Abstract
The disposition in mice of the cannabimimetics JWH-018 and JWH-073 in blood and brain following inhalation of the smoke from the herbal incense product (HIP) “Magic Gold” containing 3.6% JWH-018, 5.7% JWH-073 and less than 0.1% JWH-398 (w/w) is presented. Specimens were analyzed by HPLC/MS/MS. The validation of the method is also presented. Five C57BL6 mice were sacrificed 20 min after exposure to the smoke of 200 mg of “Magic Gold” and a second set of five exposed mice were sacrificed after 20 h. Twenty minutes after exposure to “Magic Gold” smoke, blood concentrations of JWH-018 ranged from 42 to 160 ng/mL (mean: 88 ng/mL ± 42) and those of JWH-073 ranged from 67 to 244 ng/mL (mean: 134 ng/mL ± 62). Brain concentrations 20 min after exposure to “Magic Gold” smoke for JWH-018 ranged from 225 to 453 ng/g (mean: 317 ng/g ± 81) and those of JWH-073 ranged from 412 to 873 ng/g (mean: 584 ng/g ± 163). Twenty hours after exposure to “Magic Gold” smoke, JWH-018 was detected and quantified in only two of the five blood samples. Blood concentrations of JWH-018 were 3.4 ng/mL and 9.4 ng/mL. JWH-073 was detected in only one blood specimen 20 h after exposure at 4.3 ng/mL. Brain concentrations 20 h post exposure for JWH-018 ranged from 7 to 32 ng/g (mean: 19 ng/g ± 9). JWH-073 was not detected in 20 h post exposure brain specimens. JWH-398 was not detected in any of the blood or brain samples. The disposition data presented with the limited data available from human experience provide reasonable expectations for forensic toxicologists in JWH-018 or JWH-073 cases. As with THC after smoking marijuana, blood and brain concentrations of JWH-018 and JWH-073 after HIP smoking can be expected to rise initially to readily detected values, and then drop dramatically over the next few hours to several ng/mL or ng/g, and finally to be at extremely low or undetectable concentrations by 24 h apparently due to extensive biotransformation, and redistribution to body fat.
Keywords: Cannabimimetics, JWH-018, JWH-073, Magic Gold, HPLC/MS/MS
1. Introduction
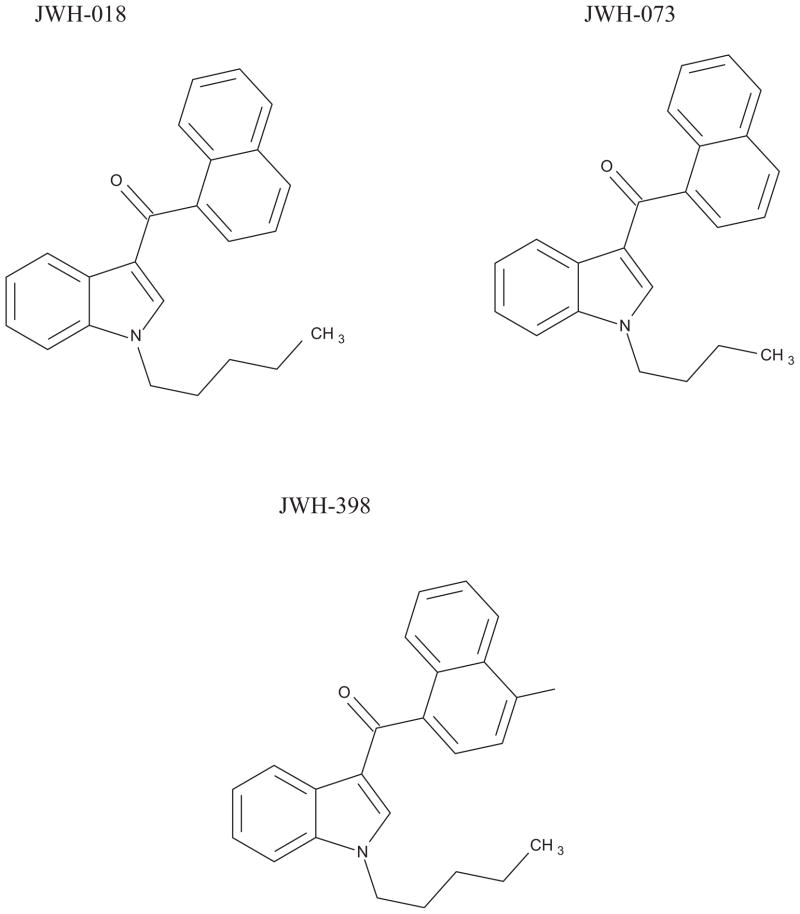
Since the mid-2000s, various herbal incense products (HIPs) became available via the internet and at various retail outlets, initially in Europe, then more recently in the United States and Japan [1]. HIPs purport to be blends of plant materials and incense and are labeled “not for human consumption” however, they are allegedly capable of producing a marijuana-like high when smoked [1]. HIPs are usually packed in colorful foil packets containing 3–5 g of plant material. DNA testing has shown that the plant species listed as ingredients were not always present [8]. HIPS are skillfully marketed under numerous, and an ever increasing number of attractive or exotic names such as Spice Gold, Magic Gold, K-2, Summit, Lion’s Tail, Buzz, Pulse, Chill Out and Yucatan Fire. Chemical analyses performed on HIPs have identified a variety of synthesized cannabimimetic compounds present [1–8]. Many of these compounds interact with the CB1 receptors in the nervous system in a similar manner to Δ9-tetrahydrocannibinol (THC), the main active compound in marijuana [9–13]. Cannabimimetics were initially synthesized as pharmacological tools to help elucidate the structure of the cannabinoid receptor(s) in the brain and other tissues. They have also been used to assist in the search for medical applications of cannabinoids [14]. The most commonly encountered drugs are the cyclohexylphenol compounds such as CP 47, 497 [15] and naphthoylindole derivatives synthesized by John W. Huffman such as JWH-018, JWH-073 and JWH-398, Fig. 1 [16].
