Abstract
Context
The phenomenology of bipolar I disorder affects treatment and prognosis.
Objective
To describe the duration of bipolar I mood episodes and factors associated with recovery from these episodes.
Design
Subjects with Research Diagnostic Criteria bipolar I disorder were prospectively followed up for as long as 25 years. The probability of recovery over time from multiple successive mood episodes was examined with survival analytic techniques, including a mixed-effects grouped-time survival model.
Setting
Five US academic medical centers.
Participants
Two hundred nineteen subjects with bipolar I disorder.
Main Outcome Measures
Level of psychopathology was assessed with the Longitudinal Interval Follow-up Evaluation every 6 months for the first 5 years of follow-up and annually thereafter.
Results
The median duration of bipolar I mood episodes was 13 weeks. More than 75% of the subjects recovered from their mood episodes within 1 year of onset. The probability of recovery was significantly less for an episode with severe onset (psychosis or severe psychosocial impairment in week 1 of the episode) (hazard ratio [HR]=0.746; 95% confidence interval [CI], 0.578–0.963; P=.02) and for subjects with greater cumulative morbidity (total number of years spent ill with any mood episode) (HR=0.917; 95% CI, 0.886–0.948; P<.001). Compared with the probability of recovery from a major depressive episode, there was a significantly greater probability of recovery from an episode of mania (HR=1.713; 95% CI, 1.373–2.137; P<.001), hypomania (HR=4.502; 95% CI, 3.466–5.849; P<.001), or minor depression (HR = 2.027; 95% CI, 1.622–2.534; P<.001) and, conversely, a significantly reduced probability of recovery from a cycling episode (switching from one pole to the other without an intervening period of recovery) (HR=0.438; 95% CI, 0.351–0.548; P<.001).
Conclusions
The median duration of bipolar I mood episodes was 13 weeks, and the probability of recovery was significantly decreased for cycling episodes, mood episodes with severe onset, and subjects with greater cumulative morbidity.
Bipolar I disorder is usually characterized by recurrent mood episodes.1 It is important to follow up with subjects over many years to study the phenomenology of these episodes given that bipolar I disorder has a mean (SD) age at onset of 18.2 (11.6) years2 and the risk of having mood episodes remains relatively high for at least 40 years after onset.3
Another issue to consider is whether the data analytic procedures can use all of the relevant data rather than selected portions. Until recently, survival analysis has been the primary means of analyzing longitudinal data in psychiatry, particularly when examining the time to an event such as recovery from a mood episode.4 However, a limitation of hypothesis testing with standard survival analytic techniques is that they assume independence among observations and therefore can include only 1 mood episode per subject. As a consequence, these techniques preclude studying the effects of successive mood episodes. Rather, the investigator must arbitrarily select only 1 of many mood episodes, such as the most recent. This approach fails to characterize the course of an episodic and recurrent illness such as bipolar I disorder and limits the generalizability of the results.
In the last 30 years, statisticians have developed analytic methods that can examine correlated observations, such as multiple within-subject mood episodes, in one model. These statistical techniques account for the correlation among multiple mood episodes and at the same time allow the number of episodes per subject to vary widely, thus making it possible to estimate the cumulative effect of prior mood episodes and better understand the overall course of illness. Examples of these relatively new analytic methods include intensity analyses,5 frailty models,6 and mixed-effects grouped-time survival models.7
To our knowledge, only 1 prior study3 has used one of these new methods to analyze recovery from multiple, within-subject, prospectively observed bipolar I mood episodes. The median duration of all mood episodes was 4.2 months. No results were given for the average duration of the different types of mood episodes observed, which included major depression, minor depression, mania, hypomania, cycling, and mixed.
Our study addresses these 2 methodological issues and the need for additional studies by applying a mixed-effects grouped-time survival model to data from the ongoing National Institute of Mental Health Collaborative Program on the Psychobiology of Depression–Clinical Studies (Collaborative Depression Study). The Collaborative Depression Study began prospectively following subjects with bipolar I disorder in 1978,8 and up to 25 years of follow-up data are now available for analysis. The methodological strengths featured by this observational study include the use of direct subject interviews, standardized diagnostic and follow-up instruments, frequent follow-up assessments, careful characterization of the different types of mood episodes observed, and length of prospective follow-up.
Our results describe the duration of bipolar I mood episodes. Initially, time to recovery was estimated without regard to mood episode type, ie, the different types of episodes were analyzed collectively. Next, time to recovery was estimated for each type of mood episode, including major depression, minor depression, mania, hypomania, cycling, and mixed.
Finally, a mixed-effects model examined the magnitude of the association between hypothesized clinical predictors and the probability of recovery from a mood episode. The predictors included type of mood episode, severe onset of mood episode (psychosis or severe psychosocial impairment in week 1 of the episode), number of prior mood episodes, and cumulative morbidity (total number of years spent ill with any mood episode). These variables were selected for testing because they are characteristic of all bipolar I mood episodes and are routinely assessed during the initial clinical evaluation of patients.
METHODS
SUBJECTS
From 1978 to 1981, the Collaborative Depression Study recruited patients receiving treatment for mood episodes at academic medical centers in Boston, Massachusetts; Chicago, Illinois; Iowa City, Iowa; New York, New York; or St Louis, Missouri. Inclusion criteria included age of at least 17 years, IQ greater than 70, ability to speak English, white race (genetic hypotheses were tested), knowledge of one’s biological parents, and no evidence that the intake mood disorder was secondary to a general medical condition. The study was approved by the institutional review board at each study site, and subjects provided written informed consent after receiving a complete description of the study.
The sample for our study included 219 subjects who (1) met Research Diagnostic Criteria9 for a major mood episode at the time of enrollment, (2) were diagnosed at study intake or during prospective follow-up as having either bipolar I disorder or schizoaffective disorder, mainly affective subtype, (3) recovered from the mood episode present at study intake, and (4) eventually had at least 1 recurrent mood episode. (Research Diagnostic Criteria9 and DSM-IV criteria10 for bipolar I disorder are identical. Research Diagnostic Criteria9 for schizoaffective disorder, mainly affective subtype, are very similar to the DSM-IV criteria10 for bipolar I disorder. The inclusion of subjects with schizoaffective disorder in this study is consistent with other longitudinal studies of bipolar I disorder.3,11,12)
Of the 219 subjects, 156 (71%) were diagnosed at study intake as having bipolar I disorder, 25 (11%) were diagnosed at study intake as having unipolar major depressive disorder but subsequently had at least 1 episode of mania during prospective follow-up, 14 (6%) were diagnosed at study intake as having bipolar II disorder but subsequently had at least 1 episode of mania during prospective follow-up, and 24 (11%) were diagnosed at study intake as having schizoaffective disorder, mainly affective subtype.
