Abstract
Excessive adverse events were encountered in a Phase I/II study of cyclophosphamide (CY) dose deescalation in a fludarabine-based conditioning regimen for bone marrow transplantation from unrelated donors in patients with severe aplastic anemia. All patients received fixed doses of antithymocyte globulin, fludarabine, and low-dose total body irradiation. The starting CY dose was 150 mg/kg, with deescalation to 100 mg/ kg, 50 mg/kg, or 0 mg/kg. CY dose level 0 mg/kg was closed due to graft failure in 3 of 3 patients. CY dose level 150 mg/kg was closed due to excessive organ toxicity (n = 6) or viral pneumonia (n = 1), resulting in the death of 7 of 14 patients. CY dose levels 50 and 100 mg/kg remain open. Thus, CYat doses of 150 mg/kg in combination with total body irradiation (2 Gy), fludarabine (120 mg/m2), and antithymocyte globulin was associated with excessive organ toxicity.
Keywords: Bone marrow transplantation, Stem cell transplantation, Matched unrelated donor, Antithymocyte globulin
INTRODUCTION
Unrelated donor bone marrow transplantation (BMT) is an important therapy for patients with severe acquired aplastic anemia (SAA) who lack suitable related donors and who fail to respond or who experience recurrence after immunosuppressive therapy [1]. Outcomes of unrelated donor BMT have improved over the last few decades [2,3], presumably due to closer donor–recipient HLA matching and improved supportive care. However, the optimal preparative regimen remains unknown.
Kojima et al. [4] reported on 154 patients who underwent BMT after conditioning with various regimens. Five-year overall survival was 56%, and 11% of the patients experienced graft failure. The conditioning regimens included total body irradiation (TBI; 200–1000 Gy or limited field irradiation), cyclophosphamide (CY; 120–200 mg/kg), and antithymocyte globulin (ATG). Deeg and coworkers [5,6] evaluated a TBI dose deescalation regimen in 87 patients and found that the optimum TBI dose was 200 cGy given along with CY (200 mg/kg) and horse ATG (ATGAM 90 mg/kg).[5,6] Graft failure occurred in 5% of patients. Overall, 55% of patients were alive; the dose-limiting toxicity was diffuse pulmonary injury. Recently, the need for TBI has been called into question, and fludarabine (FLU) has been incorporated in some conditioning regimens as a possible alternative to low-dose TBI [7–11]. Although most regimens still use CY, the drug’s role and dose in combination regimens have not been well established [1].
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) designed a multicenter Phase I/ II study to identify the optimal dose of CY in a BMT preparative regimen that also incorporates FLU, ATG, and low-dose TBI [12]. To notify the transplantation community of these critical interim results, we now report serious adverse events in the 2 of 4 study cohorts with the highest (150 mg/kg) and the lowest (0 mg/kg) CY dose levels. The trial continues to accrue patients to the 50 mg/kg and 100 mg/kg dose levels.
PATIENTS AND METHODS
BMT CTN 0301 is a Phase I/II study evaluating the optimal dose of CY within a preparative regimen of FLU, ATG, and TBI before unrelated BMT for SAA. The trial is registered at www.clinicaltrials.gov (NCT00326417). The study protocol has been approved by the Institutional Review Board at each participating center. Written informed consent is obtained in accordance with the Declaration of Helsinki before the initiation of conditioning therapy. Eligibility criteria are SAA, age up to 65 years, adequate organ function, and an available unrelated adult marrow donor matched for HLA-A, -B, -C, and -DRB1 or mismatched at a single locus. Transplantation of peripheral blood progenitor cells is prohibited, in view of the inferior patient outcome with this stem cell product in related donor transplantation [13], as was recently confirmed in a study of unrelated donor transplantation [14]. Patients with Fanconi anemia or other marrow failure syndromes are excluded.
