Abstract
CD4 helper T cells are critical for proper immune cell homeostasis and host defense, but are also major contributes to immune and inflammatory disease. Arising from a simple, biphasic model of differentiation, Th1 and Th2 cells, a bewildering number of fates seem to possible for helper T cells. To what extent different helper cell subsets maintain their characteristic gene expression profiles or exhibit functional plasticity is a hotly debated topic. In this review, we will discuss how the expression of “signature cytokines” and “master regulator” transcription factors do not neatly conform to a simple T helper paradigm. While this may seem confusing, the good news is that the newly recognized complexity fits better with our understanding of immunopathogenesis. Finally, we will discuss factors include epigenetic regulation and metabolic alterations that contribute to helper cell specific and plasticity.
Keywords: T cell plasticity, asthma, allergic disease, epigenetics, histone modification, therapy
1. Introduction
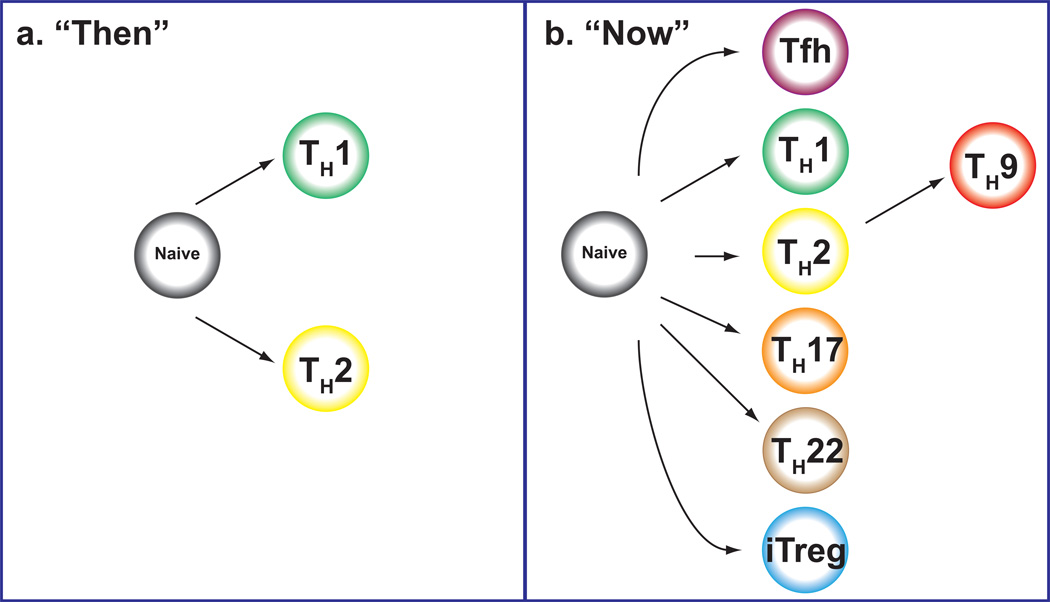
CD4 T cells are critical for host defense, but in addition to their key role as helper cells they can also be troublemakers, driving autoimmune diseases, asthma and allergies 1,2. Classically, we viewed helper T cells as having two major fates, T helper 1 (Th1) and Th2 cells (Figure 1a), but we now know that the opportunities for helper diversity are far greater than just these two outcomes. The new diversity includes Th17, Th9, and Th22, follicular helper T (Tfh) cells and different types of regulatory T cells 3,4,5,2,6 (Figure 1b). In addition though, emerging data point increased flexibility of these subsets. Fortunately, we are also beginning to understand the molecular basis of this complexity. This newer appreciation is not just pertinent for understanding basic aspects of T cell biology; on the contrary, the new insights provide a more sophisticated understanding of immune-mediated disease and new opportunities for therapy. In this review, we discuss helper cell differentiation decisions and how the regulation of helper cell specificity pertains to susceptibility to immune and inflammatory disease. We will consider the intrinsic and extrinsic factors that drive specification and the mechanisms that influence flexibility. Of particular interest with respect to the issue of plasticity are advances in epigenetic technologies as they pertain to T cell biology. The insights provided are especially relevant for immunologically mediated diseases in which both genetic and environmental factors play key roles in susceptibility.
Fig 1. Helper T cells “Then” and “Now”.
More than two decades ago, we viewed helper T cells as having two major fates, Th1 and Th2 cells. But now we recognize the new diversity of helper T cells including Th17, Th9, Th22, Tfh and different types of regulatory T cells.
2. Complexity of helper cell fate determination
For more than two decades, it has been recognized that CD4 T cells specialize in response to microbial challenges. The first subsets recognized were denoted T helper 1 (Th1) and Th2 cells, based on the selective production of two cytokines, interferon (IFN)-γ and interleukin (IL)-4, respectively 7. This “Th1/Th2 paradigm” was reasonably useful for initial categorization of mechanisms involving elimination of microbial pathogens. For instance, Th1 cells are critical for the clearance of many intracellular pathogens, like L. major and M. tuberculosis 8,9. Similarly, Th2 cells were found to be important for elimination of helminthic parasites such as Nippostrongylus brasiliensis and Schistosoma mansoni 10.
At first, the pathogenesis of immune-mediated disease also seemed to fit within this paradigm. Human asthma, as well as animal models of allergic airway inflammation, revealed the importance of cytokines produced by Th2 cells, namely IL-4, IL-5 and IL-13 11,12,13,14; the contribution of these various cytokines to the pathophysiology of airway inflammation, eosinophilia, fibrosis and other responses is well-recognized 15,16,17. Moreover, genome-wide association studies (GWAS) of asthma patients have revealed the association of DNA variants in the Th2 cytokine locus and the Il4R gene with susceptibility to asthma 18,19,20. Equally important has been the successful use of therapeutic monoclonal antibody directed against IL-5 (mepolizumab) and IL-13 (lebrikizumab) 21,22,23,24. Such discoveries clearly point to the pathophysiologic role of these cytokines; although, it is also clear that not all patients respond to these agents. Such findings clearly point to additional complexity of these diseases.
Initially viewed as one of the products of Th2 cells, IL-9 is an important factor that promotes mucus production; its expression is increased in the airways of asthmatic patients 25,26,27. Recently though, IL-9 has been found to be produced in a subset of cells that is distinct from classical Th2 cells 28,5. These cells are dubbed Th9 cells, but precisely how they relate to other subsets and the extent to which they constitute a stable subset remains to be determined.
It is also well-appreciated that IgE is a central player in the pathophysiology of allergies and asthma 24,29. While the generation of IgE-producing B cells is a well-accepted action of IL-4, it is also becoming clear that a specific population of CD4 T cells are important for providing B cell help. These cells are designated as T follicular helper cells (Tfh) and are identified based on their location in germinal centers and surface expression of the molecules CXCR5 and PD-1 4,30,31,32. IL-21 has been referred to as the signature cytokine for Tfh cells, but IL-21 is also produced by Th1 and Th17 cells 33,34. In addition, Tfh cells can produce cytokines made by other subsets including IFN-γ, IL-4, IL-17 and IL-10 4,35,36. Therefore, Tfh cells may have both overlapping and distinct contributions to disease as they can make Th1 and Th2 cytokines, but also contribute specifically to antibody formation. As they do not localize to tissues, the direct effects of their cytokine production is unlikely on tissue inflammation, but rather on isotype specific antibody production. Accordingly, genetic mutations in ICOS or SAP, genes expressed by Tfh cells that are necessary for interaction with B cells, result in a loss of Tfh cell development and thus, antibody production 37,38,39. In addition, patients with mutations in STAT3 have reduced Tfh cells, which may contribute to the altered antibody repertoire they display 40.