Fig. 1.
Chemical structures of JWH-018, JWH-073 and JWH-398.
JWH-018 has been detected recently in the serum of two subjects after smoking 100 mg (female subject) and 150 mg (male subject) of the HIP “Smoke” which contained 2.9% JWH-018 [17]. Serum concentration were 8 and 10 ng/mL within 5 min of smoking, respectively; however, they dropped below the assay’s lower limit of quantitation (LLOQ) of 0.5 ng/mL after 6 h. Another recent study of serum specimens from 80 subjects provided by various clinical and forensic sources detected JWH-018 in 9 samples at concentrations ranging from 0.30 to 8.1 ng/mL with a mean of 1.84 ng/mL [18]. JWH-073 was found in 6 samples ranging from 0.23 to 0.6 ng/mL, mean 0.42 ng/mL. Also the metabolites of JWH-018 have recently been identified in the urine of three people arrested for public intoxication [19]. Recently, Grigoryev et al. found that the major urinary metabolites of JWH-018 in man and mice are monhydroxylated metabolites and dealkylated with monhydroxylated metabolites, respectively [20]. However, there is little pharmacological or toxicological data concerning the disposition of the cannabimimetics in animals or man. To gain a better understanding of the pharmacokinetic phase of cannabimimetic intoxication, we studied the disposition of JWH-018 and JWH-073 in blood and brain of mice after controlled exposure to smoke from the HIP “Magic Gold.” These compounds were identified and quantified in whole blood and brain by high pressure liquid chromatography with ion spray positive ion triple quadruple mass spectrometry (HPLC/MS/MS). The validation of the analytical method is also presented.
2. Materials and methods
2.1. Animals and procedures
Two sets of five C57BLC/6J mice (Jackson Laboratory, Bar Harbor, ME) were housed in the animal care quarters and maintained at 22 ± 2 C on a 12 h light/dark cycle with food and water available ad libitum. They were brought to the test environment and allowed 24 h to recover from the move. The animal study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The mice were exposed to the smoke of 200 mg of the burning HIP “Magic Gold.” The “Magic Gold” was obtained by ordering it over the internet (imzonged.com, TX). Following exposure the mice were subjected to two of the Martin Tetrad tests that measure hypothermia and catalepsy, physiological responses consistent with cannabinoid receptor (CB1) binding [21]. Rectal temperatures were taken with a digital thermometer (Fisher Scientific, Pittsburgh, PA) at a depth of 2 cm and recorded to the nearest 0.1 °C 10 min exposure. Mice were evaluated for catalepsy 15 min after exposure by placing their front paws on a bar oriented parallel to and approximately 1 in. off of the ground. Twenty minutes after exposure to “Magic Gold” smoke, one set of five mice was sacrificed. Immediately after death, blood and brain were collected. The specimens were frozen at −80 °C until chemical analysis. It was intended that the additional locomotor suppression and antinociception tetrad tests be performed with the second set of mice. However, the mice remained immobile for almost the entire study time. Their responses to “Magic Gold” were so dramatic that it would have confounded the tests for antinociception, so these tests were not completed. All the mice in the second set survived 20 h post-exposure, at which time they were sacrificed. Each animal was decapitated, the blood was collected in heparinized tubes (Elkins-Sinn, Cherry Hill, NJ) and the brain tissues were removed. All samples were frozen at −80 °C until chemical analysis.
