Abstract
A bloom of Karenia brevis Davis developed in September 2007 near Jacksonville, Florida and subsequently progressed south through east Florida coastal waters and the Atlantic Intracoastal Waterway (ICW). Maximum cell abundances exceeded 106 cells L−1 through October in the northern ICW between Jacksonville and the Indian River Lagoon. The bloom progressed further south during November, and terminated in December 2007 at densities of 104 cells L−1 in the ICW south of Jupiter Inlet, Florida. Brevetoxins were subsequently sampled in sediments and seagrass epiphytes in July and August 2008 in the ICW. Sediment brevetoxins occurred at concentrations of 11–15 ng PbTx-3 equivalents (g dry wt sediment)−1 in three of five basins in the northern ICW during summer 2008. Seagrass beds occur south of the Mosquito Lagoon in the ICW. Brevetoxins were detected in six of the nine seagrass beds sampled between the Mosquito Lagoon and Jupiter Inlet at concentrations of 6–18 ng (g dry wt epiphytes)−1. The highest brevetoxins concentrations were found in sediments near Patrick Air Force Base at 89 ng (g dry wt sediment)−1. In general, brevetoxins occurred in either seagrass epiphytes or sediments. Blades of the resident seagrass species have a maximum life span of less than six months, so it is postulated that brevetoxins could be transferred between epibenthic communities of individual blades in seagrass beds. The occurrence of brevetoxins in east Florida coast sediments and seagrass epiphytes up to eight months after bloom termination supports observations from the Florida west coast that brevetoxins can persist in marine ecosystems in the absence of sustained blooms. Furthermore, our observations show that brevetoxins can persist in sediments where seagrass communities are absent.
Keywords: Brevetoxin, Karenia brevis, Seagrass epiphytes, Sediments
1. Introduction
The dinoflagellate Karenia brevis Davis (G. Hansen and Moestrupcmb. Nov.) dominates harmful algal blooms (HABs) that develop on an almost annual basis in the eastern Gulf of Mexico (GOM). Dense aggregations of K. brevis co-occur with three other Karenia species (Steidinger et al., 2008) at cell densities of 105–106 cells L−1 in west Florida (WF) coastal waters. Blooms typically develop between Tampa and Ft. Myers, FL. K. brevis produces a suite of cyclic polyether neurotoxins known as brevetoxins (PbTx); the PbTx designation is derived from Ptychodiscus brevis, a prior epithet for K. brevis (Daugbjerg et al., 2000). The two primary brevetoxins synthesized within the cell are PbTx-1 and PbTx-2, and various analogs are derived from these ‘parent’ compounds (Baden et al., 2005). Florida ‘red tides’ frequently coincide with fish kills and mortality events of marine reptiles, mammals and birds (Landsberg, 2002). Brevetoxins produced by K. brevis also impact human health when shellfish containing brevetoxins are consumed, producing Neurotoxic Shellfish Poisoning. Furthermore, brevetoxins can be aerosolized through wave action and produce respiratory irritation when inhaled (Fleming et al., 2005; Kirkpatrick et al., 2004). The overall impact of prolonged K. brevis blooms on Florida’s economy can approach $20 million per year (Anderson et al., 2000).
Brevetoxins have been detected in marine organisms weeks to months after K. brevis blooms have terminated (Flewelling et al., 2005). Seagrass communities, for example, can retain brevetoxins at concentrations of several tens of ng (g dry wt)−1 throughout the year in west Florida coastal waters, including periods when K. brevis is absent from the water column (Flewelling, 2008). In contrast to seagrass leaves, seagrass epiphytic communities were found to contain higher brevetoxins concentrations. Seagrass epiphyte communities are comprised of autotrophic (diatom, dinoflagellate, cyanobacteria) organisms as well as heterotrophs that range in size from bacteria to benthic filter-feeders such as bivalves. Planktivorous fish that ingest K. brevis, and benthic feeders that graze on seagrasses and their epiphytes, can retain brevetoxins in their tissues for weeks (Naar et al., 2007; Woofter et al., 2005). Individual brevetoxin analogs (PbTx-2 and PbTx-3) have been found in west Florida coastal sediments at concentrations of ca. 1–10 ng (g dry wt)−1 when K. brevis is absent in the overlying waters (Flewelling, 2008; Mendoza et al., 2008). Collectively these studies indicate that brevetoxins persist for weeks to months in the coastal ecosystems of west Florida.