The attempt to link common autoimmune diseases with a simple Th1/Th2 paradigm has been even more problematic 41. Certainly there is evidence that excessive activation of Th1 cells contributes to organ-specific autoimmune diseases 42. However, a number of lines of evidence suggested that autoimmune mechanisms cannot be reduced to the action of Th1 cells alone. In particular the discovery of a new cytokine, IL-23, led to the recognition of a new subset of helper T cells and their importance in autoimmunity 43.
The discovery of an IL-17-producing population of CD4 T cells, termed Th17 cells, helped clarify contrasting findings in experimental autoimmune encephalitis (EAE), a mouse model of multiple sclerosis. IL-23 was found to have a critical role in EAE pathogenicity, and selective production of IL-17 by helper T cells was linked with IL-23. Although pathogenicity of the cytokine IL-17 in arthritis has been recognized since the late 1990’s, the discovery of IL-23 led to the appreciation of Th17 cells as a distinct subset 43,44,45,46,47. Accordingly, monoclonal antibodies that interfere with IL-17 action such as ixekizumab and seckinumab appear to be useful in diseases such as rheumatoid arthritis and psoriasis 48,49,50,51. In addition to pathogenic roles in human autoimmunity and a variety of mouse models of disease, Th17 cells contribute to host defense against extracellular bacteria such as Staphylococcis aureus, Klebsiella pneumonia, as well as fungi 52,53,54.
The importance of IL-17 applies not only to autoimmune disease, but is also relevant to the pathophysiology of asthma 55,56,57. One important action of IL-17 is the recruitment of neutrophils to the lung during airway inflammation and likely plays a role in steroid-resistant asthma 55,56,57,58,59,60,61,62. IL-17 also contributes to allergen-induced airway hyperresponsiveness (AHR) through direct effects on airway smooth muscle 63. IL-17 also appears to contribute to the pathogenesis of chronic obstructive pulmonary disease (COPD) and atopic dermatitis 64,65,66,67. Conversely, IL-27 is an important negative regulator of Th17 differentiation, which also induces Th1 differentiation 68,69,70. Interestingly, IL-27-receptor deficient mice have exaggerated airway inflammation 71. Although IL-27R deficient mice displayed increases in Th2 cytokines, they also had slightly elevated IFN-γ responses to experimental asthma challenge. This suggested that IL-27 plays a role in suppression of asthma responses by inhibiting Th2 response, and this inhibition is independent from promoting Th1 responses 71.
Th17 cells can also produce IL-22, which has been shown to have important roles in protecting barrier function in the lung and the gut 72. Increased IL-22 levels are associated with severity of asthma and are present in the skin of patients with atopic dermatitis 73,74. IL-22 neutralization in mouse models reduces eosinophil recruitment in the lung 75. The identification of a population of CD4 T cells that make IL-22, but does not express IL-17, IL-4, or IFN-γ, has led to the notion of “Th22” cells 76. However, IL-22 is also made by non-T cell lineages, including innate lymphoid cells (ILCs) 77.
With respect to the pathogenesis of autoimmune disease, the recognition of the criticality of regulatory T (Treg) cells was another key discovery 78,79,80,6,81,82. Treg cells are essential for the maintenance of immunological tolerance, as is vividly documented in mice or humans lacking the transcription factor Foxp3, which drives specification of this subset 6,81. In humans, this disorder is termed immune dysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome. Treg cells can be divided into natural thymic-derived Treg (nTreg) cells and peripherally antigen-induced Treg (iTreg) cells 6,81,83,84; however, there are a paucity of markers that distinguish these subsets 85,86,87. Among the ways Treg cells suppress immune responses is through the production of anti-inflammatory cytokines IL-10 or TGF-β 82. Treg cells also suppress effector T cell responses by consuming IL-2, limiting access to this important effector CD4 T cell growth factor 82. However, IL-2 interferes with Th17 cell differentiation and there are also circumstances in which Treg cells can promote Th17 responses through the consumption of IL-2 88,89,90,91.
In summary, while the importance of CD4 T cells in host defense and immune-mediated diseases is evident, it is also clear that they execute these functions by attaining multiple distinct fates. Advances over the last few years have led to the recognition, that a simple Th1/Th2 view of helper T cells was a vast oversimplification. However, we face a new challenge in understanding T helper cell function, as simply adding more “Th” cells to our lexicon does not provide a satisfactory understanding of immune homeostasis and immune-mediated pathology 92.
3.1. Flexibility of helper cell responses
The diversity of outcomes available to naïve CD4 T cells provided new ways of conceptualizing the pathogenesis of immune-mediated disease and new opportunities for therapy. However, it is often tacitly assumed that these subsets are stable or behaved as lineages, with defined signature cytokines, distinct transcriptional profiles and unique master regulator transcription factors. Initial experiments argued that Th1 and Th2 cells did conform to this view. Recently though, the exceptions to these notion have also become evident. What was also less clear is whether the more recently described other “Th” cells behave as lineages.
