Abstract
Chemical neurotransmission may include transmission to local or remote sites. Locally, contact between ‘bare’ portions of the bulbous nerve terminal termed a varicosity and the effector cell may be in the form of either synapse or non-synaptic contact. Traditionally, all local transmissions between nerves and effector cells are considered synaptic in nature. This is particularly true for communication between neurons. However, communication between nerves and other effectors such as smooth muscles has been described as nonsynaptic or junctional in nature. Nonsynaptic neurotransmission is now also increasing recognized in the CNS. This review focuses on the relationship between structure and function that orchestrate synaptic and junctional neurotransmissions. A synapse is a specialized focal contact between the presynaptic active zone capable for ultrafast release of soluble transmitters and the postsynaptic density that cluster ionotropic receptors. The presynaptic and the postsynaptic areas are separated by the ‘closed’ synaptic cavity. The physiological hallmark of the synapse is ultrafast postsynaptic potentials lasting in milliseconds. In contrast, junctions are juxtapositions of nerve terminals and the effector cells without clear synaptic specializations and the junctional space is ‘open’ to the extracellular space. Based on the nature of the transmitters, postjunctional receptors and their separation from the release sites, the junctions can be divided into ‘close’ and ‘wide’ junctions. Functionally, the ‘close’ and the ‘wide’ junctions can be distinguished by postjunctional potentials lasting ~1 second and 10s of seconds, respectively. Both synaptic and junctional communications are common between neurons; however, junctional transmission is the rule at many neuro-non-neural effectors.
Keywords: postsynaptic potentials, junction potentials, smooth muscle neuromuscular transmission, autonomic ganglia, enteric nervous system, nonsynaptic neurotransmission, modes of neurotransmission
INTRODUCTION
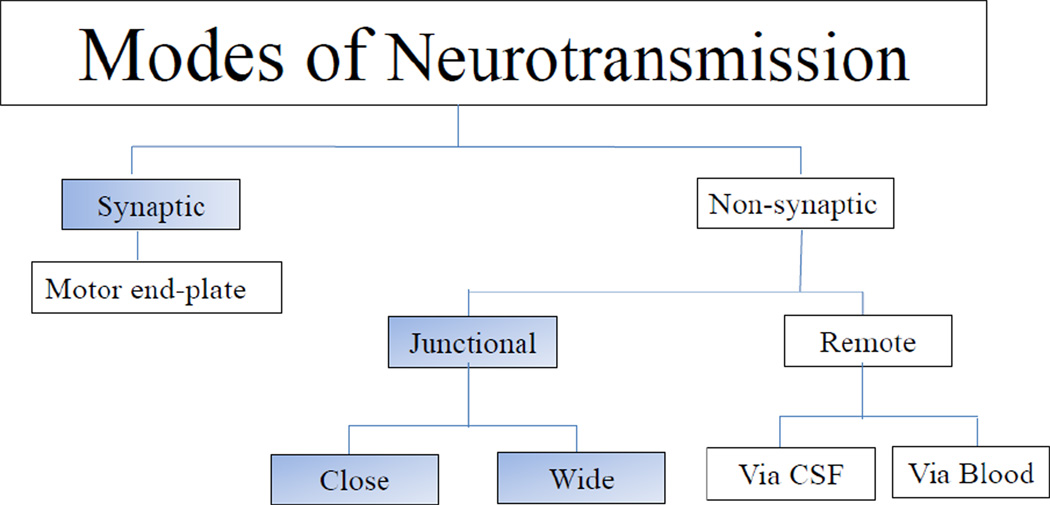
What constitutes a synapse and synaptic neurotransmission continues to be a hotly debated issue. There are both structural and functional aspects to the definition of a synapse. Traditionally, investigators have used only a functional definition and consider any neurotransmission between the nerve and a target cell as synaptic neurotransmission. In recent years there have been considerable advances in our understanding of the morphology and molecular biology of synapses. Neurotransmission at the skeletal neuromuscular junctions via the ‘motor endplate’ is a type of a synapse but is not generally included in discussion of a synapse. Here we present our analyses of the existing structure-function relationship data to support a case for restricting the term synaptic transmission to the communication that involves morphologically defined synapses during an action potential. Nonsynaptic neurotransmission, also called ‘volume’ transmission, includes a continuum of wide spectrum of modes of transmission including junctional transmission and transmission to distant and remote sites via neurotransmitter release into the cerebrospinal fluid and blood capillaries (Agnati et al., 1986). Junctional transmission involves transmission between nerve terminals and juxtaposed membranes of target cells without synaptic contacts (Agnati et al., 1986; Vizi, 1984). Junctional transmission can be arbitrarily further divided into close junctional and wide junctional transmissions (Figure 1). This review is focused on relationship of structure and activity during synaptic and junctional neurotransmission.
Figure 1.
Flow diagram of different modes of neurotransmission.
BRIEF HISTORY
How nerves communicate with other neurons or targets has preoccupied physiologists since the middle of the nineteenth century. Excellent accounts of the history of different aspects of the synapse and neurotransmission have been provided by Cowan and Kandel (Cowan W.M., 2003), Bennett (Bennett, 1997), Sudhof (Sudhof, 2008a) and Lopez-Munoz and Alamo (Lopez-Munoz et al., 2009). The earliest morphological consideration of neural communication revolved around whether the cytoplasm of a nerve was continuous with the adjacent nerve or merely discontinuous and were led by Du Bois-Raymond (1877) and Kuhn (1862) [cited by (Bennett, 2005)]. The later theory, popularly known as the neuron theory, was championed by Cajal [see (Cajal, 1954)] and it was envisioned that the tips of axons of one neuron made contacts with dendrites and cell bodies of other neurons. Subsequently, the nature of these contacts became a subject of intense discussion. Sherrington proposed that these contacts were specialized and advocated the use of the term synapse in 1897 [see (Tansey, 1997)]. The term synapse was derived from Greek, synapsis "conjunction," from synaptein, from syn- "together" + haptein "to fasten" (Kandel et al., 2000).
Functionally, communication at the synapses was believed to take place via chemicals. However, experimental proof for the existence of chemical synaptic neurotransmission came later from studies on striated neuromuscular endplates, even though the term synapse is not traditionally used in that context. As early as 1877, Du Bois-Raymond had recorded electrical potentials in the striated muscles in response to the motor nerve stimulation and had suggested the existence of chemical transmission [see (Dierig, 2000)]. The concept of chemical transmission was advanced by the work of Langley, who introduced the idea of receptors on which chemicals acted (Langley, 1921). Evidence for chemical transmission was established further by Dale, Loewi, Feldberg and their colleagues by their work on physiology and pharmacology of the neurotransmitters noradrenaline and acetylcholine (Cowan W.M., 2003; Dale, 1952).
Even after the chemical nature of synaptic neurotransmission was well established, many investigators continued to doubt whether chemical signals could be conducted fast enough to explain the ultra-short synaptic potentials (see (Eccles, 1982). These doubts were laid to rest by the classical work of Katz and his colleagues, who showed that at the striated muscle neuromuscular junctions, acetylcholine (ACh) was released in a quantal fashion and produced ultra-fast postsynaptic responses (Katz, 2003). In addition to chemical synapses, the existence of electrical synapses, called gap junctions, was also validated (Bennett, 1972b; Eccles, 1982; Furshpan et al., 1957).
Structurally, the use of electron microscopy in mid-1950s provided strong morphological basis of synapses in the central nervous system (CNS) (Couteaux, 1958; Palay et al., 1955) and the enteric nervous system (ENS) (Caesar et al., 1957; Richardson, 1958; Taxi, 1958). Further technical advances including high resolution electron microscopy and freeze fracture microscopy definitively identified and differentiated different types of specialized cell to cell contacts including synapses and gap junctions (Gray, 1976; Heuser et al., 1981; Peters et al., 1996). In the late 1950s, Whittaker and colleagues described the landmark techniques of the isolation of nerve varicosities (Whittaker, 1959). These techniques have allowed for the precise determination of synapse molecular anatomy and biochemistry (Morciano et al., 2009; Sudhof, 2008a).
In the CNS, distant nonsynaptic transmission to remote sites may include neuro-hormonal transmission where certain peptide neurotransmitters are released in blood capillaries of portal circulation and carried to distant targets (Kordon, 1985). Vizi and colleagues divided modes of neurotransmission into synaptic and nonsynaptic pathways (Vizi, 1984; Vizi et al., 2010; Vizi et al., 2004). On the other hand, Agnati and colleagues classified neurotransmission into wired transmission and volume transmission (Agnati et al., 1986; Agnati et al., 2010; Zoli et al., 1996). Wired transmission included neurotransmission at synapses and close juxtaposition of nerve terminals and the target cells. On the other hand, volume transmission included 3-dimensional diffusion of signals in the extracellular fluid (ECF) for distances larger than the synaptic cleft, including the CSF (Sykova et al., 2008; Vigh et al., 2004).
In the peripheral nervous system, local junctional transmission was recognized in the late 1960s and early 1970s (Bennett, 1997; Burnstock, 1986). Until then, all chemical neurotransmission was thought to involve synapses and the innervations of tissue were considered synonymous with the existence of a synapse. Later, it was observed that at smooth muscle neuromuscular junctions in the gut and other peripheral autonomic neuro-effector junctions, neurotransmission takes place in the absence of any synapses and it was suggested that at these sites, neurotransmission involved nonsynaptic transmission. Accordingly, nerve endings release their neurotransmitters in extracellular space in a manner similar to paracrine secretion. Target cells affected by a locally released transmitter even though located several hundreds to thousands of nanometers away from the release site are considered as being innervated [see (Burnstock, 2008)]. Thus, junctional transmission includes nonsynaptic wired neurotransmission and slightly overlaps the volume transmission (Vizi et al., 2010; Zoli et al., 1996).
NERVE TERMINALS AND VARICOSITIES
The nerve terminal is the terminal part of the axon filled with neurotransmitters and the location from which neurotransmitters are released. Nerve terminals may take different forms in different tissues. Nerve terminals appear like a button in the CNS, end plates in striated muscle and varicosities in many tissues including the gut (Jonakait et al., 1979). Buttons, endplates or varicosities all function to store and release neurotransmitters. In many peripheral tissues, the varicose axon branches in its proximal course and carries a covering of Schwann sheath, which is interrupted and finally lost in its most terminal part. The unmyelinated, preterminal axons with very long varicose branches are present in small axon bundles and varicose terminal axons are present as single isolated axons. The small axon bundles run parallel to and between muscle bundles and the “en passage“ varicose axons are the main sources of innervations to the gut smooth muscle bundles (Bennett, 1997). Neuro-neuronal synapses are also extensively present in the neuropil of the myenteric ganglia [see (Furness 2006)].
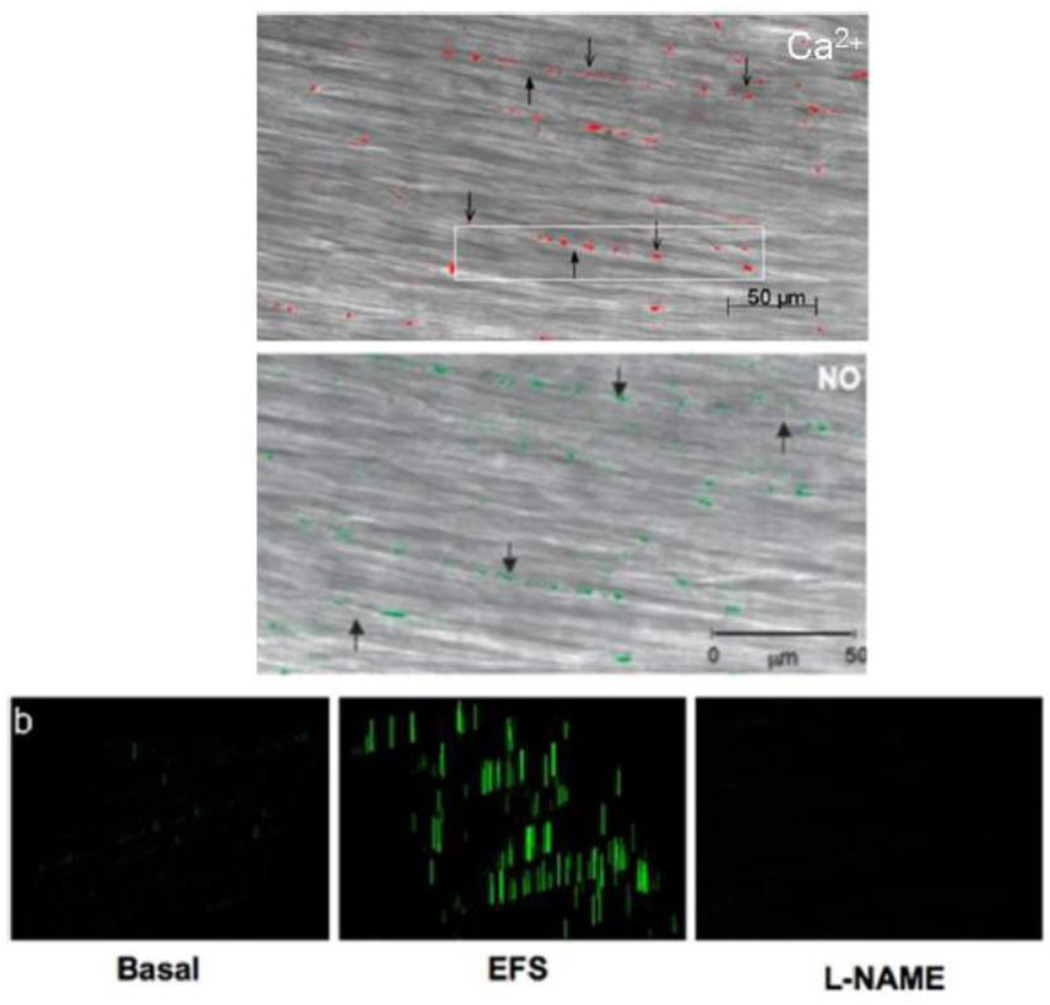
The varicose axons were first visualized for adrenergic terminals using fluorescence histochemistry described by Falck and colleagues (Falck et al., 1962; Hokfelt, 2010). These varicose axons resemble strings of beads with varicosities 0.5–2.0µ in diameter and 1 to 3 µ in length and separated by inter-varicosity axon 0.1 to 0.2 µ in diameter (Bennett, 1972a). The varicosities occur at 2–10 µm intervals and it has been estimated that a single adrenergic axon may have over 25000 varicosities on its terminal part (Bennett, 1972a). Use of dyes that penetrate and opacify terminal parts of the axons has revealed varicose endings in vagal preganglionic axons (Brookes S.J.H., 2006; Holst et al., 1997). On electron microscopy, varicosities similar to those on adrenergic fibers were also found on cholinergic axons (Richardson, 1966). Nitrergic varicosities on inhibitory motor axons in enteric muscles have recently been visualized using nitric oxide imaging in situ, providing evidence for the localized production of nitric oxide (Thatte et al., 2009) (Figure 2).
Figure 2.
Nitrergic varicosities in circular smooth muscle strip of the mouse gastric fundus. Top panel shows calcium fluorescence in the varicosities. Note that the nerve varicosities are visible as beaded structures lying along the bed of smooth muscle cells (thin arrows). The inter-varicosity axons are also clearly visible, giving a beads-on-string appearance (thick arrows). Middle panel shows DAF fluorescence indicating nitric oxide production. Note that the nerve varicosities (green) are appear as beaded structures, similar to those seen by calcium fluorescence. Bottom panel shows profiling of DAF signals. Note small signals in the basal state, intense signals after EFS and loss of signals after pretreatment with L-NAME, indicating their nitrergic nature. The fluorescence images were obtained using multiphoton microscope in muscle strips preloaded with calcium orange and DAF-2DA. EFS was applied under nonadrenergic noncholinergic conditions (From (Thatte et al., 2009), by permission).
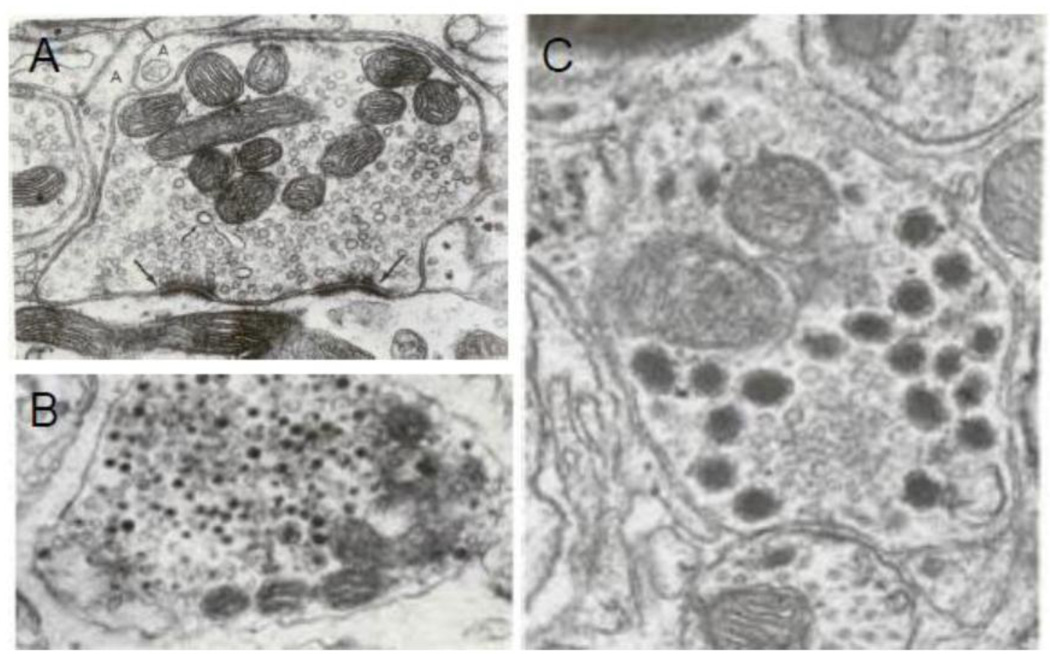
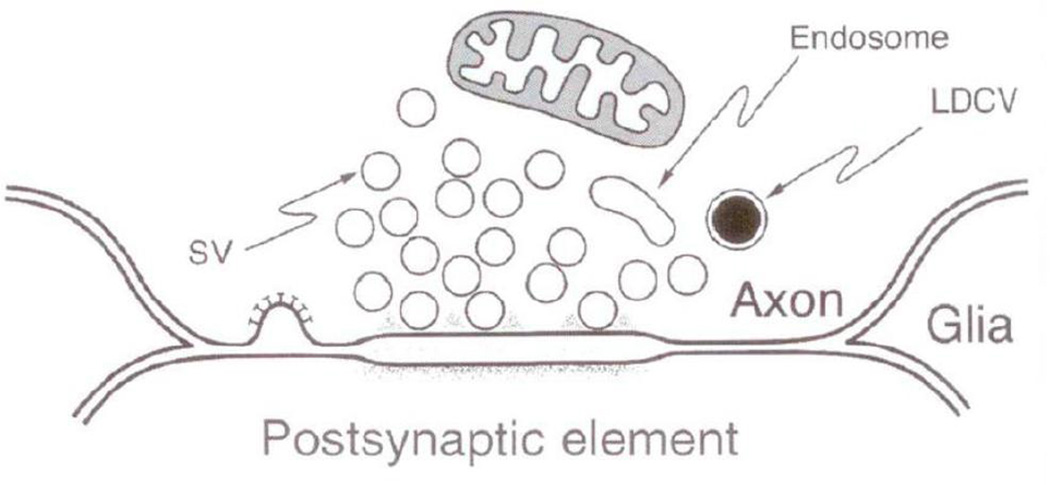
Electron microscopy reveals that varicosities are filled with vesicles. The vesicles can be divided into several different types based on their size, shape and electron density. They include: small clear vesicles (SCV), small dense core vesicles (SDCV) and large dense core vesicles (LDCV) (Song et al., 1995). Nerve varicosities that contain predominantly SCV show clear synapses but varicosities that contain mainly DCV of different sizes show no synaptic specialization (Basbaum, 1974; Heuser JE, 1977). The varicosities that contain a combination of vesicle types show an interesting distribution of vesicles types around the active zones; only the SCV cluster at the presynaptic zone and DCV are nested away from the synaptic zone. LDCV were not seen to cluster around and attach to the active zone (Cuadras, 1989; Lysakowski et al., 1999; Palay S.L., 1977; Smolen, 1988). Figure 3 presents some illustrative examples of various vesicle types and their relationship to synaptic specialization (Basbaum, 1974; Heuser JE, 1977; Klemm, 1995; Luff et al., 1987; Palay S.L., 1977). This unique distribution of vesicles in a varicosity appears to forecast that SCV and DCV vesicles may be released from synaptic and non-synaptic sites, respectively.