2.2. Exposure apparatus
The exposure system used was a modification of that described by Lichtman et al. [22]. A 15 cm corncob pipe was used to burn the herbal incense product. The smoke from the pipe was drawn through a 27.5 cm length of Tygon® tubing to the manifold at a flow rate of 35 mL/min using a vacuum pump and a flow regulator. A solenoid puffing device was used to alternate the flow of smoke and fresh air to the animals every 8 s. Tygon® tubing, containing 0.5 g of glass-wool fiber to sequester the smoke, was connected to the exhaust of the manifold. The mice were placed into holding tubes that fit snugly into the manifold, consisting of six ports for a nose-only exposure. They were exposed to the smoke until the HIP was completely consumed which occurred within a 2-min time period. If the material ceased to burn at any time, it was lit again until completely consumed.
The glass-wool fibers connected to the exhaust manifold were collected after each exposure and stored in 5.0 mL of methanol for 1 week. One hundred μL of the methanol extract were combined with 100 μL of the internal standard, then dried under nitrogen, re-constituted with mobile phase, and transferred to auto-sampler vials and analyzed using the present HPLC/MS/MS method. The glass wool contained 169 μg JWH-018, 193 μg JWH-073 and 0.8 μg JWH-398 for the 20-min samples and 213 μg JWH-018, 224 μg JWH-073 and 0.8 μg JWH-398 for the 20-h samples.
3. Methods
3.1. Reagents and supplies
The d3-tetrahydrocannabinol (d3-THC) was purchased from Cerilliant (Texas, USA). Naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018), Naphthalen-1-yl-(1-butylindol-3-yl)methanone (JWH-073) and 4-chloronaphthalen-1-yl-(1-penty-lindolin-3-yl)methanone (JWH-398), Fig. 1, were obtained from John W. Huffman (Clemson University). The methanol, acetonitrile, water and ammonium formate were purchased from Fisher Scientific (New Jersey, USA) and were HPLC grade or better. A 0.1 mg/L JWH-018 and JWH-073 working standard solution was prepared in methanol. A 0.5 mg/L d3-THC working internal standard solution was also prepared in methanol. A negative control and eight point calibration curves at concentrations of 1, 2.5, 5, 10, 20, 50, 100 and 200 ng/mL JWH-018, JWH-073 and JWH-398 in 1.0 mL of drug-free whole blood were prepared with each analytical run.
3.2. Extraction of “Magic Gold”
The cannabimimetic content of the HIP “Magic Gold” was determined by continuous extraction of 50 mg of “Magic Gold” with methanol for 72 h. A 100 μL aliquot of the extract was then mixed with 20 μL of d3-THC internal standard and then dried under dry nitrogen. The resultant residue was dissolved in 100 μL of mobile phase and analyzed using the instrument method described herein but applying non-extracted calibrators. “Magic Gold” was found to contain 3.6% JWH-018, 5.7% JWH-073 and less than 0.1% JWH-398 (w/w).
3.3. Extraction of blood and brain tissue
The extractions were performed using a modification of the procedure of Foltz et al. [23] as previously described for analysis of natural cannabinoids in brain tissue [24]. Prior to extraction, 250 μL of mouse blood was diluted with 750 μL of drug-free human blood to create a sample size of 1 mL. The brains were homogenized by hand using a glass homogenizer with deionized water at a 1:4 dilution (w/w). Half of the resulting homogenate by mass was extracted according to the procedure. To each sample was added 50 μL of the working internal standard containing 25 ng/mL of d3-THC. These samples were mixed and allowed to equilibrate overnight. The following day 2 mL of ice cold acetonitrile was added drop by drop to each sample while vortexing. The samples were then centrifuged at 3500 rpm for 10 min. After centrifuging, the samples were placed in a −40 °C freezer for at least 2 h. The top layer of each sample containing the acetonitrile was then removed via a disposable glass pipette and placed in clean test tube. Extracts were then dried using a Savant AES1000. The resultant residues were reconstituted with 100 μL of mobile phase and placed in auto-sampler vials for HPLC/MS/MS analysis.