ASSESSMENTS AND PROCEDURES
At study intake, raters interviewed subjects about their current and past psychiatric history using the Schedule for Affective Disorders and Schizophrenia.13 Raters also reviewed medical records and, whenever feasible, interviewed other informants. Diagnoses were then made according to Research Diagnostic Criteria.9
After study intake, raters assessed the level of psychopathology through direct interviews conducted every 6 months for the first 5 years of the study and annually thereafter using variations of the semi-structured Longitudinal Interval Follow-up Evaluation.14 Subjects were prospectively followed up for as long as 25 years.
At each assessment, the interviewer rated the weekly level of psychopathology for each mood syndrome that occurred since the time of the last interview and assigned a separate weekly score for each mood syndrome. To accomplish this, the rater first identified chronological anchor points such as holidays to assist the subject in remembering those times when significant clinical improvement or deterioration occurred. Whenever possible, corroborative data were obtained from medical records.
Level of psychopathology for mania or major depression was quantified on a 6-point scale in which a rating of 1 corresponded to no symptoms and 6 indicated meeting full criteria for the disorder along with psychosis or extreme impairment in functioning. For minor depression or hypomania, level of psychopathology was quantified on a 3-point scale (fewer symptoms were required to meet criteria for these disorders).
Consistent with Research Diagnostic Criteria,9 recovery from a mood episode was defined as at least 8 consecutive weeks either with no symptoms of major depression, minor depression, mania, and hypomania or with only 1 or 2 symptoms of a mild degree and no impairment of psychosocial functioning. During a mood episode, the weekly level of psychopathology may have varied anywhere between no symptoms to meeting full criteria. For example, a major depressive episode may have included periods of euthymia (lasting <8 consecutive weeks) or minor depression in addition to those weeks in which the subject met full criteria. Similarly, a manic episode may have included periods of euthymia (lasting <8 consecutive weeks) or hypomania.
An episode of minor depression was distinguished from partial remission of major depression in that the symptoms of a minor depressive episode never rose to the level of major depression from onset to offset of the minor depressive episode. If at any point during a minor depressive episode the subject met criteria for major depression, the entire episode was considered to be one of major depression. Hypomania was distinguished from partial remission of mania in a similar manner.
Recurrence or onset of a new mood episode was defined as the reappearance of major depression meeting full criteria for at least 2 consecutive weeks, mania meeting full criteria for at least 1 week, minor depression at the definite level for at least 2 consecutive weeks, or hypomania at the definite level for at least 1 week. Recurrence of a mood episode occurred only after the individual had first recovered from the preceding mood episode.
TYPES OF MOOD EPISODES
Mood episodes were classified empirically within 1 of 8 different categories, beginning with the categories of major depression, minor depression, mania, hypomania, and mixed episode. Minor depression was defined as depressed mood accompanied by 2 or more other symptoms, without psychosis or the full depressive syndrome that characterizes major depression, for at least 2 weeks. A mixed episode was defined as major depression or minor depression concurrent with mania or hypomania throughout the entire episode, with at least 1 major pole (major depression or mania) present at some point during the episode.
In addition, we empirically defined 3 categories of cycling mood episodes because of their prevalence in this study and because of their prevalence and prognostic significance in previous studies.15–27 Major cycling was defined as alternating periods of depression (major depression or minor depression) and mood elevation (mania or hypomania), immediately contiguous with each other or separated by less than 8 consecutive weeks with euthymia. In addition, at least 1 major pole (major depression or mania) was present at some point during the episode. Mixed major cycling was defined as an episode of major cycling that at some point also included a mixed state, ie, a period with concurrent depression and mood elevation. (These mixed states were distinguished from and not synonymous with the mixed episodes defined earlier). Minor cycling was defined as alternating periods of hypomania and minor depression, immediately contiguous with each other or separated by less than 8 consecutive weeks with euthymia.
TREATMENT
The Collaborative Depression Study is an observational study in that treatment is not assigned by design and not controlled by anyone connected with the study. Over time, the intensity of treatment varied within subjects as well as between subjects. The type and dose of all prescribed somatic treatment were collected with the Longitudinal Interval Follow-up Evaluation14 and corroborated with available medical records. Our results describe the somatic treatment subjects received during episodes of major depression and mania.
DATA ANALYTIC PROCEDURES
The Kaplan-Meier product-limit method28 estimated the cumulative probability of recovery over time from each of the first 5 mood episodes per subject that began after recovery from the intake mood episode. The observations within each of the 5 Kaplan-Meier analyses were independent because each subject had only 1 of each successive episode number (eg, first episode). Based on the small number of mood episodes beyond the fifth prospective recurrence, especially at longer follow-up periods, we chose to not analyze episodes beyond the first 5 mood episodes. The intake mood episode was also excluded from these analyses because it was previously described elsewhere19 and was not entirely prospective (subjects enrolled in the Collaborative Depression Study after onset of the intake mood episode). Thus, all mood episodes included in these analyses were observed prospectively from the time they commenced.
Survival time (duration of mood episode) was defined as the number of weeks until recovery from the episode, beginning with the first week of the episode. The survival analyses estimated the cumulative probability of recovery over the course of follow-up. The survival time ended with recovery from the mood episode or, for censored cases, the end of the follow-up period (25 years), withdrawal from the study, or death. In estimating the rate of recovery, the analyses made use of all available data from all subjects, including the incomplete information from censored cases. These analyses also accounted for the varying lengths of follow-up for different subjects.