Figure 3.
Ultrastructure of nerve varicosities containing different types of vesicles and their relationship to synaptic specialization in the CNS neurons. (A) Shows a varicosity loaded with SCV many of which are in close association with the varicosity membrane at presynaptic membrane specialization (large arrow). The apposing postsynaptic membrane possesses postsynaptic density. This synapse is on a dendrite in superior olive, X 60.000 (From (Heuser JE, 1977), with permission). The specialized active zone is for active synaptic exocytosis. (B) Shows a varicosity with DCV of various sizes. Specialized presynaptic zone with docked vesicles are not found in such varicosities. This illustration represents an adrenergic varicosity in rat vas deferens, X 110,000; (From (Basbaum, 1974), with permission). (C) shows a varicosity containing SCV and LDCV. Note that the synaptic junction is characterized by some widening of cleft and pre- and post-synaptic plaques. Also note an interesting distribution of the vesicles in the varicosity: whereas SCV are clustered around the presynaptic specialization, the LDCV are seen away from the synapse, X77000; From dentate nucleus of cerebellum of Macaca mullata (From (Palay S.L., 1977), with permission).
Different types of vesicles have been shown to contain different neurotransmitters (Table 1). SCV usually contain the so-called classical neurotransmitters that include glutamate, γ-aminobutyric acid (GABA), glycine, acetylcholine (ACh) and adenosine triphosphate (ATP). The vesicles are round in excitatory glutamatergic synapses, but flattened in inhibitory GABA synapses (Gray, 1959; Murphy et al., 1996; Takamori et al., 2000). Monoamines such as norepinephrine (NE), dopamine and serotonin or 5-hydroxytryptamine (5-HT) are typically contained in vesicles that appear SDCV when loaded with 5-hydroxy dopamine (5-OHDA). Dense core appearance of these vesicles is artifact of 5-OHDA loading. These vesicles are in fact clear, pleomorphic in nature and include small, flat or rounded and larger round, elongated or dumb-bell shaped vesicles (Hayakawa et al., 2008). Large dense core vesicles (LDCV) may contain a large variety of neuropeptides including cholecystokinin (CCK), galanin, neurokinin (NK), neuropeptide (NPY), neurokinin (NK) including substance P, opioids, oxytocin, somatostatin, vasoactive intestinal peptide (VIP) and vasopressin, and may also contain monoamines. ATP is contained in all types of vesicles, including SCV, pleomorphic adrenergic vesicles and LDCV (Table 1). In addition to vesicular neurotransmitters, varicosities also contain enzymes of nonvesicular neurotransmitters, an example of which is nNOSα, which generates nitric oxide (NO) (Rao et al., 2008; Thatte et al., 2009).
Table 1.
Vesicle types and main neurotransmitters they contain
| Vesicle type | Size | Neurotransmitter |
|---|---|---|
| Small clear vesicle (SCV) |
30–60 nm | Classical: ACh, GABA, Glutamate, ATP |
| Pleomorphic clear vesicle (PCV) or Small dense core vesicle (SDCV) |
50–90 nm | Amines: NE, Dopamine, 5HT, Histamine, ATP |
| Large dense core vesicle (LDCV) |
90–130 nm | Peptides: CCK, CGRP, GRP, DYN, ENK, GAL, PACAP, PYY, NPY, SOM, TK,VIP Also: NE, Dopamine, 5-HT, Histamine, ATP |
| None | nNOS (NO) |
Neuropeptides are synthesized and packaged into the dense core vesicles at the Golgi complex in the nerve cell body and transported to nerve varicosities along axons via kinesin motors traveling on microtubules. Within the varicosity, LDCV mature and are filled with ATP and other transmitters and are transported to the release site on the varicosity membrane by myosin motors traveling on actin tracts for exocytosis (Bridgman, 2009). Clear vesicles are also filled with transmitters in the varicosity and are transported to synaptic and nonsynaptic release sites on the varicosity membrane by myosin motors. It has recently been shown that the parent enzyme of the non-vesicular transmitter nitric oxide (NO), nNOSα, is transported to release sites on the varicosity membrane by motor proteins like myosin Va (Chaudhury et al., 2011).
Varicosities generally contain different types of vesicles containing different types of neurotransmitters, although one transmitter may predominate in some. Therefore, co-transmission is very common. ATP is a co-transmitter with most of the other transmitters including ACh, monoamines and neuropeptides. Moreover, various combinations of these neurotransmitters yield a large variety of chemically defined varicosities in different tissues (Chaudhury et al., 2011; Chaudhury et al., 2012; Lundberg, 1996; Mitsui et al., 2002; Qu et al., 2008).
Varicosities are in contact with all cell-types surrounding them, separated by the extravascular space. However, the close contact between a terminal button or varicosity of a neuron with a dendrite or other parts of a recipient neuron show membrane specializations called synapses (Colonnier, 1968; Gray, 1959). The site of contact between the nerve terminal and striated muscle is also a type of a synapse, although the word synapse is seldom used in this regard. In contrast, most of the varicosities making contacts with smooth muscles lack synaptic specialization, while some make synapse-like, close junctions (Burnstock, 2008; Gabella, 1995). Based on the presence or absence of synaptic specialization, varicosities can be divided into synaptic and nonsynaptic varicosities.
The release of all neurotransmitters across the varicosity membrane occurs at specialized areas of the membrane called release sites and includes both synaptic and nonsynaptic release sites (Haucke et al., 2011; Neher, 2010), although some investigators have considered all release sites to be synaptic (Vanden Berghe et al., 2007). The synaptic release sites are called presynaptic active zones (AZ) and are characterized by presence of readily releasable SCV on the AZ. The synaptic release sites are seen only in synaptic varicosities. On the other hand, nonsynaptic release sites are exclusively present on nonsynaptic varicosities, but they appear to be also present on the synaptic varicosities. The synaptic and nonsynaptic release sites share some common features to accomplish vesicular exocytosis. However, they differ substantially in details of their organization, types of vesicles they handle, their modes of exocytosis and sensitivity to stimulation (Ariel et al., 2012; Golding, 1994). Synaptic release sites primarily handle exocytosis from SCV and nonsynaptic release sites particularly handle exocytosis from LDCV (Cuadras, 1989; Hammarlund et al., 2008; Lysakowski et al., 1999; Thureson-Klein et al., 1986). The nonsynaptic release sites have molecular organization similar to that seen in endocrine cells (Stevens et al., 2011; Sudhof, 2008a; Tsuboi, 2009). For example, the deletion of synaptobrevin, SNAP25 or Munc18.1 results in the loss of all synaptic and junctional exocytosis (Verhage et al., 2000). They use both ‘kiss and run’ as well as ‘complete vesicular collapse’ modes of exocytosis that determine the types of transmitters that are released. ‘Kiss and run’ exocytosis predominantly releases fully soluble transmitters and full collapse exocytosis also releases proteinaceous neuropeptides from DCV (Fulop et al., 2005; Harata et al., 2006). The mode of exocytosis is determined by stimulus intensity and the type of transmitter released (Fulop et al., 2005). Nonsynaptic neurotransmitter release involves all peptide neurotransmitters (Salio et al., 2006; Zupanc, 1996), catecholamines (Basbaum, 1974; Stjarne et al., 1994) and nonvesicular transmitters (Steinert et al., 2008), as well as the classical synaptic transmitters (Olah et al., 2009; Sarter et al., 2009). Nonsynaptic varicosities may have distinct release sites for vesicular and non-vesicular transmitters such as nitric oxide (Chaudhury et al., 2011; Chaudhury et al., 2012). Note that postsynaptic clustering scaffold proteins like PSD95 may actually exist at extrasynaptic sites, in addition to their prominent locations in the asymmetric synapses (Aoki et al., 2001) and nonsynaptic varicosities (Chaudhury et al., 2009).
The probability of transmitter secretion has been investigated in sympathetic nerve terminals. It was found that the varicosities of single sympathetic nerve terminals showed different probabilities of transmitter secretion which correlated with different syntaxin zones and synaptotagmin content of different varicosities (Brain et al.,1997).
CONTACTS BETWEEN NERVE TERMINALS AND EFFECTOR CELLS
The contact between a nerve terminal and the target cell is a 3 dimensional structure, but is often described in two dimensions. Typically the bare nerve terminal and the target receptor bearing membrane are less than 200 nm apart. The nature of contact between nerve terminal and effector cell has been investigated by light microscopy, electron microscopy and confocal microscopy. Light microscopy is limited by its resolution (~200nm) and its two dimensional capability. Confocal microscopy has advantage of providing information in 3 dimensions, but is limited by its resolution (~200nm). Confocal microscopy, however, remains a convenient method of scanning for contacts between nerve terminals and effector cells (Mann et al., 1997). Electron microscopy may have the needed spatial resolution but is only effective in two dimensions unless tedious serial sections are obtained. High resolution electron microscopy and freeze fracture techniques are needed to unequivocally distinguish between synapses and synapse-like close contacts.
POSTSYNAPTIC AND POSTJUNCTIONAL RECEPTORS
Postsynaptic receptors are almost exclusively ligand-gated ion channels (ionotropic receptors) for glutamate, GABA, ACh, certain monoamines and ATP, which are designed for eliciting very rapid response to a classical transmitter (Sudhof et al., 2008). Nonsynaptic postjunctional receptors are mostly G-protein coupled metabotropic receptors that produce a slower response. They include metabotropic receptors for the classical neurotransmitters, monoamines, norepinephrine, purines and peptide transmitters (Kandel et al., 2000). Postjunctional receptors also include some ionotropic receptors such as nicotinic receptors in the central nervous system (CNS) (Dani et al., 2007) as well as the autonomic nervous system (ANS) (Fernandes et al., 2010). For example, in chicken ciliary ganglion, postganglionic neurons have been show to possess a high concentration of nicotinic receptors at the synapse and a lower concentration of nicotinic receptors outside the synapse (ectopic site) (Coggan et al., 2005). Similar observations have been made in the skeletal neuromuscular junctions, as well as autonomic neuromuscular junctions in heart and blood vessels (Hirst et al., 1992; Sargent et al., 1989). Interestingly, the synaptic and nonsynaptic nicotinic receptors was found to be molecularly different, α3-nAChR in synapses and α7-nAChR outside the synapse, each having different functional behavior (Coggan et al., 2005).
POSTSYNAPTIC AND POSTJUNCTIONAL POTENTIALS
Neurotransmitters released from nerve terminals produce changes in the membrane potential of target cells that are called either postsynaptic or junction potentials, terminologies coined based on the investigators’ belief as to whether they are due to synaptic or junctional transmission. In the CNS, the ANS and the enteric nervous system (ENS), these electrical voltages are traditionally called postsynaptic potentials. At the smooth muscle neuromuscular junction, these potentials are celled junction potentials. These potentials propagate passively and may result from either depolarization (called excitatory potential) or hyperpolarization (called inhibitory potential). The effector cells may show only excitatory, inhibitory or both excitatory and inhibitory potentials. Moreover, they are graded and show temporal and spatial summation resulting in net change in membrane potential. Duration of the action potential generated by Na+ channels as in neurons is around 1ms. On the other hand, duration of the action potential generated by Ca2+ channels in muscles may be as long as 100ms (Kandel et al., 2000).
Nerve stimulation simultaneously releases many neurotransmitters that produce overlapping postsynaptic or postjunctional potentials in the effector cells. Individual potential changes are pharmacologically isolated by the use of receptor antagonists of the overlapping potentials. The potentials are also classified based on their time courses including latency, time to peak, decay time and total duration. These potentials can be arbitrarily grouped into those lasting ~10–50ms, ~250ms-2s, and several seconds to minutes.
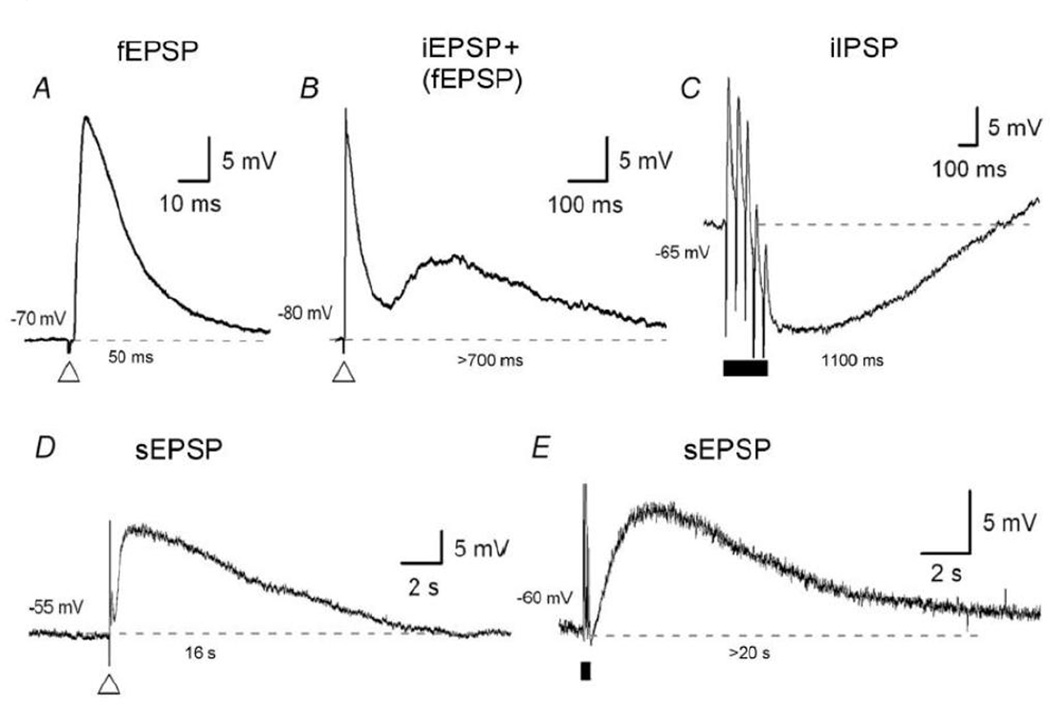
The ultrafast potential lasting 10–50 ms is only seen in synapses that are present between neurons. It is not seen at other neural targets such as smooth neuromuscular junctions. The synaptic potential is called fast postsynaptic potential (fPSP) or simply postsynaptic potential (PSP). PSP may be excitatory called EPSP or inhibitory called IPSP. Both of these are mediated by ionotropic responses, but the IPSPs have slower time courses than fast EPSPs with durations up to 150 ms. The slow potentials lasting several seconds to minutes are seen in neurons as well as non-neural targets. They have been traditionally called sPSP in the neurons and may be excitatory or sEPSP and inhibitory or sIPSP. At the smooth muscle neuromuscular junction, these slow potentials have been called sJP and may be excitatory or sEJP and inhibitory or sIJP. The potentials lasting 250ms-2s have been recognized at the neuromuscular junctions for a long time and have also been recognized in the neurons (Kobayashi et al., 1968). At the smooth muscle neuromuscular junction, they are called fast junction potentials that may be excitatory or fEJP or inhibitory or fIJP. In the ENS, potentials with similar durations have recently been recognized but are called intermediate PSP, because the true synaptic ultrafast PSP has been called fPSP. Monro et al. (Monro et al., 2004) have described purine mediated fast, intermediate and slow PSP in submucous as well as myenteric neurons (Gwynne et al., 2009a) (Figure 4). A critical review of the available data suggests that the potential lasting 10ms-50ms is truly a synaptic response. iPSPs and sPSPs in neurons have origins that are similar to fJPs and sJPs seen at neuromuscular junctions.
Figure 4.
Three time courses of postsynaptic potentials in enteric neurons. (A) Shows fast excitatory postsynaptic potential (fEPSP) with duration of ~50ms. (B) Shows intermediate postsynaptic potentials (IPSP) having duration of ~600 ms; B1) Shows intermediate excitatory postsynaptic potential (iEPSP) and B2) Shows inhibitory postsynaptic potential (iIPSP). The iIPSP shown here was elicited by a train of stimuli; however, a single pulse also produces a similar IPSP. (C) Shows slow post synaptic potential (sPSP) with a duration of >12,000 ms. C1) shows sEPSP elicited by a single pulse and C2) shows a sEPSP elicited by a train of stimuli. Note long duration of this sEPSP. Thus durations of intermediate potentials are 20-times longer and that of sEPSPs is >400-times longer than that of the fEPSP. The time course of iIPSP and sIPSP are similar to those of fastJP and slowJP respectively (From (Monro et al., 2004), with permission).
In neurons, excitability induced electrical signals facilitate neurotransmitter release. In smooth muscles, the released neurotransmitters influence the contractile state of smooth muscles by a process called electromechanical coupling. Neurotransmitters may also cause muscle contraction or relaxation by directly affecting contractile processes without any change in membrane potential by a process called pharmacomechanical coupling. However, transmitters usually cause muscle contraction or relaxation via both electromechanical and pharmacomechanical coupling mechanisms (Bolton et al., 1986; Somlyo et al., 1968). Therefore, membrane potentials may not fully reflect the effect of a transmitter on muscle.
The time course of the synaptic/junctional response is dependent upon several factor including: 1) time course of neurotransmitter release which itself is dependent upon the nature of the neurotransmitter and the release mechanism; 2) characteristics of the space where the neurotransmitter is released such as ‘closed’ or ‘open’ space and the distance between the release site; and 3) the target receptors; and density, distribution and kinetics of the receptor and the signaling pathway. These individual factors are appropriately packaged together into synaptic and junctional modes of neurotransmission with distinct structural units that can be distinguished by the time course of the response. Morphological and molecular components of the synapses and correlative synaptic potentials are best described in the CNS and are being investigated in the ANS including the ENS. On the other hand, structural and functional features of the junctional neurotransmission are best described at the peripheral autonomic neuromuscular junctions.
SYNAPTIC NEUROTRANSMISSION
Synaptic neurotransmission exclusively involves the so called synaptic varicosities. Sometimes, conclusions about the presence of a synapse are made on light or confocal microscopy. High resolution electron microscopy with proper tissue fixation and orientation is necessary for identification of a synapse (Colonnier, 1968; Gray, 1976; Westphal et al., 2008). Synaptic transmission typically occurs between neurons. The advances in molecular anatomy and pharmacology of a synapse, which have been made in the CNS, are largely lacking in the study of the peripheral autonomic nervous system including the ENS and AG. Therefore, structure to function relationship of synaptic neurotransmission is mostly derived from studies in the CNS neurons.
CENTRAL NERVOUS SYSTEM
On electron microscopy, a synapse is recognized as a focal area of less than a square micrometer that is characterized by variably thickened presynaptic and postsynaptic membranes, separated by a synaptic cleft (Peters et al., 1996). However, the prominence of the thickening may vary in different types of synapses (Klemann et al., 2011) and there are many pitfalls in definitively identifying a synapse from other synapse-like specializations (Gray, 1976). The use of freeze fracture or freeze etching techniques is necessary for the unequivocal identification of a synapse from other synapse-like junctions (Gray, 1976; Peters et al., 1996).
Presynaptic membrane specialization
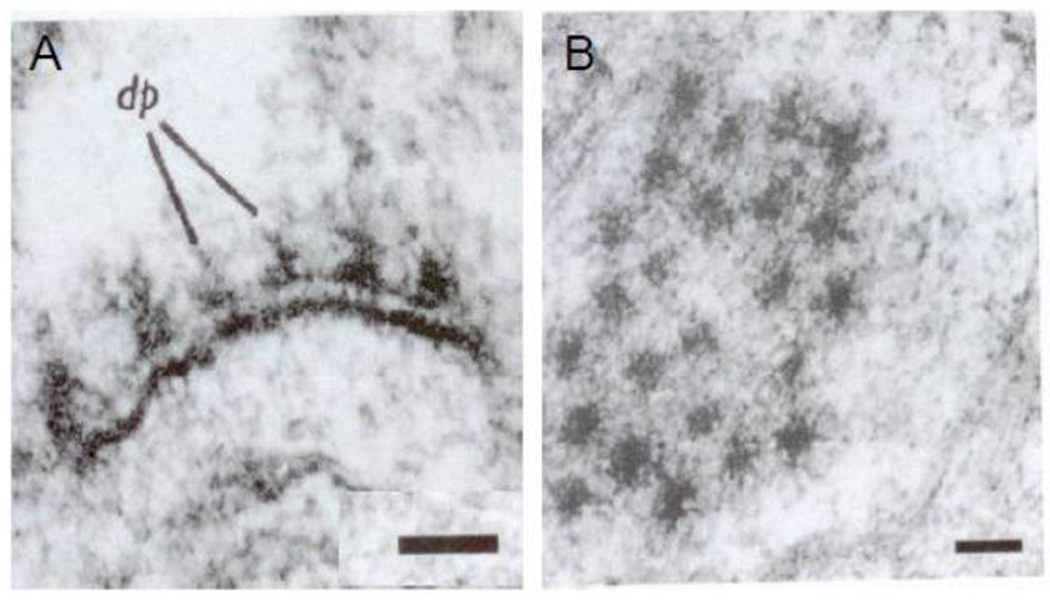
The focal area of membrane at the synapse in the presynaptic terminal or varicosity is called the presynaptic “active zone”. It is characterized by the presence of a synaptic grid and SCV docked on the membrane. The synaptic grid was initially described by Gray in 1963 and subsequently in more detail by others using careful transverse and tangential sections of synapses (Gray, 1963; Pfenninger et al., 1972). The grid is also called cytomatrix of active zone (CAZ) and has been described in detail (Gray, 1963; Phillips et al., 2001; Zamorano et al., 2001). It appears as an interrupted presynaptic thickening on a transverse section of a synapse and closely spaced hexagonal electron dense areas in a tangential section (Gray, 1963) (Figures 5A& 5B).