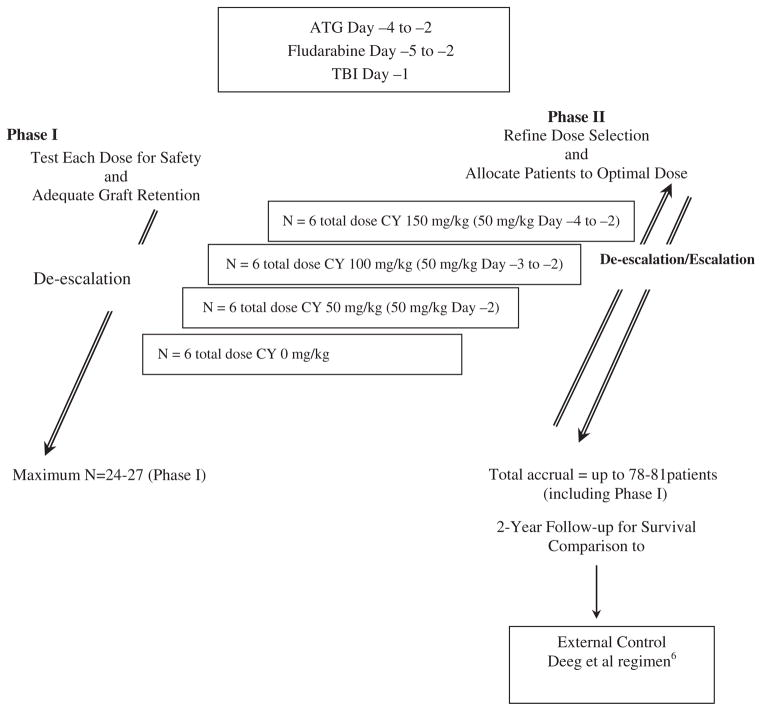
All patients receive a fixed dose of ATG (either Thymoglobulin 3 mg/kg i.v. or ATGAM 30 mg/kg i.v. daily on days −4 to −2), FLU (30 mg/m2 i.v. daily on days −5 to −2), and low-dose TBI (200 cGy on day −1) (Figure 1). Graft-versus-host disease prophylaxis is provided by a calcineurin inhibitor (cyclosporine or tacrolimus) and methotrexate. Day 0 is the day of marrow infusion.
Figure 1. Study design.
BMT CTN: Blood and Marrow Transplant Clinical Trials Network; CY: Cyclophosphamide; ATG: antithymocyte globulin; TBI: Total Body Irradiation.
The Phase I component of the trial tested 4 CY dose levels—150 mg/kg (50 mg/kg/day on days −4 to −2), 100 mg/kg (on days −3 to −2), 50 mg/kg (on day −2), and 0 mg/kg—to establish the optimal CY dose. The intention was to test each dose level in 6 patients, unless graft failure or toxicity boundaries were crossed, and then enroll additional patients to the optimal dose level in the Phase II portion of the trial, using adaptive Bayesian criteria to rank the desirability of the CY doses (Figure 1). Patients were evaluated for graft failure and early mortality at days 42 and 100. The aim of the safety monitoring was to minimize the number of patients treated at a dose level at which the probability of engraftment is too low or the probability of severe toxicity (including death) is too high, and to maximize the number of patients treated at the “optimal” dose. Stopping guidelines were devised incorporating both endpoints. The Phase II portion of the trial was designed to refine the dose selection and to provide a more precise estimate of efficacy at the optimal dose.
RESULTS AND DISCUSSION
The study opened for accrual in January 2006. As of June 26, 2011, a total of 61 patients had been enrolled. Characteristics of patients enrolled at the 0 mg/kg and 150 mg/kg CY dose levels are summarized in Table 1. The Phase I portion was completed (21 patients treated) in August 2007. CY dose level 0 mg/kg was closed in August 2007 during Phase I, because of an excessive number of graft failures. All 3 enrolled patients (all treated with Thymoglobulin) developed secondary graft failure (at days +29, +52, and +84), defined as initial neutrophil engraftment followed by a decline in the neutrophil count to < 0.5 × 109/L for 3 consecutive measurements on different days, unresponsive to growth factor therapy. All 3 patients received a second allograft. One patient is alive at 23 months after BMT, and 2 patients died, one due to interstitial pneumonia on day +114 and the other from adult respiratory distress syndrome on day +200 (Table 1).
Table 1.