A mixed-effects grouped-time survival model29 estimated the magnitude of the association between various clinical predictors and the probability of recovery over time. The predictors included mood episode type (described earlier), severe onset of mood episode, number of prior mood episodes, and cumulative morbidity. Severe onset of mood episode was defined such that in week 1 of the episode, the subject met full criteria for major depression or mania along with psychosis or extreme impairment in functioning. Number of prior mood episodes included only those episodes observed during prospective follow-up, beginning with the intake mood episode and ending with the mood episode immediately prior to the episode that was analyzed. Cumulative morbidity was defined as the total number of years spent ill with any type of mood episode during prospective follow-up, beginning at study intake and ending at the week prior to onset of the mood episode that was analyzed.
The mixed-effects model accounted for the correlation among multiple, within-subject mood episodes. In this grouped-time survival model, the mood episode durations were categorized as follows: 1 to 2, 3 to 4, 5 to 13, 14 to 26, 27 to 39, 40 to 52, 53 to 104, and longer than 104 weeks. It was implicitly assumed that the hazard (likelihood of recovery) was constant within any one categorized time interval. The mixed model also calculated an intraclass correlation coefficient, which estimated the within-subject consistency in duration of mood episodes, across multiple episodes (without regard to mood episode type). Each statistical test used a 2-tailed α level of .05.
RESULTS
SUBJECTS AND LENGTH OF FOLLOW-UP
Table 1 displays the sociodemographic and clinical characteristics of the sample at study intake. The mean (SD) length of prospective follow-up was 17.3 (7.9) years, and the median (range) was 20 (0.5–25) years. Of the 219 subjects, 196 (90%) were followed up for at least 5 years, 169 (77%) for at least 10 years, 144 (66%) for at least 15 years, and 122 (56%) for at least 20 years. Attrition resulted from withdrawal of consent, an inability to locate or contact the subject, and death.
Table 1.
Sociodemographic and Clinical Characteristics at Study Intake for 219 Subjects With Bipolar I Disorder
| Characteristic | Value |
|---|---|
| Age, mean (SD), y | 37 (13) |
| Sex, No. (%) | |
| Male | 97 (44) |
| Female | 122 (56) |
| Marital status, No. (%) | |
| Never married | 84 (38) |
| Married or live together | 85 (39) |
| Divorced, separated, or widowed | 50 (23) |
| Socioeconomic status, No. (%)a | |
| I | 8 (4) |
| II | 32 (15) |
| III | 69 (32) |
| IV | 63 (29) |
| V | 47 (21) |
| Intake medical center, No. (%) | |
| Boston, Massachusetts | 34 (16) |
| Chicago, Illinois | 43 (20) |
| Iowa City, Iowa | 62 (28) |
| New York, New York | 31 (14) |
| St Louis, Missouri | 49 (22) |
| Status at intake, No. (%) | |
| Inpatient | 196 (89) |
| Outpatient | 23 (11) |
| Polarity of mood state at study intake, No. (%) | |
| Mania | 142 (65) |
| Major depression | 62 (28) |
| Mixed | 15 (7) |
| Psychosis, No. (%) | |
| Present | 121 (55) |
| Absent | 98 (45) |
| Global Assessment Scale score, mean (SD)b | 32 (11) |
| Age at onset for first lifetime mood episode, mean (SD), yc | 24 (10) |
| No. of mood episodes prior to intake episode, No. (%) | |
| 0 | 27 (12) |
| 1 | 20 (9) |
| 2 | 26 (12) |
| ≥3 | 146 (67) |
Based on the Hollingshead-Redlich scale, with I indicating the highest socioeconomic status and V indicating the lowest.30 The percentages do not sum to 100 because of rounding.
The range for the Global Assessment Scale is 1 to 100; higher scores indicate less psychopathology and better psychosocial functioning.31
Major depression, minor depression, mania, or hypomania.
DURATION OF MOOD EPISODES ANALYZED WITHOUT REGARD TO MOOD EPISODE TYPE
A total of 1208 mood episodes were observed during follow-up. The median number of mood episodes per subject was 4 (range, 1–21). The mean (SD) number of episodes per subject was 5.5 (4.6), and the mean (SD) number of episodes per subject per year of follow-up was 0.4 (0.3). The mean (SD) percentage of follow-up time spent ill with a mood episode was 31% (27%), and the median was 23%.
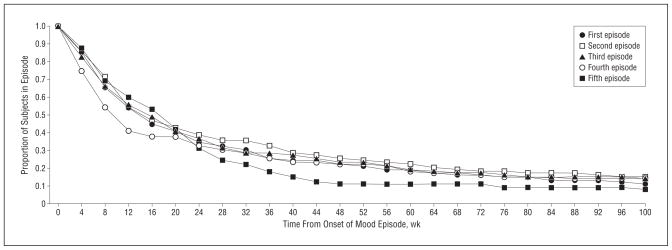
Initially, we examined time to recovery without regard to mood episode type, ie, the different types of episodes were analyzed collectively. Table 2 displays the proportion of subjects recovering from each of the first 5 successive recurrent mood episodes. These first 5 episodes comprised 768 of the 1208 mood episodes (64%). There were 219 subjects who had at least 1 recurrent mood episode. Based on Kaplan-Meier estimates, 89% recovered from the first recurrent episode within 2 years of onset of the episode. Of the 181 subjects with a second recurrent episode, 85% recovered from that episode within 2 years. Of those with a third or fourth recurrent episode, 87% recovered within 2 years. The Figure shows the corresponding survival curves for the duration of the first 5 recurrent mood episodes based on cumulative recovery probabilities (Kaplan-Meier estimates). The 5 curves are similar, indicating that time to recovery in the sample as a whole was consistent across multiple mood episodes.
Table 2.