3.4. Instrumental analysis
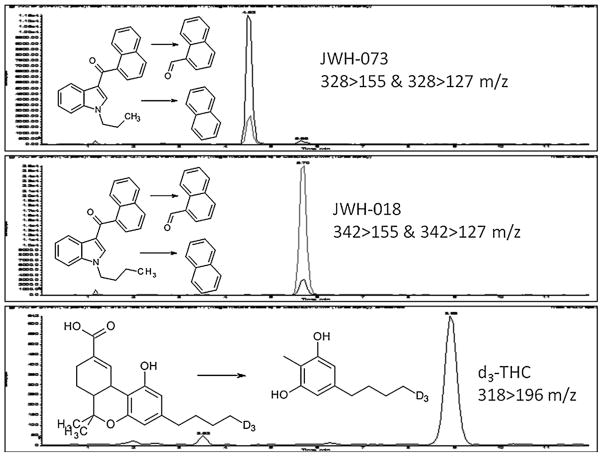
The HPLC/MS/MS system used was an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. The chromatographic separation was performed using a Zorbaz eclipse XDB-C18 column, 4.6 mm × 75 mm, 3.5 μm (Agilent Technologies, USA). The mobile phase contained water/acetonitrile (20:80, v/v) with 0.1 mM ammonium formate and was delivered at a flow rate of 0.5 mL/min. The source temperature was set at 650 °C and had a curtain gas at a flow rate of 30 mL/min. The ionspray voltage was 5000 V, with the ion source gases 1 and 2 at flow rates of 60 mL/min. The acquisition mode used was multiple reaction monitoring (MRM). Table 1 lists the transition ions monitored and the corresponding deprotonation (DP) and collision energies (CE) used for each target cannabimimetic. The chromatographic method resolved d3-THC, JWH-018 and JWH-073, Fig. 2. The total run time for the analytical method was 12 min.
Table 1.
The transition ions and their corresponding deprotonation (DP) and collision energy (CE).
| Compound | Retention times (min) | Transition ions (m/z) | DP (V) | CE (eV) |
|---|---|---|---|---|
| d3-THC | 9 | 318 >196 | 46 | 27 |
| JWH-018 | 5.7 | 342 >155 | 65 | 65 |
| 342 >127 | 65 | 50 | ||
| JWH-073 | 4.5 | 328 >155 | 60 | 63 |
| 328 >127 | 60 | 36 |
Fig. 2.
SIM chromatograph of the transition ions of JWH-018, JWH-073 and the internal standard d3-THC in mouse blood.
3.5. Preparation of calibration standards and quality control (QC) samples in blood and brain tissue
Drug-free blood and brain tissue provided the matrix for the study. Appropriate volumes of the working solutions of JWH-018 and JWH-073 were added to the blood to obtain an eight-point calibration of 1, 2.5, 5, 10, 20, 50, 100 and 200 ng/mL of each cannabimimetic. Calibration standards were prepared before each analytical run. Using a different lot of solutions of JWH-018 and JWH-073, QC samples representing the LLOQ, low (LQC), medium (MQC) and high (HQC) quality controls were prepared in human blood at 1, 2.5, 20 and 75 ng/mL, respectively. LQC, MQC and HQC controls were prepared in 1:4 mouse:human blood. Upper limit of quantitation (ULQ) samples above the high calibration point were prepared at concentrations of 500 and 1000 ng/mL. Tissue QC samples of JWH-018 and JWH-073, 10 ng/g for the LQC, 80 ng/g for the MQC and 100 ng/g for the HQC, were prepared using drug-free 1:4 (tissue:water) mouse brain tissue homogenates. All QC samples were prepared as a single lot and were stored at −20 °C until analysis. Calibrators and QC samples were extracted using the same procedure as described for the blood and brain tissue samples.
4. HPLC/MS/MS validation
The method validation was performed as follows. Validation runs containing calibration standards, blank samples, blank sample with internal standard added and replicates of LLOQ, LQC, MQC and HQC samples were prepared and run on three separate days.