Although K. brevis blooms develop frequently along the west Florida coast, blooms also occur infrequently along the southeastern Atlantic coast (e.g., Murphey et al., 1975; Roberts, 1979). In 1987, a west Florida bloom was transported along the edge of the GOM Loop Current and the Florida Current-Gulf Stream system, and eventually developed as a ‘red tide’ off South Carolina and North Carolina. Similar blooms occurred in the South Atlantic Bight (SAB) in 1976, 1978, 1987 (Tester et al., 1991), and 1990 (Tester et al., 1993). A circulation model applied to the WF shelf indicates that winds, buoyancy, and the proximity to the GOM Loop Current are critical factors in the export of K. brevis from west Florida coastal waters (Weisberg et al., 2009).
In summer 2007 a bloom of K. brevis developed along the WF coast under conditions that favored the export of cells to the Loop Current with eventual transport to the SAB (Walsh et al., 2009). A subsequent K. brevis bloom was reported near Jacksonville, FL in mid-September 2007. By mid-October K. brevis had spread from St. Augustine south through the Intracoastal Waterway (ICW) and into the Indian River Lagoon (see Fig. 1). The Indian River Lagoon (IRL) is composed of inter-connected estuarine systems: the Mosquito Lagoon, The Banana River, which is within Cape Canaveral, and the Indian River. The Indian River is composed of an elongate basin inshore of barrier islands that connects to the Mosquito Lagoon on the north and terminates at St. Lucie Inlet. The Atlantic ICW extends through the IRL and continues along the east Florida (EF) coast. Fish kills were reported along the EF coast through December 2007 that were attributed to the presence of the ichthyotoxic K. brevis (Walsh et al., 2009).
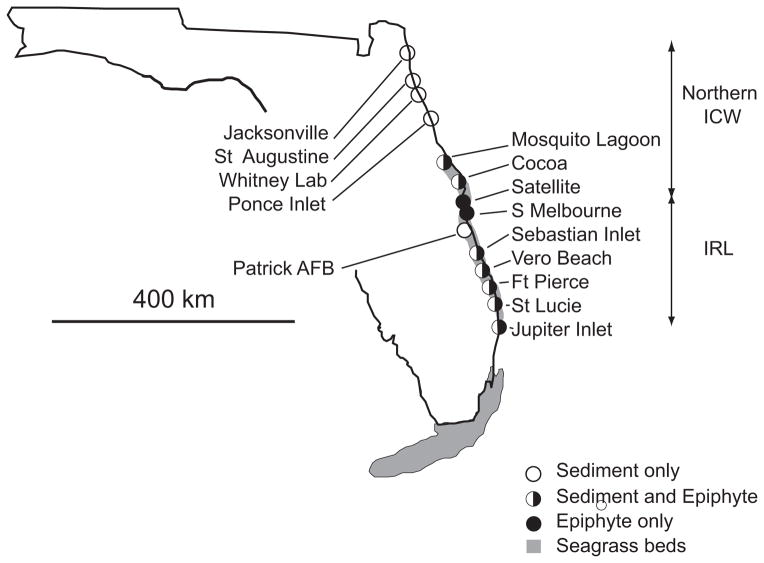
Fig. 1.
Map of the Florida east coast. The Intracoastal Waterway (ICW) extends along the Florida coastline inshore of barrier islands. The northern ICW is designated as the portion from the state border, near Jacksonville FL (J), to the southern end of the Mosquito Lagoon (ML). St. Augustine (StA) and Ponce Inlet (P) are within the Northern ICW. The Indian River Lagoon connects to the Mosquito Lagoon at its northern end and extends south to St. Lucie Inlet. The Banana River (BR) and Patrick Air Force Base (PAF) are on Cape Canaveral. The IRL extends south of the Cape to Sebastian Inlet (Seb), Ft. Pierce Inlet (FtP) St. Lucie Inlet (StL) and Jupiter Inlet (Jup).
Here we describe the relationship between the spatial distribution of the 2007 EF coastal bloom of K. brevis and the persistence of brevetoxins in sediment and seagrass epiphytes within the ICW in summer 2008. The southerly progression of the K. brevis bloom is described from weekly surveys conducted by the Florida Fish and Wildlife Conservation Commission. We sampled brevetoxins in the sediments and seagrass epiphyte communities in the ICW in July–August, 2008 between Jacksonville, FL and Jupiter Inlet, FL, a distance of ca. 400 km.
2. Materials and methods
2.1. K. brevis abundance
Samples are collected weekly from Florida coastal waters for the HAB monitoring program of the Florida Fish and Wildlife Conservation Commission (FWC) (Heil and Steidinger, 2009). Surface waters are sampled each week by local, State, and Federal government agencies, as well as academic institutions and nongovernmental organizations. Water samples are preserved with Lugol’s solution and sent to the Florida Wildlife Research Institute in St. Petersburg, FL. The species identification and enumeration of HAB species is conducted by microscopic examination.