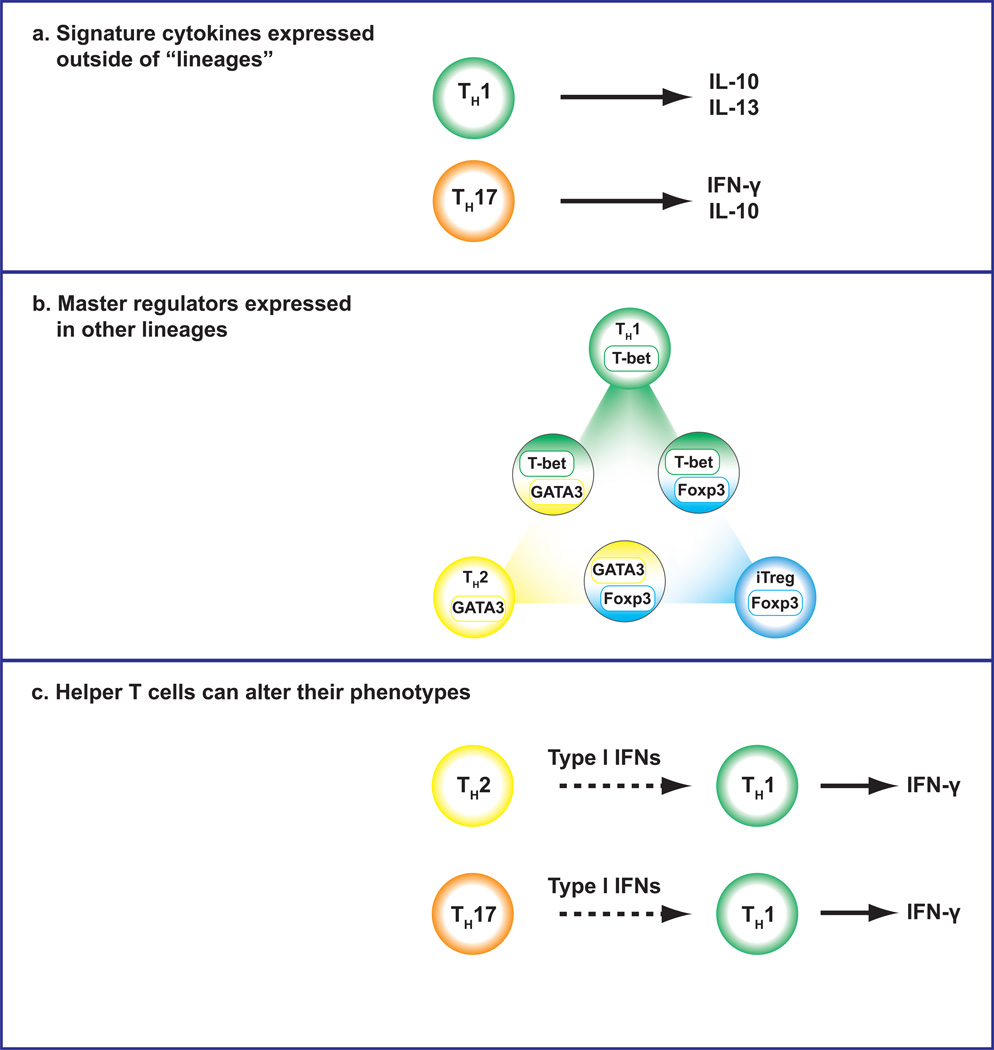
Indeed, it is now very clear that CD4 T cells can be remarkably flexible in their responses. It is not infrequent for CD4 T cells to co-express more than one “signature” cytokine, particularly in vivo (Figure 2a). Th17 cells, for example, readily become IFN-γ producers 93,94,95,96,97. In fact, the conversion of IL-17 producers to IFN-γ producers is an important aspect of immunopathogenesis in disease models and likely in human autoimmune disease 95. Even polarized Th2 cells can acquire the ability to produce IFN-γ 98. Similarly, in asthma memory/effector cells can be identified that produce both Th17 and Th2 cytokines 99. These hybrid cells appear to be important drivers of pathology. This fits with the immunopathological features of diseases like atopic dermatitis, which exhibit Th2 characteristics early on, but later show Th1-like disease 100.
Fig 2. Plasticity and Promiscuity in Helper T cells.
Recent advances have allowed us to point out several evidences of plasticity of helper T cells. Helper T cells can express the signature cytokines (a) and the master regulators (b) outside of lineages. Moreover, helper T cells can alter their phenotypes by the environmental stimulations (c).
While they are clearly important in promoting B cell responses, Tfh cells do not produce a single “signature” cytokine; on the contrary, they can express a range of different cytokines in different circumstances. Ordinarily, Tregs cells do not produce effector cytokines; however, there are data arguing that Treg cells can be unstable and can acquire the ability to produce such cytokines. This though remains a controversial topic 101,102,103,104,105,106,107.
Initially, “Th2 cytokines” included IL-4, IL-5, IL-13 and IL-10. However, we now appreciate that IL-10 is broadly produced by multiple types of helper T cells including Th1 and Th17 cells 108,109,110. In addition, we also now recognized that IL-13 can be made without IL-4 111,112. Th2 cells, may in fact, be heterogeneous 113,114,115 and cells that produce IL-5 and not IL-4 may be more differentiated cells. Similarly, “Th17” cells are also heterogeneous; some are pathogenic in autoimmune disease and other are not 116.
3.2. Factors that regulate plasticity versus phenotypic stability
Given that CD4 T cells can exhibit features of stability but also apparently retain the potential for flexible responses, the question arises as to how immediately phenotypic conversion occurs and what mechanisms promote flexibility in differentiating helper T cells?
3.3. Flexible expression of multiple master regulators
In the conventional view, a given helper T cell subset is defined based on its ability to produce a single signature cytokine as well as corresponding a single master transcription factor. Th1, Th2, Th9, Th17, Tfh and Treg cells express T-bet (encoded by Tbx21) 117,118, GATA-binding protein 3 (GATA3) 119, SFFV proviral integration 1 (PU.1) 120, retinoic acid receptor-related orphan receptor-γt (Rorγt) 47, B cell lymphoma 6 (Bcl6) 30,32, and forkhead box P3 (FoxP3) 121,79 respectively.
However, recent data show that, like the signature cytokines, the expression of T cell master regulators is far more complicated than originally appreciated. For example, T-bet and GATA3 are transiently co-expressed in recently activated CD4 T cells and can functionally interact, limiting the action of GATA3 122. In addition, following viral infection, T-bet and GATA3 can also be stably co-expressed in previously committed Th2 cells, to create a population termed Th2+1 that has features of both Th1 and Th2 cells 98. This provides a means by which previously committed CD4 T cells can maintain flexibility in functional responses.
T-bet and Bcl6 can also be simultaneously expressed 33,123,124,125. In fact, the same cytokines that induce Bcl6 can also induce T-bet 33. As T-bet is induced, it can bind Bcl6 and interfere with the ability of Bcl6 to act on its target genes. In this case, master regulators can fine tune function for each other. Thus, the balance between T-bet and Bcl-6 expression could be important for the decision between a Th1 and Tfh cell.
Simultaneous expression of FoxP3 with T-bet, GATA3 or Bcl6 has also been documented in Treg cells. This has been argued to be functionally relevant for control of Th1, Th2 and Tfh cell responses respectively 104,126,127,128,129,130,131. In this way, the expression of multiple master regulators can be viewed as a means of specialization of regulatory responses. In other cases, master regulators are important for more than one “lineage”. Th9 cells express the transcription factors IRF4 and PU.1 132,133,120,134. Further more, IRF4 is proven to be important for Th2, Th9 and Th17 cells 135.
These examples of co-expression make it appropriate to re-visit our views of “master regulator”. There are now clear examples in which helper T cells express more than one master regulator (Figure 2b). In fact, the complex modes of expression can modulate function, specialize responses or preserve flexibility.
4.1. Sensing the environment
The dynamic expression of master regulators is controlled both positively and negatively by other factors. Among the more relevant factors are STAT family DNA binding proteins. Typically, we associated STAT4, STAT6, STAT3, and STAT5 with Th1, Th2, Th17 and Treg cells, respectively. However, unlike the master regulators, STATs are not necessary differentially expressed amongst subsets. Cytokine receptor expression can be down-modulated, but the action of STATs can be redundant. For instance, IL-12 acting via STAT4 is an important driver of T-bet expression. In Th2 cells, IL-12Rβ2 is downregulated, thus making Th2 cells resistant to the effect of IL-12. However, type I IFNs acting via STAT1 can also induce T-bet expression. In this way, IFNs can “reprogram” Th2 cells in the setting of viral infection 98 (Figure 2c).
Similarly, STAT3, being activated by IL-6 and IL-21 promotes Th17 differentiation. However, STAT5, in response to IL-2, can bind the same sites in the Il17 locus and inhibit IL-17 expression 89. IL-2 acting on STAT5 also inhibits Bcl6 expression 136.
STAT5 is a critical positive regulator of Foxp3 expression; in fact, the phenotypic stability of Treg cells requires the expression of the high affinity IL-2 receptor 107. Conversely, activation of STAT3 can limit Foxp3 expression; helper T cells that lack STAT3 exhibit a more stable Foxp3 expression 137.
