variables are presented as medians and interquartile ranges, unless otherwise stated. Comparisons were designed to detect data inhomogeneity. The Kruskal-Wallis test was used in Tables 1 and 2 for probability values for continuous variables. Tables 1, 2, and 3 used Chi-square tests for categorical variables with a null hypothesis that all rates across columns are equal. Patients with missing data were excluded when calculating P values. Unadjusted event rates were computed for mortality (including operative and 30-day) and morbidity (including renal failure, hemodialysis, atrial fibrillation, reoperation, acute limb ischemia, deep sternal wound infection, perioperative myocardial infarction, and need for postoperative blood products). The analysis of postoperative renal failure excluded those patients with preoperative renal failure, and the analysis of postoperative atrial fibrillation excluded patients with preoperative atrial fibrillation or flutter.
TABLE 1.
Patient/operative characteristics by high, medium, and low inotrope use all values are percentages for dichotomous variables and median [IQR] for continuous variables)
| Characteristic | Overall (n=2390) |
High (n=808) |
Medium (n=819) |
Low (n=763) |
P-value |
|---|---|---|---|---|---|
| Age | 72.0 [66,77] | 72.0 [66,77] | 72.0 [66,77] | 72.0 [67,78] | 0.116 |
| Female | 33.6 | 34.4 | 32.5 | 33.9 | 0.692 |
| Diabetes | 52.6 | 52.7 | 53.7 | 51.4 | 0.339 |
| Morbid obesity | 13.1 | 12.1 | 12.4 | 12.7 | 0.895 |
| Dyslipidemia | 75.6 | 75.3 | 77.2 | 75.5 | 0.630 |
| Current smoker | 17.6 | 16.7 | 18.2 | 18.0 | 0.376 |
| Severe chronic lung disease | 5.1 | 5.2 | 5.7 | 4.3 | 0.245 |
| Preoperative creatinine | 1.10 [1.0,1.4] | 1.10 [1.0,1.4] | 1.10 [1.0,1.4] | 1.20 [1.0,1.4] | 0.444 |
| Hypertension | 83.9 | 81.3 | 85.1 | 85.3 | 0.049 |
| Arrhythmia | 17.2 | 19.8 | 15.3 | 16.5 | 0.046 |
| PVD | 19.9 | 20.7 | 16.7 | 22.5 | 0.012 |
| History of stroke | 11.5 | 13.5 | 9.3 | 11.8 | 0.075 |
| Previous MI | 47.7 | 51.2 | 44.1 | 47.7 | 0.015 |
| Prior CV surgery | 29.5 | 33.4 | 25.5 | 29.8 | 0.002 |
| Urgent procedure | 48.8 | 51.4 | 50.2 | 44.6 | 0.016 |
| CABG only | 74.7 | 71.3 | 76.0 | 77.0 | 0.036 |
| IABP use | 0.007 | ||||
| Preoperative | 6.6 | 6.4 | 8.8 | 4.5 | |
| Postoperative | 3.4 | 3.8 | 4.2 | 2.4 | |
| Postoperative blood product transfusion | 52.1 | 57.4 | 51.4 | 47.3 | 0.001 |
| CPB time (minutes) | 110 [85,143] | 115 [90,150] | 110 [89,144] | 110 [85,143] | <0.0001 |
| Cross-clamp time (minutes) | 76 [55,107] | 78 [57,114] | 77 [57,108] | 71 [51,100] | <0.0001 |
CABG=coronary artery bypass grafting; CPB=cardiopulmonary bypass; CV=cardiovascular; IABP=intra-aortic balloon pump; IQR=interquartile range; MI=myocardial infarction; PVD=peripheral vascular disease
TABLE 2.
Hospital characteristics by high, medium, and low inotrope use (all values are percentages for dichotomous variables and median [IQR] for continuous variables)
| Characteristic | Overall (n=55) |
High (n=19) |
Medium (n=20) |
Low (n=16) |
P-value |
|---|---|---|---|---|---|
| Teaching hospital | 18.2 | 15.8 | 20.0 | 18.7 | 0.914 |
| Annual CABG volume | 328 [222, 530] | 349 [168, 454] | 402 [279, 655] | 283 [201, 460] | 0.159 |
| Intensivist staffing | 54.6 | 57.9 | 45.0 | 62.5 | 0.859 |
| JCAHO accreditation | 83.6 | 89.5 | 75.0 | 87.5 | 0.365 |
| Total hospital beds | 0.738 | ||||
| 100–199 | 5.4 | 10.53 | 5.0 | 0.0 | |
| 200–299 | 21.8 | 21.0 | 20.0 | 25.0 | |
| 300–399 | 25.4 | 26.3 | 15.0 | 37.5 | |
| 400–499 | 10.9 | 5.3 | 15.0 | 12.5 | |
| 500 or > | 25.4 | 26.3 | 30.0 | 18.8 |
CABG=coronary artery bypass grafting; IQR=interquartile range; JCAHO=Joint Commission on Accreditation of Healthcare Organizations
TABLE 3.