Figure 5.
The presynaptic active zone (A) and (B) are electron micrographs of cat spinal cord synapse cut perpendicularly (A) and tangentially (B), respectively. Note the regularly arranged dense projections in the presynaptic active zone. The electron density of the grid was enhanced by the use of 1% phosphotungstic acid. Vesicle membranes were not preserved by the fixative; Scale bar 100nm (From (Gray, 1963), with permission).
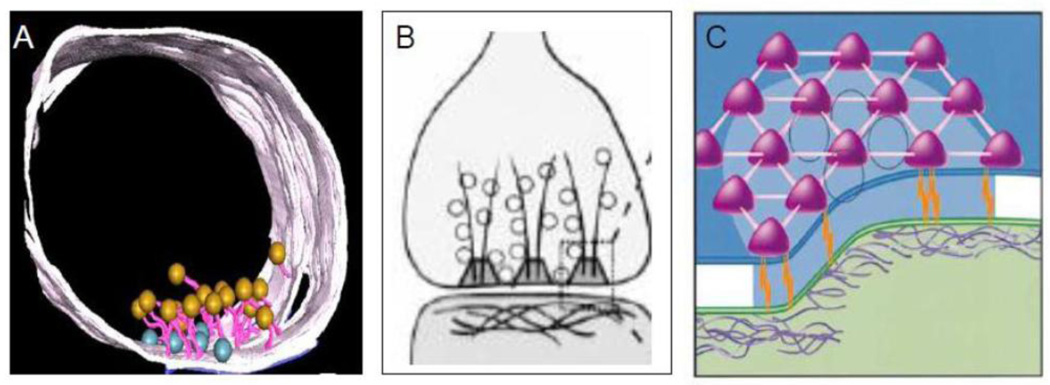
Recent studies have further established the focal nature of the presynaptic active zone and the presynaptic grid. Figure 6A shows a localized synaptic active zone with attached SCV that can be easily distinguished from the extensive nonsynaptic region of the varicosity (Siksou el al 2007). Figures 6B and 6C present a model of presynaptic web or grid at the active zone (Phillips et al 2001). The presynaptic grid consists of dense fibrils and particles. The grid contains a fibrillar component that projects into the cytosol and a particulate component that forms a sievelike structure (Bloom et al., 1968a; Bloom et al., 1968b; Landis et al., 1988). The grid particles are 50–80nm and are located 50–100nm apart. They are connected to each other by 10nm fibrils forming a web or sieve-like grid. They are also connected to the PSD across the synaptic cleft via adhesion molecules. The presynaptic active zone shows secretory granules and voltage dependent Ca2+ channels (Haucke et al., 2011). The active zone grid is thought to serve several functions: 1) exclude larger-sized DCVs from the active zone and allow only SCV access to the active zone; 2) guide SCV towards the presynaptic membrane; 3) register the active zone in perfect alignment with the postsynaptic density; and 4) provide housing for proteins involved in exo-endocytosis of SCV (Ariel et al., 2012; Gray, 1963; Pfenninger et al., 1972; Waites et al., 2011; Zamorano et al., 2001).
Figure 6.
The presynaptic active zone and the synaptic grid (A) is a 3D reconstruction of transverse section through a varicosity Note that active zone with docked vesicles at the varicosity membrane (blue) forms a small localized zone of the varicosity membrane (From (Siksou et al., 2007), with permission). (B) Represents a model of the presynaptic grid. Fibrillar components originating at the presynaptic membrane project into the cytoplasm. The presynaptic particles with spaces in between are present next to the membrane. (C) Three-dimensional model of the presynaptic grid formed by the particles. The particles form a hexagonal array with sieve like formation. The particle grid supports selective access of small vesicles to the plasma membrane for their subsequent fusion The particles are linked to PSD of the postsynaptic cell across the synaptic cleft by adhesion molecules. Presynaptic particles also contain components necessary for the retrieval of vesicle membrane proteins after their fusion with the plasma membrane. (From (Phillips et al., 2001), with permission).
The presynaptic active zone is characterized by the presence of SCV docked on the cytoplasmic side of the synaptic membrane. Such membrane docked SCV are not seen in nonsynaptic release sites, at least during thorough review of widely available electron photomicrographs. SCV attached to the cytoplasmic side of the membrane at the synaptic cleft were first observed at motor endplates in striated muscles (Peters et al., 1996). Similar observations were made in the varicosities at the glutamatergic synapse in the CNS (Peters et al., 1996).
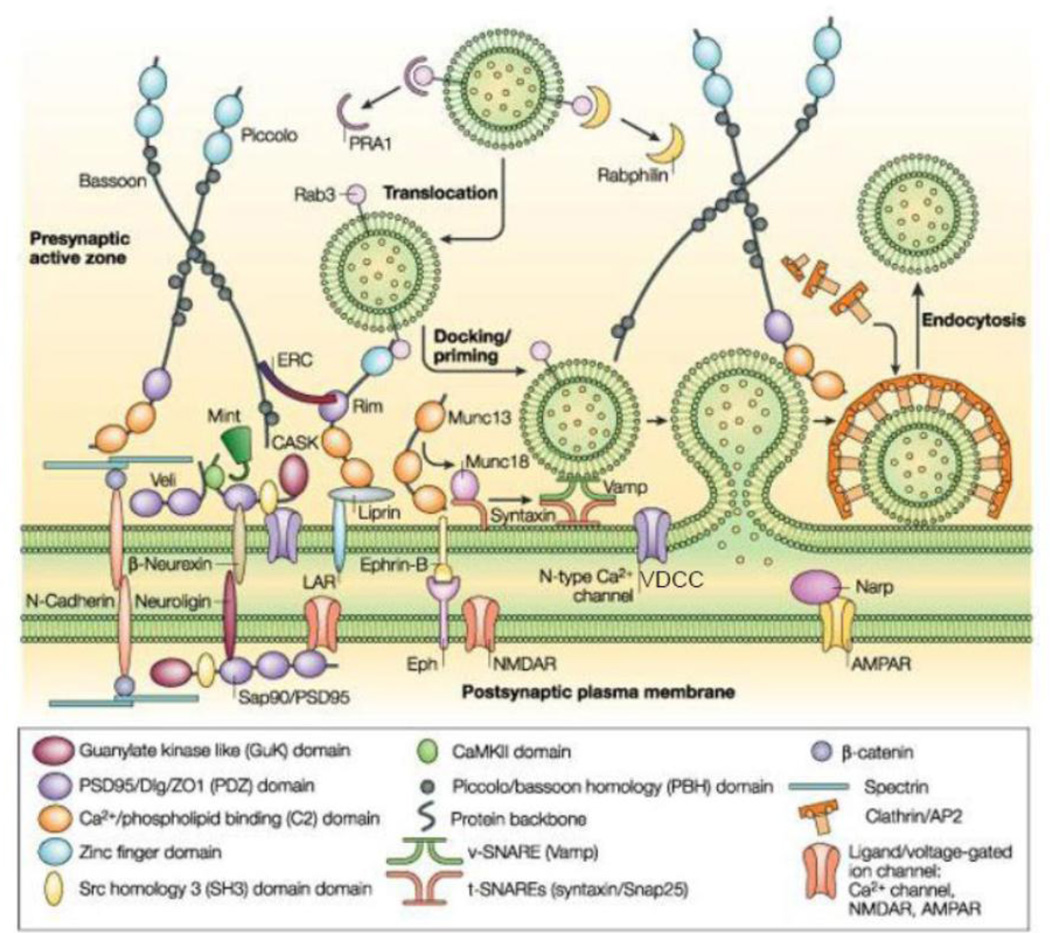
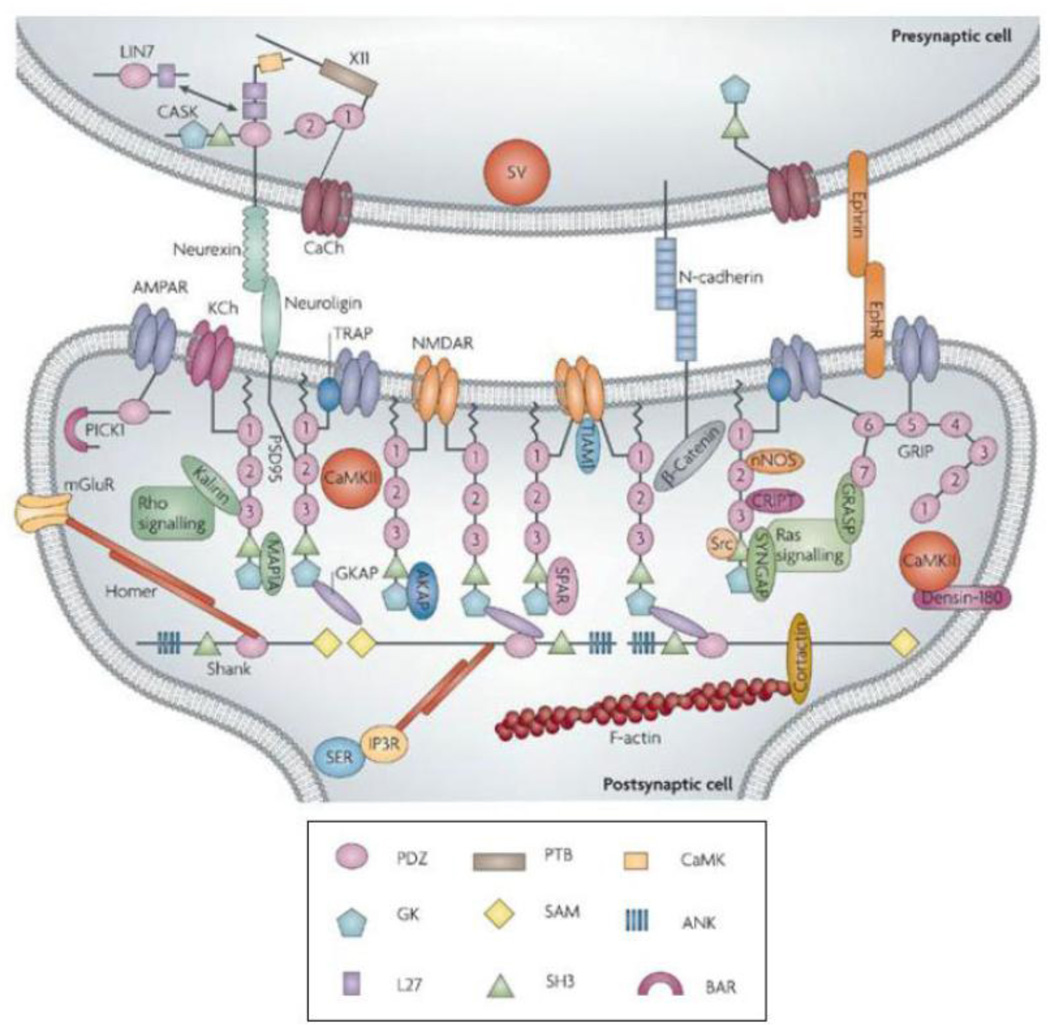
The proteome and the 3D cytomatrix of the presynaptic active zone in CNS neurons reveal several hundred proteins that serve specific functions during the release of neurotransmitters (Morciano et al., 2009; Siksou et al., 2007). Three major protein complexes with overlapping functions define the presynaptic active zone (Figure 7). 1) The first complex is largely structural that includes proteins associated with the presynaptic grid and registration with the postsynaptic membrane. It includes: cell adhesion molecules such as neurexins, ephrin and SynCAM that bind with postsynaptic proteins, neuroligins and N-cadherin, ephrin receptor and NCAM, respectively,to maintain the structure of a synapse. Out of these adhesion proteins, neuroligins and neurexins may be unique to a synapse, and N-cadherin and integrins anchor together the perisynaptic areas around the synapse to “lock-in” the synaptic cavity (Fannon et al., 1996)‥ Cytoskeletal proteins, such as piccolo, bassoon, ERC/Cast, liprins; PDZ domain containing proteins such as CASK (calcium/calmodulin-dependent serine protein kinase), veli and mint, which help cluster transmembrane proteins including VDCCs (Fejtova et al., 2006). The filamentous component of the grid includes actin, spectrin and structural proteins such as bassoon, piccolo and rim. The filaments help regulate the delivery of SCV to their presynaptic location (Cingolani et al., 2008; Hilfiker et al., 1999). The particle is a supramolecular structure formed by structural proteins and is rich in proteins involved in synaptic vesicular exocytosis and endocytosis (Phillips et al., 2001; Zamorano et al., 2001). 2) The second complex is involved in synaptic vesicle docking and fusion. It includes Rim, Rab3a and Munc13, components of the SNARE complex, including syntaxin, SNAP 25, and Munc18 and VDCCs. 3) The third complex is involved in synaptic vesicle endocytosis. It includes clathrin, dynamin and a family of SH3-domain-containing adaptor proteins (Dittman et al., 2009; Sorensen, 2009).
Figure 7.
Molecular anatomy of the presynaptic active zone. Three distinct complexes help to define the active zone (described in text). The first complex is largely structural, and is thought to hold the active zone in register with the postsynaptic density (PSD) and clusters calcium channels within the active plasma membrane. The second complex is involved in synaptic vesicle docking and fusion. The third complex is involved in synaptic vesicle endocytosis. (From (Qui, 2004), with permission).
A large number of proteins are involved in the exocytosis of SCV at the presynaptic active zone (Haucke et al., 2011; Sudhof et al., 2008). In varicosities, SCV anchors to the actin forming fibrils in the core of the varicosity via synapsin (Hilfiker et al., 1999). These vesicles form the ‘reserve pool’ of the SCV (Rizzoli et al., 2005). Phosphorylation of synapsin detaches SVC from the actin grid and vesicles enter the ‘recycling pool’ as they are guided to the varicosity membrane with the help of motor proteins like myosin Va (Bridgman, 2009; Rizzoli et al., 2005). Tethering and docking proteins of the active zone include the RIM complex that includes RIM, ELKS, Munc13, α-liprins, piccolo, and bassoon. Rab proteins including Rab3 and Rab27 are GTPases that mediate the anchoring of vesicles to the RIM proteins. Docking of the vesicles to the active zone is also facilitated by active zone actin (Cingolani et al., 2008).
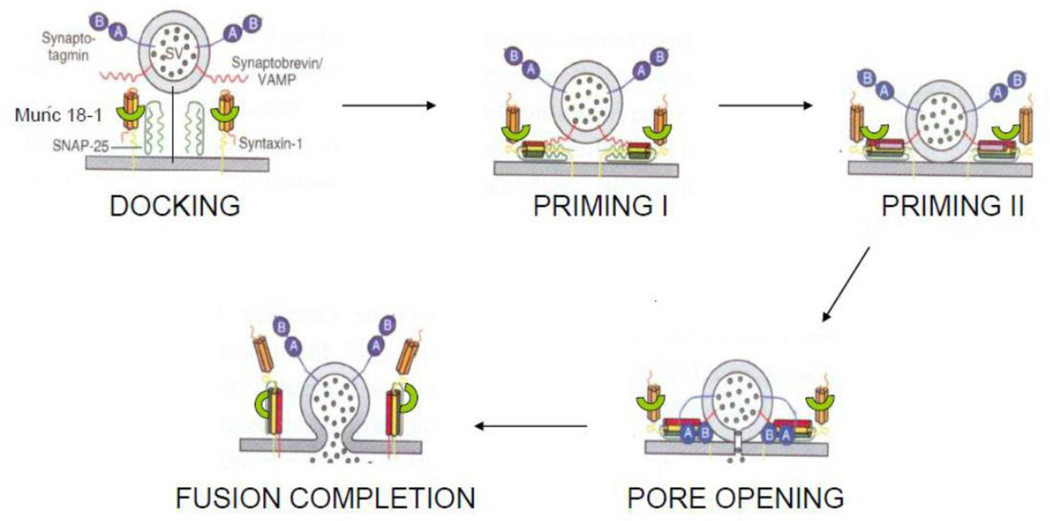
Neurotransmitters contained in secretory vesicles are released by exocytosis as packets in a quantal fashion (Katz, 2003). The steps involved in synaptic exocytosis have been described by Sudhof and colleagues (Sudhof, 2008a) (Figure 8). After vesicle docking, the priming proteins force the docked vesicles and the active zone of the varicosity membrane together and prepare them for immediate exocytosis. These vesicle constitute the so called ‘release ready pool’(Sudhof, 2008a). The priming proteins include the SNARE complex, Munc18.1 and complexin. The SNARE complex includes synaptobrevin on synaptic vesicle and SNAP25 and syntaxin-1 on the plasma membrane. Both the SNARE complex and clasp like Munc18 are necessary for early stages of priming (Sudhof, 2008a). A soluble protein, complexin, binds with the SNARE complex for further priming and preparing of the vesicles for Ca2+-induced exocytosis (Tang et al., 2006). Membrane-associated actin plays the additional regulatory role of providing physical and molecular barrier to priming and therefore possibly acts to hinder exocytosis (Cingolani et al., 2008).
Figure 8.
Schematic diagram of key steps involved in exocytosis of vesicles at the active zone. For details, refer text. (Modified from (Tang et al., 2006) and (Sudhof, 2008b), with permission).
At the presynaptic active zone, there are two types of Ca2+-induced exocytosis. At resting Ca2+ concentrations, there is a spontaneous low-rate neurotransmitter release called “asynchronous exocytosis” that is synaptotagmin and complexin independent. With Ca2+ influx, associated with an invading action potential, there is a rapid neurotransmitter release called “synchronous release”, which is synaptotagmin and complexin dependent and accounts for >90% of total transmitter release. During synchronous release, the rate of neurotransmitter release increases >10,000 fold in less than a millisecond. This amazing speed is consistent with the view that Ca2+ acts as a trigger for the release of the vesicles that are already docked and primed at the presynaptic active zone (Sudhof, 2008a). Genetic deletions of synaptotagmin-1 and complexin cause the loss of synaptic synchronous vesicular release while normal asynchronous vesicular exocytosis is preserved (Tang et al., 2006).
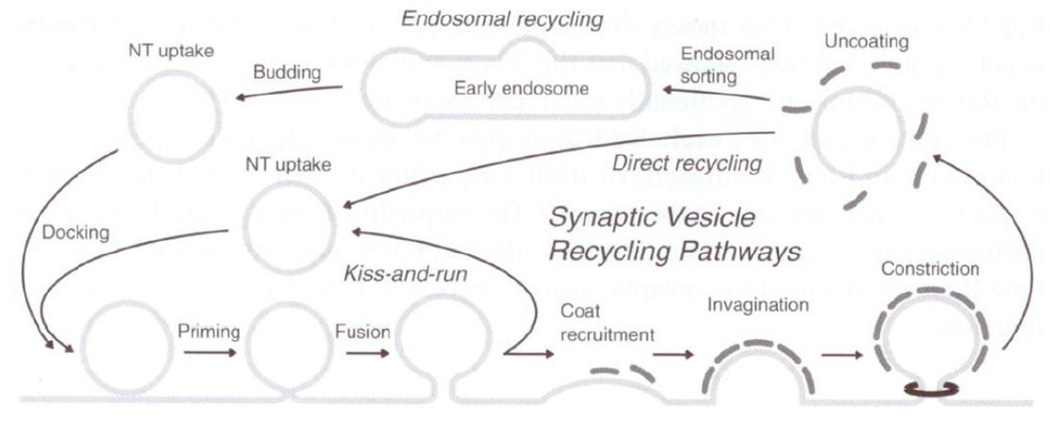
Endocytosis and recycling events follow exocytosis (Lang et al., 2008) (Figure 9). The prominent form of exo-endocytosis in synaptic neurotransmission is “kiss and run type” or Ω fusion, as it is the fastest type of recycling (Harata et al., 2006). In kiss and run type exo-endocytosis, complete fusion of the vesicle does not occur. After complete fusion of the vesicles, endocytosis of synaptic vesicles involves clathrin coating, membrane deformation, budding, fission and uncoating that occurs in the perisynaptic region of the varicosity membrane (Dittman et al., 2009).