Time to Recovery From Successive Prospectively Observed Bipolar I Mood Episodesa
| Mood Episode No. | Subjects With Mood Episode, No. | Elapsed Time From Onset of Mood Episode and Proportion of Subjects Who Recovered (95% CI)b,c
|
|||||
|---|---|---|---|---|---|---|---|
| 1 mo | 3 mo | 6 mo | 1 y | 2 y | 5 y | ||
| 1 | 219 | 0.150 (0.072–0.299) | 0.470 (0.332–0.632) | 0.670 (0.524–0.809) | 0.790 (0.652–0.901) | 0.890 (0.765–0.965) | 0.950 (0.846–0.992) |
| 2 | 181 | 0.150 (0.066–0.320) | 0.480 (0.328–0.659) | 0.630 (0.472–0.787) | 0.760 (0.607–0.887) | 0.850 (0.707–0.947) | 0.900 (0.770–0.973) |
| 3 | 146 | 0.180 (0.078–0.385) | 0.450 (0.286–0.653) | 0.650 (0.474–0.820) | 0.770 (0.599–0.906) | 0.870 (0.710–0.965) | 0.950 (0.807–0.996) |
| 4 | 120 | 0.260 (0.126–0.491) | 0.590 (0.400–0.789) | 0.700 (0.510–0.869) | 0.780 (0.594–0.921) | 0.870 (0.702–0.968) | 0.910 (0.745–0.986) |
| 5 | 102 | 0.120 (0.035–0.369) | 0.410 (0.229–0.657) | 0.710 (0.500–0.890) | 0.900 (0.718–0.985) | NAd | NAd |
Abbreviations: CI, confidence interval; NA, not applicable.
Recovery from a mood episode was defined according to Research Diagnostic Criteria,9 which require at least 8 consecutive weeks either with no symptoms of major depression, minor depression, mania, and hypomania or with only 1 or 2 symptoms of a mild degree and no impairment of psychosocial functioning.
Proportions were derived from Kaplan-Meier product-limit estimates.
Of 219 subjects with 1 prospectively observed mood episode, 79% (95% CI, 65%–90%) recovered within 1 year. Of 181 subjects with a second prospectively observed mood episode, 76% (95% CI, 61%–89%) recovered from that episode within 1 year. Of 146 subjects with a third prospectively observed mood episode, 77% (95% CI, 60%–91%) recovered from that episode within 1 year. Of 120 subjects with a fourth prospectively observed mood episode, 78% (95% CI, 59%–92%) recovered from that episode within 1 year. Of 102 subjects with a fifth prospectively observed mood episode, 90% (95% CI, 72%–99%) recovered from that episode within 1 year.
Estimates were not calculated because of limited numbers of subjects at risk for recovery.
Figure.
Time to recovery from the first 5 prospectively observed mood episodes. The survival curves depict the duration of the first 5 prospectively observed mood episodes and are based on cumulative recovery probabilities (Kaplan-Meier estimates). The 5 curves are similar, indicating that time to recovery in the sample as a whole was consistent across multiple mood episodes.
The quartiles for duration of mood episode for each of the first 5 prospectively observed mood episodes were also examined. Overall, across the entire set of these first 5 mood episodes, 25% of the subjects recovered within 5 weeks of onset of the episode (ie, the first quartile), 50% of the subjects recovered within 13 weeks of onset (ie, the median), and 75% recovered within 38 weeks (ie, the third quartile).
DURATION OF EACH TYPE OF MOOD EPISODE
Another set of analyses examined time to recovery from each type of bipolar I mood episode. Table 3 shows the quartiles for the duration of the different types of mood episodes. The median duration of major depressive episodes, the most common type, was 15.0 weeks (SE, 1.1 weeks). Recovery from 75% of the major depressive episodes occurred within 35.0 weeks (SE, 3.7 weeks) of onset of the episode.
Table 3.
Time to Recovery From Each Type of Bipolar I Mood Episodea
| Mood Episode Type | Episodes, No. (%) | Time Until Recovery (SE), wk
|
||
|---|---|---|---|---|
| First Quartileb | Second Quartileb | Third Quartileb | ||
| Major depression | 373 (31) | 6.0 (0.6) | 15.0 (1.1) | 35.0 (3.7) |
| Minor depression | 157 (13) | 3.0 (0.3) | 7.0 (0.8) | 15.0 (2.0) |
| Mania | 246 (20) | 4.0 (0.3) | 7.0 (0.7) | 15.0 (2.0) |
| Hypomania | 126 (10) | 2.0 (0.2) | 3.0 (0.4) | 6.0 (0.9) |
| Major cycling | 182 (15) | 21.0 (1.4) | 42.0 (4.2) | 111.0 (23.5) |
| Mixed major cycling | 94 (8) | 25.0 (4.6) | 61.0 (19.9) | 368.0 (104.7) |
| Minor cycling | 28 (2) | 9.0 (1.4) | 13.0 (4.9) | 35.0 (20.7) |
| Mixed | 2 (<1) | 7.0c | 7.0c | 76.0c |
| Total | 1208 (100) | |||
Consistent with Research Diagnostic Criteria,9 recovery from a mood episode was defined as at least 8 consecutive weeks either with no symptoms of major depression, minor depression, mania, and hypomania or with only 1 or 2 symptoms of a mild degree and no impairment of psychosocial functioning.
The first quartile indicates 25% of episodes; the second quartile, 50% of episodes; and the third quartile, 75% of episodes. As an example, there were 373 prospectively observed episodes of bipolar I major depression. Recovery from 25% of these episodes occurred within 6.0 (SE, 0.6) weeks of onset, recovery from 50% occurred within 15.0 (SE, 1.1) weeks, and recovery from 75% occurred within 35.0 (SE, 3.7) weeks.
The sample sizes are too small to estimate the SEs.
The median duration of major cycling episodes was approximately 3 to 14 times longer than that of episodes of pure depression or pure mood elevation, and the median duration of mixed major cycling episodes was approximately 4 to 20 times longer. One-fourth of the mixed major cycling episodes lasted more than 7 years (368.0 weeks [SE, 104.7 weeks]).
CYCLING MOOD EPISODES
The 304 cycling mood episodes (182 major cycling, 94 mixed major cycling, and 28 minor cycling) were composed of 4 possible component mood states: depression (major or minor depression), mood elevation (mania or hypomania), mixed state (concurrent depression and mood elevation), and euthymia lasting less than 8 consecutive weeks. During these cycling episodes, the mean (SD) durations were 84 (160) weeks (median, 23 weeks) for depression, 28 (76) weeks (median, 7 weeks) for mood elevation, 8 (52) weeks (median, 0 weeks) for mixed states (ie, more than half the cycling episodes did not include a mixed state), and 11 (44) weeks (median, 2 weeks) for euthymia.