Counts of K. brevis and congeners are disseminated weekly on the World Wide Web, and the data is archived in the Florida HAB Historical Database. Current and archived data are available at http://research.myfwc.com/features/default.asp?id=1018. Although samples are sporadically collected from east Florida coastal waters, an intensive sampling program was conducted during summer 2007 to monitor the bloom distribution. Surface samples were collected weekly at more than 30 locations in the ICW and EF beaches between September and December. Sampling has continued in EF coastal waters since the termination of the 2007 bloom, although on a reduced scale.
2.2. Sediment sampling
Sediment samples were collected from 17 sites on the EF coast in July and August 2008 (Table 1). Weather events, such as thunderstorms, dictated when and where samples could be collected at individual sites with a small boat. Surface sediments were collected by hand after wading into shallow waters. Care was taken not to disturb sediments before sampling. Material was taken from the upper 5 cm with a 2.5-cm diameter open-end plastic syringe that was capped as it was pulled from the sediments. Sediments were extruded from the syringe into a solvent-cleaned glass jar, capped, placed on ice for transport, and frozen at the laboratory at the Universityof Miami. The contents of the jars were transported on dry ice to the laboratory at Wilmington, NC for analysis following the protocol of Mendoza et al. (2008).
Table 1.
Sample location with latitude, longitude, date and local time (EDT) of collection for sediment and seagrass epiphyte materials assayed for brevetoxin concentration in east Florida coastal waters, summer 2008. The samples are listed by latitude.
| Location | Latitude | Longitude | Date | Time |
|---|---|---|---|---|
| Jacksonville | 30.3699 | 81.3966 | July 23 | 0945 |
| St. Augustine | 29.7682 | 81.2579 | July 22 | 1825 |
| Whitney ML | 29.6702 | 81.2160 | July 22 | 1746 |
| Ponce Inlet | 29.0735 | 80.9169 | July 23 | 1520 |
| NewSmyrna Beach | 29.0715 | 80.9104 | July 23 | 1552 |
| Mosquito Lagoon* | 28.8090 | 80.7817 | August 5 | 1220 |
| Cocoa Beach* | 28.3572 | 80.6286 | August 5 | 1420 |
| Patrick AFB | 28.2331 | 80.5996 | July 22 | 1422 |
| Satellite Beach* | 28.1781 | 80.5897 | July 22 | 1406 |
| Melbourne | 28.0700 | 80.5790 | August 5 | 1730 |
| S. Mello Beach | 27.9560 | 80.5009 | July 22 | 1328 |
| South Melbourne* | 27.9520 | 80.5163 | August 6 | 1130 |
| Sebastian* | 27.7890 | 80.4400 | August 6 | 1315 |
| Vero Beach* | 27.6840 | 80.3860 | August 6 | 1430 |
| Ft. Pierce* | 27.4820 | 80.3330 | August 6 | 1700 |
| St. Lucie Inlet* | 27.1793 | 80.1818 | August 7 | 0845 |
| Jupiter Inlet* | 26.9740 | 80.0831 | August 6 | 1130 |
Site where epiphytes were collected.
After the sediments were lyophilized and dry weights obtained, the brevetoxins were extracted with a solvent of 1:1 dichloromethane:acetone and sonication. An internal standard of PbTx-9 was added to the dried sample before the extraction procedure. Following centrifugation the solvent layer was transferred to a glass vial, and the process repeated three more times. The pooled sample was dried under high purity N2 and held at −20° C. The dried material was suspended in 5 ml of a 85:15 (v/v) mixture of methanol:water with sonication for HPLC analysis.
The LC–MS analyses conditions were modified after Cheng et al. (2005). The extracts were separated by an Agilent 1100 LC series using a Phenomenex, LUNA, 3 μm, 50 mm × 2.0 mm, C18 column. The mobile phase was 50:50 acetonitrile:water (0.3% acetic acid) for the first 40 min, then changed to 5:95 acetonitrile:water for 2 min and finally back to 50:50 acetonitrile:water for 8 min. The flow rate was set at 0.2 ml min−1.