Patient Characteristics and Outcomes
| Patient | CY Dose, mg/kg | Time from Diagnosis to BMT, Months | Age, Years | HLA Match | Previous Immunosuppressive Treatment | TNC Dose Infused, × 107/kg | Recipient–Donor Sex Match | Recipient–Donor CMV Match | Engraftment/ Graft Failure | Survival Status | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 17 | 22 | 7/8 | ATG, S | 23.5 | M/M | P/N | Engrafted at day 22; secondary graft failure at day 29 | Died, day 114 | ARDS (diffuse alveolar damage at postmortem) |
| 2 | 0 | 62 | 17 | 8/8 | ATG, S, CSA | 10.8 | F/M | P/P | Engrafted at day 23; secondary graft failure at day 84 | Alive, day 732 | — |
| 3 | 0 | 7 | 61 | 8/8 | ATG, CSA | 0.29 | F/F | P/P | Engrafted at day 24; secondary graft failure at day 52 | Died, day 200 | CMV pneumonia |
| 4 | 150 | 5 | 16 | 8/8 | ATG, S, CSA | 36.0 | M/M | P/P | Engrafted, day 21 | Alive, day 838 | — |
| 5 | 150 | 7 | 5 | 8/8 | No treatment | 30.0 | F/M | P/N | Engrafted, day 20 | Alive, day 742 | — |
| 6 | 150 | 7 | 8 | 8/8 | ATG, S, CSA | 55.4 | F/F | N/N | Engrafted, day 26 | Alive, day 704 | — |
| 7 | 150 | 19 | 18 | 8/8 | ATG, S, CSA | 44.5 | F/F | P/N | Engrafted, day 22 | Alive, day 763 | — |
| 8 | 150 | 21 | 18 | 8/8 | ATG, CSA, MMF | 19.8 | F/F | P/N | Engrafted, day 26 | Alive, day 703 | — |
| 9 | 150 | 12 | 15 | 8/8 | ATG, S, CSA | 38.8 | F/F | N/P | Engrafted, day 31 | Died, day 135 | Pulmonary failure |
| 10 | 150 | 44 | 17 | 7/8 | Androgens, ATG, S, CSA, sirolimus | 32.1 | F/F | P/U | Engrafted, day 22 | Died, day 92 | ARDS |
| 11 | 150 | 77 | 20 | 7/8 | Unknown | 12.1 | M/F | N/N | Engrafted, day 20 | Died, day 82 | Multiorgan failure |
| 12 | 150 | 15 | 61 | 8/8 | ATG, CSA, | 20.0 | M/M | P/N | Primary graft failure by day 42 | Died, day 62 | Cardiac failure |
| 13 | 150 | 15 | 23 | 8/8 | ATG, CSA, alemtuzumab | 19.7 | M/F | N/N | Engrafted, day 23 | Alive, day 817 | — |
| 14 | 150 | 7 | 44 | 8/8 | ATG, S, CSA | 21.9 | F/F | P/P | Engrafted, day 26 | Died, day 148 | Parainfluenza virus type 3 pneumonia |
| 15 | 150 | 12 | 52 | 8/8 | ATG, CSA | 23.1 | F/M | P/N | NA | Died, day 1 | ARDS (diffuse acute lung injury postmortem) |
| 16 | 150 | 20 | 9 | 7/8 | ATG, S, CSA | NA | M/F | P/P | NA | Died 1 day before planned BMT | Pulmonary failure |
| 17 | 150 | 8 | 15 | 7/8 | ATG, S, CSA | 0.07 | F/M | N/N | Engrafted, day 22 | Alive, day 897 | — |
CSA indicates cyclosporine; S, corticosteroids; ATG, antithymocyte globulin; MMF, mycophenolate mofetil; SAA, severe aplastic anemia; CMV, cytomegalovirus; P, positive; N, negative; U, unknown; ARDS, adult respiratory distress syndrome; IP, interstitial pneumonia; BMT, bone marrow transplantation; CY, cyclophosphamide; TNC, total nucleated cells; NA, not applicable.
The transplantation in patient 1 was mismatched at the HLA-DRB1 locus. Engraftment was defined as the achievement of an absolute neutrophil count (ANC) ≥0.5 × 109/L for 3 consecutive measurements on different days. Primary graft failure was defined by a lack of neutrophil engraftment, that is, ANC < 0.5 × 109/L on 3 consecutive measurements on different days by 100 days after transplantation. See the text for the definition of secondary graft failure.
At the completion of Phase I, CY dose level 150 mg/ kg was chosen as the likely optimal dose, and 8 additional patients were accrued at this level (total n = 14). This dose level was closed in March 2008 because of excessive organ toxicity. Seven of the 14 patients died (including 7 of the last 8 enrolled). Four died from organ failure, 2 from acute respiratory distress syndrome, and 1 from pneumonia due to parainfluenza virus type 3 infection (Table 1). Accrual continues at CY 100 mg/kg (currently 35 patients). Enrollment is also allowed at the CY 50 mg/kg dose level (currently 13 patients), whereas CY 100 mg/kg has been paused to allow follow-up of the enrolled patients through the predefined 42- and 100-day monitoring points. To ensure that the CY 50 mg/kg dose level undergoes adequate testing and to achieve a more balanced accrual in the 2 remaining groups, we have proposed a revised patient allocation plan that favors the latter dose level to the study Data Safety Monitoring Board.