PROBABILITY OF RECOVERY OVER TIME
The association between the hypothesized predictors and the probability of recovery from a mood episode was analyzed with a mixed-effects grouped-time survival model. This analysis included 1178 of the 1208 prospectively observed mood episodes (98%) (the 2 mixed episodes and 28 minor cycling episodes were not included because of small sample sizes).
Table 4 shows that the probability of recovery from a mood episode with severe onset (major depression or mania in week 1 of the episode, along with psychosis or extreme impairment in functioning) was about 25% smaller than that for an episode with less severe onset (hazard ratio [HR]=0.746; 95% confidence interval [CI], 0.578–0.963). Cumulative morbidity (total number of years spent ill with any mood episode during prospective follow-up) was also associated with a significantly decreased probability of recovery, such that with each additional year of illness, the likelihood of recovery from the current mood episode was reduced by 8% (HR=0.917; 95% CI, 0.886–0.948).
Table 4.
Association Between Clinical Predictors and the Probability of Recovery From Bipolar I Mood Episodesa
| Predictor Variable | HR (95% CI) | Z Score | P Value |
|---|---|---|---|
| No. of prior mood episodesb | 1.005 (0.979–1.031) | 0.351 | .72 |
| Severe onset of mood episodec | 0.746 (0.578–0.963) | −2.250 | .02 |
| Cumulative morbidityd | 0.917 (0.886–0.948) | −5.058 | <.001 |
| Mood episode typee | |||
| Minor depression | 2.027 (1.622–2.534) | 6.205 | <.001 |
| Mania | 1.713 (1.373–2.137) | 4.767 | <.001 |
| Hypomania | 4.502 (3.466–5.849) | 11.270 | <.001 |
| Major cycling | 0.438 (0.351–0.548) | −7.250 | <.001 |
| Mixed major cycling | 0.335 (0.251–0.446) | −7.459 | <.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Results are derived from mixed-effects grouped-time survival analysis. Each predictor except number of prior mood episodes was significantly associated with the probability of recovery.
Included only those episodes observed during prospective follow-up, beginning with the intake mood episode and ending with the mood episode immediately prior to the episode that was analyzed.
Defined such that in week 1 of the episode, the subject met the full criteria for major depression or mania, along with psychosis or extreme impairment in functioning. The probability of recovery from an episode with severe onset was 25% less than that for an episode with less severe onset.
Defined as the total number of years spent ill with any type of mood episode during prospective follow-up, beginning at study intake and ending at the week prior to onset of the mood episode that was analyzed. For each additional year that a subject had affective illness, the probability of recovery from the current episode was reduced by 8%.
The probability of recovery from each mood episode type was compared with the probability of recovery from an episode of major depression. Recovery from an episode of minor depression was 2 times more likely to occur than recovery from an episode of major depression, recovery from hypomania was 4.5 times more likely, and recovery from mania was 71% more likely. Recovery from an episode of major cycling was reduced by 56% relative to recovery from an episode of major depression, and recovery from mixed major cycling was reduced by 67%.
In addition, mood episode type was significantly associated with the probability of recovery. Compared with an episode of major depression, the probability of recovery from minor depression was 2 times greater (HR=2.027; 95% CI, 1.622–2.534), from mania was 71% greater (HR=1.713; 95% CI, 1.373–2.137), and from hypomania was more than 4 times greater (HR=4.502; 95% CI, 3.466–5.849). Conversely, the probability of recovery from an episode of major cycling was reduced by 56% relative to an episode of major depression (HR=0.438; 95% CI, 0.351–0.548), and recovery from an episode of mixed major cycling was reduced by 67% (HR=0.335; 95% CI, 0.251–0.446).
The mixed-effects model examined within-subject variability in time to recovery from one mood episode to the next (without regard to mood episode type). The model yielded an intraclass correlation coefficient of 0.124, meaning that for each subject with 2 or more prospectively observed mood episodes, the duration of these multiple episodes was inconsistent.
TREATMENT
We examined the number of major depressive episodes and manic episodes that were treated with somatic therapy for at least 4 consecutive weeks or, in the case of episodes lasting less than 4 weeks, those that were treated for the entire duration of the episode. For subjects with a study intake diagnosis of (1) unipolar major depressive disorder, (2) schizoaffective disorder, major depression, or (3) bipolar II disorder, the treatment analyses did not include mood episodes that occurred prior to the first prospectively observed episode of mania.
During 326 episodes of major depression, 179 (55%) were treated with at least 1 mood stabilizer (aripiprazole, carbamazepine, clozapine, lamotrigine, lithium carbonate, olanzapine, oxcarbazepine, quetiapine fumarate, risperidone, valproate sodium, or ziprasidone hydrochloride), 111 (34%) were treated with at least 1 mood stabilizer plus an antidepressant, and 89 (27%) were treated with an antidepressant in the absence of a mood stabilizer. During 246 manic episodes, 181 (74%) were treated with at least 1 mood stabilizer.
COMMENT
The results describe the duration of bipolar I mood episodes and factors significantly associated with the probability of recovery from a mood episode.
CLINICAL IMPLICATIONS
The mixed-effects model (Table 4) provided a number of clinically relevant results. First, the probability of recovery from a mood episode with severe onset was significantly decreased compared with the probability of recovery from a mood episode with less severe onset. This finding raises the possibility that mood episodes with severe onset may be more difficult to treat. Future treatment studies or secondary analyses of archival randomized controlled trial data should examine whether severe onset moderates recovery from mood episodes.
The mixed-effects model and other survival analyses (Table 3) also demonstrated that there are clinically meaningful and statistically significant differences in the probability of recovery from different types of mood episodes. In particular, major cycling and mixed major cycling episodes were much longer than other types of mood episodes, consistent with previous studies showing that cycling episodes are associated with poorer outcomes compared with episodes of pure major depression or pure mania.25 The observed prevalence and poorer prognosis of cycling episodes help validate the concept of such episodes. Thus, we encourage the work groups revising the DSM-IV10 and the International Statistical Classification of Diseases and Related Health Problems, 10th revision32 to add cycling as a subtype for bipolar I mood episodes.