Another example of cytokines mediating an antagonism between STAT molecules, which alters T helper fate, can be found in patients with gain-of function STAT1 mutations who have mucocutaneous candidiasis. These patients have a deficiency in Th17 cells, which are also key to anti-candida defense 138. It has been suggested that because IL-27 can suppress Th17 differentiation, and because IL-27 signals through STAT1, these patients have a failure to differentiate into Th17 cells because of over-exuberant STAT1 signaling triggered at least in part by IL-27 139.
4.2. The Helper T Cell Transcription Factor Network
While STATs and master regulators are critical for helper cell differentiation, it is overly simplistic to try to explain the diverse functionalities of helper T cells based on these two classes of factors. In reality, specification requires a cohort of critical transcription factors working in concert. During T cell development, CD4 T cells express an array of transcription factors that dynamically change over the course of commitment in the thymus, allowing them to diverge from CD8 T cells 140. Factors such as Thpok, Runx3, Runx1, Ets1, Tox and the E proteins E2A and HEB are all induced at discrete steps to drive commitment to either the CD4 or CD8 lineage. It is in the context of these other transcription factors that master regulators and STATs exert their effect. Even GATA3 has critical roles in thymic development, aside from its function in Th2 cells 141. Thus, any given transcription factor can have stage-specific functions.
In addition to factors required for development, several other transcription factors have been described that are critical for CD4 T cells, but are not specifically required for only one subset. Interferon regulatory factor (IRF) 4 is important for the differentiation of Th2, Treg, Th17, Th9 and Tfh cells 142,143,134,144,145. To function, IRF4 complexes with members of the AP1 family, making family members like BATF and c-Maf necessary for several CD4 T cell subsets 146,147,148,149,150.
Additional transcription factors that are important for Th1 cells include Hlx, Runx3, and the Ets family members 151,152,153. In addition to c-Maf, the AP1 family member JunB is required for Th2 cells, as well as the transcription factor Gfi-1 154,155. The transcription factor NFIL3 (E4BP4) is a key regulator of IL-13 production 156,111. Recently, HIF1, Runx1, Aiolos and Fosl2 have all been demonstrated to be important for Th17 cells 148,157,158,159. Coupled to this, we appreciate that factors like Klf2, Bcl6 and Blimp1 (encoded by PRDM1) influence the extent to which cells exhibit features of effector cells 160,161. Despite the importance of Foxp3, other factors are important contributors to the phenotype of various regulatory cells 162,163,164,165,166,167.
Given that helper T cells express a panoply of key transcription factors it is naïve to interrogate expression of one transcription factor, master regulator or otherwise, and make inferences about functionality. There is not a simple correlation between one helper cell “lineage” and expression of a single master regulator transcription 168. Multiple transcription factors work in concert to effect complex cellular decisions; fortunately, the technological advances in imaging and sequencing facilitate measuring numerous factors simultaneously.
5.1. Transcriptomic views of helper T cell specification
While T helper subsets were initially defined based on their selective cytokine production, the advent of microarray technology provided the opportunity to define the global patterns of gene expression or “transcriptomes” of helper T cell subset. What became obvious is that different types of helper T cells exhibit a large cassette of genes that contribute to their functionalities. For instance, the regulation of chemokine and chemokine receptor expression is an important feature of different subsets of helper T cells. Th1 cells preferentially express CCR5 and CXCR3, while Th2 cells are characterized by the expression of CCR4 and CCR8 169. CCR6 and CXCR5 are important for Th17 and Tfh cells, respectively 169. In addition, deep sequencing technology coupled to chromatin immunoprecipitation techniques has allowed the first views of how various helper cell-expressed transcription factors act on a genome wide scale to contribute to helper cell transcriptomes. This is important because we can begin to determine direct versus indirect effects. If a transcription factor is important, we can more precisely dissect why this is the case.
What we have learned is that many of the key genes associate with particular fates are direct targets of STATs and master regulators. These transcription factors bind at thousands of sites in the genome in a sequence-specific manner and regulate the transcription of their target genes. STATs and master regulators are known to activate many genes, although they are also responsible for silencing of genes expressed in other cell fates. Disrupted binding of transcription factors by disease-associated SNPs are now being linked to changes in the transcriptional profile in relevant cell types 170. It must be emphasized however, that when we consider stability versus flexibility of helper T cells, more often than not, only a few genes are interrogated, not entire transcriptomes. However, as the technology for measuring global gene expression become available, it will be important to factor in global information on all genes as we consider to what extent different populations of cells appear to be stable or not.
5.2. Epigenomic views of helper T cells stability and flexibility
While the specific functions of different helper T cells are obviously a reflection of distinctive patterns of global gene expression and the action of transcription factors, this is not the whole story. Transcription factors do not act in isolation; in order for them to exert their effect, the region of the genome they are acting upon must be accessible. We are now beginning to understand on genome wide scales what this means and what are the biochemical and cell biological underpinnings of what it means for a gene and its regulatory elements to be accessible. Classically, the term epigenetic has been used to refer to heritable changes in gene expression that are not due to changes in the DNA sequence. It is now clear that many factors contribute to how and when different portions of the DNA code can be read. From this perspective a modern view of epigenetics can be viewed as encompassing the combined action of the many factors that will be further discussed below. On a global scale this can be referred to as epigenomics.
DNA is bound to histone molecules to form nucleosomes, which can exist in accessible states (euchromatin) or inaccessible states (heterochromatin). The histone components of the nucleosome are associated with an array of post-translational covalent modifications. For example, histone 3 lysine 4 trimethylation (H3K4me3) is associated with active promoters and histone 3 lysine 36 trimethylation (H3K36me3) is indicative of active transcription of genes. In contrast, histone 3 lysine 27 trimethylation (H3K27me3) and histone 3 lysine 9 trimethylation (H3K9me3) are marks of silenced genes. For example, the H3K4me3 and H3K27me3 status of PU.1 promoter acts as a unique regulator of Th9 memory acquisition and Th9 immunity 171.
A variety of enzyme complexes (Trithorax and Polycomb complexes) deposit these “marks” and other enzymes (Jmjd3 etc.) can remove these marks; these though are just a few of the many modifications that have been described. The movement of nucleosomes is also regulated by ATP-dependent enzymes (Brg1, the Swi-Snf complex and other factors). DNA methylation is another important factor that dictates whether genes can be “read” or not. All these factors working in concert influence the accessibility of genes to the action of transcription factors.
It needs to be emphasized though - genes represent only 2% of the genome. Equally impressive is recent discoveries provided by the Encode project that has shown that 80% of the so called “junk DNA” is active. Among the vast numbers of “switches” and regulatory hubs that reside in the junk DNA are enhancers. We have known for many years that the regulation of a limited number of genes is carefully regulated by a complex architecture of distal enhancers. For instance, many studies focused on the distal cis-regulatory elements of lineage-specific cytokine genes. The IFNG/Ifng locus encompasses approximately 200 kb with multiple distal enhancers 172. The IL4/Il4 locus also comprises multiple enhancers as well as a silencer element 173. Enormous stretches of DNA are required for proper regulation of these key genes. Similarly, the Foxp3 gene is also regulated by distinct enhancer elements 174. Current technology now allows enumeration of active and poised enhancers on a genome wide scale. What is becoming clear is that the enhancer landscape is cell-specific and in this way, cell identity is not just a reflection of genes expressed at any given time, but what genes may be expressed based on accessibility of key regulatory elements. Finally, gene expression is also regulated by the actions of long noncoding RNAs (lncRNAs) and microRNAs (miRNAs).