Unadjusted event rates by high, medium, and low inotrope use (all values are percentages)
| Outcome | Overall (n=2390) |
High (n=808) |
Medium (n= 819) |
Low (n= 763) |
Unadjusted P-value |
|---|---|---|---|---|---|
| Operative mortality | 4.98 | 4.46 | 5.25 | 5.24 | 0.702 |
| 30-day mortality | 4.56 | 4.08 | 4.64 | 4.98 | 0.690 |
| Stroke | 2.47 | 2.60 | 2.44 | 2.36 | 0.953 |
| Renal failure | 7.66 | 7.18 | 9.16 | 6.55 | 0.124 |
| Dialysis | 2.97 | 2.97 | 3.79 | 2.10 | 0.142 |
| Acute limb ischemia | 0.54 | 0.62 | 0.49 | 0.52 | 0.945 |
| Atrial fibrillation | 25.3 | 23.0 | 27.2 | 25.6 | 0.106 |
| Deep sternal wound infection | 0.54 | 0.74 | 0.49 | 0.39 | 0.620 |
| Reoperation* | 9.33 | 9.16 | 10.0 | 8.78 | 0.687 |
| Postoperative blood product transfusion | 52.1 | 57.4 | 51.4 | 47.3 | 0.001 |
Reoperation includes take-back for bleeding or any other cause in the postoperative period
Multivariable logistic regression, with generalized estimating equation (GEE) modeling with compound symmetric working correlation matrix and empirical (sandwich) standard error estimates, was used to examine the association between inotrope level used and outcomes of operative mortality, atrial fibrillation, and postoperative renal failure. The GEE method was used to account for within-hospital clustering as patients at the same hospital are more likely to have similar responses, relative to patients in other hospitals (i.e., within-center correlation for response). Patient-level covariates in the model for risk adjustment consisted of age, sex, race, history of stroke, arrhythmia, atrial fibrillation, peripheral vascular disease, cerebrovascular disease, creatinine level, valve procedure, other procedures, on-pump procedure, prior cardiac surgery, prior myocardial infarction, 3-vessel coronary artery disease, smoking history, family history of coronary artery disease, diabetes, dyslipidemia, hypertension, heart failure, New York Heart Association classification, mitral regurgitation, left ventricular ejection fraction, and chronic lung disease.
In addition, we repeated the above multivariable analysis using all STS eligible high risk patients from all CAPS-Care hospitals between January 2004 and December 2006. Missing data were rare (<1% for any variables). In multivariable modeling, missing values of the continuous variables were imputed to the median value and missing values of the categorical variables were imputed to their most common value. All analyses were performed using SAS software version 8.2 (SAS Institute, Cary, NC).