4.1. Linearity, LLOQ and ULQ
Eight calibration standards with concentrations of 1, 2.5, 5, 10, 20, 50, 100 and 200 ng/mL were prepared in drug-free blood (in house certified) for the determination of JWH-018 and JWH-073 concentrations. A linear regression of the ratio of the peak area response of JWH-018 or JWH-073 and internal standard (d3-THC) versus concentration was determined. For each calibration curve, the back calculated standard concentrations were within 15% deviation from the nominal value (DFN) except the LOQ, where it could be within 20%. The R2 value for each curve was 0.99 or better. The LLOQ of JWH-018 and JWH-073 was administratively set at 1 ng/mL and had response at least five times the signal to noise ratio of the blank response. The ULQ samples of 500 and 1000 ng/mL of JWH-018 and JWH-073 were prepared to evaluate accuracy above the highest calibrator (200 ng/mL). These controls were analyzed and determined to be within 15% deviation from the DFN.
4.2. Accuracy and precision
Accuracy and precision were determined from the prepared QC blood samples for three different validation runs. The concentrations of the prepared QC samples (n = 6) were calculated from the calibration curves analyzed in the same run. A criterion of 20% of the DFN (expected values of the prepared QC samples) was used to assess accuracy, while precision expressed as coefficient of variation (CV) did exceed 15% (20% for LOQ). Both intra- and inter-assay accuracy and precision were determined not exceed a 15% CV and 20% for the LOQ. Accuracy/bias and inter-day precision for 1:4 mouse:human blood and mouse brain tissue QC samples were evaluated with three aliquots of each control. The criteria for acceptable assay accuracy/bias were quantified results within +20% of the target value of the prepared QC tissue samples and the precision expressed as CV less than 20% for these controls.
4.3. Selectivity
Six different lots of cannabimimetic-free blood were used to assess selectivity. Each individual lot was analyzed with and without internal standard. No peaks co-eluting with JWH-018, JWH-073 or the internal standard (d3-THC) were detected for any the six lots of cannabimimetic-free blood. Three different lots of cannabimimetic-free 1:4 mouse:human blood and mouse brain tissue were used to assess selectivity in mouse blood and tissue samples. Each individual lot was analyzed with and without internal standard. No co-eluting peaks were detected in any of the 1:4 mouse:human blood or mouse brain tissue lots. This ensured that the endogenous blood components do not interfere with the assay.
4.4. Matrix
Matrix effect from blood and mouse brain tissue was determined as follows. Six different lots of cannabimimetic-free blood were fortified with 4 ng/mL JWH-018 and JWH-073 and analyzed in triplicate. Three different lots of each of 1:4 mouse:human blood fortified with 4 ng/mL JWH-018 and JWH-073 and mouse brain tissue fortified with 16 ng/mg JWH-018 and JWH-073 were analyzed. The results were determined to have less than a 20% deviation from the DFN.
4.5. Carryover
Sample carryover was assessed by injecting a drug-free blood or tissue homogenate samples immediately after an ULQ sample (1000 ng/mL JWH-018 and JWH-073) injection. The acceptance criteria for carryover were set at ≤20% of the LLOQ. No JWH-018 or JWH-073 was detected in the drug-free samples. Sample carryover during the run was also assessed by injecting a HQC sample immediately followed by a LQC sample six times for blood and three times for 1:4 mouse:human blood and mouse brain tissue samples. The LQC was evaluated for any bias. Lack of carryover was confirmed with a bias within 15% of the LQC concentration.
4.6. Stability
Stability experiments in blood were performed at three QC concentrations (2.5, 20 and 75 ng/mL) with six replicates at each concentration. The stability in blood was assessed during storage and after three freeze–thaw cycles at −20 °C with at least 24 h in between two cycles. These freeze–thaw QC samples were then run against freshly prepared calibration standards. The bench-top stability at room temperature was assessed in blood for 24 h to simulate sample processing time including transportation. These samples were considered to be stable in blood and the JWH-018 and JWH-073 concentrations were within 15% of the nominal concentration for the QC samples tested. The post-preparative stability or the auto-sampler stability was assessed from re-injection reproducibility after storage of the samples in the auto-sampler for 72 h at room temperature. JWH-018 samples showed between 45% to 30% degradation and JWH-073 had approximately 20% degradation.
5. Results and discussion
The presented HPLC/MS/MS method for analysis of blood and brain specimens applied an administrative LOQ for JWH-018 and JWH-073 of 1.0 ng/mL for blood and 4.0 ng/g for brain tissue. The methods of Teske et al. [17] and Sebastian et al. [18] applied to serum analysis were more sensitive with LOQs of 0.5 ng/mL and 0.1 ng/mL, respectively. However, blood and brain are more complex matrices than serum from which to extract and purify lipid soluble compounds such as JWH-018 and JWH-073. Therefore, it was deemed that the analytical parameters of the presented method were sufficient for the goals of this study.