Functionally, we already know epigenetic regulation of helper T cells is important 175,176. Deletion of BRG1 interferes with IFN-γ production 177. Similarly, the cohesin protein complex is also important for the maintenance of gene architecture; deletion of component of this complex also blocks IFN-γ 178,179. Disruption of Trithorax causes aberrant Th2 differentiation 180,181 and deletion of the H3K9 methylase results in inappropriate expression of Th1 genes in Th2 cells 182. Deletion of the DNA methyltransferase, DNMT1 in T cells leads loss of silencing of Th1 and Th2 genes in the opposing subset 183,184,185.
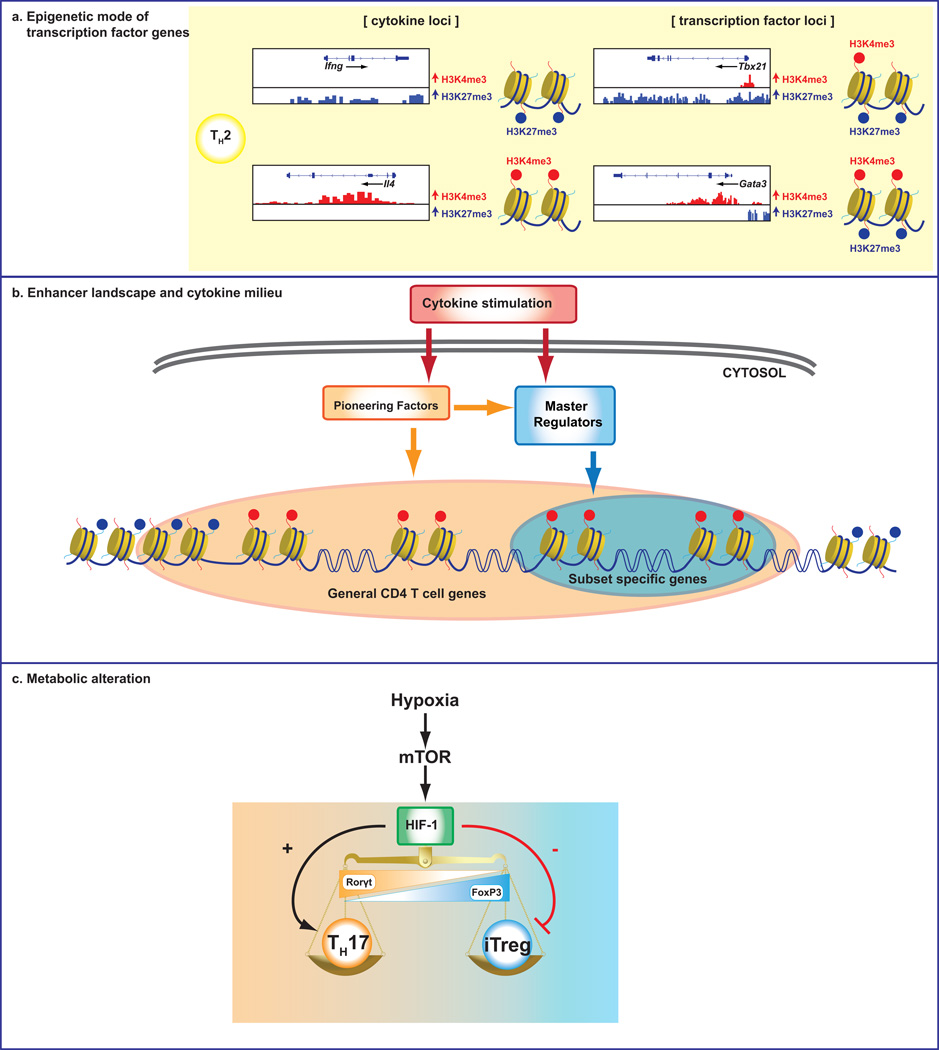
Thanks to technological advances, genome-wide mapping of histone modifications in helper T cells has been accomplished 103. Consistent with the standard “lineage commitment” view of helper cell differentiation, signature cytokine genes exhibit unopposed permissive marks (H3K4me3) in the appropriate subsets (e.g. Ifng in Th1 cells) and repressive marks (H3K27me3) in other subsets that do not express these cytokines (Figure 3a). Contrary to expectation, genes encoding master regulators including Tbx21, Gata3, Bcl6, Runx3, and Prdm1 have complex marks: the genes exhibit both accessible and repressive marks 103 (Figure 3a). This helps explain the flexible expression of master regulator genes. The action of master regulators and STAT proteins on global histone modifications has also been elucidated. STATs can impact target genes by affecting transcription and epigenetic modifications or by only affecting transcription (~11%). For a sizable number of genes, STAT only affect epigenetic modifications (~20%). It is clear that deletion of some master regulators has an impact on the epigenome; however, not all lineage-specific factors are essential for the epigenetic status: T-bet and GATA3 clearly have global epigenetic effects, but other factors such as Rorγt and Foxp3, have little effect on the epigenetic landscapes of their respective subsets. Overall, it appears that target genes of master regulators like FoxP3 and Rorγt are prepared by other transcription factors and these master regulators are mostly modulators of expression and have focused impacts on promoters 148,163,186.
Fig 3. Mechanisms underlying plasticity of Helper T cells.
(a) Specific modifications of the histone molecules that make up the nucleosomes are associated with accessibility. (b) Cytokine milieu can induce “pioneering factors” and activate different STATs, which are indispensable for establishing the genomic epigenetic landscapes of developing helper T cells and dictating the accessibility of key target genes. Master regulators may be better viewed as critical modulators, but not drivers of the chromatin architecture underlying helper cell identity. (c) Alterations in T cell metabolism also have critical roles in the plasticity of helper T cell lineages.
The global enhancer landscapes of helper T cell subsets have begun to be elucidated. This has already provided a number of surprises. First is the extent to which the active enhancer landscapes are distinct. Th1 and Th2 cells exhibit roughly 10,000 of these elements of which only half are shared. If macrophages are also analyzed, the number of elements drops to roughly 1000 active elements. This is very consistent with evolving notions of the cell-specific nature of enhancer landscapes. The action of STATs and master regulators are also quite different. STATs have a major effect on the acquisition of active elements. In contrast, the effect of the master regulators is much more restricted; although master regulators vary in their capacity to impact global landscapes. Some master regulators like T-bet, Rorγt and Foxp3 have very small effects on creating active enhancer elements; rather these factors appear to highjack existing sites that are already created by other factors 148,163,186. Studies on these three transcription factors support the idea that these so-called master regulators have focused roles particularly on the promoter of their target genes and are not capable of globally changing the chromatin signature of enhancers.
In summary, cytokines change behavior of cells and work in conjunction with other transcription factors to effect changes in chromatin and gene expression (Figure 3b). The extent to which cytokines acting via STATs influence the global chromatin organization was unexpectedly broad. Alterations in gene expression have long been understood to be the result of signaling events as transcription factors can be activated or induced by exogenous factors. However, the epigenome is increasingly understood also to be the outcome of signal transduction events. If we consider cell identity as a reflection of what functionalities are apparent and those that are possible based on accessibility to alternative gene programs, it is clear that alterations in the cytokine milieu can have substantial impacts on cell identity.