RESULTS
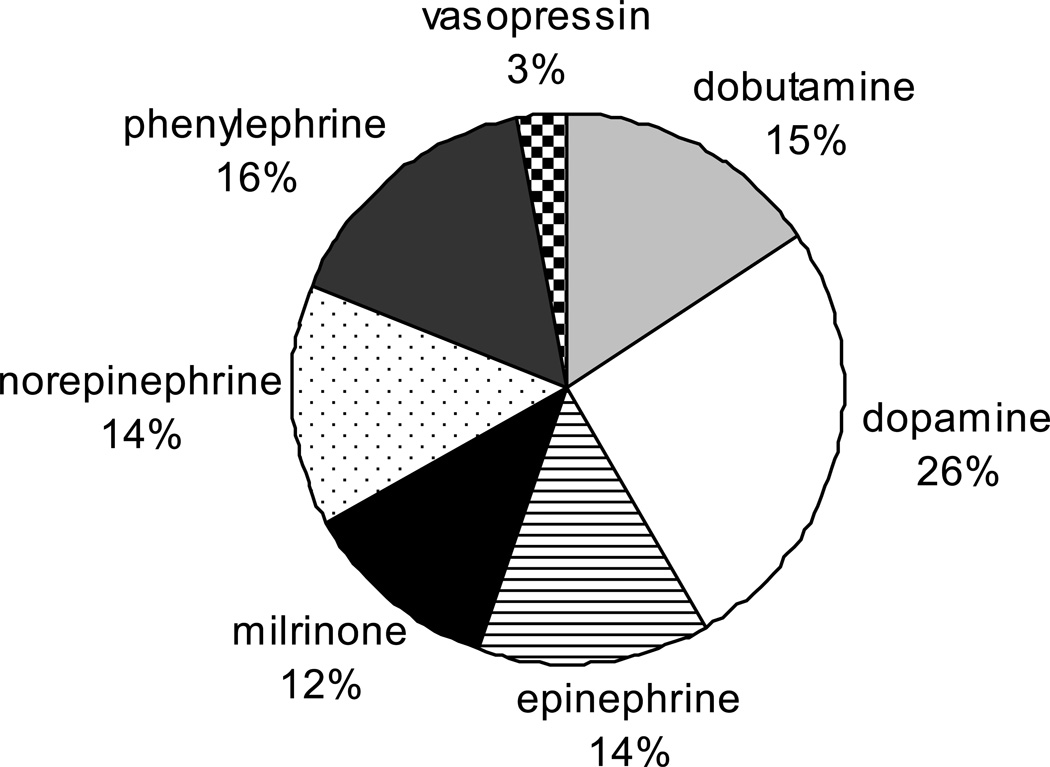
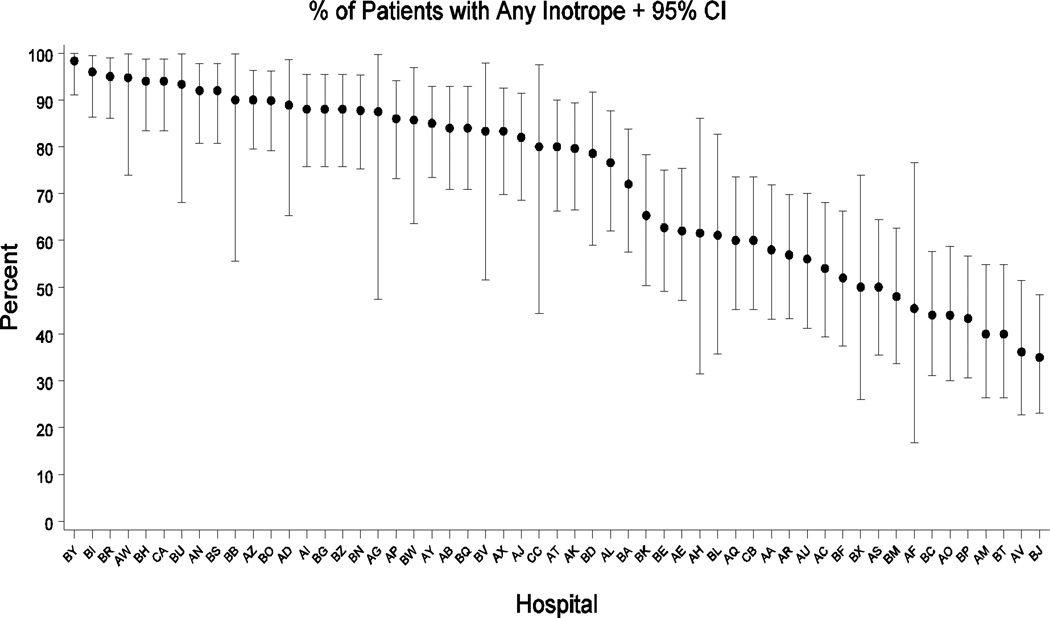
Available for data abstraction and fulfilling the selection criteria were 2,390 cases from 55 hospitals and 48 separate STS NCD database participants. The median number of patients per hospital was 50. Figure 1 illustrates the proportions of various inotropic agents used in the CAPS-Care study sample: dopamine accounted for 26% of all positive vasoactive therapies used, phenylephrine 16%, dobutamine 15%, epinephrine 14%, norepinephrine 14%, milrinone 12%, and vasopressin 3%. Figure 2 displays the wide inter-hospital variation in positive vasoactive agent use. Among the selectively high-risk CAPS-Care patients, inotropes/vasopressors were used perioperatively in 72% (1705/2390) of cases overall and hospital-level risk-adjusted rates of any inotrope/vasopressor use varied from 100% to 35%. With respect to individual agents and centers, the variability is described more fully elsewhere.9
Figure 1.