The responses of all the mice to the Tetrad tests were consistent with CB1 activity. The body temperature of the mice dropped 6–7 °C from the base measurements. By comparison, mice exposed to smoke from 200 mg of marijuana containing 3.5% THC, had their body temperatures drop only 3–4 °C [22]. Additionally, each mouse remained immobile on the bar for longer than 20 s. In fact, the mice remained cataleptic until the first set was sacrificed.
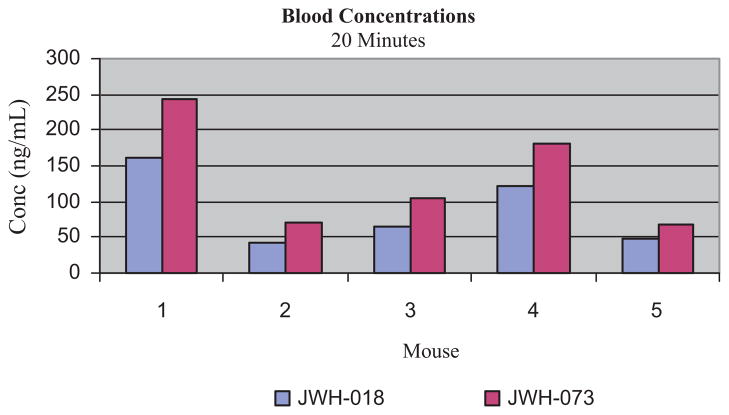
Twenty minutes after exposure to “Magic Gold” smoke, blood concentrations of JWH-018 ranged from 42 to 160 ng/mL (mean: 88 ng/mL ± 42) and those of JWH-073 ranged from 67 to 244 ng/mL (mean: 134 ng/mL ± 62), Fig. 3. In all specimens JWH-073 concentrations were higher than those of JWH-018: mean increase 53%; ranging from 34% to 71% higher. This is consistent with the relative concentrations of the cannabimimetics in the “Magic Gold” product which contained 58% more JWH-073 than JWH-018. JWH-398, found in “Magic Gold” at <1%, was not detected in any of the blood or brain samples.
Fig. 3.
Blood concentrations of JWH-018 and JWH-073 in mice after exposure to “Magic Gold” smoke.
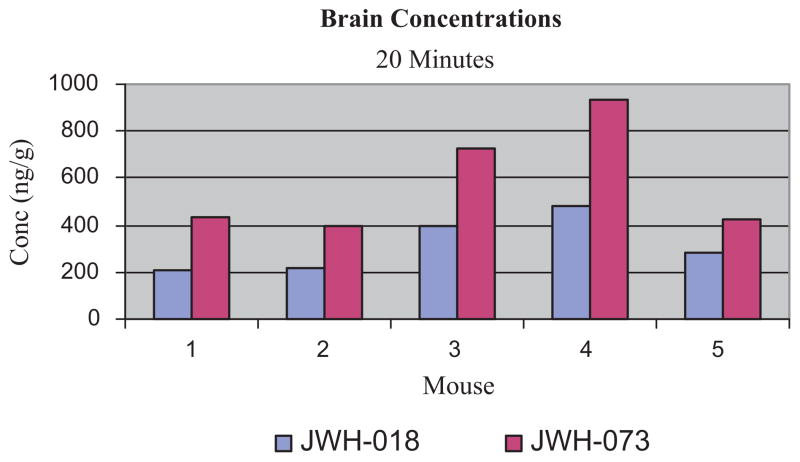
Brain concentrations 20 min after exposure to “Magic Gold” smoke for JWH-018 ranged from 225 to 453 ng/g (mean: 317 ng/g ± 81) and those of JWH-073 ranged from 412 to 873 ng/g (mean: 584 ng/g ± 163), Fig. 4. As with blood, JWH-073 concentrations were higher than JWH-018 concentrations in all brain specimens: mean increase 84%; ranging from 51% to 112% higher. This higher concentration in brain not only reflects the relative concentration of JWH-073 in “Magic Gold,” but also may be due to the greater lipophilic character of JWH-073 as compared to JWH-018. The presence in brain of both cannabimimetics and the response of the mice to the Tetrad tests are consistent with the hypothesis that the behavioral effects of “Magic Gold” are attributable to JWH-018 and JWH-073. The mean blood to brain ratio for JWH-018 was 4.4, ranging from 1.4 to 5.9. For JWH-073, the mean blood to brain ratio was 5.2 and ranged from 2 to 6.6. While it is difficult to make direct comparisons, it is interesting to note the relative disposition of these cannabimimetics and THC. Indeed these observations suggest that the cannabimemetic effects of HIPs should be evaluated at lower doses of HIPs. In the same exposure system with marijuana containing approximately 4% THC, at 20 min post exposure the JWH-018 and JWH-073 blood concentrations are much lower than those of THC, 200–300 ng/mL [22,24], while the brain values are similar, 300–400 ng/g [24,25].