The low intraclass correlation coefficient of 0.124 is consistent with many different clinical situations. For example, the intraclass correlation coefficient will be low if mood episode duration progressively decreases over time or if mood episodes are initially shorter, then longer, then shorter again. In addition, it will be low if mood episode duration progressively increases over time. The kindling model, which predicts among other things that mood episode duration will increase from one episode to the next,33 is thus one of many competing explanations for the low intraclass correlation coefficient. This raises the possibility that there is a subgroup of subjects with kindling that can be identified and clinically characterized.
COURSE OF ILLNESS AND PREVIOUS STUDIES
The Figure shows that the rate of recovery was fairly consistent across multiple episodes. Similar results were previously obtained in a case-register study that used hospitalization as a proxy for mood episodes.34
Subjects were affectively ill for 31% of the follow-up time. Previous analyses of Collaborative Depression Study subjects with bipolar I disorder found that they were affectively ill for a mean of 47% of follow-up.35 The primary reason for this discrepancy is that the 2 studies handled subsyndromal symptoms differently. Subsyndromal symptoms were defined as 1 or 2 symptoms of a mild degree and no impairment of psychosocial functioning. The previous study35 counted a week with sub-syndromal symptoms as a week in which the subject was affectively ill, whereas the present study did not (consistent with the procedures specified by Research Diagnostic Criteria9).
At study intake, the mood state was mania for 65% of the subjects and mixed in another 7% (Table 1). This may have yielded a sample that was predisposed to have fewer depressive episodes during follow-up compared with the number of depressive episodes that occur in the general bipolar I population. Of the 1208 prospectively observed mood episodes, 44% (Table 3) were depressive (major or minor depression). This is considerably less than what was observed in the Systematic Treatment Enhancement Program for Bipolar Disorder, which found that 72% of recurrences were depressive.36 In contrast, however, the Stanley Foundation Bipolar Network found that the mean number of manic recurrences actually exceeded the mean number of depressive recurrences,26 and another group found that the number of manic and depressive recurrences were approximately equal.37 In addition, the 44% figure from our study is comparable to the Zurich study,16 which found that depressive mood episodes composed 51% of the mood episodes observed in 26 years of follow-up.
During prospective follow-up lasting up to 25 years, the mean number of mood episodes per subject in our study was 5.5. In evaluating this number, it is worth noting certain aspects of the methods. First, the definition of recovery from a mood episode was relatively rigorous: at least 8 consecutive weeks with 2 or fewer mood symptoms of a mild degree and no impairment in psychosocial functioning. Second, alternating syndromes of depression and mood elevation separated by less than 8 consecutive weeks with euthymia were counted as a single cycling mood episode. In other studies, these alternating syndromes are usually counted as separate mood episodes regardless of how little time, if any, elapses between the offset of one syndrome and the onset of the next (consistent with the DSM-IV10 and the International Statistical Classification of Diseases and Related Health Problems, 10th revision32). The third issue is subject selection. Some studies recruit patients from bipolar disorder specialty clinics and may thus enroll subjects who are more resistant to maintenance treatment and therefore more vulnerable to recurrences. By contrast, the Collaborative Depression Study is strictly an observational study—treatment is not controlled by anyone associated with the study and subjects are free to pursue treatment anywhere or to forego treatment.
LIMITATIONS
One limitation is that there may be other types of mood episodes beyond the ones described in this article, eg, atypical depression. Another limitation is the absence of subjects with bipolar II disorder, who may be quite vulnerable to mixed states. In addition, some subjects were recruited closer to onset of the illness than others. Generalizability is limited in that 89% of the subjects were inpatients at academic medical centers at study intake. Also, the time that elapsed between assessments (initially 6 months and subsequently 12 months) may have introduced recall bias and limited the accuracy of the results.
The diagnosis of hypomania required a minimal duration of 1 week. This contrasts with the DSM-IV,10 in which the minimal duration is 4 days. More so, there is evidence that an adequate threshold is 2 days.38 Thus, our study may have missed several episodes of hypomania.
Another limitation is that treatment was not controlled and may not have been optimal. For example, contrary to treatment guidelines,39,40 27% of the bipolar I major depressive episodes were treated with an antidepressant in the absence of a mood stabilizer. However, this finding is very similar to what was observed in a nationally representative sample of outpatients with bipolar disorder.41
Yet another limitation of this longitudinal study is attrition, which could have influenced some of the findings. However, mixed-effects models yield valid inferences assuming ignorable attrition.42 The mixed-effects model that examined clinical factors associated with recovery from mood episodes did not include chronic or episodic life stressors. New studies seeking to further specify the factors associated with recovery should consider measuring such stressors.
CONCLUSIONS
The median duration of bipolar I mood episodes was 13 weeks, and more than 75% of subjects recovered from their episodes within 1 year of onset. Factors associated with a significantly decreased probability of recovery from a mood episode included severe onset of the mood episode, cycling from one pole to the other (with no intervening period of recovery), and greater cumulative morbidity. Future analyses will examine risk factors for recurrence of bipolar I mood episodes and will use propensity score analyses to study the effectiveness of treatment.
Acknowledgments
Funding/Support: This study was supported by grant MH25478-29A2 from the National Institute of Mental Health.
Role of the Sponsor: The National Institute of Mental Health had no role in the design or conduct of this study; in the collection, management, analysis, and interpretation of the data; and in the preparation, review, or approval of the manuscript.
Footnotes
Deceased.
Author Contributions: All authors had full access to all of the data in the study. Dr Solomon takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Information: The manuscript was reviewed by the Publication Committee of the Collaborative Depression Study and has its endorsement. The data for this article came from the National Institute of Mental Health Collaborative Program on the Psychobiology of Depression–Clinical Studies. The Collaborative Program was initiated in 1975 to investigate nosologic, genetic, family, prognostic, and psychosocial issues of mood disorders and is an ongoing, long-term, multidisciplinary investigation of the course of mood and related affective disorders. The original principal and coprincipal investigators were from 5 academic centers and included Gerald Klerman, MD† (co-chairperson), Martin B. Keller, MD, and Robert Shapiro, MD† (Massachusetts General Hospital, Harvard Medical School, Boston); Eli Robbins, MD,† Paula Clayton, MD, Theodore Reich, MD,† and Amos Wellner, MD† (Washington University Medical School, St Louis, Missouri); Jean Endicott, PhD, and Robert Spitzer, MD (Columbia University, New York, New York); Nancy Andreasen, MD, PhD, William Coryell, MD, and George Winokur, MD† (University of Iowa, Iowa City); and Jan Fawcett, MD, and William Scheftner, MD (Rush-Presbyterian-St Luke’s Medical Center, Chicago, Illinois). The National Institute of Mental Health Clinical Research Branch was an active collaborator in the origin and development of the Collaborative Program with Martin M. Katz, PhD, branch chief, as the co-chairperson and Robert Hirschfeld, MD, as the program coordinator. Other past collaborators include Jack Croughan, MD, M. Tracie Shea, PhD, Robert D. Gibbons, PhD, Michael A. Young, PhD, and David C. Clark, PhD.