Individual inotrope/vasopressor agents as percentage of total
Pie chart showing individual inotrope/vasopressor agents given to all study patients, as a percentage of the total overall inotrope usage.
Figure 2.
Variation in inotrope/vasopressor use by hospital
Plot showing the variation in inotrope/vasopressor use between hospitals. Point estimates and 95% confidence intervals are given for the percentage (y-axis) of patients receiving any inotrope at each study hospital (x-axis).
Table 1 shows the patient and operative characteristics of the study hospitals by high (≥86%), medium (≥60% and <86%), and low (<60%) inotrope use. No difference between the upper, middle, and lower tertiles was observed in age, gender, smoking status, diabetes mellitus, morbid obesity, baseline creatinine, hypertension, history of stroke, or severe chronic lung disease. Hospitals in the highest tertile of inotrope use had more patients with moderate or greater mitral regurgitation compared to medium or low use (7.8% vs. 6.5% vs. 6.4%; P<0.001), higher rates of previous cardiovascular interventions (33.4% vs. 25.5% vs. 29.8%; P=0.002), longer cardiopulmonary bypass (130 vs. 122 vs. 108 minutes; P<0.001), longer cross-clamp times (92 vs. 86 vs. 78 minutes, P<0.001), and required more postoperative blood product transfusion (57% vs. 51% vs. 47%; P=0.001). Other patient characteristics having univariate association with hospitals in the highest tertile included heart failure, severe mitral regurgitation, preoperative arrhythmia, and low ejection fraction.
Table 2 shows hospital characteristics stratified by high, medium, and low use of inotropes. Univariate analysis of teaching status, annual CABG volume, presence of intensivist staffing, Joint Commission on Accreditation of Healthcare Organizations certification, and hospital size revealed no statistically significant differences between the three tertiles of inotrope use.
Table 3 displays unadjusted event rates. Despite the significant differences in patient baseline characteristics, unadjusted outcomes were statistically indistinguishable between high-, medium-, and low-use hospitals for operative mortality (4.5% vs. 5.3% vs. 5.2%; P=0.702); 30-day mortality (4.1% vs. 4.6% vs. 5.0%; P=0.690); stroke (2.6% vs. 2.4% vs. 2.4%; P=0.953); postoperative renal failure (7.2% vs. 9.2% vs. 6.6%; P=0.142); atrial fibrillation (23.0% vs. 27.2% vs. 25.6%; P=0.106); and acute limb ischemia (0.6% vs. 0.5% vs. 0.5%; P=0.945). Unadjusted event rates that are not statistically significant for operative and 30-day mortality show a tendency towards higher mortality at both end points in the low (<60%) inotrope use hospitals.
Table 4 reveals adjusted odds radios for mortality, renal failure, and atrial fibrillation comparing high- and medium-use centers vs. low-use centers. Table 4 results include all patients sampled in the CAPS-Care study (N=2390). The confidence interval for the adjusted odds ratio of each inter-tertile comparison includes 1; none of the P values are statistically significant. A logistic regression model for all CABG patients from the CAPS-Care study hospitals (January 2004–December 2006 [N=18688]) found that for every 5% increase in hospital inotrope use, risk-adjusted OR=0.97, 95% CI [0.94, 1.00], P=0.086 for operative mortality and risk-adjusted OR=1.00, 95% CI [0.96, 1.04], P=0.974 for postoperative renal failure.
TABLE 4.
Odds ratios for outcomes of patients in high- and medium-use centers vs. low-use centers
| Outcome | Variable | Total N | Adjusted odds ratio |
95% CI | Adjusted P-value |
|---|---|---|---|---|---|
| Operative mortality | High-use centers | 808 | 0.82 | 0.47, 1.44 | 0.491 |
| Medium-use centers | 819 | 1.23 | 0.77, 1.96 | 0.393 | |
| Renal failure | High-use centers | 734 | 1.16 | 0.60, 2.26 | 0.654 |
| Medium-use centers | 744 | 1.42 | 0.75, 2.70 | 0.286 | |
| Atrial fibrillation | High-use centers | 808 | 0.87 | 0.57, 1.32 | 0.517 |
| Medium-use centers | 818 | 1.02 | 0.72, 1.44 | 0.917 |
CI=confidence interval
CONCLUSIONS
The hospitals in the highest tertile of inotrope use had more patients with moderate or greater mitral regurgitation, higher rates of previous cardiovascular interventions, longer cardiopulmonary bypass, longer cross-clamp times, and required more perioperative blood product transfusions. These differences indicate that sicker patients were recovered following CABG in the high-use centers. Nonetheless, even unadjusted outcomes were similar across the three tertiles of inotrope use. In fact, there was a trend towards lower mortality, both operative and 30-day, and lower rates of renal failure, dialysis, acute limb ischemia, and atrial fibrillation among the highest tertile of inotrope use (with the highest tertile representing those centers treating the sickest patients in this study). Using risk-adjusted measures, our logistic regression models detected no difference between the high, medium, and low inotrope use centers for mortality, atrial fibrillation, or postoperative renal failure.