Figure 9.
Recycling of the synaptic vesicle. For details, refer text (From (Lang et al., 2008), with permission).
Synaptic cavity
A synaptic cleft separates the pre- and post- synaptic membranes. The usual width of the synaptic cavity is 20–30 nm (De Camilli et al., 2003) (Figure 10). At the motor endplate, which is a type of synapse at the skeletal neuromuscular junction, the synaptic cavity is as wide as 100 nm (Cowan W.M., 2003). The synaptic cavity in the CNS is usually wider than that of the adjoining junctional space (De Camilli et al., 2003). Therefore the proximity of membranes of the prejunctional varicosity and the target cell is not a reliable morphological marker of a synapse. The synaptic cavity is characterized by anchoring proteins that bind the presynaptic and postsynaptic membranes together (Fannon et al., 1996). In freeze-substituted and in deep etched material, 4–6 nm diameter fibrils can be seen to bridge between the pre- and postsynaptic membranes (Gray, 1976). The synaptic cavity also contains short fibrillar structures that are 4–6 nm in diameter and 8–15 nm in length that may represent ionotropic receptors in the membrane of the postsynaptic element. The concentration of neurotransmitters in the synaptic cleft may reach millimolar range and decays rapidly with a time constant, tdecay of 0.1–1 ms.
Figure 10.
Schematic illustration of synaptic cleft Synaptic cleft separates the presynaptic active zone with SCV docked on the varicosity membrane and synaptic grid and the postsynaptic density. Note that the synaptic cleft may be wider (20–30nm) than the adjoining nonsynaptic, junctional space (10–20nm). However, width of the junctional space is highly variable (From De Camilli P, 2003, with permission).
Postsynaptic specialization
Presynaptic nerve terminals make focal synaptic contacts with different parts of the postsynaptic neuron including dendrites, cell bodies and axons. The post synaptic membrane is highly specialized and precisely aligned with the presynaptic release site.
The postsynaptic site is characterized by the postsynaptic density, which is a dense plate of actin frame work and scaffolding proteins called PSD proteins, bearing transmembrane ionotropic channel receptors (Okabe, 2007). The size, shape, and prominence of the postsynaptic density differ at different synapses (Klemann et al., 2011). The postsynaptic density may be oval, perforated or annular in shape and is prominent in glutamatergic excitatory synapses (Okabe, 2007). Glutamatergic synapses have a prominent postsynaptic density, whereas GABA inhibitory synapses have a less prominent postsynaptic density. Glutamatergic synapses with postsynaptic prominence are celled asymmetrical synapses whereas GABA inhibitory synapses without postsynaptic prominence have been called symmetrical synapses (Colonnier, 1968; Gray, 1963; Peters et al., 1996).
The main scaffolding protein at the glutamatergic synapse is PSD95, which assembles ionotropic glutamate receptors, nNOSα and neuroligins. PSD95 is dynamically anchored to the membrane via palmitoylation. The proteome of the postsynaptic density of a glutamatergic synapse includes several hundred proteins (Feng et al., 2009) (Figure 11). The nature of the synapse-associated proteins may differ in different types of synapses. At the cholinergic synapse in the parasympathetic ganglion, PSD93 may play an important role in the stabilization of nicotinic receptors (Conroy et al., 2003; Neff et al., 2009; Parker et al., 2004). The ionotropic receptors are anchored directly or indirectly to the postsynaptic density by scaffolding proteins containing PDZ binding domains (Conroy et al., 2003; Kim et al., 2004). Ionotropic receptors are ligand gated ion channels that allow for the rapid activation of ionic currents in the postsynaptic membrane. Note the interesting observation that despite the diversity of the neurotransmitter-specific receptors, there is huge homology in the scaffolding proteins in different varieties of the synapses.
Figure 11.
Molecular anatomy of the postsynaptic density For details, refer text (From Feng et al., 2009, with permission).
Postsynaptic potentials
The postsynaptic electrical response is very fast and called the fPSP. The fPSP is characterized by a small delay of 1–3 ms and lasts up to 30 ms. Duration of the postsynaptic current can vary more than an order of magnitude depending upon the kinetics of the postsynaptic receptors (Scimemi et al., 2009). In the CNS, the fPSP may be either depolarizing (excitatory) or hyperpolarizing (inhibitory) in nature (Kandel et al., 2000). The fPSP correlates with the structural features of a synapse such as the release-ready vesicles, a synaptic cavity where sudden high concentrations of released transmitter can be achieved and rapidly responding transmitter-gated ion channels. The fEPSP and fIPSP may be surrogate electrophysiological markers of a synapse.
The fast excitatory postsynaptic potentials (fEPSP) in the CNS are usually due to glutamate acting on its ionotropic receptors called NMDA and non-NMDA (AMPA and kainate) receptor (Frank, 2011). The non-NMDA ionotropic receptors are permeable to Na+ and K+ and are responsible for fast early peak of the fEPSP. The NMDA receptor has the additional property of being voltage dependent and Ca2+ permeable. NMDA ionotropic receptor contributes to the late component of the fEPSP and the Ca2+ mediated effects in the postsynaptic neuron. The time constant of decay of fEPSPs mediated by AMPA receptors is 1–2 ms and is much longer for NMDA receptor, which reflects the channel kinetics.
The fast inhibitory postsynaptic potential (fIPSP) in the CNS is mediated by GABA and glycine acting on their ionotropic receptors, respectively, that conduct Cl− (Arancibia-Carcamo et al., 2006). The influx of Cl− in central neurons causes membrane hyperpolarization (Smith et al., 2010).
ENTERIC NERVOUS SYSTEM
The electron microscopic details of ENS and CNS synapses are very similar. However, the molecular anatomy and physiology of synapses in the ENS remain to be fully investigated. Functionally, synapses of ENS exhibit synaptic fast excitatory potentials similar to those of the CNS synapses, but the chemical nature of their synaptic transmitters are different (Bornstein J.C., 2002; Galligan, 2002a; Galligan, 2002b; Gwynne et al., 2007). Fast IPSPs have not been described in the enteric neurons.
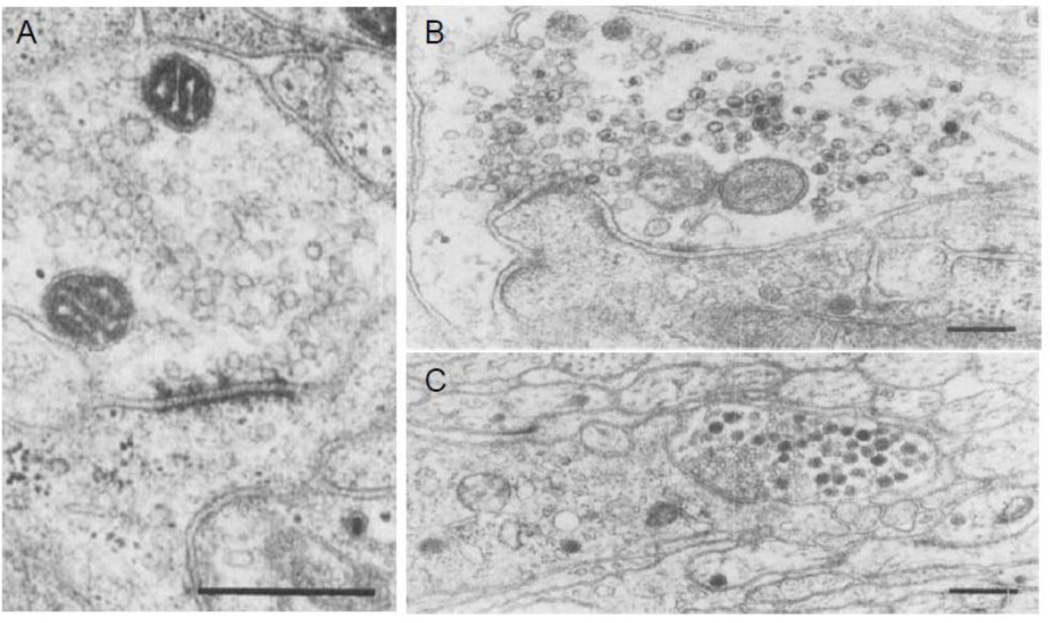
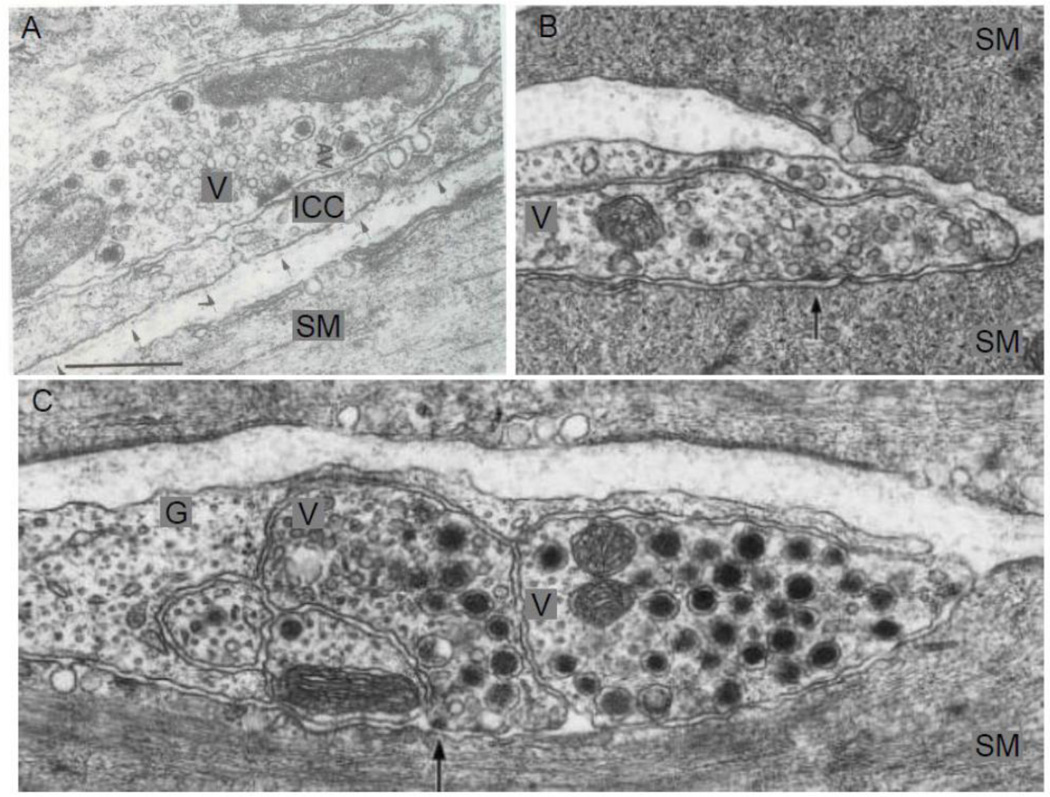
In the ENS, presynaptic varicosities contain several different types of vesicles (Baumgarten et al., 1970; Gabella, 1972; Richardson, 1966). In the myenteric plexus of the guinea pig ileum, Gabella (Gabella, 1979) reported that the nerve profiles that contained SCV showed synaptic specializations that varied from a moderate thickening of pre- and post-synaptic membranes to prominent dense presynaptic projections. In the ENS, SCV are filled with ACh. This is in contrast to the CNS where most SCV are filled with amino acids. Nerve profiles that mainly contained LDCV are nonsynaptic and contain neuropeptides such as VIP and SP as well as ATP (Furness, 2006). Moreover, LDCV were not observed closer than 200 nm to the presynaptic membrane (Baumgarten et al., 1970; Gabella, 1979) (Figure 12). These observations are similar to those made in the CNS neurons.
Figure 12.
Distribution of vesicles around synaptic specialization in enteric varicosities. (A) Shows a varicosity containing SCV and presynaptic active zone with prominent cytoplasmic projections. Also note the associated postsynaptic density on a dendrite. (From myenteric plexus of the guinea pig ileum) Marker: 0.5 µm. (B) Shows a varicosity containing SGV contacting with an intramural neuron. Note the absence of well-defined synapse. Marker 0.2 µm (From myenteric plexus of the guinea pig ileum). (C) Shows a nerve varicosity with different types of synaptic vesicles. Note that SCV are clustered around an area of active zone and the SCV seem to be interposed between the DCV and the active zone. Marker: 0.5 µm (From submucosal enteric plexus of the guinea pig ileum). Note that the distribution of the vesicles in enteric varicosities is similar to that in the CNS varicosities (From (Gabella, 1979), with permission).
Hayakawa and colleagues (Hayakawa et al., 2008) reported that in the neuropil of myenteric ganglia in rat duodenum, half of the nerve terminals contained pleomorphic vesicles. A considerable number of tyrosine hydroxylase immunoreactive terminals made asymmetrical synaptic contacts with dendrites, spine or soma of myenteric ganglia. It was also found that 16% of the total number of axosomatic terminals showed tyrosine hydroxylase immunoreactivity. These studies show that myenteric neurons receive direct input from adrenergic terminal containing pleomorphic vesicles, which contain NE, 5HT and also ATP. However, it is unclear if there are well defined synapses between the adrenergic nerve terminals and the myenteric neurons.
Furness and colleagues have investigated connections between the immunohistochemically identified enteric neurons, using high resolation confocal microscopy and electron microscopy. They have described many synapses in these circuits (Li and Furness 2000; Pompolo and Furness 1998; Mann et al., 1997; Portbury et al. 1995). Such studies will help define the relative abundance and the role of synapses in well defined neural circuits in the ENS.
The enteric neurons can be divided based on morphology, neurochemistry, pharmacology and function. Electrophysiologically, they can be divided into AH neurons that are characterized by prolonged hyperpolarization following their action potentials and S-neurons that show prominent fast EPSPs (Bornstein J.C., 2002; Galligan et al., 2000; Lomax et al., 1999). AH neurons have Dogiel type II type morphology and serve as intrinsic primary sensory neurons (IPANs). S neurons serve as interneurons, motor neurons and secretomotor neurons. However, some AH neurons also receive synaptic input and serve as interneurons.
Limited information is currently available on the molecular anatomy and biochemistry of neurotransmitter release from the active zone and nature and physiology of postsynaptic density in the enteric synapses. However, the synaptic adhesion molecules, neurexins and neuroligins that are known to be present in the CNS synapse, have also been reported in the ENS (Gershon and Ratcliffe, 2004). Moreover, several types of ligand gated ion channels are expressed in the enteric neurons. These include: nACh receptors, P2X receptors, 5-HT3 receptors, GABA-A receptors, NMDA-and AMPA receptors and glycine receptors (Galligan, 2002a). All these receptors are expressed in AH neurons and many of them are localized to extrasynaptic sites. In all S-type and some AH neurons, nACh receptors, P2X receptors and 5-HT3 receptors mediate fast synaptic potentials (Galligan, 2002b). The functional role of GABA, glutamate and glycine receptors in the enteric neurons is unclear at this time.
In contrast to the paucity of structural data, a considerable amount of functional data is available in the ENS. In the ENS, fast EPSPs are observed, which have a latency of 1 to 3 ms and last around 30 ms (Galligan, 2002a; Gwynne et al., 2007). This response pattern is very similar to synapse-mediated fPSPs in the CNS. However, unlike the central neurons, the enteric neurons exhibit only fEPSPs, no fIPSPs. fEPSPs are present in 70% of the myenteric and 90% of submucous neurons (Gwynne et al., 2007). Fast EPSPs are uncommon in AH neurons, but are present in almost all musculomotor neurons, secretomotor neurons and interneurons (Castelucci et al., 2002; Gwynne et al., 2007).
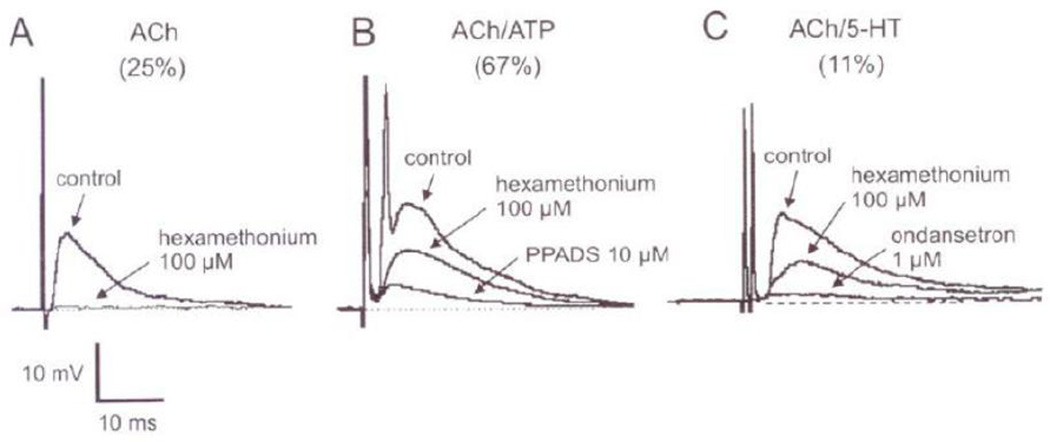
Acetylcholine acting on nicotinic receptors is the major synaptic neurotransmitter in the ENS. However, P2X and 5HT3 receptor mediated purinergic and serotoninergic neurotransmission also plays a role (Galligan 2002b; Monro et al., 2002) (Figure 13). The nicotinic receptors on the enteric neurons have subunit composition of α3α5β4 that is effectively blocked by hexamethonium (Galligan, 2002b; Zhou et al., 2002). Hexamethonium-sensitive fEPSPs are demonstrable in almost all S-type neurons (Zhou et al., 2002). Consistent with this observation, most of the identified enteric neurons express nicotinic receptors and nicotinic fEPSP (Bian et al., 2004; Gwynne et al., 2007; Kirchgessner et al., 1998; Monro et al., 2002; Nurgali et al., 2004; Zhou et al., 2002). More recent quantitative studies revealed that exclusive nicotinic transmission was seen in only one quarter of unselected myenteric S-type neurons. However, in the majority (> three quarters) of neurons, nicotinic transmission appeared to account for a portion of the fEPSP. Despite the paucity of information on the molecular biology of nicotinic synapses, there appear to be important differences between synaptic proteins at nicotinic and glutamatergic synapses (Conroy et al., 2003; Neff et al., 2009; Parker et al., 2004).
Figure 13.
Pharmacological identification of chemical nature of the fast EPSP in S neurons the myenteric plexus of guinea pig ileum. (A) An exclusive cholinergic fEPSP that is blocked by hexamethonium. (B) A mixed cholinergic and purinergic fEPSP that is blocked only be a combination of hexamethonium and ionotropic P2X receptor antagonist, PPADS (C) A mixed cholinergic and serotonergic fEPSP that is only blocked by a combination of hexamethonium and ondansetron. The percentages of synaptic responses of each type are indicated. (From Galligan, 2002a, with permission).
ATP or a related purine and one of the several subtypes of P2X receptors contribute to the fEPSP in two-thirds of unselected enteric neurons (Galligan, 2002b; Ren et al., 2008). Until recently, purinergic nerves involved in specific locations could not be identified because reliable markers of purinergic varicosities or vesicle had not been available. However, recently, a vesicular ATP/ADP transporter has been identified as the protein, SLC17A9 (Sawada et al., 2008). Immunolocalization of SLC17A9 should enhance our ability to identify purinergic nerve terminals, as has been recently shown in enteric varicosities (Chaudhury et al., 2012). ATP is co-stored with most other neurotransmitters including acetylcholine. The involvement of P2X receptors in fEPSP is inferred by pharmacologic studies and by studies in mice lacking P2X2 receptor (Burnstock, 2007; Galligan et al., 1994; Ren et al., 2008). Ionotropic P2X receptors and purinergic fEPSP have been found to be widely distributed in the ENS (Castelucci et al., 2002).