Structural identification, detection and quantification was carried out using a Thermo Finnigan TSQ Quantum triple quadrupole mass spectrometer, equipped with the electrospray ionization source (Thermo Fisher Scientific, Inc., USA). Nitrogen gas was used as both drying gas and nebulizing gas. The instrument was run using the selective reaction monitoring (SRM) mode. Optimized collision induced decomposition MS/MS spectra for each of the PbTx transitions were acquired at 4 V (PbTx-2), 18 V (PbTx-3) using argon as the collision gas at a pressure of 1.0 mTorr. The SRM transitions of the two types of toxins were set at 895.5–877.5 m/z (PbTx-2) and 897.5–725.3 m/z (PbTx-3). Scan events of product ions were set at scan width of 1.0 s with scan time of 0.25 s. Q1 and Q3 peak width were set at 0.70 s. Total acquired segment time ran for 40 min. The HPLC–MS/MS limit of detection was similar to Cheng et al. (2005) at 2.5 ng ml−1.
2.3. Seagrass epiphyte samples
Seagrass epiphytes were present at a sufficient density to provide samples at nine locations (Table 1). The epiphyte samples were collected concurrently with sediment samples. Shoots of Syringodium filiforme and Halodule wrightii were gently harvested by hand from depths of <1 m, placed in plastic bags, and stored on ice for transport to shore. In WF coastal ecosystems Thalassiates tudinum is often the most abundant dominant seagrass, with H. wrightii and S. filiforme comprising a lower proportion of the seagrass community. testudinum is much less abundant in the EF coastal ecosystems we sampled than in the WF coastal ecosystems.
Epiphytes were harvested within eight hours of collection by scraping the surface community from each shoot with a clean razor blade. The scraped material was collected on waterproof paper and transferred to a cleaned glass test tube with a Teflon-lined cap. Samples were frozen for transport to the lab. The epiphyte material was lyophilized and dry weights determined prior to a triple extraction with HPLC-grade methanol. The combined extracts were dried under N2 gas in solvent-cleaned test tubes. Each composite sample was then assayed for PbTx-3 equivalent concentrations in the laboratory of Dr. Kathleen Rein at Florida International University using the competitive enzyme-linked immunosorbent assay (ELISA) procedure of Naar et al. (2002).
The ELISA method provides a ‘total’ equivalent normalized to PbTx-3 (Naar et al., 2002). The competitive ELISA method has been successfully applied to quantify brevetoxin tissue burdens in shellfish (Naar et al., 2002), marine mammals (Flewelling et al., 2005), and fish (Fire et al., 2008; Naar et al., 2007), as well as seagrass and epiphytes (Flewelling et al., 2005; Flewelling, 2008), seawater, and marine aerosols (Pierce et al., 2005). Antibodies for the assay were derived from goats immunized with PbTx-3 conjugates (see Naar et al., 2007; Trainer and Baden, 1991). Although the method does not permit quantification of individual brevetoxins and their analogs, there is agreement between ELISA and HPLC–MS determined brevetoxins quantities in tissue samples from shellfish (Plakas et al., 2004; Pierce et al., 2006).
3. Results
3.1. The 2007 K. brevis bloom
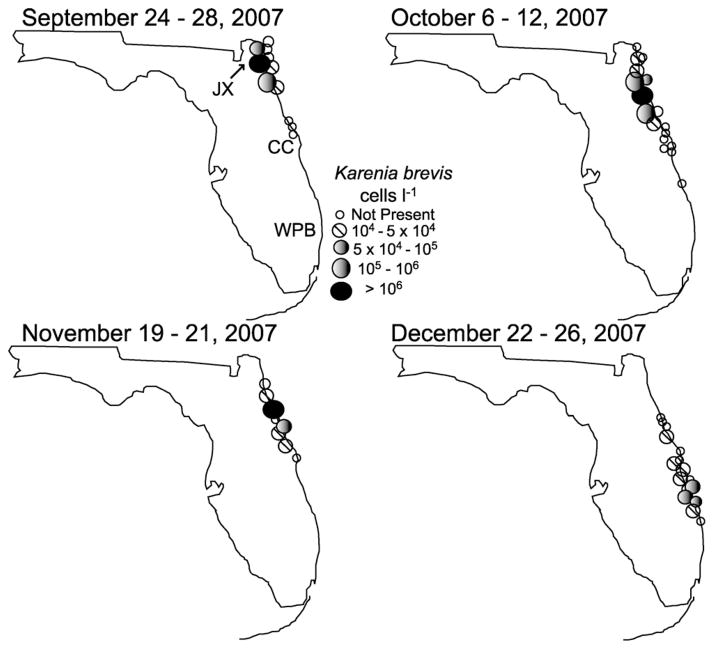
The 2007 bloom of K. brevis along the east coast of Florida was first observed in early September near Jacksonville, FL (Walsh et al., 2009). Thereafter, the bloom expanded to the south as summarized by the east coast distribution from the FWC HAB monitoring program (Fig. 2). There were relatively few stations with detectable K. brevis in the first three weeks of September. In the last week of September, a bloom was evident in the ICW at Hanna Park, near Jacksonville, FL where cell abundances exceeded 106 L−1 (Fig. 2). Following the initial detection of the bloom, the highest densities were found progressively south throughout October and November. By mid-October maximum densities along the east coast were recorded near St. Augustine (Figs. 1 and 2). Maximum cell concentrations exceeding 106 cells L−1 occurred in the ICW throughout October. While K. brevis was detectable along the beaches, the highest concentrations were in samples collected from the ICW.