Fig. 4.
Brain concentrations of JWH-018 and JWH-073 in mice after exposure to “Magic Gold” smoke.
Twenty hours after exposure to “Magic Gold” smoke, JWH-018 was detected and quantified in only two of the five blood samples. Blood concentrations of JWH-018 were 3.4 ng/mL and 9.4 ng/mL. JWH-073 was detected in only one blood specimen 20 h after exposure at 4.3 ng/mL. These data are consistent with the findings of Teske et al. in their two subjects [17]. Serum JWH-018 concentrations dropped as low as 0.13 ng/mL 6 h after smoking and were undetectable in the next specimen collected 18 h later. Mouse brain concentrations 20 h post exposure for JWH-018 ranged from 7 to 32 ng/g (mean: 19 ng/g ± 9). JWH-073 was not detected in 20 h post exposure brain specimens. This indicates that JWH-018 may have a longer half-life than JWH-073. However, pharmacokinetic constants including plasma half-lives have not been determined for cannabimimetics such as the JWH series. Recent metabolic studies using human liver microsomes and urines collected from persons smoking HIPs have shown that JWH-018 is extensively biotransformed to multiple hydroxylated and conjugated metabolites such that the parent drug is not detected in urine [26,27]. Again, it is difficult to make direct comparisons, but it is interesting to note that the loss of the cannabimimetics from brain over the 20-h sampling period is similar to the disappearance of THC from brain and visceral organs due to biotransformation, elimination and redistribution to body fat [28].
While in a small animal, the disposition data presented does, when compared to the limited data available from human experience, provide reasonable expectations for forensic toxicologists in JWH-018 or JWH-073 cases. As with THC after smoking marijuana, blood and brain concentrations of JWH-018 and JWH-073 after HIP smoking can be expected to rise initially to readily detected values, several hundred ng/mL, and then drop dramatically over the next few hours to several ng/mL or ng/g, and, finally, be at extremely low or undetectable concentrations by 24 h. This pattern of concentrations is apparently due to extensive biotransformation and re-distribution to body fat.
Acknowledgments
This project was supported by the National Institute on Drug Abuse (NIDA) Center for Drug Abuse Grants R01DA02396, R01DA03672, and P50DA005274.
Footnotes
This data was presented, in part, at the Annual Meeting, Society of Forensic Toxicologist, Richmond, VA October 21, 2010.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.European Monitoring Centre for Drugs and Drug Addiction. Action on New Drugs Briefing Paper: Understanding the Spice Phenomenon, a Report from an EMCDDA Expert Meeting; 6 March 2009; Lisbon. [accessed 25.05.10]. Final Version 2009. http://www.emcd-da.europa.edu/html.cfm/index78154EN.html. [Google Scholar]
- 2.Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N. Spice and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;45 doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- 3.Uchiyama N, Kikura-Hanajiri R, Kawahara N, Haishima Y, Goda Y. Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chem Pharm Bull. 2009;57:439–441. doi: 10.1248/cpb.57.439. [DOI] [PubMed] [Google Scholar]
- 4.Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y. Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol. 2009;27(2):61–66. [Google Scholar]
- 5.Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggerman M, Ernst L, Beuerle T. Spice: a never ending story? Forensic Sci Int. 2009;191(1–3):58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Hudson S, Ramsey J, King L, Maynard S, Dargan PJ, Wood DM. Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “Herbal High” products. J Anal Toxicol. 2010;24:252–260. doi: 10.1093/jat/34.5.252. [DOI] [PubMed] [Google Scholar]
- 7.Zuba D, Byrska B, Maclow M. Comparison of “Herbal Highs” composition. Anal Bioanal Chem. 2011;400:119–126. doi: 10.1007/s00216-011-4743-7. [DOI] [PubMed] [Google Scholar]
- 8.Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198:31–38. doi: 10.1016/j.forsciint.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend. 2000;60:133–140. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 10.Huffman JW, Dai D. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1994;4:563–566. [Google Scholar]