AUTONOMIC GANGLIA
Motor neurons in sympathetic and parasympathetic ganglia receive synaptic inputs from preganglionic neurons. Many neurons in prevertebral sympathetic ganglia receive additional synaptic inputs from intestinofugal neurons located in the enteric plexuses. Interestingly, only a minority of varicosities make synaptic contacts (Gibbins et al., 2006). The presynaptic varicosities are filled with SCV containing predominantly ACh. The postsynaptic nicotinic receptors are of different subunit compositions (Skok, 2002). Little information on electron microscopy and the molecular nature of synapses in the autonomic ganglia is currently available. Electrical potentials in the postsynaptic neuron elicited by presynaptic nerve stimulation in the autonomic ganglia are similar to those described in neural synapses in the CNS and the ENS. They are characterized by fEPSP with synaptic delays of less than a millisecond and usually lasts over 10 to 30 milliseconds (Ascher et al., 1979). The fEPSP is mediated by ACh acting on nicotinic receptors and the duration of fEPSP may depend on the subunit composition of nicotinic receptors (Skok, 2002). It has been shown that in chicken ciliary ganglion, α3-nAChR are concentrated at the PSD and α7-nAChR migrate laterally to nonsynaptic areas (Coggan et al., 2005). ATP and other transmitters, acting via their ionotropic receptors, also play a role in synaptic transmission in the autonomic ganglia (Galligan et al., 2000; Nakazawa, 1994).
JUNCTIONAL NEUROTRANSMISSION
In the past, based on the dogma that all neurotransmission between motor varicosities and smooth muscles were synaptic in nature, all types of electrical responses to nerve stimulation in smooth muscle cells were called postsynaptic potentials (Bennett, 1997).
Morphology of the Junctions is quite different from that of the synapses. Electron microscopic studies of the neuro-smooth muscle junctions show that the bare en passage nerve varicosities in the axon bundle lie at variable distances from the smooth muscle cells, with the apposition interval varying from 100–200 nm and greater than 2 mm (Rogers et al., 1966; Thaemert, 1966). Studies using serial EM sections have provided us with useful limited information on the contacts between the bare varicosities and the smooth muscles(Hirst et al., 1992; Klemm, 1995; Luff, 1996; Luff et al., 1987). Klemm (Klemm 1996) reported that reconstruction of serial sections of neuromuscular junctions of the longitudinal muscle, reveal two types of contacts. These contacts were called large and small contacts, respectively. In the large contacts, the bare varicosities and the smooth muscles were separated by ~60nm and the small contacts the two were separated by ~400nm . These varicosities show no synaptic specializations of the membranes (Thaemert, 1966). Therefore, neurotransmission between motor nerves and smooth muscle bundles is nonsynaptic in nature. In contrast to the synaptic cavity, the junctional space is not ‘closed’ and is –’open’ to extracellular space. Overall, nonsynaptic junctional space between the neural release site and the postjunctional receptors may show variable degrees of separation (from <20 nm to >2000 nm) between the release site on the prejunctional nerve terminal and the postjunctional receptors on the target cell.
Electrical potentials in smooth muscle cells in response to motor nerve stimulation are called junction potentials rather than postsynaptic potentials (Bennett, 1997). Functionally, junctional neurotransmission is distinguished from synaptic transmission by the time course of the response. Whereas synaptic transmission is measured in milliseconds, junctional transmission is measured in second to minutes. The time course of the junctional potential has been divide into two most frequently observed time courses representing ‘close and ‘wide’ junctional transmissions. The “close” junctional transmission associated with fast junction potential and “wide” junctional transmission associated with slow junction potential. The slow electrical potentials reach a peak in about 150 ms and then declines with a time constant between 250 and 500 msec. These responses typically last several seconds to minutes and may be hyperpolarizing or inhibitory and depolarizing or excitatory, and have been called slow EJP or slow IJP, respectively. The time scale of the response is dependent on many factors including distance of anatomic separation of the junctional space. The time course is determined by the nature of the neurotransmitters and kinetics of their release, distance between the release site and the postjunctional receptors, and nature of the receptors and the signaling pathway. Nature of the receptors play a critical role in the time course with metabotropic receptors being associated with much slow time courses than ionotropic receptors, a natural consequence of the way these receptors operate. The fast and the slow junction potentials are usually observed together in the same target cell.
Nonsynaptic junctional transmission is the only mode of transmission involving the varicosities that show no synaptic contacts that includes almost all nerve terminals whose target is not a neuron. Most smooth muscles exhibit both fast and slow junction potentials typically mediated by different classes of metabotropic receptors (although some P2X receptors are involved) with different kinetics (Bennett, 1972a). However, the synaptic varicosities are not only involved in synaptic transmission, but also partake in junctional neurotransmission. Junctional transmission also coexists with synaptic transmission in the central and the peripheral nervous systems including the enteric nervous system. In these cases, the neurotransmitter producing junctional, extrasynaptic effects may represent the neurotransmitter that may overflow out of the synaptic cavity or released from extrasynaptic release sites. All receptors involved in junctional transmission are present in the extrasynaptic region.
CLOSE JUNCTIONAL NEUROTRANSMISSION
The close junctional neurotransmission is characterized by synapse like close contact between the prejunctional release site and the postjunctional receptors. However, unlike the synapse, the junctional space is open to the extravascular space; the prejunctional release site lack the distinguishing features of the presynaptic active zone and release of the soluble transmitters; and the post junctional receptors include metabotropic receptors or slower acting ionotropic receptors. Functional marker of close junctional transmission is post junctional potential lasting ~1 second.
At many neuroeffector contacts, there is a very close juxtaposition of membranes of the nerve terminal and the effector cell. Electron microscopic evaluation of thin serial sections of an entire varicosity is necessary to define all contacts of a varicosity with effector cells in 3 dimensions (Hirst et al., 1992; Luff, 1996). However, random sections have identified very close (< 20 nm) junctions at some smooth muscle neuromuscular junctions (Hirst et al., 1992; Richardson, 1962). This interval is similar to or even smaller than that of a synapse.
At the close neuromuscular junctions, there may be some presynaptic specialization but indistinct postsynaptic membrane specialization and without an organized synaptic cavity. Brain et al. (1997) reported that adrenergic varicosities in the vas deferens possessed active zones about 250 – 700 nm in diameter, that are delineated by syntaxin, SNAP25 and N-type calcium channels and vesicular protein VAMP (Brain et al., 1997). These focal zones of release were precisely opposed to large clusters of P2X1 receptors on the smooth muscle membrane (Bennett et al., 1995), suggesting the presence of close contact between the adrenergic nerve ending and the smooth muscle. These junctions may have some resemblance to synapses in the CNS (Colonnier, 1968) and, therefore, have been called “synapse-like junctions.” Close junctional transmitters may also include synaptic transmitters that leak out of the synaptic cleft and act on extra-synaptic receptors located outside the synapse (Scimemi et al., 2009).
Several studies have reported localized clustering of receptors on the postjunctional membrane at the close junctions and small patches of diffusely distributed receptors at the distant junctions (Bennett et al., 1995; Hansen et al., 1998). Moreover, the nature of the receptors at the synapselike close junctions also appears to be different from those at the synapse and nonsynaptic distant junctions. Postjunctional receptors at the synapse-like close junctions are mostly metabotropic ACh and ATP receptors, ionotropic receptors of ATP and certain types of monoamine receptors.
Functionally, the close junctional transmission appear to be associated with whose time course (~ 1 sec) is much faster than the slow junction potentials at the smooth muscle neuromuscular junctions. In the neurons, this time course is intermediate between the time courses of the fPSPs and sEPSPs.
CLOSE JUNCTIONAL NEUROTRANSMISSION AT DIFFERENT SITES
Close junctions or fJP and its equivalent have been described in smooth muscles of vas deferens, urinary bladder, blood vessels and gut, as well as neurons of the ENS, AG and the CNS.
SMOOTH MUSCLES OF VAS DEFERENS, URINARY BLADDER AND BLOOD VESSELS
In the vas deferens, there is a predominance of close contacts between the adrenergic varicosities and smooth muscles, but no synapses (Bennett, 1972a; Burnstock et al., 2010). However, release sites are precisely apposed to large clusters of P2X1 receptors on the smooth muscle membrane (Bennett et al., 1995; Brain et al., 1997). High-resolution microscopy and biochemical and molecular studies of these pre-junctional varicosity membrane have not been performed. Functional studies have shown that in the mouse or rat vas deferens, sympathetic nerve stimulation produces a fEJP, which reaches a peak value in around 20 ms and decay with a time constant between 20 and 100ms, with an overall duration of around 1 sec. The fEJP is mediated by ATP acting on P2X purino-receptors.
In the urinary bladder, a close relationship between nerve terminals and smooth muscles in the absence of clear synaptic contacts has been recognized for a long time (El-Badawi, 1988). Gabella (1995) reported that in rat detrusor muscle, part of the varicosities in axons may be devoid of Schwann cells and varicosity membranes come within 10 nm to 100nm of the muscle membrane (Gabella, 1995). When there is no Schwann cell covering, the entire nerve terminal may be in contact with the membrane of the smooth muscle. Hansen and colleagues (1998) reported that P2X1 receptors on detrusor muscle formed clusters of two sizes (Hansen et al., 1998). One of them forms a large ellipse of around 1 micrometer and small spherical clusters of around 0.4 micrometer. Whereas the larger clusters were found juxtaposed to the nerve varicosities, smaller ones were not. The post-junctional clusters of P2X1 receptors without a synapse may represent a pattern of close contacts. It has also been shown that the varicosities contain both ACh and ATP. Functional studies have shown that in the rat urinary bladder, parasympathetic (cholinergic) nerve stimulation elicits a fEJP that lasts around 500 ms to 1 sec and is mediated by ATP acting on P2X1 purino-receptors (Burnstock, 2007; Hashitani et al., 2000).
The vascular smooth muscles of the resistance arteries and veins are richly innervated by sympathetic nerves. Terminals of sympathetic nerves make close contacts with smooth muscles (Luff, 1996). Adrenergic varicosities are filled with pleomorphic clear vesicles which appear as SDCV upon loading with 5-OHDA and contain 5-HT and ATP in addition to NE. Sympathetic nerve stimulation also elicits fEJPs that are mediated by ATP acting on P2X receptors in these smooth muscles (Luff, 1996). Stjarne and colleagues (Stjarne et al., 1994) reported that although there are no morphological synapses between adrenergic nerve terminals and vascular smooth muscles, small amounts of NE release by low frequency stimulation results in its actions on muscle cells close to and directly facing the varicosity via α2 receptors whereas high discharge rate results in actions of NE in more distant α1 receptors.
SMOOTH MUSCLES AND PDGFRα+ INTERSTITIAL CELLS IN THE GUT
In the gut, nerve terminals make close junctions with smooth muscles and transmural stimulation produces fast junction potentials (Bennett, 1972a). In the guinea pig tenia ceci, electrical field stimulation (EFS) elicits an ATP and P2X receptor mediated fEJP (Zhang et al., 2005). At enteric neuromuscular junctions in most of the gut, transmural stimulation elicits fEJPs which are mediated by ACh acting on metabotropic muscarinic M3 receptors (Inoue et al., 1993; Maggi et al., 1997). Gut smooth muscles also exhibit fIJPs which have a similar time course to the fEJP. The fIJP is mediated by ATP or related purines acting on P2Y1 receptors (Gallego et al., 2012; Goyal, 2011).
At the contact sites between nerve terminals and fibroblast-like cells positive for platelet derived growth factor receptor α fibroblast-like cell (PDGFRα+FLC), synapse-like close contacts (<20 nm) have been described using confocal microscopy that has resolution of only ~200 nm (Kurahashi et al., 2011). No molecular morphological evidence supporting the presence of synapses between the nerve varicosities and PDGFRα+FLC is currently available. Moreover, synaptic potentials have not been reported from these cells. The functional role of these cells in purinergic fIJP recorded from smooth muscle cells is not fully understood (Goyal et al., 2013).
ENTERIC NERVOUS SYSTEM, AUTONOMIC GANGLIA AND CENTRAL NERVOUS SYSTEM
In the synaptic varicosities that mediate true ultrafast synaptic potentials, junctional potential lasting few seconds have not been well recognized and have been given different names. In the ENS, synapse-like close junctions have also been described between adrenergic varicosities and enteric ganglia in guinea pigs as well as humans, which have a rich supply of adrenergic varicosities. On electron microscopy, 5-OHDA loaded adrenergic varicosities were found to contain SDCV and make nonsynaptic contacts with enteric neurons (Llewellyn-Smith et al., 1984). It is now known that 5-OHDA loading causes artifactual appearance of dense core vesicle and destroys synaptic structures (Hayakawa et al., 2008). Recent studies have shown that the adrenergic terminals are filled with pleomorphic clear vesicles and make synapse-like close contacts with enteric neurons (Hayakawa et al., 2008). There are limited studies examining synapse-like close junctions of other motor varicosities of enteric neurons (Mann et al., 1997; Pompolo et al., 1995; Young, 1983; Young et al., 1995). Electrophysiological studies have shown that submucous and myenteric neurons in guinea pigs exhibit an EPSP and IPSP lasting 150 ms to 2.5sec. This duration is longer than the longest fEPSPs (<50 ms) and much shorter than that of the sPSPs (several seconds). Therefore, it was named “intermediate” EPSP (iEPSP). Intermediate EPSP is mediated by ATP acting on P2Y receptors; and interestingly, iIPSP is also mediated by ATP (Gwynne et al., 2007; Monro et al., 2002). The time courses of iEPSPs or iIPSPs are similar to that of the fast EJPs at the neuromuscular junctions that have synapse-like close contacts. Further studies are needed to establish the association of close junctions with iEPSPs and iIPSPs in the enteric neurons.
In the autonomic ganglia, prejunctional nerve stimulation produces inhibitory and excitatory post synaptic potentials that last ~1 second each. Although they are called slow, their time course may resemble the ‘fast’ potentials described in other systems (Dun et al, 1993). The so called ‘slow’ IPSP is due to catecholamines that exert their effect by stimulating adrenergic receptirs that are metabotropic in nature (Eranko et al., 1980). This sEPSP is expressed after the sIPSP (Adams et al., 1986; Gibbins et al., 2006; Mochida et al., 1988). It is mediated by ACh acting on muscarinic M1 receptors.
In the CNS, postsynaptic potentials lasting 200ms-1.6 sec and time to peak duration of 10ms-120ms have been described in subsets of neurons in the CNS (Benardo, 1994; Byrne, 2009). Moreover, phasic release of ACh, at the scale of seconds, produces precisely defined cognitive responses (Sarter et al., 2009). These potentials lasting a few seconds may be due to close junctional transmission. However, further studies are needed to test this speculation.
WIDE JUNCTIONAL NEUROTRANSMISSION
The wide junctional neurotransmission is characterized by widely separated prejunctional release site and the postjunctional receptors. The junctional space is freely continuous with the extravascular space; the prejunctional release sites have features similar to the neuroendocrine cells and can also release NE and peptide neurotransmitters; and the post junctional receptors include metabotropic receptors, including those for neuropeptides that are diffusely distributed at distant sites. Functional marker of close junctional transmission is post junctional potential lasting several seconds.
Almost all tissues that exhibit close junctional neurotransmission also show wide junctional neurotransmission. Thus, wide junctional transmission has been described in many smooth muscles such as vas deferens, urinary bladder, blood vessels, gut as well as the nervous systems including ENS, autonomic ganglia and the CNS. Wide junctional neurotransmission is also seen in all other innervated cells including epithelial and secretory cells (Burnstock, 2008; Owyang et al., 2004), endocrine cells (Grunditz et al., 1988), endothelial cells (Draid et al., 2005) and immune cells (Selmeczy et al., 2008). It is interesting to note that the effect of cholinergic nerves on the immune cells is mediated by nicotinic receptors which may explain the beneficial effect of smoking in ulcerative colitis (Ben-Horin et al., 2008)
SMOOTH MUSCLES OF VAS DEFERENS, URINARY BLADDER AND BLOOD VESSELS
In the vas deferens, there is a predominance of close contacts between the adrenergic varicosities and smooth muscles. Functional studies have shown that in the mouse or rat vas deferens, sympathetic nerve stimulation produces a sEJP mediated by NE acting on α-adrenergic receptors, in addition to a fEJP mediated by ATP acting on P2X purino-receptors (Muir et al., 1988).
In the urinary bladder, there are large areas of distant junctions, in addition to the predominance of close junctions (El-Badawi, 1988; Gabella, 1995). Functional studies have shown that apart from producing ATP mediated fEJPs, parasympathetic nerve stimulation also produces sEJPs. The sEJP lasts several seconds and is mediated by ACh acting on metabotropic M3 muscarinic receptors (Krell et al., 1981). The sEJP was associated with late contraction (El-Badawi, 1988; Krell et al., 1981).
In resistance blood vessels and veins, adrenergic innervation to smooth muscle is very prominent. Adrenergic varicosities are filled with pleomorphic clear vesicles containing NE, 5-HT and ATP. Whereas ATP produces fEJPs, NE acting on α-adrenoreceptors mediates slow EJPs. It has been shown that α adrenergic receptors are only located on the junctional regions of the smooth muscle membrane and adrenergic neurotransmission is junctional in nature (Luff, 1996).
SMOOTH MUSCLES AND c-KIT+ INTERSTITIAL CELLS IN THE GUT
In the gut, prejunctional nerves endings contain neuropeptides including VIP and NK, transmitters such as ACh and ATP, and nNOSα that is the enzymatic source of nonvesicular transmitters such as NO. ACh and NK are colocalized in the excitatory varicosities and VIP and nNOSα are colocalized in inhibitory varicosities. Electrical field stimulation of intramural nerves in the gastrointestinal muscle strips produces sEJPs that are mediated by NK acting on its metabotropic receptors (Furness, 2006). On the other hand, sIJPs are mediated by the VIP and the nonvesicular neurotransmitter NO that stimulates NO-sensitive G-cyclase (Mashimo et al., 1996; Thatte et al., 2009). VIP may act presynaptically to enhance NO release and may also cause relaxation without directly contributing to the genesis of the inhibitory potential (Mashimo et al., 1996; Van Geldre et al., 2004).
There has been considerable recent debate regarding the nature of the contacts between enteric varicosities and interstitial cells of Cajal (ICC) or c-kit positive interstitial cells and their role in junction potentials recorded from the smooth muscles. Based on anatomic location and function, two main types of ICC have been described: myenteric ICC (ICC-MY) and intramuscular ICC (ICC-IM) (Sanders et al., 2010). ICC-MY are present around the myenteric plexus and thought to be pacemaker cells for slow waves in the smooth muscles cells (Hirst et al., 2006). Calcium imaging studies in the colon have shown that ICC-MY is innervated by nitrergic and cholinergic nerve terminals, though the nature of the contacts has not been well defined. (Bayguinov et al., 2010).
ICC-IM is located in between the smooth muscle cells. Enteric nerves have been reported to make synaptic contacts with ICC-IM. These contacts include areas of electron dense lining on the inner aspect of the varicosity membrane without any postsynaptic density on the membrane of ICC. Such contacts were not reported between the nerves and the smooth muscles (Horiguchi et al., 2003; Sanders et al., 2010). Most of these recent studies that used confocal microscopy (resolution ~200 nm) to identify ~20 nm synapses or close-contacts raise concerns about their validity. Moreover, careful comparative studies report that the contacts between the nerve terminals and the ICC are not truly synaptic nature and are similar to those between nerve terminal and smooth muscle cells (Mitsui et al., 2002) (Figure 14). Synaptic ultrafast potentials have not been recorded in the ICC. It has been proposed that electrical signals generated in the ICC are passively transferred to the smooth muscles. If enteric nerve stimulation produced synaptic potential in the ICC and transferred to the smooth muscle, ultrafast synaptic potential should be recorded in the smooth muscle. However, cholinergic sEJPs and nitrergic sIJPs are recorded in smooth muscle cells. Slow junction potentials are hallmark of junctional neurotransmission. Moreover, recent studies have shown that the nitrergic junction potentials are actively generated in the smooth muscle cells (He and Goyal, 2012). The functional role of ICC-IM in neuromuscular transmission is currently unproven.
Figure 14.
Close contacts between nerve varicosities and ICC and smooth muscle cell in the gut. (A) Shows a varicosity containing SCV and some LDCV. This varicosity makes a close ~20nm contact with a thin process of ICC and end of a smooth muscle cell. Also note membrane densities on the presynaptic membrane but no postsynaptic specialization. The section in a single plane section gives the illusion that the thin processes of ICC form a barrier between the varicosity and the smooth muscle cell that is seen several hundred nm away (scale, 0.5µ) (B) Shows 10–20nm close contact between a circular muscle and a varicosity filled with SCV The presynaptic electron-dense lining is indicated by an arrow, X41,000. (C) Shows close contact 10–20nm between a longitudinal muscle cell and two axon terminals: one containing a mixture of SCV and LDCV and the other containing only LDCV. Electron dense lining is marked by an arrow, X28,000 (A is from Rumessen et al., 1982, with permission; B and C are from Mitsui et al., 2002, with permission).