Additional Contributions: This study was conducted with the current participation of the following investigators: Martin B. Keller, MD (chairperson), Providence, Rhode Island; William H. Coryell, MD (co-chairperson), Iowa City, Iowa; David A. Solomon, MD, Providence; William Scheftner, MD, Chicago, Illinois; Jean Endicott, PhD, Andrew C. Leon, PhD, and Joellen Loth, MSW, New York, New York; and John Rice, PhD, St Louis, Missouri. Other current contributors include Hagop S. Akiskal, MD, Jan Fawcett, MD, Lewis L. Judd, MD, Philip W. Lavori, PhD, Jack D. Maser, PhD, and Timothy I. Mueller, MD.
Financial Disclosure: Dr Solomon has served as an investigator for research funded by the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, Janssen, Merck, and Wyeth-Ayerst; as a consultant to Novartis, Shire, and Solvay Pharmaceuticals; and on the lecture bureaus of AstraZeneca, GlaxoSmithKline, Pfizer, and Shire. Dr Leon has served as an investigator for research funded by the National Institute of Mental Health and the National Institute on Drug Abuse; is on data safety monitoring boards for Dainippon Sumitomo Pharma America, Pfizer, Neuronetics, Organon, and Vanda; and has recently served as a consultant to the US Food and Drug Administration, the National Institute of Mental Health, Avera, Best Practices, Cadence, Concordant Raters, Cortex Pharmaceuticals, Cyberonics, Eli Lilly, MedAvante, and Noven. Dr Endicott has been an investigator for research funded by 4 of the National Institutes of Health and the New York State Department of Mental Health; has received research support from Abbott, Bristol-Myers Squibb, Cyberonics, Interneuron, Merck, Parke-Davis, Pfizer, Upjohn, and Wyeth-Ayerst; and has served as a consultant or advisory board member for Abbott, AstraZeneca, Bayer Schering, Berlex, Bristol-Myers Squibb, Cyberonics, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Otsuka, Ovation, Pfizer, Sanofi-Synthélabo, and Wyeth-Ayerst. Dr Fiedorowicz has served as an investigator for research funded by Eli Lilly. Dr Keller has received consulting fees or honoraria from Abbott, Bristol-Myers Squibb, Cenerex, Cephalon, Collegium, Cyberonics, Cypress Bioscience, Eli Lilly, Forest Laboratories, Janssen, JDS, Medtronic, Merck, Organon, Otsuka, Novartis, Pfizer, Pharmacia, Pharmastar, Roche, Sepracor, Sierra Pharmaceuticals, Solvay, Vela Pharmaceuticals, and Wyeth-Ayerst; has received grants or research support from Eli Lilly, Forest Laboratories, Organon, Pfizer, and Wyeth-Ayerst; and has served on advisory boards for Abbott, Bristol-Myers Squibb, Cenerex, Cephalon, Cyberonics, Cypress Bioscience, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Janssen, Merck, Mitsubishi Pharma, Neuronetics, Novartis, Organon, Pfizer, Sanofi-Synthélabo, Scirex, Sepracor, Somerset Pharmaceuticals, Vela Pharmaceuticals, and Wyeth-Ayerst.
References
- 1.Zis AP, Goodwin FK. Major affective disorder as a recurrent illness: a critical review. Arch Gen Psychiatry. 1979;36(8 spec No):835–839. doi: 10.1001/archpsyc.1979.01780080009002. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angst J, Gamma A, Sellaro R, Lavori PW, Zhang H. Recurrence of bipolar disorders and major depression: a life-long perspective. Eur Arch Psychiatry Clin Neurosci. 2003;253(5):236–240. doi: 10.1007/s00406-003-0437-2. [DOI] [PubMed] [Google Scholar]
- 4.Leon AC, Friedman RA, Sweeney JA, Brown RP, Mann JJ. Statistical issues in the identification of risk factors for suicidal behavior: the application of survival analysis. Psychiatry Res. 1990;31(1):99–108. doi: 10.1016/0165-1781(90)90112-i. [DOI] [PubMed] [Google Scholar]
- 5.Aalen OO, Borgan O, Keiding N, Thormann J. Interaction between life history events: nonparametric analysis for prospective and retrospective data in the presence of censoring. Scand J Stat. 1980;7:161–171. [Google Scholar]
- 6.Kessing LV, Hansen MG, Andersen PK. Course of illness in depressive and bipolar disorders: naturalistic study, 1994–1999. Br J Psychiatry. 2004;185:372–377. doi: 10.1192/bjp.185.5.372. [DOI] [PubMed] [Google Scholar]
- 7.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2(1):64–78. [Google Scholar]
- 8.Katz MM, Secunda SK, Hirschfeld RMA, Koslow SH. NIMH Clinical Research Branch Collaborative Program on the Psychobiology of Depression. Arch Gen Psychiatry. 1979;36(7):765–771. doi: 10.1001/archpsyc.1979.01780070043004. [DOI] [PubMed] [Google Scholar]
- 9.Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35(6):773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 11.Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Biol Psychiatry. 2003;53(11):1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 12.Nolen WA, Luckenbaugh DA, Altshuler LL, Suppes T, McElroy SL, Frye MA, Kupka RW, Keck PE, Jr, Leverich GS, Post RM. Correlates of 1-year prospective outcome in bipolar disorder: results from the Stanley Foundation Bipolar Network. Am J Psychiatry. 2004;161(8):1447–1454. doi: 10.1176/appi.ajp.161.8.1447. [DOI] [PubMed] [Google Scholar]
- 13.Endicott J, Spitzer RL. A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry. 1978;35(7):837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 14.Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcomes in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 15.Kraepelin E. In: Manic-Depressive Insanity and Paranoia. Robertson GM, editor; Barclay RM, translator. Vol. 115. Edinburgh, Scotland: Livingstone; 1921. pp. 135–136. [Google Scholar]