- 11.Atwood BK, Huffman J, Straiker A, Mackie K. JWH-018, a common constituent of “Spice” herbal blends, is a potent and efficacious cannabinoid CB1 receptor agonist. Br J Pharm. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiley JL, Compton DR, Dai D, Huffman JW, Martin BR. Structure–activity relationships of indole and pyrrole-derived cannabinoids. J Pharm Exp Ther. 1998;285:994–1004. [PubMed] [Google Scholar]
- 13.Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharm Exp Ther. 1992;260:201–209. [PubMed] [Google Scholar]
- 14.Zhang Q, Ma P, Cole RB. Identification of in vitro metabolites of JWH-015, an aminoalkylindole agonist for the peripheral cannabinoid receptor (CB2) by HPLC–MS/MS. Anal Bioanal Chem. 2006;386:1345–1355. doi: 10.1007/s00216-006-0717-6. [DOI] [PubMed] [Google Scholar]
- 15.Weissmann A, Milne GM, Melvin LS., Jr Cannabimimetic activity from CP-47, 497, a derivative of 3-phenylcyclohexanol. Pharm Exp Ther. 1982;223:516–523. [PubMed] [Google Scholar]
- 16.Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MIP, Wiley JL, Martin BR. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005;15:4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Teske J, Weller J, Fieguth A, Rothamel T, Schulz Y, Troger HD. Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalene-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2010;878:2659–2663. doi: 10.1016/j.jchromb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Sebastian D, Kneisel S, Weinmann W, Zimmermann R, Auwärter V. Development and validation of a liquid chromatography–tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J Mass Spectrom. 2011;46:163–171. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- 19.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200:141–147. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Grigoryev, Savchuk S, Melnik A, Moskaleva N, Dzhurko J, Ershov M, Nosyrev A, Vedenin A, Izotov B, Zabirova I, Rozhanets V. Chromatography–mass-spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking. J Chromatogr B. 2011;879:1126–1136. doi: 10.1016/j.jchromb.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharm Exp Ther. 1988;247:1046–1051. [PubMed] [Google Scholar]
- 22.Lichtman AH, Poklis JL, Wilson DM, Poklis A, Martin BR. The pharmacological activity of inhalation exposure to marijuana in mice. Drug Alcohol Depend. 2001;63:107–116. doi: 10.1016/s0376-8716(00)00205-2. [DOI] [PubMed] [Google Scholar]
- 23.Foltz RL, McGinnis KM, Chinn DM. Quantitative measurement of Delta-9-tetrahydrocannabinol and two major metabolites in physiological specimens using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Biomed Mass Spectrom. 1983;10:316–323. doi: 10.1002/bms.1200100503. [DOI] [PubMed] [Google Scholar]
- 24.Poklis JL, Thompson CC, Long KA, Lichtman AH, Poklis A. Disposition of cannabichromene cannabidiol and delta9-tetrahydrocannibinol and its metabolites in mice brain following marijuana inhalation determined by HPLC/MS/MS. J Anal Toxicol. 2010;34:516–520. doi: 10.1093/jat/34.8.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varvel S, Wiley J, Yang R, Bridgen D, Long K, Lichtman A, Martin B. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology (Berl) 2006;186:226–234. doi: 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- 26.Wintermeyer A, Moller I, Thevis M, Jubner M, Beike J, Rothschild MA, Bender K. In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal Bioanal Chem. 2010;398:2143. doi: 10.1007/s00216-010-4171-0. [DOI] [PubMed] [Google Scholar]
- 27.Moller I, Wintermeyer A, Bender K, Jubner M, Thomas A, Krug O, Schanzer W, Thevis M. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Anal. 2011 doi: 10.1002/dta.158. [DOI] [PubMed] [Google Scholar]
- 28.Brunet B, Doucet C, Venisse N, Hauet T, Hebrard W, Papet Y, Mauco G, Mura P. Validation of Large White Pig as an animal model for study of cannabinoid metabolism: application to the study of THC distribution in tissues. Forensic Sci Int. 2006;161:169–174. doi: 10.1016/j.forsciint.2006.04.018. [DOI] [PubMed] [Google Scholar]