Fig. 2.
The distribution of Karenia brevis along the east Florida coast in 2007.
Source: FWRI HAB Monitoring Program. The location of Jacksonville (JX), Cape Canaveral (CC) and West Palm Beach (WPB) are provided for reference.
In late November Karenia abundance still exceeded 106 cells L−1 near Cape Canaveral with >5 × 106 cells L−1 adjacent to Patrick Air Force Base. Cell densities remained at >106 cells L−1 in the first week of December in the Indian River Lagoon, Banana River, and ICW adjacent to Cape Canaveral. By mid-December the maximum cell concentrations in the southern ICW were 105–106 cells L−1 (Fig. 2). By late December, the maximum cell concentrations detected in EF coastal waters were 3 × 105 cells L−1, with highest concentrations between St. Lucie and Jupiter Inlet. Maximum abundances recorded at the southern terminus of the 2007 bloom were 103 cells L−1 at Jupiter Inlet FL. By early January 2008 K. brevis was nearly absent from the ICW, with isolated patches present at cell abundances of only 102–103 cells L−1 (data not shown). No K. brevis was found in the ICW after February 2008.
3.2. Sediment brevetoxin distribution
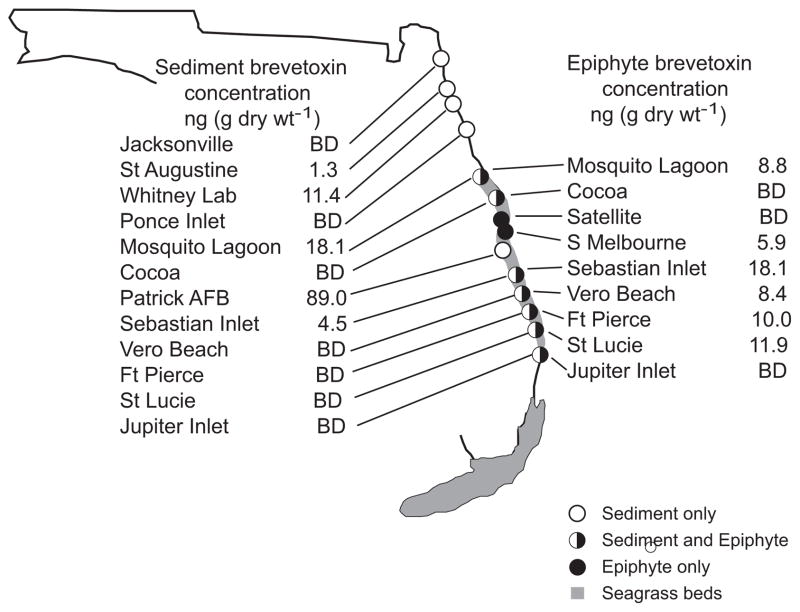
Detectable brevetoxins concentrations were present in five of the sediment samples collected in July and August 2008, although the distributions were spatially heterogenous (Fig. 3). The northernmost station at Hanna Park, near Jacksonville, FL, had water column cell concentrations exceeding 106 cells L−1 in September 2007 with no detectable sediment brevetoxins in July 2008. K. brevis cell concentrations also exceeded 106 cells L−1 in water samples collected in July 2007 between St. Augustine and the Mosquito Lagoon. However, sediment brevetoxin concentrations during the following summer were 1.3 ng (g dry wt)−1 and 11.4 ng (g dry wt)−1 at St. Augustine and the Whitney Laboratory, respectively. Further south, brevetoxins were absent from sediment samples at Ponce Inlet, but were present at 18.1 ng (g dry wt)−1 in the Mosquito Lagoon.
Fig. 3.
Brevetoxin concentrations in sediment and seagrass epiphytes collected during July and August 2008 along the east Florida coast. Concentrations are expressed as ng PbTx-3 equivalents (g dry wt)−1 of sample for sediments (left) and seagrass epiphytes (right).BD = below detection limit.