6. Back to the future: Metabolic Regulation of Helper T cells
A surprising new development is the appreciation that alterations in T cell metabolism have critical roles in selective regulation of cytokine production. Alterations in the availability of nutrients has long been known to affect immune reponses, for example in patients with type II diabetes or after profound starvation. The transition from a resting naive T cell into an activated, rapidly proliferating effector T cell requires a substantial change in the metabolic machinery within the cell. However, it was not recognized that these were two distinct effects. We now appreciate that the metabolism of a proliferating lymphoblast resembles that of a cancer cell with an ability to rapidly consume both glucose and amino acids chiefly for the purpose of creating new protein and nucleotides for cell growth 187; consequently, little of the ATP generated by glucose metabolism is provided by aerobic metabolism. The majority is generated by anaerobic glycolysis 187.
The elevated uptake of glucose and glutamine in activated lymphocytes is to be mediated by the Ras/ MAPK and PI3'K pathways, which are activated by T cell receptor- and IL-2-dependent signals. Activation of either receptor is capable of sustaining T cell proliferation in vitro and both are able to activate the kinase mTOR, which comprises two functionally distinct signaling complexes: mTOR complex 1 (mTORC1) and complex 2 (mTORC2) 188. mTORC1 is activated by anabolic PI3K/ AKT signaling pathway and is inactivated in the absence of available nutrients. It is a checkpoint regulator of protein synthesis through the phosphorylation of p70 S6 kinase and 4E-BP1 188. In contrast the mTORC2 is required for the phosphorylation of several AGC family kinases including AKT, SGK1 and some PKC family members 189,190.
Genetic deletion of mTOR or inhibition by rapamycin in mouse T cells inhibits effector cell differentiation and promotes generation of Treg cells 191. It is of note that Treg cells do not depend on anaerobic glycolysis to the same degree as the effector cell lineages 192. T cells that lack RAPTOR a key component of mTORC1 demonstrate impaired Th1 and Th17 differentiation 193 whereas T cells that lack RICTOR a key component of mTORC2 have impaired Th2 differentiation 194. Hypoxia-inducible factor (HIF) 1 is a key metabolic sensor. HIF promotes Th17 differentiation and attenuates Treg cell development 157,195 (Figure 3c). The extent to which metabolic perturbations might impact polarized subsets of helpers is only just beginning to be studied. However, the idea that acute metabolic changes can selectively impact helper T cell genetic programs has profound implications.
7. Conclusions
Ultimately, why should these plasticity and epigenomic studies matter to clinical allergists? Understanding the complex programs of differentiation of helper T cells in response to intrinsic and extrinsic clues is a fascinating basic science problem; however, there are also several benefits. From the point of view of someone interested in the genetics of allergy, a better view of the landscape of critical transcription binding sites might open a new paradigm for finding genetic causes of common allergic diseases. Instead of overt syndromes, polymorphisms in individual transcription factor binding sites might alter the risk for disease and provide a very specific cause for certain symptom constellations, and ultimately, hint towards better therapeutic interventions. Finding polymorphisms in “junk DNA” might initially seem to be unrevealing; however, as we understand the functional relevance of the “junk” some surprises may emerge. Such examples have been found in non-allergic settings already 196.
Although genetics plays a large part in the susceptibility to autoimmune and allergic disease, environmental factors are also major contributors 197,198,199. Better understanding of the factors that influences the epigenomes of CD4 T cells could allow us to develop interventions which keep the “good” phenotypes of T helper cells while skewing the “bad” ones away from their pathogenic effector capacities, even after they have already differentiated from the naïve state. This is exciting because we have targeted therapies, both biologics and oral agents that can influence responsiveness to cytokines 200,201; precisely how these therapies influence epigenomes and transcriptomes of immune cells and how this relates to cellular plasticity will be exciting to ascertain. Will we be able to thoughtfully “reprogram” immune cells? Perhaps immunotherapy, or some of the cytokine or anti-cytokine therapies in place now do this. But a more sophisticated understanding will help us develop cellular assays, which could more accurately predict their efficacy in a perspective patient, and/or follow the treatment to determine whether it is working before clinical improvement is noted. In an era of “personalized medicine” such insight and tools will become more and more important. The exciting part is that we have the tools are now available to get started.
JACI Glossary: Mechanisms Underlying Helper T cell Plasticity: Implications for Immune-Mediated Disease
| TERM | DEFINITION |
|---|---|
| Locus | The position in a chromosome of a particular gene or allele |
| Genome Wide Association Studies (GWAS) | Cohorts of patients with and without a given disease are examined across the entire genome for single nucleotide polymorphisms (SNPs) that are over represented in patients with the disease. This identifies regions of the genome that contain a variant gene or genes that confer disease susceptibility. Candidate genes are then selected based on how closely they are associated with the disease and whether their biologic function correlates with the disease under study. |
| Germinal Center | An area within a lymphoid follicle where affinity maturation occurs. B cells activated by antigen and helper T cells migrate into germinal centers. Somatic mutation of V region genes in these B cells generates antibodies with different affinity for antigen. Binding of B cells to antigen presented on follicular dendritic cells rescues these B cells from apoptosis. B cells with the highest affinity for antigen will have a survival advantage and results in an average increase in the affinity of antibodies for antigen during the immune response. |
| ICOS (Inducible Costimulator) | ICOS is a member of the CD28 family of costimulatory receptors on T cells. ICOS binds to ICOS ligand on antigen-presenting cells and promotes effector responses. Mutations in the ICOS gene have been reported in patients with common variable immunodeficiency. |
| Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX) Syndrome | FOXP3 mutations lead to immune system dysregulation in this disorder. Features include early onset diabetes, diarrhea, and failure to thrive. Newborns have an eczematous rash. Serious infections can occur. Laboratory abnormalities include high IgE, normal IgG, normal IgM, and normal IgA. T and B subsets are also normal. Autoimmune hemolytic anemia, neutropenia, and thrombocytopenia can occur. Female carriers are usually healthy. |
| Naïve CD4 T cells | T cells that have completed maturation in the thymus, but have not yet encountered foreign antigen. They are characterized by no effector function, no cell cycling, and high expression of CCR7 and CD62L (L selectin, peripheral lymph node homing receptor). Their major CD45 isoform is CD45RA. |
| STAT (signal transducers and activators of transcription) | Transcription factors that are a part of the Jak (Janus kinase)-STAT pathway. Many cytokines use Jak-STAT pathways for signaling. There are seven STATs (1–4, 5a, 5b, and 6). The discovery of the Jak- STAT pathway came from analyses of interferon signaling. |
| Mucocutaneous candidiasis | Persistent superficial candidal infections of mucous membranes, skin, and nails. Other defects that are associated with mucocutaneous candidiasis include CARD9/Dectin-1 deficiency and AIRE gene defects. Patients have selective anergy to candida on delayed type hypersensitivity testing. |
| Blimp1 | A key transcription factor for the differentiation of B cells into antibody secreting plasma cells within lymphoid organs. |
| Rapamycin | An immunosuppressant drug used in renal transplantation as prophylaxis against organ rejection. |
| Epigenetics | The term “epigenetics” was coined by Waddington prior to the era of modern molecular biology, it has come to denote hereditable changes in phenotype or gene expression without changes in DNA sequence. |
| Epigenome | The term “epigenome” indicates that the status of the genomewide chemical changes to the DNA and histone proteins. |
Acknowledgements
This work was supported by NIH/NIAMS Intramural Research Program (IRP), the JSPS Research Fellowship for Japanese Biomedical and Behavioral Researchers at NIH (K.H.) and PRAT (A.P.).