The excessive graft failures occurred when CY was omitted from the regimen. One of the 3 transplants was mismatched at the HLA-DRB1 locus, and the other 2 patients received relatively low graft cell doses. Graft rejection is a recognized complication in these settings [1]. The lethal organ toxicities (primarily pulmonary) seen in the CY 150 mg/kg cohort were unexpected, given that the safe use of similar (and even higher) doses of CY has been reported in other transplantation regimens and settings [1,4]. Thus, this toxicity might have been related to the other agents in the conditioning regimen. Of note, pulmonary toxicity is the main adverse effect of even low-dose TBI [5,6]. Furthermore, the administration of FLU with high-dose CY may lead to interactions that magnify the known effects of low-dose TBI.
In conclusion, our early analysis of this trial has revealed 2 important findings regarding the regimen tested in this study. First, the omission of CY was associated with a higher-than-expected secondary graft failure rate, although 2 of 3 patients in this group received lower-than-recommended cell doses. Second, a CY dose of 150 mg/kg was associated with higher-than-expected transplantation-related mortality. The current data suggest caution in combining 150 mg/ kg CY, ATG, FLU, and TBI (or in omitting the CY entirely) in transplantation regimens targeting SAA, and highlight the need for well-controlled and carefully monitored multicenter studies of new dosing regimens before widespread implementation.
Acknowledgments
We thank Juan Wu and Yanli Wang for help with manuscript preparation, Erica Sanchez for secretarial assistance, and the clinical investigators who entered and managed the patients in this study. The BMT CTN is supported in part by Grant EQ1 U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute.
Footnotes
Financial disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Peinemann F, Grouven U, Kröger N, et al. Unrelated donor stem cell transplantation in acquired severe aplastic anemia: a systematic review. Haematologica. 2009;94:1732–1742. doi: 10.3324/haematol.2009.007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maury S, Balére-Appert ML, Chir Z, et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica. 2007;92:589–596. doi: 10.3324/haematol.10899. [DOI] [PubMed] [Google Scholar]
- 3.Viollier R, Socié G, Tichelli A, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41:45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 4.Kojima S, Matsuyama T, Kato S, et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood. 2002;100:799–803. doi: 10.1182/blood.v100.3.799. [DOI] [PubMed] [Google Scholar]
- 5.Deeg HJ, Amylon ID, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208–215. doi: 10.1053/bbmt.2001.v7.pm11349807. [DOI] [PubMed] [Google Scholar]
- 6.Deeg HJ, O’Donnell M, Tolar J, et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood. 2006;108:1485–1491. doi: 10.1182/blood-2006-03-005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacigalupo A, Locatelli F, Lanino E, et al. Fludarabine, cyclophosphamide and antithymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005;36:947–950. doi: 10.1038/sj.bmt.1705165. [DOI] [PubMed] [Google Scholar]
- 8.Bacigalupo A, Locatelli F, Lanino E, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low-dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica. 2010;95:976–982. doi: 10.3324/haematol.2009.018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Choi SJ, Lee JH, et al. Non–total body irradiation–con taining preparative regimen in alternative donor bone marrow transplantation for severe aplastic anemia. Bone Marrow Transplant. 2005;35:755–761. doi: 10.1038/sj.bmt.1704880. [DOI] [PubMed] [Google Scholar]
- 10.Kang HJ, Shin HY, Park JE, et al. Successful engraftment with fludarabine, cyclophosphamide, and thymoglobulin conditioning regimen in unrelated transplantation for severe aplastic anemia: a Phase II prospective multicenter study. Biol Blood Marrow Transplant. 2010;16:1582–1588. doi: 10.1016/j.bbmt.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Anderlini P, Acholonu SA, Okoroji GJ, et al. Fludarabine, cyclophosphamide, and antithymocyte globulin for matched related and unrelated allogeneic stem cell transplant in severe aplastic anemia. Leuk Lymphoma. 2011;52:137–141. doi: 10.3109/10428194.2010.524328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisdorf D, Carter S, Confer D, et al. Blood and Marrow Transplant Clinical Trials Network (BMT CTN): addressing unanswered questions. Biol Blood Marrow Transplant. 2007;13:257–262. doi: 10.1016/j.bbmt.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Schrezenmeier H, Passweg JR, Marsh JCW, et al. Worse outcome and more chronic GHVD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007;110:1397–1400. doi: 10.1182/blood-2007-03-081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after adult unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618–2621. doi: 10.1182/blood-2011-05-354001. [DOI] [PMC free article] [PubMed] [Google Scholar]