ENTERIC NERVOUS SYSTEM
Neurons in the enteric ganglia receive rich projections of varicosity-bearing axons from many different populations of neurons. Some varicosities contain SCV or a mixture of SCV and LDCV. These varicosities show synaptic specialization. The varicosities containing a mixture of SCV and LDCV revealed that in the region of the varicosity near the presynaptic active zone, only SCV and LDCV were present (Figure 11) (Baumgarten et al., 1970; Gabella, 1979). These observations are similar to those made in the CNS neurons. The SCV contain ACh and ATP that may participate in synaptic neurotransmission. LDCV are filled with neuropeptides such as VIP and NK and LDCV that do not participate in synaptic neurotransmission and their release may be at non-synaptic contacts or junctions.
Stimulation of nerves innervating enteric neurons also elicit sPSPs. Slow EPSPs and sIPSPs are mediated by tachyinins, mGluR1, mGluR5 and other receptor subtypes including muscarinic and 5-HT7 receptors and somatostatin, respectively [see (Gwynne et al., 2007)]. These neuropeptides are contained in LDCV, released at nonsynaptic junctions and produce the slow potentials by stimulating their metabotropic receptors. ACh, ATP and 5-HT produce fIPSP by acting on their ionotropic receptors and sEPSP by stimulating metabotropic receptors on postjunctional membranes (Foong et al., 2010). NE produces sIPSPs in submucosal neurons acting via α2-adrenoceptors (Bornstein et al., 2002).
Durations of the sEPSPs vary from tens of second to minutes and are dependent on many factors including temporal summation of stimuli affecting transmitter release, anatomic distribution of the receptors, and properties of the metabotropic receptors which is the dominant factor. The ionic basis of sEPSP is different in different tissues (Wood et al., 2004). Slow EPSP are seen in virtually all enteric neurons. As many as twenty different neurotransmitters are candidates for producing sEPSP. However, functional studies identify a handful of transmitters. They include NK acting on NK1 and NK3 receptors, 5-HT acting on 5HT1P, 5HT7, ACh acting on M1 muscarinic receptors and ATP acting on P2Y1 receptors.
AUTONOMIC GANGLIA
In the autonomic ganglia, a potential called ‘late slow’EPSP is expressed that lasts over 30 to 60 seconds to several minutes (Dun et al, 1993). Its time course may resemble the sEPSP described in other systems. It is mediated by neuropeptides. A variety of peptides including SP, VIP, NPY, ENK, CGRP and angiotensin have been localized to SIF nerve cell bodies or nerve fibers (Tanaka et al., 1996). They have been shown that the neuropeptides are e released on nerve stimulation and may mediate the ‘late slow’ EPSP. The action of neuropeptides is exerted on their respective metabotropic receptors involving a large field of action. ACh and the peptides may be released from the same varicosity or interneuron in certain ganglia (Samano et al., 2006).
CENTRAL NERVOUS SYSTEM
In the CNS, nonsynaptic neurotransmission is now increasing been recognized and has also been called ‘volume’ transmission (Agnati et al., 1986; Vizi, 1984). It includes a wide spectrum of modes of transmissions varying from transmission to remote sites via neurotransmitter release into the cerebrospinal fluid, blood capillaries and junctional transmission. The junctional neurotransmission involves transmission between nerve terminals and juxtaposed membranes of target cells without synaptic contacts
Junctional neurotransmission in the CNS is mediated by various neuropeptides, catecholamines, purines as well as classical neurotransmitters. Junctional transmitters include those released from the release sites on the nonsynaptic membrane of the varicosities and also those that may leak out of the synaptic cleft to exert effects on extra-synaptic receptors (Clark et al., 2002; Scimemi et al., 2009). In the CA1 pyramidal cells, repetitive electrical stimulation of stratum oriens produces a series of fEPSPs followed by an inhibitory potential. With higher intensity stimulation these potentials were followed by a sEPSP lasting 20–30s (Cole et al., 1984). It has also been reported that GABA released from synaptic cleft or GABA release from nonsynaptic release sites of neuroglia can affect extrasynaptic receptors to exert their effect (Olah et al., 2009).
In the CNS, most of the cholinergic buttons in the CNS have been shown to be nonsynaptic and release acetylcholine (ACh) into the extracellular space (Coggan et al., 2005; Sarter et al., 2009; Vizi et al., 2004). Non-synaptically released ACh exerts its effect by stimulating muscarinic M1 receptors which have been localized to nonsynaptic sites (Yamasaki et al., 2010). Moreover, nicotinic acetylcholine receptors have been shown to be localized outside the synapses at postjunctional and presynaptic sites (Vizi et al., 2004). These postjunctional nicotinic receptors are of high affinity and are of different subtypes than the synaptic nicotinic receptors (Conroy et al., 1995; Fernandes et al., 2010). Nonsynaptic nicotinic transmission plays a role in both neural plasticity and cellular learning processes, as well as in long-term changes in basic activity through fast activation, desensitization of receptors, and fluctuations of the steady-state levels of ACh. The effects of smoking and nicotine in the CNS are mediated by the nonsynaptic nicotinic receptors (Lendvai et al., 2008).
MODULATION OF SYNAPTIC AND JUNCTIONAL NEUROTRANSMISSION
Both synaptic and nonsynaptic neurotransmission are highly regulated. These regulatory mechanisms are major targets of therapy to enhance neurotransmission. The modulation of neurotransmission is affected by two broad mechanisms (Vizi et al., 2010).
One of the important mechanisms of modulation of synaptic activity is by the termination of the action of the neurotransmitter. It is brought about by: 1) Rapid reuptake or enzymatic degradation of the transmitter. Reuptake by transporters located outside the synapse on the presynaptic terminal and glial cells are major regulators of glutamate and GABA neurotransmission and are targets of therapy in glutamatergic and GABA-ergic neurotransmission in the CNS (Bunch et al., 2009; Madsen et al., 2010). Inhibitors of the active reuptake of monoamine (monoamine transporters) inhibit the transport of norepinephrine, dopamine and 5-HT and play important roles in augmenting actions of these monoamines. Nonselective serotonin uptake inhibitors such as tricyclic antidepressants (TCA) and selective serotonin uptake inhibitors (SSRI) are extensively used in treatment of anxiety, depression and irritable bowel syndrome (Haenisch et al., 2011; Kaminski et al., 2011). 2) Enzymatic degradation such as that of ACh by cholinesterase that may be present within the synapse or outside the synapse. Cholinesterase inhibitors are used to enhance cholinergic neurotransmission in Alzheimer disease (Geula et al., 2004). 2) The synaptic strength is strongly modulated by excitatory or inhibitory presynaptic receptors. Presynaptic receptors may be the targets of the transmitter released by the same terminal (autocrine) or from a postsynaptic site (retrograde neurotransmission). Mediators that modulate presynaptic receptors may also be derived from different nerve terminals or autocoids released by other cell types. At glutamatergic central synapses, NO serves as a retrograde modulator of synaptic transmission. Similarly, at GABA synapses in the CNS, endocannabinoids have shown to be retrograde neurotransmitters that modulate the activity of the GABA synapses (Lovinger, 2008; Steinert et al., 2008). Retrograde neurotransmitters, NO and cannabinoids, have been shown to play an important role in the long term plasticity of their respective synapses (Galligan, 2009; Lovinger, 2008).
The ‘presynaptic’ receptors usually include both metabotropic and ionotropic receptors (Marchi et al., 2010; Parnas et al., 2010) and their actions are usually brought about by increasing or decreasing activity of the presynaptic Ca2+ channels and the regulation of cycling of the neurotransmitter vesicles and by regulation of the size of the pool undergoing exocytosis (Sudhof, 2008a).
In the gut, there are several examples of modulation of neurotransmitter release by presynaptic receptors. Neurotransmitter release by nicotinic fEPSPs has been shown to be augmented by presynaptic muscarinic M1 receptors (North et al., 1985a). Such synergistic augmentation of neurotransmitter release may be necessary for full expression of a functional response. It has been reported that a combination of nicotinic and muscarinic antagonists was necessary to abolish vagal stimulation mediated relaxation of the lower esophageal sphincter relaxation (Goyal et al., 1975). Presynaptic excitation of VIP autoreceptors at smooth muscle neuromuscular junctions may act to enhance NO release or ACh release (He et al., 1993; Lundberg, 1996; Van Geldre et al., 2004). The activation of presynaptic CCK receptors has been shown to facilitate nicotinic synaptic neurotransmission in the gall bladder (Mawe, 1991). Activation of 5-HT4 receptors enhances ACh release and augments synaptic transmission via presynaptic actions (Neal et al., 2006; Tonini et al., 1989), though they may also inhibit synaptic transmission via 5HT1A receptors. (North et al., 1980) and inhibit neurotransmission at nerve endings of the intrinsic sensory neurons (Schikowski et al., 2002). 5-HT4 receptor antagonists and partial agonists have been shown to enhance synaptic ACh release and enhance sensory input and thus act as prokinetic agents to enhance gastrointestinal transit (Fang et al., 2008; Schikowski et al., 2002).
The inhibition of synaptic and nonsynaptic neurotransmission by the stimulation of presynaptic inhibitory receptors is due to a wide variety of transmitters and chemical agents. Some of the metabotropic presynaptic inhibitory receptors include: 5-HT1 receptors, M2 muscarinic receptors, α2 adrenergic receptors, A1 adenosine receptors, H3 histamine receptors, receptors for pancreatic polypeptide family (PP, NPY, PYY) and receptors for inflammatory mediators such as H3 histamine, IL-1β, IL-6 and platelet activating factor. Presynaptic inhibition by norepinephrine (NE) acting on α2 receptors occurs at both fast EPSP and slows EPSP in the enteric neurons and neuro-effector junctions and is responsible for inhibition of intestinal motility and secretion during states of adrenergic stimulation (North et al., 1985a; North et al., 1985b; Wood, 2006). Presynaptic inhibition by inflammatory mediators may be responsible for the suppression of intestinal motility in states of intestinal inflammation. Retrograde transmission by endocannabinoids may be an important inhibitory modulators of synaptic neurotransmission in the ENS (Galligan, 2009).
CONCLUSIONS
Traditionally, all chemical communication between neurons had been assumed to be synaptic in nature. In the last few decades, there have been important advances in our understanding of the molecular anatomy, biology and physiology of synapses in the neurons that suggest a strong structure-function correlation. Based on structure to function relationship studies, this review proposes three types of contacts between nerve terminals and target cells. They include: synapse, close junction and wide junction (Table 2). Morphologically, a synapse is: 1) a small, less than 1 micrometer square area of close contact between specialized active zone of the varicosity and the PSD on the postsynaptic membrane. 2) The synaptic membranes are separated by a 20–30nm ‘closed’ synaptic cavity which makes it unaccessible to exogenous chemicals. 3) The active zone of the varicosity membrane is characterized by the presynaptic grid and membrane docked ‘release ready’ small clear vesicles with exclusion of the large vesicles. 4) The postsynaptic membrane has a postsynaptic density, bearing densely packed ionotropic receptors. 5) The molecular anatomy of a synapse is designed to support a pinpoint, precise, and fast neurotransmission. 6) Electrophysiological hallmark of a synapse is a fPSP that lasts ~30ms. 7) Synapses are typically present between neurons in the CNS, the ANS and the ENS.
Table 2.
Summary of key structure-activity differences in neurotransmission at synapse nonsynaptic, close and wide junctions
| SYNAPSE | JUNCTION | ||
|---|---|---|---|
| CLOSE | WIDE | ||
| STRUCTURE | |||
| Nature of the contact | ~1 µm2 closed cavity | Open to extracellular space |
Open to extracellular space |
| Separation of release sites and their target receptors |
20 nm –30 nm | 10 nm –20 nm | >100nm- >2000 nm |
| Morphology | Pre- and postsynaptic specialization |
Subtle pre- and post-junctional specialization |
Some pre- and postjunctional specialization |
| Transmitter release site |
Actin grid & Membrane docked SCV (Active zone) |
No actin grid or membrane docked SCV |
No actin grid or membrane docked SCV |
| Vesicles | SCV | SCV, SDCV (pleomorphic) |
DCV, nonvesicular, other |
| Transmitters released | Classical transmitters (amino acids, ACh, ATP) |
Classical transmitters and 5HT |
Neuropeptides, nonvesicular and others |
| Postsynaptic/ postjunctional receptor clustering |
Dense clustering in the synapse receptors |
Localized areas of clustering |
Widespread distribution |
| Typical receptor type | Ionotropic, for classical transmitters |
Metabotropic for classical transmitters |
Metabotropic for neuropeptides and all other transmitters |
| ACTIVITY | |||
| Potentials (synaptic varicosities) |
fPSP (fEPSP, fIPSP) | iPSP (iEPSP, iIPSP) | sEPSP (sEPSP, sIPSP) |
| Potentials (nonsynaptic varicosities) |
none | fJP (fEJP, fIJP) | sJP (sEJP, sIJP) |
| Duration of response | 10 ms– 30 ms | 250ms-2.5s | several second - minutes |
| EFFECTOR CELL TYPE | |||
| Usual effector cell types |
Neurons | Neurons, smooth muscles |
Neurons, smooth muscles, secretory cells |
Nonsynaptic, junctional transmission can be divided into close and wide junctional transmissions. The close junctional neurotransmission is characterized by: 1) synapses-like narrow junctional space separating the prejunctional release site and the postjunctional receptors. However, the junctional space is open to the extravascular space. 2) The post junctional receptors include metabotropic receptors or slower acting ionotropic receptors. 3) The neurotransmitters include the synaptic transmitters that leak out of the synapses and those released from the prejunctional release sites. The prejunctional release sites lack the distinguishing features of the presynaptic active zone. 4) The prejunctional transmitters are soluble transmitters including the classical transmitters and others such as catecholamines. 5) Functional marker of close junctional transmission are fIJPs as described in the smooth muscles, and iPSPs in neurons (this distinguishes it from the synaptic ultrafast potential that has been called fPSP). The fIJPs or iPSPs last round ~1 second or 30-fold longer than the fPSP. 6) The close junctional transmission may be seen along with the synaptic potentials in neurons and in the absence of synaptic potentials at the smooth muscle neuromuscular junctions.
The wide junctional neurotransmission is characterized by: 1) wide junctional space separating the prejunctional release site and the postjunctional receptors, ‘open’ to the extravascular space. 2) Metabotropic receptors with slow kinetics that are widely distributed on the post junctional membrane. 3) The prejunctional release sites resemble peptide release sites seen in neuroendocrine cells and non-vesicular release sites such as nitric oxide release sites. 4) The neurotransmitters include all types of transmitters including neuropeptides and nonvesicular neurotransmitters such as nitric oxide. 5) Functional markers of the wide junctional transmission are sJPs or sPSPs (in neurons) that may last 10s of seconds. 6) In neurons, the sJPs are often seen with the fPSPs, whereas at smooth muscle neuromuscular junctions, sJPs are observed in association with the fJPs. Slow junctional transmission is very widespread among almost all tissues. Salient differences between neurotransmission at a synapse, close junction and wide junctions are summarized in table 2.
Acknowledgments
This work was supported by a National Institute of Diabetes and Digestive and Kidney Diseases grant (DK-062867). The authors wish to thank Antonietta D’Urso for help with preparation of manuscript. We also thank Dr Joel Bornstein for his constructive criticisms.
GRANT SUPPORT: This work was supported by a NIDDK grant (DK 062867).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: Authors have no financial, professional or personal conflicts.