The highest sediment concentrations along the EF coast occurred in the IRL near Patrick Air Force Base (Fig. 3). This location corresponded to the October 2007 bloom epicenter (Fig. 2). The LC–MS analyses revealed that Patrick Air Force Base was the only location at which both parent brevetoxins were present. This sediment sample contained detectable concentrations of PbTx-1 at 0.9 ng (g dry wt)−1, a parent brevetoxin with the A backbone (Baden et al., 2005). A second parent brevetoxin, PbTx-2 with the B backbone, was also present at 15.0 ng (g dry wt)−1. Three additional brevetoxins found at this location were PbTx-3 (89.0 ng g dry wt)−1, PbTx-6 (1.0 ng g dry wt)−1 and PbTx-9 (4 ng g dry wt)−1, which are all analogs of PbTx-2. Much lower brevetoxin concentrations were found further south at Sebastian Inlet, where there was only 4.5 ng (g dry wt)−1. No brevetoxins were detected further south in the IRL sediments.
3.3. Seagrass epiphyte brevetoxin distribution
In general, epiphyte brevetoxins were not uniformly distributed in the ICW. Concentrations ranged from non-detectable to a maximum of 18 ng PbTx-3 (g dry wt)−1. In the northernmost sample from the Mosquito Lagoon brevetoxin was present at 8.8 ng (g dry wt)−1. In the vicinity of Cape Canaveral, seagrass epiphytes at Cocoa Beach and Satellite Beach had no detectable brevetoxins, while epiphyte communities sampled at South Melbourne contained 0.9 ng (g dry wt)−1.
The five seagrass beds sampled in the southern ICW contained detectable epiphyte brevetoxins at four of the five stations. These beds contain higher seagrass abundances than those located further north (Morris et al., 2000). Concentrations varied from 8.4 to 18.1 ng (g dry wt)−1 in four seagrass beds sampled between Sebastian Inlet and St. Lucie Inlet where sediment brevetoxins were absent (Fig. 3). The southernmost sample collected at Jupiter Inlet had no detectable brevetoxin in either the seagrass epiphytes or the sediments.
4. Discussion
4.1. Sediment brevetoxin distributions
Concentrations of sediment brevetoxins along the EF coast ranged from below detection to a maximum of 89 ng (g dry wt)−1, with most common values <20 ng (g dry wt)−1. These concentrations are comparable to, but generally less than, those in WF coastal sediments during non-bloom periods. A sediment survey utilizing ELISA analyses was completed in sediments adjacent to Charlotte Harbor in March through August 2002, and found concentrations from non-detectable to 62 ng (g dry wt)−1 with most values at 12–17 ng (g dry wt)−1 during non-bloom periods (Flewelling, 2008). Values exceeded 1000 ng (g dry wt)−1 during K. brevis blooms in 2002 (Flewelling, 2008). A subsequent study in April–December 2005 found 60–170 ng (g dry wt)−1 in sediments following the decline of a dense K. brevis bloom. In regions where the dinoflagellate was not present, sediment concentrations were undetectable to 43 ng (g dry wt)−1. Further south, near Ft. Myers, Mendoza et al. (2008) reported <1–12 ng (g dry wt)−1 in sediments from three locations in December 2006. As in our study, Mendoza and colleagues utilized liquid chromatography–mass spectrometry analyses to identify analogs. They reported 0.8–3.6 ng PbTx-2 (g dry wt)−1 at two stations, and 2.7–9.7 ng PbTx-3 (g dry wt)−1. Differences in analog concentrations in the west Florida sediments were attributed to the relative energy environment of sedimentary environments near Passes and at beaches.
Along the EF coast, high sediment brevetoxin occurred in the interior IRL (Patrick Air Force Base), and the Mosquito Lagoon. The highest sediment brevetoxins in the ICW occurred at Patrick Air Force Base where high cell concentrations (105–106 cells L−1) persisted in the IRL for more than a month in 2007. The presence of additional analogs in sediments at Patrick Air Force Base suggests that forms other than PbTx-2 and PbTx-3 could persist under favorable conditions in addition to PbTx-2 and PbTx-3. Brevetoxins were not detected in the sediments near inlets with extensive seagrass beds in the southern IRL. Our results support the hypothesis posed by Mendoza that less energetic conditions in lagoons may enhance deposition and the long-term persistence of brevetoxins, relative to the higher energy environment found near inlets.