Abbreviation
- Th
T helper
- Tfh
Follicular helper T
- IFN-γ
Interferon-γ
- IL
Interleukin
- GWAS
Genome-wide association studies
- EAE
Experimental autoimmune encephalitis
- AHR
Airway hyperresponsiveness
- COPD
Chronic obstructive pulmonary disease
- ILCs
Innate lymphoid cells
- Treg
Regulatory T
- nTreg
natural thymic-derived Treg
- iTreg
peripherally antigen-induced Treg
- IPEX
Immune dysregulation polyendocrinopathy enteropathy X-linked
- GATA3
GATA-binding protein 3
- PU.1
SFFV proviral integration 1
- Rorγt
Retinoic acid receptor-related orphan receptor-γt
- Bcl6
B cell lymphoma 6
- FoxP3
Forkhead box P3
- IRF
Interferon regulatory factor
- H3K4me3
Histone 3 lysine 4 trimethylation
- H3K27me3
Histone 3 lysine 27 trimethylation
- H3K9me3
Histone 3 lysine 9 trimethylation
- IncRNAs
long noncoding RNAs
- miRNAs
microRNAs
- mTORC
mTOR complex
- HIF
Hypoxia-inducible factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126:1081–1091. doi: 10.1016/j.jaci.2010.06.025. quiz 92-3. [DOI] [PubMed] [Google Scholar]
- 2.Akdis M, Palomares O, van de Veen W, van Splunter M, Akdis CA. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J Allergy Clin Immunol. 2012;129:1438–1449. doi: 10.1016/j.jaci.2012.05.003. quiz 50-1. [DOI] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 4.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 5.Tan C, Gery I. The unique features of Th9 cells and their products. Crit Rev Immunol. 2012;32:1–10. doi: 10.1615/critrevimmunol.v32.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 8.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 9.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 10.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 12.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 13.Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirahara K, Yamashita M, Iwamura C, Shinoda K, Hasegawa A, Yoshizawa H, et al. Repressor of GATA regulates TH2-driven allergic airway inflammation and airway hyperresponsiveness. J Allergy Clin Immunol. 2008;122:512–520. e11. doi: 10.1016/j.jaci.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 17.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Howard TD, Zheng SL, Haselkorn T, Peters SP, Meyers DA, et al. Genome-wide association study of asthma identifies RAD50-IL13 and HLA-DR/DQ regions. J Allergy Clin Immunol. 2010;125:328–335. e11. doi: 10.1016/j.jaci.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Doi S, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet. 2011;43:893–896. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 22.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 24.Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354:2689–2695. doi: 10.1056/NEJMct055184. [DOI] [PubMed] [Google Scholar]
- 25.Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol. 2000;105:108–115. doi: 10.1016/s0091-6749(00)90185-4. [DOI] [PubMed] [Google Scholar]
- 26.Vermeer PD, Harson R, Einwalter LA, Moninger T, Zabner J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol. 2003;28:286–295. doi: 10.1165/rcmb.4887. [DOI] [PubMed] [Google Scholar]
- 27.Ciprandi G, De Amici M, Giunta V, Marseglia A, Marseglia G. Serum Interleukin-9 Levels Are Associated With Clinical Severity in Children With Atopic Dermatitis. Pediatr Dermatol. 2012 doi: 10.1111/j.1525-1470.2012.01766.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt E, Bopp T. Amazing IL-9: revealing a new function for an "old" cytokine. J Clin Invest. 2012;122:3857–3859. doi: 10.1172/JCI65929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011;35:919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. The Journal of biological chemistry. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature immunology. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature immunology. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 37.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 38.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 42.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 43.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 44.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 45.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 47.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 48.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 49.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 50.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001107. 52ra72. [DOI] [PubMed] [Google Scholar]
- 51.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- 52.Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 56.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 57.Laan M, Palmberg L, Larsson K, Linden A. Free, soluble interleukin-17 protein during severe inflammation in human airways. Eur Respir J. 2002;19:534–537. doi: 10.1183/09031936.02.00280902. [DOI] [PubMed] [Google Scholar]
- 58.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 59.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 60.Gold DR, Fuhlbrigge AL. Inhaled corticosteroids for young children with wheezing. N Engl J Med. 2006;354:2058–2060. doi: 10.1056/NEJMe068058. [DOI] [PubMed] [Google Scholar]
- 61.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 66.Oyoshi MK, Elkhal A, Kumar L, Scott JE, Koduru S, He R, et al. Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proc Natl Acad Sci U S A. 2009;106:14954–14959. doi: 10.1073/pnas.0904021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124:485–493. 93, e1. doi: 10.1016/j.jaci.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 69.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 70.Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miyazaki Y, Inoue H, Matsumura M, Matsumoto K, Nakano T, Tsuda M, et al. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175:2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 72.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151:297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 74.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252. e2. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schnyder B, Lima C, Schnyder-Candrian S. Interleukin-22 is a negative regulator of the allergic response. Cytokine. 2010;50:220–227. doi: 10.1016/j.cyto.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 77.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 79.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 80.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nature reviews Immunology. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 81.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hawrylowicz CM, OɿGarra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 85.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1–19. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–1742. S1. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 89.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature immunology. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, et al. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, et al. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 92.Eyerich S, Onken AT, Weidinger S, Franke A, Nasorri F, Pennino D, et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. N Engl J Med. 2011;365:231–238. doi: 10.1056/NEJMoa1104200. [DOI] [PubMed] [Google Scholar]
- 93.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hegazy AN, Peine M, Helmstetter C, Panse I, Fr√∂dhlich A, Bergthaler A, et al. Interferons Direct Th2 Cell Reprogramming to Generate a Stable GATA-3+T-bet+ Cell Subset with Combined Th2 and Th1 Cell Functions. Immunity. 2010;32:116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oyoshi MK, He R, Kumar L, Yoon J, Geha RS. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol. 2009;102:135–226. doi: 10.1016/S0065-2776(09)01203-6. [DOI] [PubMed] [Google Scholar]
- 101.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 103.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, et al. Global Mapping of H3K4me3 and H3K27me3 Reveals Specificity and Plasticity in Lineage Fate Determination of Differentiating CD4+ T Cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. lnterleukin-12 converts Foxp3+ regulatory T cells to interferon-gamma-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140:2031–2043. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 108.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 109.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing antiinflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 110.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 111.Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011;12:450–459. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gallo E, Katzman S, Villarino AV. IL-13-producing Th1 and Th17 cells characterize adaptive responses to both self and foreign antigens. Eur J Immunol. 2012;42:2322–2328. doi: 10.1002/eji.201142227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Upadhyaya B, Yin Y, Hill BJ, Douek DC, Prussin C. Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J Immunol. 2011;187:3111–3120. doi: 10.4049/jimmunol.1101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Endo Y, Iwamura C, Kuwahara M, Suzuki A, Sugaya K, Tumes DJ, et al. Eomesodermin controls interleukin-5 production in memory T helper 2 cells through inhibition of activity of the transcription factor GATA3. Immunity. 2011;35:733–745. doi: 10.1016/j.immuni.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 115.Prussin C, Yin Y, Upadhyaya B. T(H)2 heterogeneity: Does function follow form? J Allergy Clin Immunol. 2010;126:1094–1098. doi: 10.1016/j.jaci.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghoreschi K, Laurence A, Yang XP, Hirahara K, O'Shea JJ. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011;32:395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cho JY, Grigura V, Murphy TL, Murphy K. Identification of cooperative monomeric Brachyury sites conferring T-bet responsiveness to the proximal IFN-gamma promoter. Int Immunol. 2003;15:1149–1160. doi: 10.1093/intimm/dxg113. [DOI] [PubMed] [Google Scholar]