REFERENCES
- 1.Adams PR, Jones SW, Pennefather P, Brown DA, Koch C, Lancaster B. Slow synaptic transmission in frog sympathetic ganglia. The Journal of experimental biology. 1986;124:259–285. doi: 10.1242/jeb.124.1.259. [DOI] [PubMed] [Google Scholar]
- 2.Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta physiologica Scandinavica. 1986;128:201–207. doi: 10.1111/j.1748-1716.1986.tb07967.x. [DOI] [PubMed] [Google Scholar]
- 3.Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001;40:239–257. doi: 10.1002/syn.1047. [DOI] [PubMed] [Google Scholar]
- 5.Arancibia-Carcamo IL, Moss SJ. Molecular organization and assembly of the central inhibitory postsynapse. Results Probl Cell Differ. 2006;43:25–47. doi: 10.1007/400_017. [DOI] [PubMed] [Google Scholar]
- 6.Ariel P, Ryan TA. New insights into molecular players involved in neurotransmitter release. Physiology (Bethesda) 2012;27:15–24. doi: 10.1152/physiol.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ascher P, Large WA, Rang HP. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. The Journal of physiology. 1979;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basbaum CB. Effects of magnesium ions on adrenergic nerve terminals. Journal of neurocytology. 1974;3:753–762. doi: 10.1007/BF01097196. [DOI] [PubMed] [Google Scholar]
- 9.Baumgarten HG, Holstein AF, Owman C. Auerbach's plexus of mammals and man: electron microscopic identification of three different types of neuronal processes in myenteric ganglia of the large intestine from rhesus monkeys, guinea-pigs and man. Z Zellforsch Mikrosk Anat. 1970;106:376–397. doi: 10.1007/BF00335780. [DOI] [PubMed] [Google Scholar]
- 10.Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. The Journal of physiology. 2010;588:4453–4474. doi: 10.1113/jphysiol.2010.196824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Horin S, Chowers Y. Neuroimmunology of the gut: physiology, pathology, and pharmacology. Current opinion in pharmacology. 2008;8:490–495. doi: 10.1016/j.coph.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Benardo LS. Separate activation of fast and slow inhibitory postsynaptic potentials in rat neocortex in vitro. The Journal of physiology. 1994;476:203–215. doi: 10.1113/jphysiol.1994.sp020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett M. History of the Synapse. Amsterdam: Harwood Academic Publishers; 2005. The Early History of the Synapse: from Plato to Sherrington; pp. 1–5. [Google Scholar]
- 14.Bennett MR. Autonomic Neuromuscular Transmission. In: Davson H, Greenfield ADM, Whittam R, Brindley GS, editors. Monographs of the Physiological Society. London: Cambridge University Press; 1972a. pp. 1–279. [PubMed] [Google Scholar]
- 16.Bennett MR. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Prog Neurobiol. 1997;52:159–195. doi: 10.1016/s0301-0082(97)00012-9. [DOI] [PubMed] [Google Scholar]
- 17.Bennett MR, Gibson WG. On the contribution of quantal secretion from close-contact and loose-contact varicosities to the synaptic potentials in the vas deferens. Philos Trans R Soc Lond B Biol Sci. 1995;347:187–204. doi: 10.1098/rstb.1995.0021. [DOI] [PubMed] [Google Scholar]
- 18.Bennett MVL. A comparison of electrically and chemically mediated transmission. In: Pappas GD, Purpura DP, editors. Structure and Function of Synapses. New York: Raven Press; 1972b. [Google Scholar]
- 19.Bian X, Zhou X, Galligan JJ. R-type calcium channels in myenteric neurons of guinea pig small intestine. American journal of physiology. Gastrointestinal and liver physiology. 2004;287:G134–G142. doi: 10.1152/ajpgi.00532.2003. [DOI] [PubMed] [Google Scholar]
- 20.Bian XC, Bornstein JC, Bertrand PP. Nicotinic transmission at functionally distinct synapses in descending reflex pathways of the rat colon. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2003;15:161–171. doi: 10.1046/j.1365-2982.2003.00393.x. [DOI] [PubMed] [Google Scholar]
- 21.Bloom FE, Aghajanian GK. An electron microscopic analysis of large granular synaptic vesicles of the brain in relation to monoamine content. The Journal of pharmacology and experimental therapeutics. 1968a;159:261–273. [PubMed] [Google Scholar]
- 22.Bloom FE, Aghajanian GK. Fine structural and cytochemical analysis of the staining of synaptic junctions with phosphotungstic acid. J Ultrastruct Res. 1968b;22:361–375. doi: 10.1016/s0022-5320(68)90027-0. [DOI] [PubMed] [Google Scholar]
- 23.Bolton TB, Large WA. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986;71:1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- 24.Bornstein JC, F JB, Kunze WA, Bertrand PP. Enteric Reflexes that influence motility. In: Brookes SJH, Costa M, editors. Innervation of the gastrointestinal tract. London: Taylor & Francis; 2002. pp. 1–55. 1–55. [Google Scholar]
- 25.Bornstein JC, Marks KA, Foong JP, Gwynne RM, Wang ZH. Nitric oxide enhances inhibitory synaptic transmission and neuronal excitability in Guinea-pig submucous plexus. Front Neurosci. 2010;4:30. doi: 10.3389/fnins.2010.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brain KL, Cottee LJ, Bennett MR. Varicosities of single sympathetic nerve terminals possess syntaxin zones and different synaptotagmin N-terminus labelling following stimulation. Journal of neurocytology. 1997;26:491–500. doi: 10.1023/a:1018533524643. [DOI] [PubMed] [Google Scholar]
- 27.Bridgman PC. Myosin motor proteins in the cell biology of axons and other neuronal compartments. Results Probl Cell Differ. 2009;48:91–105. doi: 10.1007/400_2009_10. [DOI] [PubMed] [Google Scholar]
- 28.Brookes SJH, C M. Functional Histoanatomy of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Academic Press; 2006. pp. 577–602. [Google Scholar]
- 29.Bunch L, Erichsen MN, Jensen AA. Excitatory amino acid transporters as potential drug targets. Expert Opin Ther Targets. 2009;13:719–731. doi: 10.1517/14728220902926127. [DOI] [PubMed] [Google Scholar]
- 30.Burnstock G. The changing face of autonomic neurotransmission. Acta Physiol Scand. 1986;126:67–91. doi: 10.1111/j.1748-1716.1986.tb07790.x. [DOI] [PubMed] [Google Scholar]
- 31.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. Non-synaptic transmission at autonomic neuroeffector junctions. Neurochem Int. 2008;52:14–25. doi: 10.1016/j.neuint.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Burnstock G, Verkhratsky A. Vas deferens--a model used to establish sympathetic cotransmission. Trends Pharmacol Sci. 2010;31:131–139. doi: 10.1016/j.tips.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Byrne J. Postsynaptic potentials and synaptic integration. In: Byrne JH, Roberts JL, editors. From Molecules to Networks: An introduction to cellular and molecular neuroscience. Elsevier; 2009. pp. 469–488. [Google Scholar]
- 35.Caesar R, Edwards GA, Ruska H. Architecture and nerve supply of mammalian smooth muscle tissue. The Journal of biophysical and biochemical cytology. 1957;3:867–878. doi: 10.1083/jcb.3.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cajal SR. Objective Evidence of the Anatomical Unity of Nerve Cells. In: Cajal SR, editor. Neuron Theory or Reticular Theory? Madrid: Consejo Superior de Investigaciones Cientificas; 1954. pp. 1–10. [Google Scholar]
- 37.Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652–668. doi: 10.1053/gast.2001.21908. [DOI] [PubMed] [Google Scholar]
- 38.Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X(2) receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117:415–422. doi: 10.1007/s00418-002-0404-4. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhury A, He XD, Goyal RK. Role of PSD95 in membrane association and catalytic activity of nNOSalpha in nitrergic varicosities in mice gut. American journal of physiology. Gastrointestinal and liver physiology. 2009;297:G806–G813. doi: 10.1152/ajpgi.00279.2009. Erratum in: Am J Physiol Gastrointest Liver Physiol. 2010 Oct;299(4):G100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhury A, He XD, Goyal RK. Myosin Va plays a key role in nitrergic neurotransmission by transporting nNOS{alpha} to enteric varicosity membrane. American journal of physiology. Gastrointestinal and liver physiology. 2011;301:G498–G507. doi: 10.1152/ajpgi.00164.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhury A, He XD, Goyal RK. Role of myosin Va in purinergic vesicular neurotransmission in the gut. American journal of physiology. Gastrointestinal and liver physiology. 2012;302:G598–G607. doi: 10.1152/ajpgi.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 43.Clark BA, Cull-Candy SG. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:4428–4436. doi: 10.1523/JNEUROSCI.22-11-04428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, Berg DK, Ellisman MH, Sejnowski TJ. Evidence for ectopic neurotransmission at a neuronal synapse. Science. 2005;309:446–451. doi: 10.1126/science.1108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole AE, Nicoll RA. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. The Journal of physiology. 1984;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colonnier M. Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain research. 1968;9:268–287. doi: 10.1016/0006-8993(68)90234-5. [DOI] [PubMed] [Google Scholar]
- 47.Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- 48.Conroy WG, Liu Z, Nai Q, Coggan JS, Berg DK. PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons. Neuron. 2003;38:759–771. doi: 10.1016/s0896-6273(03)00324-6. [DOI] [PubMed] [Google Scholar]
- 49.Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn-Schmiedeberg's archives of pharmacology. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- 50.Couteaux R. Morphological and cytochemical observations on the post-synaptic membrane at motor end-plates and ganglionic synapses. Experimental cell research. 1958;14:294–322. [PubMed] [Google Scholar]
- 51.Cowan WM, K ER. A brief history of synapses and synaptic transmission. In: Cowan WM, Sudhof TC, Stevens CF, editors. Synapses. Baltimore: The Johns Hopkins University Press; 2003. pp. 1–88. [Google Scholar]
- 52.Cuadras J. Non-synaptic release from dense-cored vesicles occurs at all terminal types in crayfish neuropile. Brain research. 1989;477:332–335. doi: 10.1016/0006-8993(89)91423-6. [DOI] [PubMed] [Google Scholar]
- 53.Dale H. Transmission of effects from nerve-endings: a survey of the significance of present knowledge University Press. 1952 [Google Scholar]
- 54.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 55.De Camilli P HV, Takei K, Mugnaini E. The structure of synapses. Baltimore: The Johns Hopkins University Press; 2003. pp. 89–133. [Google Scholar]
- 56.Dierig S. Urbanization, place of experiment and how the electric fish was caught by Emil du Bois-Reymond. J Hist Neurosci. 2000;9:5–13. doi: 10.1076/0964-704X(200004)9:1;1-2;FT005. [DOI] [PubMed] [Google Scholar]
- 57.Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- 58.Draid M, Shiina T, El-Mahmoudy A, Boudaka A, Shimizu Y, Takewaki T. Neurally released ATP mediates endothelium-dependent hyperpolarization in the circular smooth muscle cells of chicken anterior mesenteric artery. British journal of pharmacology. 2005;146:983–989. doi: 10.1038/sj.bjp.0706413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dun NJ, Karczmar AG, Wu SY, Shen E. Putative transmitter systems of mammalian sympathetic preganglionic neurons. Acta neurobiologiae experimentalis. 1993;53:53–63. [PubMed] [Google Scholar]
- 60.Eccles JC. The synapse: from electrical to chemical transmission. Annu Rev Neurosci. 1982;5:325–339. doi: 10.1146/annurev.ne.05.030182.001545. [DOI] [PubMed] [Google Scholar]
- 61.El-Badawi A. Neuromuscular mechanisms of micturition. In: Yalla SV, McGuire EJ, El-Badawi A, Blaivas JG, editors. Neurology and Urodynamics Principles and Practice. New York: MacMillan Publishing Company; 1988. pp. 3–35. [Google Scholar]
- 62.Eranko O, Eranko L. Induction of SIF cells by hydrocortisone or human cord serum in sympathetic ganglia and their subsequent fate in vivo and in vitro. Adv Biochem Psychopharmacol. 1980;25:17–26. [PubMed] [Google Scholar]
- 63.Falck B, Torp A. New evidence for the localization of noradrenalin in the adrenergic nerve terminals. Medicina experimentalis. International journal of experimental medicine. 1962;6:169–172. doi: 10.1159/000135153. [DOI] [PubMed] [Google Scholar]
- 64.Fang X, Liu S, Wang XY, Gao N, Hu HZ, Wang GD, Cook CH, Needleman BJ, Mikami DJ, Xia Y, Fei GJ, Hicks GA, Wood JD. Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20:80–93. doi: 10.1111/j.1365-2982.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 65.Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 66.Fejtova A, Gundelfinger ED. Molecular organization and assembly of the presynaptic active zone of neurotransmitter release. Results Probl Cell Differ. 2006;43:49–68. doi: 10.1007/400_012. [DOI] [PubMed] [Google Scholar]
- 67.Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- 68.Fernandes CC, Berg DK, Gomez-Varela D. Lateral mobility of nicotinic acetylcholine receptors on neurons is determined by receptor composition, local domain, and cell type. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8841–8851. doi: 10.1523/JNEUROSCI.6236-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foong JP, Bornstein JC. mGluR(1) Receptors Contribute to Non-Purinergic Slow Excitatory Transmission to Submucosal VIP Neurons of Guinea-Pig Ileum. Frontiers in neuroscience. 2009;3:46. doi: 10.3389/neuro.21.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foong JP, Parry LJ, Gwynne RM, Bornstein JC. 5-HT(1A), SST(1), and SST(2) receptors mediate inhibitory postsynaptic potentials in the submucous plexus of the guinea pig ileum. American journal of physiology. Gastrointestinal and liver physiology. 2010;298:G384–G394. doi: 10.1152/ajpgi.00438.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frank RA. Endogenous ion channel complexes: the NMDA receptor. Biochem Soc Trans. 2011;39:707–718. doi: 10.1042/BST0390707. [DOI] [PubMed] [Google Scholar]
- 72.Frieling T, Cooke HJ, Wood JD. Serotonin receptors on submucous neurons in guinea pig colon. Am J Physiol. 1991;261:G1017–G1023. doi: 10.1152/ajpgi.1991.261.6.G1017. [DOI] [PubMed] [Google Scholar]
- 73.Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7324–7332. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furness JB. The Enteric Nervous System Blackwell Publishing. 2006:1–274. [Google Scholar]
- 75.Furshpan EJ, Potter DD. Mechanism of nerve-impulse transmission at a crayfish synapse. Nature. 1957;180:342–343. doi: 10.1038/180342a0. [DOI] [PubMed] [Google Scholar]
- 76.Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat. 1972;111:69–97. [PMC free article] [PubMed] [Google Scholar]
- 77.Gabella G. Innervation of the gastrointestinal tract. Int Rev Cytol. 1979;59:129–193. doi: 10.1016/s0074-7696(08)61662-9. [DOI] [PubMed] [Google Scholar]
- 78.Gabella G. The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. Journal of neurocytology. 1995;24:159–187. doi: 10.1007/BF01181533. [DOI] [PubMed] [Google Scholar]
- 79.Gallego D, Gil V, Martinez-Cutillas M, Mane N, Martin MT, Jimenez M. Purinergic neuromuscular transmission is absent in the colon of P2Y1 knocked out mice. The Journal of physiology. 2012 doi: 10.1113/jphysiol.2011.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2002a;14:611–623. doi: 10.1046/j.1365-2982.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- 81.Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Current opinion in pharmacology. 2002b;2:623–629. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 82.Galligan JJ. Cannabinoid signalling in the enteric nervous system. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21:899–902. doi: 10.1111/j.1365-2982.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 83.Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. Journal of the autonomic nervous system. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 85.Gershon MD, Ratcliffe EM. Developmental biology of the enteric nervous system: pathogenesis of Hirschsprung's disease and other congenital dysmotilities. Semin Pediatr Surg. 2004;13:224–235. doi: 10.1053/j.sempedsurg.2004.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geula C, Darvesh S. Butyrylcholinesterase, cholinergic neurotransmission and the pathology of Alzheimer's disease. Drugs Today (Barc) 2004;40:711–721. doi: 10.1358/dot.2004.40.8.850473. [DOI] [PubMed] [Google Scholar]
- 87.Gibbins IL, Morris JL. Structure of peripheral synapses: autonomic ganglia. Cell and tissue research. 2006;326:205–220. doi: 10.1007/s00441-006-0233-1. [DOI] [PubMed] [Google Scholar]
- 88.Golding DW. A pattern confirmed and refined--synaptic, nonsynaptic and parasynaptic exocytosis. Bioessays. 1994;16:503–508. doi: 10.1002/bies.950160710. [DOI] [PubMed] [Google Scholar]
- 89.Goyal RK. Evidence for beta-nicotinamide adenine dinucleotide as a purinergic, inhibitory neurotransmitter in doubt. Gastroenterology. 2011;141:e27. doi: 10.1053/j.gastro.2011.07.047. author reply e27–28. [DOI] [PubMed] [Google Scholar]
- 90.Goyal RK, Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest. 1975;55:1119–1126. doi: 10.1172/JCI108013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goyal RK, Sullivan MP, Chaudhury A. Viewpoint: Progress in understanding of inhibitory purinergic neuromuscular transmission in the gut. Neurogastroenterology Motility. 2013 doi: 10.1111/nmo.12090. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- 93.Gray EG. Electron microscopy of presynaptic organelles of the spinal cord. J Anat. 1963;97:101–106. [PMC free article] [PubMed] [Google Scholar]
- 94.Gray EG. Problems of understanding the substructure of synapses. Prog Brain Res. 1976;45:207–234. doi: 10.1016/S0079-6123(08)60992-9. [DOI] [PubMed] [Google Scholar]
- 95.Grunditz T, Hakanson R, Sundler F, Uddman R. Neuronal pathways to the rat thyroid revealed by retrograde tracing and immunocytochemistry. Neuroscience. 1988;24:321–335. doi: 10.1016/0306-4522(88)90334-x. [DOI] [PubMed] [Google Scholar]
- 96.Gwynne RM, Bornstein JC. Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol. 2007;5:1–17. doi: 10.2174/157015907780077141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gwynne RM, Bornstein JC. Electrical stimulation of the mucosa evokes slow EPSPs mediated by NK1 tachykinin receptors and by P2Y1 purinoceptors in different myenteric neurons. American journal of physiology. Gastrointestinal and liver physiology. 2009a;297:G179–G186. doi: 10.1152/ajpgi.90700.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gwynne RM, Ellis M, Sjovall H, Bornstein JC. Cholera toxin induces sustained hyperexcitability in submucosal secretomotor neurons in guinea pig jejunum. Gastroenterology. 2009b;136:299–308. doi: 10.1053/j.gastro.2008.09.071. e294. [DOI] [PubMed] [Google Scholar]
- 99.Haenisch B, Bonisch H. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Ther. 2011;129:352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Hammarlund M, Watanabe S, Schuske K, Jorgensen EM. CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J Cell Biol. 2008;180:483–491. doi: 10.1083/jcb.200708018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hansen MA, Balcar VJ, Barden JA, Bennett MR. The distribution of single P2X1-receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. Journal of neurocytology. 1998;27:529–539. doi: 10.1023/a:1006908010642. [DOI] [PubMed] [Google Scholar]
- 102.Harata NC, Aravanis AM, Tsien RW. Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. Journal of neurochemistry. 2006;97:1546–1570. doi: 10.1111/j.1471-4159.2006.03987.x. [DOI] [PubMed] [Google Scholar]
- 103.Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. The Journal of physiology 524 Pt. 2000;2:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nature reviews. Neuroscience. 2011;12:127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- 105.Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M. Fine structural survey of tyrosine hydroxylase immunoreactive terminals in the myenteric ganglion of the rat duodenum. Journal of chemical neuroanatomy. 2008;36:191–196. doi: 10.1016/j.jchemneu.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 106.He XD, Goyal RK. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. The Journal of physiology. 1993;461:485–499. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He XD, Goyal RK. CaMKII inhibition hyperpolarizes membrane and blocks nitrergic IJP by closing a Cl- conductance in intestinal smooth muscle. American Journal of Physiology Gastrointestinal and Liver Physiology. 2012;303:G240–G246. doi: 10.1152/ajpgi.00102.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heuser JE, Reese TS. Structural changes after transmitter release at the frog neuromuscular junction. The Journal of cell biology. 1981;88:564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heuser JE RT. Structure of the Synapse. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology Section 1: The Nervous System. Bethesda: American Physiological Society; 1977. pp. 261–294. [Google Scholar]
- 110.Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hirst GD, Bramich NJ, Edwards FR, Klemm M. Transmission at autonomic neuroeffector junctions. Trends Neurosci. 1992;15:40–46. doi: 10.1016/0166-2236(92)90024-3. [DOI] [PubMed] [Google Scholar]
- 112.Hirst GD, Edwards FR. Electrical events underlying organized myogenic contractions of the guinea pig stomach. The Journal of physiology. 2006;576:659–665. doi: 10.1113/jphysiol.2006.116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hokfelt T. Looking at neurotransmitters in the microscope. Progress in neurobiology. 2010;90:101–118. doi: 10.1016/j.pneurobio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 114.Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. The Journal of comparative neurology. 1997;381:81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 115.Horiguchi K, Sanders KM, Ward SM. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell and tissue research. 2003;311:299–313. doi: 10.1007/s00441-002-0657-1. [DOI] [PubMed] [Google Scholar]
- 116.Inoue R, Chen S. Physiology of muscarinic receptor-operated nonselective cation channels in guinea-pig ileal smooth muscle. Exs. 1993;66:261–268. doi: 10.1007/978-3-0348-7327-7_20. [DOI] [PubMed] [Google Scholar]
- 117.Jonakait GM, Gintzler AR, Gershon MD. Isolation of axonal varicosities (autonomic synaptosomes) from the enteric nervous system. Journal of neurochemistry. 1979;32:1387–1400. doi: 10.1111/j.1471-4159.1979.tb11076.x. [DOI] [PubMed] [Google Scholar]
- 118.Kaminski A, Kamper A, Thaler K, Chapman A, Gartlehner G. Antidepressants for the treatment of abdominal pain-related functional gastrointestinal disorders in children and adolescents. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008013.pub2. CD008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4th ed. McGraw-Hill; 2000. [Google Scholar]
- 120.Katz B. Neural transmitter release: from quantal secretion to exocytosis and beyond. Journal of neurocytology. 2003;32:437–446. doi: 10.1023/B:NEUR.0000020603.84188.03. [DOI] [PubMed] [Google Scholar]
- 121.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 122.Kirchgessner AL, Liu MT. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. The Journal of comparative neurology. 1998;390:497–514. [PubMed] [Google Scholar]
- 123.Klemann CJ, Roubos EW. The gray area between synapse structure and function-Gray's synapse types I and II revisited. Synapse. 2011;65:1222–1230. doi: 10.1002/syn.20962. [DOI] [PubMed] [Google Scholar]
- 124.Klemm MF. Neuromuscular junctions made by nerve fibres supplying the longitudinal muscle of the guinea-pig ileum. Journal of the autonomic nervous system. 1995;55:155–164. doi: 10.1016/0165-1838(95)00036-w. [DOI] [PubMed] [Google Scholar]