4.2. Seagrass epiphyte communities
Epiphyte brevetoxin concentrations of 6–18 ng (g dry wt)−1 primarily occurred in seagrass beds in the southern IRL. These seagrass beds are densest at inlets that connect the IRL to the Atlantic Ocean (Morris et al., 2000). On the WF coast, seagrass epiphyte concentrations in the 2002 and 2005 studies were generally <50 ng (g dry wt)−1 during periods when K. brevis was present at <103 cells L−1 (Flewelling, 2008). During blooms, in contrast, epiphyte concentrations attain values as high as 1000–3000 ng (g dry wt)−1 in 2002 and 2005, respectively (Flewelling, 2008). Thus, brevetoxin concentrations in the east Florida seagrass epiphyte communities were within the range, though generally lower, than that in WF seagrass beds during non-bloom conditions.
Seagrass species composition could have influenced epiphyte brevetoxin concentrations in our samples from EF coastal populations, where H. wrightii and S. filiforme are the most abundant species. On the WF coast, in contrast, seagrass beds frequently have T. testudinum as the most abundant species, with H. wrightii and S. filiforme present at lower abundances. Brevetoxin concentrations are higher per unit dry weight in T. testudinum epiphyte communities than in other seagrasses, and brevetoxin may persist in T. testudinum for longer periods following bloom termination (Flewelling, 2008). The differences were attributed to morphology of Thalassia blades, which are wider, and could potentially provide enhanced retention of detritus.
Brevetoxin persistence in seagrass epiphytes for periods of several months following bloom termination suggests that some vector or processes transfers toxin between individual leaves. The average age of leaves for T. testudinum (Patriquin, 1973; Tomasko et al., 1996) and H. wrightii (Morgan and Kitting, 1984) are on the order of 30–40 days. S. filiforme blades have a comparable life span at 50 days (Zieman et al., 1979). The maximum life span of seagrass leaves of H. wrightii and S. filiforme is less than 50 days, while that of T. tesudinum is <100 days (see Fig. 1, Borowitzka et al., 2006).
The presence of brevetoxins six months after bloom termination in the ICW indicates that the toxins must be transferred within the seagrass bed as individual blades die, as supported by observations from west Florida coastal ecosystems (Flewelling, 2008). Motile organisms are likely one potential mechanism for brevetoxin transfer between individual seagrass blades. Motile invertebrate mesograzers such as copepods, isopods, and amphipods live several months and are primary grazers of seagrass epiphytes (Jernakoff et al., 1996). Amphipods are relatively resistant to brevetoxin toxicity (Sotka et al., 2009) and could likely serve as a vector transferring toxins in seagrass communities. In addition, some mobile filter feeders like the wing oyster Pinctada imbricata, can detach from senescing seagrass blades and reattach to living blades in the canopy (J. Fourqurean, personal observations), providing another potential vector.
4.3. Conclusions
The persistence of brevetoxins in sediments and epiphytes has led to the hypothesis that brevetoxins could serve as markers for prior blooms (Flewelling, 2008; Mendoza et al., 2008). The processes mediating brevetoxin transfer from K. brevis to sediments and seagrass epiphyte communities is largely unknown, as are in situ rates of toxin degradation. While routes of brevetoxin transfer have been documented in experimental pelagic communities (Turner and Tester, 1997; Tester et al., 2000) there are, to our knowledge, no comparable studies of toxin transfer in sediments or seagrass epiphyte communities.
One process that may contribute to toxin transfer to benthic communities is swimming behavior of bloom organisms. K. brevis frequently undergoes a diurnal vertical migration, as cells frequently swim descend from the surface near dusk (e.g., Heil, 1986). In an experiment, cultured K. brevis has been observed to swim into the interstitial space between sand grains (Sinclair and Kamykowski, 2006). This behavior could provide a vector for transfer of brevetoxins to sediments. Additionally, K. brevis has been reported to form a cyst in culture (Walker, 1982), with an additional report of a palmelloid stage in culture and in sediments from the WF shelf (Steidinger et al., 1998). While the full life history of this species has yet to be fully documented, the collective observations suggest that behavior and the life cycle could contribute to the transfer of brevetoxins into benthic communities. Experimental studies with cultures and mesocosms should address these potential routes of transfer, and further validate the use of sediment and epiphyte brevetoxins as biomarkers for this harmful algal species.