The mortality and postoperative event rates in this study were high for CABG, which was expected—given that only high-risk CABG patients were included, those patients more likely to suffer LCOS and require inotrope and/or vasopressor support. We chose to evaluate the early postoperative period of the first 12 hours after operation in order to focus upon practices that more likely occur systematically within a hospital. The immediate postoperative period is a time when the inotropic agents are more likely to be used as part of standard practice or protocol in transitioning a patient from cardiopulmonary bypass to recovery. Since differences in patterns of use may be influenced by center-level variables, including the presence of intensivists, teaching status, size, and annual CABG volume, we evaluated these hospital characteristics between the tertiles.
Despite delivery of post-surgical critical care being a common component of cardiac surgery practices, recent clinical data regarding the outcomes of positive vasoactive agent use remains sparse.13 In contrast to our findings, two small cardiac anesthesia studies have suggested the potential for increased myocardial oxygen consumption from frequent inotrope use, resulting in increased ischemia or arrhythmias.8,14 The Prophylactic Intravenous Use of Milrinone After Cardiac Operation in Pediatrics (PRIMACORP) study was a randomized, placebo-controlled trial that investigated the efficacy and safety of empiric milrinone in pediatric patients at risk for developing LCOS following congenital cardiac operations.15 Investigators observed a 64% relative risk reduction in the development of LCOS in infants with the prophylactic use of high-dose milrinone. However, investigators in the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study, a large placebo-controlled clinical trial evaluating the use of milrinone in patients hospitalized for heart failure exacerbation, found an increase in early adverse events in the form of hypotension and atrial arrhythmias following initiation of inotrope therapy.16
The present study, representing the largest examination of inotrope use outcomes in high-risk patients undergoing CABG, should not be interpreted to support protocols of high inotrope use. The recent trends towards increased use of inotropes/vasopressors in routine clinical practice are not driven by robust clinical data or necessarily reflective of goal-directed therapy for LCOS. These trends are reflective of efforts to move patients more quickly through the processes of extubation17 and intensive care unit discharge, and to improve hemodynamic performance in an increasingly older, more complex patient population that is at greater risk for LCOS.13 While in the present study, high-use centers showed no increase in atrial fibrillation, renal failure, or acute limb ischemia, one must remain cognizant of the fact that conclusions regarding the safety or postoperative inotrope use cannot be made. Focused prospective clinical studies are needed to appropriately evaluate the varying patterns of use for current vasoactive therapies, and the opportunity exists for cardiac surgeons to lead efforts in outlining best practice in post-surgical use of positive vasoactive therapies and critical care delivery.
Limitations
Since our study is observational, unmeasured confounders could have influenced our findings. Our data were primarily limited to those available from the subset of STS NCD centers participating in CAPS-Care. However, patient and hospital characteristics of CAPS-Care participants are similar to the other United States centers participating in the STS NCD, and the quality of the STS NCD data has been validated in a regional independent chart abstraction study.11 We did not examine the use of various cardioprotective or anesthetic strategies and the need for inotropic support. Further, the multivariable models are limited to the baseline and perioperative characteristics available in the STS NCD and the CAPS-Care data. We were unable to account for those variables in clinical practice that influence decisions in inotrope/vasopressor use which are not routinely captured by the medical record, such as the amount of viable myocardium. Finally, doses and effects of individual agents were not assessed and, as such, it remains possible that sites in the lowest tertile of inotrope use may have treated patients with more potent agents or higher doses in those cases in which an inotrope was applied. Thus, while our study is a large sampling of overall clinical outcomes of positive vasoactive therapy use in high-risk CABG patients, exact outcomes may vary from our results.
Conclusions
While there is considerable inter-hospital variability in the use of positive vasoactive agents following CABG in high-risk patients, outcomes were similar in the present study among hospitals with high, medium, and low inotrope use. These findings support the need for prospective clinical data to outline best practice with regards to pattern of inotrope use following CABG.