- 118.Sekimata M, Perez-Melgosa M, Miller SA, Weinmann AS, Sabo PJ, Sandstrom R, et al. CCCTC-binding factor and the transcription factor T-bet orchestrate T helper 1 cell-specific structure and function at the interferon-gamma locus. Immunity. 2009;31:551–564. doi: 10.1016/j.immuni.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 120.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 122.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 123.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oestreich KJ, Huang AC, Weinmann AS. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J Exp Med. 2011;208:1001–1013. doi: 10.1084/jem.20102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg Cells Undergo Abortive Th1 Cell Differentiation due to Impaired Expression of IL-12 Receptor beta2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 133.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 135.Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008;105:20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Milner JD. IL-17 producing cells in host defense and atopy. Curr Opin Immunol. 2011;23:784–788. doi: 10.1016/j.coi.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 139.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang JA, Mortazavi A, Williams BA, Wold BJ, Rothenberg EV. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell. 2012;149:467–482. doi: 10.1016/j.cell.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xiong Y, Bosselut R. CD4-CD8 differentiation in the thymus: connecting circuits and building memories. Current opinion in immunology. 2012;24:139–145. doi: 10.1016/j.coi.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ahyi AN, Chang HC, Dent AL, Nutt SL, Kaplan MH. IFN regulatory factor 4 regulates the expression of a subset of Th2 cytokines. J Immunol. 2009;183:1598–1606. doi: 10.4049/jimmunol.0803302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 145.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Glasmacher E, Agrawal S, Chang AB, Murphy TL, Zeng W, Vander Lugt B, et al. A Genomic Regulatory Element That Directs Assembly and Function of Immune-Specific AP-1-IRF Complexes. Science. 2012 doi: 10.1126/science.1228309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012;490:543–546. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, et al. A validated regulatory network for th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ho IC, Hodge MR, Rooney JW, Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- 150.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, et al. Hlx is induced by and genetically interacts with T-bet to promote heritable THI gene induction. Nature Immunology. 2002;3:652–658. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 152.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 153.Grenningloh R, Kang BY, Ho IC. Ets-1, a functional cofactor of T-bet, is essential for Th1 inflammatory responses. J Exp Med. 2005;201:615–626. doi: 10.1084/jem.20041330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 1999;18:420–432. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu J, Guo L, Min B, Watson CJ, Hu-Li J, Young HA, et al. Growth factor independent-1 induced by IL-4 regulates Th2 cell proliferation. Immunity. 2002;16:733–744. doi: 10.1016/s1074-7613(02)00317-5. [DOI] [PubMed] [Google Scholar]
- 156.Kashiwada M, Cassel SL, Colgan JD, Rothman PB. NFIL3/E4BP4 controls type 2 T helper cell cytokine expression. EMBO J. 2011;30:2071–2082. doi: 10.1038/emboj.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch'en IL, Stockmann C, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schuster M, Glauben R, Plaza-Sirvent C, Schreiber L, Annemann M, Floess S, et al. IkappaB(NS) Protein Mediates Regulatory T Cell Development via Induction of the Foxp3 Transcription Factor. Immunity. 2012;37:998–1008. doi: 10.1016/j.immuni.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 168.Crotty S. The 1-1-1 fallacy. Immunol Rev. 2012;247:133–142. doi: 10.1111/j.1600-065X.2012.01117.x. [DOI] [PubMed] [Google Scholar]
- 169.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 170.Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ramming A, Druzd D, Leipe J, Schulze-Koops H, Skapenko A. Maturation-related histone modifications in the PU.1 promoter regulate Th9-cell development. Blood. 2012;119:4665–4674. doi: 10.1182/blood-2011-11-392589. [DOI] [PubMed] [Google Scholar]
- 172.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 173.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annual Review of Immunology. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 174.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Naito T, Taniuchi I. Roles of repressive epigenetic machinery in lineage decision of T cells. Immunology. 2012 doi: 10.1111/imm.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 177.Zhang F, Boothby M. T helper type 1-specific Brg1 recruitment and remodeling of nucleosomes positioned at the IFN-gamma promoter are Stat4 dependent. Journal of Experimental Medicine. 2006;203:1493–1505. doi: 10.1084/jem.20060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422–433. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 179.Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, et al. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–U130. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yamashita M, Hirahara K, Shinnakasu R, Hosokawa H, Norikane S, Kimura MY, et al. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity. 2006;24:611–622. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 181.Onodera A, Yamashita M, Endo Y, Kuwahara M, Tofukuji S, Hosokawa H, et al. STAT6-mediated displacement of polycomb by trithorax complex establishes long-term maintenance of GATA3 expression in T helper type 2 cells. J Exp Med. 2010;207:2493–2506. doi: 10.1084/jem.20100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487:249–253. doi: 10.1038/nature11173. [DOI] [PubMed] [Google Scholar]
- 183.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 184.Makar KW, Pérez-Melgosa M, Shnyreva M, Weaver WM, Fitzpatrick DR, Wilson CB. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nature Immunology. 2003;4:1183–1190. doi: 10.1038/ni1004. [DOI] [PubMed] [Google Scholar]
- 185.Hutchins AS, Mullen AC, Lee HW, Sykes KJ, High FA, Hendrich BD, et al. Gene silencing quantitatively controls the function of a developmental trans-activator. Molecular Cell. 2002;10:81–91. doi: 10.1016/s1097-2765(02)00564-6. [DOI] [PubMed] [Google Scholar]
- 186.Vahedi G, Takahashi H, Nakayamada S, Sun HW, Sartorelli V, Kanno Y, et al. STATs Shape the Active Enhancer Landscape of T Cell Populations. Cell. 2012;151:981–993. doi: 10.1016/j.cell.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 188.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 190.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 191.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep. 2012;1:360–373. doi: 10.1016/j.celrep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 194.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–2303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Harismendy O, Notani D, Song X, Rahim NG, Tanasa B, Heintzman N, et al. 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signalling response. Nature. 2011;470:264–268. doi: 10.1038/nature09753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Patel DR, Richardson BC. Epigenetic mechanisms in lupus. Curr Opin Rheumatol. 2010;22:478–482. doi: 10.1097/BOR.0b013e32833ae915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ruiz-Esquide V, Sanmarti R. Tobacco and other environmental risk factors in rheumatoid arthritis. Reumatol Clin. 2012;8:342–350. doi: 10.1016/j.reuma.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 199.Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. 2010;126:453–465. doi: 10.1016/j.jaci.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.O’Shea JJ, Laurence A, McInnes IB. Back to the future: oral targeted therapy for RA and other autoimmune diseases. Nature Rev Rheum. 2013;9:173–182. doi: 10.1038/nrrheum.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]