- 16.Angst J. The course of affective disorders, II: typology of bipolar manic-depressive illness. Arch Psychiatr Nervenkr. 1978;226(1):65–73. doi: 10.1007/BF00344125. [DOI] [PubMed] [Google Scholar]
- 17.Kukopulos A, Reginaldi D, Laddomada P, Floris G, Serra G, Tondo L. Course of the manic-depressive cycle and changes caused by treatments. Pharmakopsychiatr Neuropsychopharmakol. 1980;13(4):156–167. doi: 10.1055/s-2007-1019628. [DOI] [PubMed] [Google Scholar]
- 18.Roy-Byrne P, Post RM, Uhde TW, Porcu T, Davis D. The longitudinal course of recurrent affective illness: life chart data from research patients at the NIMH. Acta Psychiatr Scand Suppl. 1985;317:1–34. doi: 10.1111/j.1600-0447.1985.tb10510.x. [DOI] [PubMed] [Google Scholar]
- 19.Keller MB, Lavori PW, Coryell W, Andreasen NC, Endicott J, Clayton PJ, Klerman GL, Hirschfeld RMA. Differential outcome of pure manic, mixed/cycling, and pure depressive episodes in patients with bipolar illness. JAMA. 1986;255(22):3138–3142. [PubMed] [Google Scholar]
- 20.Angst J. Switch from depression to mania, or from mania to depression. J Psychopharmacol. 1987;1(1):13–19. doi: 10.1177/026988118700100104. [DOI] [PubMed] [Google Scholar]
- 21.Keller MB, Lavori PW, Coryell W, Endicott J, Mueller TI. Bipolar I: a five-year prospective follow-up. J Nerv Ment Dis. 1993;181(4):238–245. doi: 10.1097/00005053-199304000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Cole AJ, Scott J, Ferrier IN, Eccleston D. Patterns of treatment resistance in bipolar affective disorder. Acta Psychiatr Scand. 1993;88(2):121–123. doi: 10.1111/j.1600-0447.1993.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 23.Turvey CL, Coryell WH, Solomon DA, Leon AC, Endicott J, Keller MB, Akiskal H. Long-term prognosis of bipolar I disorder. Acta Psychiatr Scand. 1999;99(2):110–119. doi: 10.1111/j.1600-0447.1999.tb07208.x. [DOI] [PubMed] [Google Scholar]
- 24.Angst J, Sellaro R. Historical perspectives and natural history of bipolar disorder. Biol Psychiatry. 2000;48(6):445–457. doi: 10.1016/s0006-3223(00)00909-4. [DOI] [PubMed] [Google Scholar]
- 25.Maj M, Pirozzi R, Magliano L, Bartoli L. The prognostic significance of “switching” in patients with bipolar disorder: a 10-year prospective follow-up study. Am J Psychiatry. 2002;159(10):1711–1717. doi: 10.1176/appi.ajp.159.10.1711. [DOI] [PubMed] [Google Scholar]
- 26.Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Rush AJ, Keck PE, Jr, McElroy SL, Luckenbaugh DA, Pollio C, Kupka R, Nolen WA. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64(6):680–690. doi: 10.4088/jcp.v64n0610. [DOI] [PubMed] [Google Scholar]
- 27.Solomon DA, Leon AC, Endicott J, Coryell WH, Li C, Fiedorowicz JG, Keller MB. Empirical typology of bipolar I mood episodes. Br J Psychiatry. 2009;195(6):525–530. doi: 10.1192/bjp.bp.108.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 29.Hedeker D, Siddiqui O, Hu FB. Random-effects regression analysis of correlated grouped-time survival data. Stat Methods Med Res. 2000;9(2):161–179. doi: 10.1177/096228020000900206. [DOI] [PubMed] [Google Scholar]
- 30.Miller DC. Handbook of Research Design and Social Measurement. 4. White Plains, NY: Longman; 1983. [Google Scholar]
- 31.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. [Accessed June 4, 2009];International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Version for 2007. http://www.who.int/classifications/apps/icd/icd10online/gf30.htm.
- 33.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2. New York, NY: Oxford University Press; 2007. p. 130. [Google Scholar]
- 34.Kessing LV, Mortensen PB. Recovery from episodes during the course of affective disorder: a case-register study. Acta Psychiatr Scand. 1999;100(4):279–287. doi: 10.1111/j.1600-0447.1999.tb10862.x. [DOI] [PubMed] [Google Scholar]
- 35.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 36.Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, Ketter TA, Miklowitz DJ, Otto MW, Gyulai L, Reilly-Harrington NA, Nierenberg AA, Sachs GS, Thase ME. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163(2):217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 37.Gitlin MJ, Swendsen J, Heller TL, Hammen C. Relapse and impairment in bipolar disorder. Am J Psychiatry. 1995;152(11):1635–1640. doi: 10.1176/ajp.152.11.1635. [DOI] [PubMed] [Google Scholar]
- 38.Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rössler W. Toward a definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord. 2003;73(1–2):133–146. doi: 10.1016/s0165-0327(02)00322-1. [DOI] [PubMed] [Google Scholar]
- 39.American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002;159(4 suppl):1–50. [PubMed] [Google Scholar]
- 40.Suppes T, Dennehy EB, Hirschfeld RM, Altshuler LL, Bowden CL, Calabrese JR, Crismon ML, Ketter TA, Sachs GS, Swann AC Texas Consensus Conference Panel on Medication Treatment of Bipolar Disorder. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870–886. doi: 10.4088/jcp.v66n0710. [DOI] [PubMed] [Google Scholar]
- 41.Blanco C, Laje G, Olfson M, Marcus SC, Pincus HA. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005–1010. doi: 10.1176/appi.ajp.159.6.1005. [DOI] [PubMed] [Google Scholar]
- 42.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7(1–2):305–315. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]