Acknowledgements
Funding sources: Dr. Williams is supported in part by training grant T32-HL069749 from the National Institutes of Health and Drs. Williams, Smith, and Ferguson are supported in part by grant U01-HL088953 from the Cardiothoracic Surgical Trials Network. The study was funded with an unrestricted research grant from Scios, Inc. (Mountain View, CA) to the Society of Thoracic Surgeons. All components of the study including the analyses and manuscript preparation were done independently at the Duke Clinical Research Institute.
The authors are grateful to Erin LoFrese, MS for editorial support in completing this work.
References
- 1.Williams JB, Delong ER, Peterson ED, et al. Secondary prevention after coronary artery bypass graft surgery: findings of a national randomized controlled trial and sustained society-led incorporation into practice. Circulation. 2011;123:39–45. doi: 10.1161/CIRCULATIONAHA.110.981068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman WL, Sansom M, Kiefe CI, et al. Alabama coronary artery bypass grafting project: results from phase II of a statewide quality improvement initiative. Ann Surg. 2004;239:99–109. doi: 10.1097/01.sla.0000103065.17661.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed I, House CM, Nelson WB. Predictors of inotrope use in patients undergoing concomitant coronary artery bypass graft (CABG) and aortic valve replacement (AVR) surgeries at separation from cardiopulmonary bypass (CPB) J Cardiothorac Surg. 2009;4:24. doi: 10.1186/1749-8090-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterworth JF, Legault C, Royster RL, et al. Factors that predict the use of positive inotropic drug support after cardiac valve surgery. Anesth Analg. 1998;86:461–467. doi: 10.1097/00000539-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Royster RL, Butterworth JF, Prough DS, et al. Preoperative and intraoperative predictors of inotropic support and long-term outcome in patients having coronary artery bypass grafting. Anesth Analg. 1991;72:729–736. doi: 10.1213/00000539-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Maganti MD, Borger MA, Ivanov J, et al. Predictors of Low Cardiac Output Syndrome After Isolated Aortic Valve Surgery. Circulation. 2005;112(9 Suppl):I448–I452. doi: 10.1161/CIRCULATIONAHA.104.526087. [DOI] [PubMed] [Google Scholar]
- 7.Gillies M, Bellomo R, Doolan L, et al. Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery -- a systematic literature review. Crit Care. 2005;9:266–279. doi: 10.1186/cc3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobato EB, Urdaneta F, Martin TD, et al. Effects of milrinone versus epinephrine on grafted internal mammary artery flow after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2000;14:9–11. doi: 10.1016/s1053-0770(00)90047-8. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez AF, Li S, Dokholyan RS, O'Brien SM, et al. Variation in perioperative vasoactive therapy in cardiovascular surgical care: data from the Society of Thoracic Surgeons. Am Heart J. 2009;158:47–52. doi: 10.1016/j.ahj.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Bastien O, Vallet B. French multicentre survey on the use of inotropes after cardiac surgery. Critical Care. 2005;9:241–242. doi: 10.1186/cc3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welke KF, Ferguson TB, Coombs LP, et al. Validity of the Society of Thoracic Surgeons National Adult Cardiac Surgery Database. Ann Thorac Surg. 2004;77:1137–1139. doi: 10.1016/j.athoracsur.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Welke KF, Peterson ED, Vaughan-Sarrazin MS, et al. Comparison of cardiac surgery volumes and mortality rates between the Society of Thoracic Surgeons and Medicare databases from 1993 through 2001. Ann Thorac Surg. 2007;84:1538–1546. doi: 10.1016/j.athoracsur.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Kastrup M, Markewitz A, Spies C, et al. Current practice of hemodynamic monitoring and vasopressor and inotropic therapy in post-operative cardiac surgery patients in Germany: results from a postal survey. Acta Anaesthesiol Scand. 2007;51:347–358. doi: 10.1111/j.1399-6576.2006.01190.x. [DOI] [PubMed] [Google Scholar]
- 14.Kikura M, Sato S. The efficacy of preemptive milrinone or amrinone therapy in patients undergoing coronary artery bypass grafting. Anesth Analg. 2002;94:22–30. doi: 10.1097/00000539-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 16.Cuffe MS, Califf RM, Adams KF, Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 17.Sostaric M, Gersak B, Novak-Jankovic V. Early extubation and fast-track anesthetic technique for endoscopic cardiac surgery. Heart Surg Forum. 2010;13:190–194. doi: 10.1532/HSF98.20091151. [DOI] [PubMed] [Google Scholar]