- 125.Kobayashi H, Libet B. Generation of slow postsynaptic potentials without increases in ionic conductance. Proceedings of the National Academy of Sciences of the United States of America. 1968;60:1304–1311. doi: 10.1073/pnas.60.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kordon C. Neural mechanisms involved in pituitary control. Neurochemistry international. 1985;7:917–925. doi: 10.1016/0197-0186(85)90140-8. [DOI] [PubMed] [Google Scholar]
- 127.Krell RD, McCoy JL, Ridley PT. Pharmacological characterization of the excitatory innervation to the guinea-pig urinary bladder in vitro: evidence for both cholinergic and non-adrenergic-non-cholinergic neurotransmission. British journal of pharmacology. 1981;74:15–22. doi: 10.1111/j.1476-5381.1981.tb09950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S, Sanders KM. A functional role for the 'fibroblast-like cells' in gastrointestinal smooth muscles. The Journal of physiology. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Landis DM, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1:201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- 130.Lang T, Jahn R. Core proteins of the secretory machinery. Handb Exp Pharmacol. 2008:107–127. doi: 10.1007/978-3-540-74805-2_5. [DOI] [PubMed] [Google Scholar]
- 131.Langley J. The Autonomic Nervous System. W. Heffer & Sons; 1921. [Google Scholar]
- 132.Lendvai B, Vizi ES. Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol Rev. 2008;88:333–349. doi: 10.1152/physrev.00040.2006. [DOI] [PubMed] [Google Scholar]
- 133.Li ZS, Furness JB. Inputs from intrinsic primary afferent neurons to nitric oxide synthase-immunoreactive neurons in the myenteric plexus of guinea pig ileum. Cell Tissue Res. 2000 Jan;299(1):1–8. doi: 10.1007/s004419900125. [DOI] [PubMed] [Google Scholar]
- 134.Liu M, Kirchgessner AL. Agonist- and reflex-evoked internalization of metabotropic glutamate receptor 5 in enteric neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3200–3205. doi: 10.1523/JNEUROSCI.20-09-03200.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Llewellyn-Smith IJ, Furness JB, O'Brien PE, Costa M. Noradrenergic nerves in human small intestine. Distribution and ultrastructure. Gastroenterology. 1984;87:513–529. [PubMed] [Google Scholar]
- 136.Lomax AE, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. Journal of the autonomic nervous system. 1999;76:45–61. doi: 10.1016/s0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 137.Lopez-Munoz F, Alamo C. Historical evolution of the neurotransmission concept. J Neural Transm. 2009;116:515–533. doi: 10.1007/s00702-009-0213-1. [DOI] [PubMed] [Google Scholar]
- 138.Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- 139.Luff SE. Ultrastructure of sympathetic axons and their structural relationship with vascular smooth muscle. Anat Embryol (Berl) 1996;193:515–531. doi: 10.1007/BF00187924. [DOI] [PubMed] [Google Scholar]
- 140.Luff SE, McLachlan EM, Hirst GD. An ultrastructural analysis of the sympathetic neuromuscular junctions on arterioles of the submucosa of the guinea pig ileum. The Journal of comparative neurology. 1987;257:578–594. doi: 10.1002/cne.902570407. [DOI] [PubMed] [Google Scholar]
- 141.Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- 142.Lysakowski A, Figueras H, Price SD, Peng YY. Dense-cored vesicles, smooth endoplasmic reticulum, and mitochondria are closely associated with non-specialized parts of plasma membrane of nerve terminals: implications for exocytosis and calcium buffering by intraterminal organelles. The Journal of comparative neurology. 1999;403:378–390. doi: 10.1002/(sici)1096-9861(19990118)403:3<378::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 143.Madsen KK, White HS, Schousboe A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol Ther. 2010;125:394–401. doi: 10.1016/j.pharmthera.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 144.Maggi CA, Giuliani S, Zagorodnyuk V. Sequential activation of the triple excitatory transmission to the circular muscle of guinea-pig colon. Neuroscience. 1997;79:263–274. doi: 10.1016/s0306-4522(96)00659-8. [DOI] [PubMed] [Google Scholar]
- 145.Mann PT, Southwell BR, Young HM, Furness JB. Appositions made by axons of descending interneurons in the guinea-pig small intestine, investigated by confocal microscopy. Journal of chemical neuroanatomy. 1997;12:151–164. doi: 10.1016/s0891-0618(96)00189-5. [DOI] [PubMed] [Google Scholar]
- 146.Marchi M, Grilli M. Presynaptic nicotinic receptors modulating neurotransmitter release in the central nervous system: functional interactions with other coexisting receptors. Prog Neurobiol. 2010;92:105–111. doi: 10.1016/j.pneurobio.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 147.Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest. 1996;98:8–13. doi: 10.1172/JCI118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mawe GM. The role of cholecystokinin in ganglionic transmission in the guinea-pig gall-bladder. The Journal of physiology. 1991;439:89–102. doi: 10.1113/jphysiol.1991.sp018658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mitsui R, Komuro T. Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell and tissue research. 2002;309:219–227. doi: 10.1007/s00441-002-0592-1. [DOI] [PubMed] [Google Scholar]
- 150.Mochida S, Libet B. Secondary late components of the muscarinic postsynaptic potentials, in rabbit superior cervical ganglion. Journal of the autonomic nervous system. 1988;24:41–49. doi: 10.1016/0165-1838(88)90133-6. [DOI] [PubMed] [Google Scholar]
- 151.Monro RL, Bertrand PP, Bornstein JC. ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2002;14:255–264. doi: 10.1046/j.1365-2982.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- 152.Monro RL, Bertrand PP, Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. The Journal of physiology. 2004;556:571–584. doi: 10.1113/jphysiol.2004.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morciano M, Beckhaus T, Karas M, Zimmermann H, Volknandt W. The proteome of the presynaptic active zone: from docked synaptic vesicles to adhesion molecules and maxi-channels. Journal of neurochemistry. 2009;108:662–675. doi: 10.1111/j.1471-4159.2008.05824.x. [DOI] [PubMed] [Google Scholar]
- 154.Muir TC, Wardle KA. The electrical and mechanical basis of co-transmission in some vascular and non-vascular smooth muscles. Journal of autonomic pharmacology. 1988;8:203–218. doi: 10.1111/j.1474-8673.1988.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 155.Murphy SM, Pilowsky PM, Llewellyn-Smith IJ. Vesicle shape and amino acids in synaptic inputs to phrenic motoneurons: do all inputs contain either glutamate or GABA? The Journal of comparative neurology. 1996;373:200–219. doi: 10.1002/(SICI)1096-9861(19960916)373:2<200::AID-CNE4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 156.Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neal KB, Bornstein JC. Serotonergic receptors in therapeutic approaches to gastrointestinal disorders. Current opinion in pharmacology. 2006;6:547–552. doi: 10.1016/j.coph.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 158.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. The Journal of physiology. 2009;587:567–586. doi: 10.1113/jphysiol.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Neff RA, 3rd, Conroy WG, Schoellerman JD, Berg DK. Synchronous and asynchronous transmitter release at nicotinic synapses are differentially regulated by postsynaptic PSD-95 proteins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15770–15779. doi: 10.1523/JNEUROSCI.4951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Neher E. What is Rate-Limiting during Sustained Synaptic Activity: Vesicle Supply or the Availability of Release Sites. Frontiers in synaptic neuroscience. 2010;2:144. doi: 10.3389/fnsyn.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.North RA, Henderson G, Katayama Y, Johnson SM. Electrophysiological evidence for presynaptic inhibition of acetylcholine release by 5-hydroxytryptamine in the enteric nervous system. Neuroscience. 1980;5:581–586. doi: 10.1016/0306-4522(80)90055-x. [DOI] [PubMed] [Google Scholar]
- 162.North RA, Slack BE, Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. The Journal of physiology. 1985a;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.North RA, Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. The Journal of physiology. 1985b;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. The Journal of comparative neurology. 2004;468:112–124. doi: 10.1002/cne.10948. [DOI] [PubMed] [Google Scholar]
- 165.Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 166.Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957–969. doi: 10.1053/j.gastro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 168.Palay SL, C -PV. General Morphology of neurons and neuroglia. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology Section 1: The Nervous System. Bethesda: American Physiological Society; 1977. pp. 5–37. [Google Scholar]
- 169.Palay SL, Palade GE. The fine structure of neurons. J Biophys Biochem Cytol. 1955;1:69–88. doi: 10.1083/jcb.1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Parker MJ, Zhao S, Bredt DS, Sanes JR, Feng G. PSD93 regulates synaptic stability at neuronal cholinergic synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:378–388. doi: 10.1523/JNEUROSCI.3865-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parnas I, Parnas H. Control of neurotransmitter release: From Ca2+ to voltage dependent G-protein coupled receptors. Pflugers Arch. 2010;460:975–990. doi: 10.1007/s00424-010-0872-7. [DOI] [PubMed] [Google Scholar]
- 172.Peters A, Palay SL. The morphology of synapses. Journal of neurocytology. 1996;25:687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- 173.Pfenninger K, Akert K, Moor H, Sandri C. The fine structure of freeze-fractured presynaptic membranes. Journal of neurocytology. 1972;1:129–149. doi: 10.1007/BF01099180. [DOI] [PubMed] [Google Scholar]
- 174.Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- 175.Pompolo S, Furness JB. Sources of inputs to longitudinal muscle motor neurons and ascending interneurons in the guinea-pig small intestine. Cell and tissue research. 1995;280:549–560. doi: 10.1007/BF00318359. [DOI] [PubMed] [Google Scholar]
- 176.Pompolo S, Furness JB. Quantitative analysis of inputs to somatostatin-immunoreactive descending interneurons in the myenteric plexus of the guinea-pig small intestine. Cell Tissue Res. 1998 Nov;294(2):219–26. doi: 10.1007/s004410051171. [DOI] [PubMed] [Google Scholar]
- 177.Portbury AL, Pompolo S, Furness JB, Stebbing MJ, Kunze WA, Bornstein JC, Hughes S. Cholinergic, somatostatin-immunoreactive interneurons in the guinea pig intestine: morphology, ultrastructure, connections and projections. J Anat. 1995 Oct;187(Pt 2):303–21. [PMC free article] [PubMed] [Google Scholar]
- 178.Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell and tissue research. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 179.Qui J. Synaptic physiology: A scaffolder at the active zone. Nature Reviews Neuroscience. 2004;5:602. [Google Scholar]
- 180.Rao YM, Chaudhury A, Goyal RK. Active and inactive pools of nNOS in the nerve terminals in mouse gut: implications for nitrergic neurotransmission. American journal of physiology. Gastrointestinal and liver physiology. 2008;294:G627–G634. doi: 10.1152/ajpgi.00519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ren J, Bertrand PP. Purinergic receptors and synaptic transmission in enteric neurons. Purinergic Signal. 2008;4:255–266. doi: 10.1007/s11302-007-9088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ren J, Galligan JJ. Dynamics of fast synaptic excitation during trains of stimulation in myenteric neurons of guinea-pig ileum. Auton Neurosci. 2005;117:67–78. doi: 10.1016/j.autneu.2004.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Richardson KC. Electronmicroscopic observations on Auerbach's plexus in the rabbit, with special reference to the problem of smooth muscle innervation. Am J Anat. 1958;103:99–135. doi: 10.1002/aja.1001030105. [DOI] [PubMed] [Google Scholar]
- 184.Richardson KC. The fine structure of autonomic nerve endings in smooth muscle of the rat vas deferens. J Anat. 1962;96:427–442. [PMC free article] [PubMed] [Google Scholar]
- 185.Richardson KC. Electron microscopic identification of autonomic nerve endings. Nature. 1966;210:756. doi: 10.1038/210756a0. [DOI] [PubMed] [Google Scholar]
- 186.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 187.Rogers DC, Burnstock G. The interstitial cell and its place in the concept of the autonomic ground plexus. The Journal of comparative neurology. 1966;126:255–284. doi: 10.1002/cne.901260207. [DOI] [PubMed] [Google Scholar]
- 188.Rumessen JJ, Thuneberg L, Mikkelsen HB. Plexus muscularis profundus and associated interstitial cells. II. Ultrastructural studies of mouse small intestine. Anat Rec. 1982;203:129–146. doi: 10.1002/ar.1092030112. [DOI] [PubMed] [Google Scholar]
- 189.Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell and tissue research. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- 190.Samano C, Zetina ME, Marin MA, Cifuentes F, Morales MA. Choline acetyl transferase and neuropeptide immunoreactivities are colocalized in somata, but preferentially localized in distinct axon fibers and boutons of cat sympathetic preganglionic neurons. Synapse. 2006;60:295–306. doi: 10.1002/syn.20300. [DOI] [PubMed] [Google Scholar]
- 191.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. The Journal of physiology. 2010;588:4621–4639. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sargent PB, Pang DZ. Acetylcholine receptor-like molecules are found in both synaptic and extrasynaptic clusters on the surface of neurons in the frog cardiac ganglion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9:1062–1072. doi: 10.1523/JNEUROSCI.09-03-01062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schemann M, Wood JD. Synaptic behaviour of myenteric neurones in the gastric corpus of the guinea-pig. The Journal of physiology. 1989;417:519–535. doi: 10.1113/jphysiol.1989.sp017816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schikowski A, Thewissen M, Mathis C, Ross HG, Enck P. Serotonin type-4 receptors modulate the sensitivity of intramural mechanoreceptive afferents of the cat rectum. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2002;14:221–227. doi: 10.1046/j.1365-2982.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 197.Scimemi A, Beato M. Determining the neurotransmitter concentration profile at active synapses. Molecular neurobiology. 2009;40:289–306. doi: 10.1007/s12035-009-8087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Selmeczy Z, Vizi ES, Csoka B, Pacher P, Hasko G. Role of nonsynaptic communication in regulating the immune response. Neurochem Int. 2008;52:52–59. doi: 10.1016/j.neuint.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shen KZ, Surprenant A. Common ionic mechanisms of excitation by substance P and other transmitters in guinea-pig submucosal neurones. The Journal of physiology. 1993;462:483–501. doi: 10.1113/jphysiol.1993.sp019565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtova A, Kao HT, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-dimensional architecture of presynaptic terminal cytomatrix. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Skok VI. Nicotinic acetylcholine receptors in autonomic ganglia. Auton Neurosci. 2002;97:1–11. doi: 10.1016/s1566-0702(01)00386-1. [DOI] [PubMed] [Google Scholar]
- 202.Smith KR, Kittler JT. The cell biology of synaptic inhibition in health and disease. Curr Opin Neurobiol. 2010;20:550–556. doi: 10.1016/j.conb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 203.Smolen AJ. Morphology of synapses in the autonomic nervous system. J Electron Microsc Tech. 1988;10:187–204. doi: 10.1002/jemt.1060100205. [DOI] [PubMed] [Google Scholar]
- 204.Somlyo AV, Somlyo AP. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. The Journal of pharmacology and experimental therapeutics. 1968;159:129–145. [PubMed] [Google Scholar]
- 205.Song ZM, Brookes SJ, Llewellyn-Smith IJ, Costa M. Ultrastructural studies of the myenteric plexus and smooth muscle in organotypic cultures of the guinea-pig small intestine. Cell and tissue research. 1995;280:627–637. doi: 10.1007/BF00318365. [DOI] [PubMed] [Google Scholar]
- 206.Sorensen JB. Conflicting views on the membrane fusion machinery and the fusion pore. Annu Rev Cell Dev Biol. 2009;25:513–537. doi: 10.1146/annurev.cellbio.24.110707.175239. [DOI] [PubMed] [Google Scholar]
- 207.Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RA, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID. Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron. 2008;60:642–656. doi: 10.1016/j.neuron.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 208.Stevens DR, Schirra C, Becherer U, Rettig J. Vesicle pools: lessons from adrenal chromaffin cells. Front Synaptic Neurosci. 2011;3:2. doi: 10.3389/fnsyn.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stjarne L, Astrand P, Bao JX, Gonon F, Msghina M, Stjarne E. Spatiotemporal pattern of quantal release of ATP and noradrenaline from sympathetic nerves: consequences for neuromuscular transmission. Adv Second Messenger Phosphoprotein Res. 1994;29:461–496. doi: 10.1016/s1040-7952(06)80030-3. [DOI] [PubMed] [Google Scholar]
- 210.Sudhof T. Neurotransmitter release Springer-Verlag, Heidelberg. 2008a:1–22. [Google Scholar]
- 211.Sudhof T. Neurotransmitter release. In: Sudhof TC, Starke K, editors. Pharmacology of Neurotransmitter Release. Heidelberg: Springer; 2008b. pp. 1–22. [Google Scholar]
- 212.Sudhof TC, Malenka RC. Understanding synapses: past, present, and future. Neuron. 2008;60:469–476. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tanaka K, Chiba T. Microvascular organization of sympathetic ganglia, with special reference to small intensely-fluorescent cells. Microscopy research and technique. 1996;35:137–145. doi: 10.1002/(SICI)1097-0029(19961001)35:2<137::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 216.Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 217.Tansey EM. Not committing barbarisms: Sherrington and the synapse, 1897. Brain Res Bull. 1997;44:211–212. doi: 10.1016/s0361-9230(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 218.Taxi J. [The structure of Auerbach's plexus in the mouse studied with the electron microscope] Comptes rendus hebdomadaires des seances de l'Academie des sciences. 1958;246:1922–1925. [PubMed] [Google Scholar]
- 219.Thaemert JC. Ultrastructural interrelationships of nerve processes and smooth muscle cells in three dimensions. J Cell Biol. 1966;28:37–49. doi: 10.1083/jcb.28.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thatte HS, He XD, Goyal RK. Imaging of nitric oxide in nitrergic neuromuscular neurotransmission in the gut. PLoS One. 2009;4:e4990. doi: 10.1371/journal.pone.0004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Thureson-Klein A, Klein RL, Zhu PC. Exocytosis from large dense cored vesicles as a mechanism for neuropeptide release in the peripheral and central nervous system. Scan Electron Microsc. 1986:179–187. [PubMed] [Google Scholar]
- 222.Tong Q, Kirchgessner AL. Localization and function of metabotropic glutamate receptor 8 in the enteric nervous system. American journal of physiology. Gastrointestinal and liver physiology. 2003;285:G992–G1003. doi: 10.1152/ajpgi.00118.2003. [DOI] [PubMed] [Google Scholar]
- 223.Tonini M, Galligan JJ, North RA. Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology. 1989;96:1257–1264. doi: 10.1016/s0016-5085(89)80012-5. [DOI] [PubMed] [Google Scholar]
- 224.Tsuboi T. Molecular mechanism of attachment process of dense-core vesicles to the plasma membrane in neuroendocrine cells. Neurosci Res. 2009;63:83–88. doi: 10.1016/j.neures.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 225.Van Geldre LA, Lefebvre RA. Interaction of NO and VIP in gastrointestinal smooth muscle relaxation. Curr Pharm Des. 2004;10:2483–2497. doi: 10.2174/1381612043383890. [DOI] [PubMed] [Google Scholar]
- 226.Vanden Berghe P, Klingauf J. Spatial organization and dynamic properties of neurotransmitter release sites in the enteric nervous system. Neuroscience. 2007;145:88–99. doi: 10.1016/j.neuroscience.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 227.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 228.Vigh B, Manzano e Silva MJ, Frank CL, Vincze C, Czirok SJ, Szabo A, Lukats A, Szel A. The system of cerebrospinal fluid-contacting neurons. Its supposed role in the nonsynaptic signal transmission of the brain. Histology and histopathology. 2004;19:607–628. doi: 10.14670/HH-19.607. [DOI] [PubMed] [Google Scholar]
- 229.Vizi ES. Physiological role of cytoplasmic and non-synaptic release of transmitter. Neurochemistry international. 1984;6:435–440. doi: 10.1016/0197-0186(84)90112-8. [DOI] [PubMed] [Google Scholar]
- 230.Vizi ES, Fekete A, Karoly R, Mike A. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. British journal of pharmacology. 2010;160:785–809. doi: 10.1111/j.1476-5381.2009.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vizi ES, Kiss JP, Lendvai B. Nonsynaptic communication in the central nervous system. Neurochem Int. 2004;45:443–451. doi: 10.1016/j.neuint.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 232.Waites CL, Garner CC. Presynaptic function in health and disease. Trends Neurosci. 2011;34:326–337. doi: 10.1016/j.tins.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 233.Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320:246–249. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- 234.Whittaker VP. The isolation and characterization of acetylcholine-containing particles from brain. Biochem J. 1959;72:694–706. doi: 10.1042/bj0720694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wood JD. Cellular Physiology of Enteric Neurons. In: Johnson LR, editor. Physiology of the Gastointestinal Tract. 4th ed. Associate Press; 2006. [Google Scholar]
- 236.Wood JD, Kirchgessner A. Slow excitatory metabotropic signal transmission in the enteric nervous system. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(Suppl 1):71–80. doi: 10.1111/j.1743-3150.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 237.Yamasaki M, Matsui M, Watanabe M. Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:4408–4418. doi: 10.1523/JNEUROSCI.5719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Young HM. Ultrastructure of catecholamine-containing axons in the intestine of the domestic fowl. Cell and tissue research. 1983;234:411–425. doi: 10.1007/BF00213778. [DOI] [PubMed] [Google Scholar]
- 239.Young HM, Furness JB, Povey JM. Analysis of connections between nitric oxide synthase neurons in the myenteric plexus of the guinea-pig small intestine. Journal of neurocytology. 1995;24:257–263. doi: 10.1007/BF01186538. [DOI] [PubMed] [Google Scholar]
- 240.Zamorano PL, Garner CC. Unwebbing the presynaptic web. Neuron. 2001;32:3–6. doi: 10.1016/s0896-6273(01)00458-5. [DOI] [PubMed] [Google Scholar]
- 241.Zhang Y, Paterson WG. Excitatory purinergic neurotransmission in smooth muscle of guinea-pig [corrected] taenia caeci. The Journal of physiology. 2005;563:855–865. doi: 10.1113/jphysiol.2004.077636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhou X, Ren J, Brown E, Schneider D, Caraballo-Lopez Y, Galligan JJ. Pharmacological properties of nicotinic acetylcholine receptors expressed by guinea pig small intestinal myenteric neurons. The Journal of pharmacology and experimental therapeutics. 2002;302:889–897. doi: 10.1124/jpet.102.033548. [DOI] [PubMed] [Google Scholar]
- 243.Zoli M, Agnati LF. Wiring and volume transmission in the central nervous system: the concept of closed and open synapses. Progress in neurobiology. 1996;49:363–380. doi: 10.1016/0301-0082(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 244.Zupanc GK. Peptidergic transmission: from morphological correlates to functional implications. Micron. 1996;27:35–91. doi: 10.1016/0968-4328(95)00028-3. [DOI] [PubMed] [Google Scholar]