Acknowledgments
This research was supported through the Advanced Research Cooperation in Environmental Health (ARCH) Program jointly located at the Florida International University and University of Miami. This program is funded by the National Institute of Environmental Health Sciences through award S11 ES11181. Additional support was provided through NSF and NIEHS awards to the University of Miami Center for Oceans and Human Health (NIEHS #1 P50 ES12736 and NSF #OCE0432368/0911373). Professor Kelly Rein conducted the assays for epiphyte brevetoxin. Dr. D. Frazel provided logistic and technical support for collection of samples in the field.[SS]. This is contribution 532 from the Southeast Environmental Research Center at Florida International University
References
- Anderson DM, Hoagland P, Kaoru Y, White AW. Estimated Annual Economic Impacts from Harmful Algal Blooms (HABs) in the United States. WHOI-2000-11. Department of Biology, Woods Hole Oceanographic Institution; Woods Hole, MA: 2000. [Google Scholar]
- Baden DG, Bourdelais AJ, Jacocks H, Michelliza S, Naar J. Natural and derivative brevetoxins: historical background, multiplicity, and effect. 2005 doi: 10.1289/ehp.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka MA, Lavery PS, van Keulen M. Epiphytes of seagrass. In: Larkum AWD, editor. Seagrasses: Biology, Ecology and Conservation. Springer; Netherlands: 2006. pp. 441–461. [Google Scholar]
- Cheng YS, Villareal TA, Zhou Y, Gao J, Pierce RH, Wetzel D, Naar J, Baden DG. Characterization of red tide aerosol on the Texas coast. Harmful Algae. 2005;4:87–94. doi: 10.1016/j.hal.2003.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugbjerg N, Hansen G, Larsen J, Moestrup Ø. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia. 2000;39:302–317. [Google Scholar]
- Fire SE, Flewelling LJ, Naar J, Twiner MJ, Henry MS, Pierce RH, Gannon DP, Wang Z, Davidson L, Wells RS. Prevalence of brevetoxins in prey fish of bottlenose dolphins in Sarasota Bay, Florida. Mar Ecol Prog Ser. 2008;368:283–294. [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Dalpra D, Tamer R, Zaias J, Cheng YS, Pierces YS, Naar R, Abraham J, Clark W, Zhou R, Henry Y, Johnson MS, Van de Bogart G, Bossart GD, Harrington M, Baden DG. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Environ Health Perspect. 2005;113:650–657. doi: 10.1289/ehp.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling LJ. PhD Dissertation. University of South Florida; Tampa, FL: 2008. Vectors of brevetoxins to marine mammals; p. 144. [Google Scholar]
- Flewelling LJ, Naar JP, Abbott JP, Baden DG, Barros NB, Bossart GD, Bottein MY, Hammond DG, Haubold EM, Heil CA, Henry MS, Jacocks HM, Leighfield TA, Pierce RH, Pitchford TD, Rommel SA, Scott PS, Steidinger KA, Truby EW, Van Dolah FM, Landsberg JH. Red tides and marine mammal mortalities. Nature. 2005;435:755–756. doi: 10.1038/nature435755a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil CA. MS Thesis. University of South Florida; Tampa: 1986. Vertical migration of Ptycodiscus brevis (Davis) 575 Steidinger; p. 118. [Google Scholar]
- Heil CA, Steidinger KA. Monitoring, management, and mitigation of Karenia blooms in the eastern Gulf of Mexico. Harmful Algae. 2009;8:611–617. [Google Scholar]
- Jernakoff PA, Brearsley A, Nielsen J. Factors affecting grazer–epiphyte interactions in temperate seagrass meadows. Oceanogr Mar Biol Ann Rev. 1996;34:109–362. [Google Scholar]
- Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R, Zaias J, Bossart G, Baden DG. Literature review of Florida red tide: implications for human health. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci. 2002;10:113–390. [Google Scholar]
- Mendoza WG, Mead RN, Brand LE, Shea D. Determination of brevetoxin in recent marine sediments. Chemosphere. 2008;73:1373–1377. doi: 10.1016/j.chemosphere.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MD, Kitting CL. Productivity and utilization of the seagrass Halodule wrightii and its attached epiphytes. Limnol Oceanogr. 1984;29:1069–1079. [Google Scholar]
- Morris LJ, Virnstein RW, Miller JD, Hall LM. Monitoring seagrass changes in the Indian River lagoon Florida using fixed transects. In: Bortone SA, editor. Seagrass Monitoring, Ecology, Physiology, and Management. CRC Press; Boca Raton: 2000. pp. 167–175. [Google Scholar]
- Murphey EB, Steidinger KA, Roberts BA, Williams J, Jolley JW., Jr An explanation for the Florida east coast Gymnodinium breve red tide of November 1972. Limnol Oceanogr. 1975;20:481–486. [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, et al. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar JP, Flewelling LJ, Lenzi A, Abbott JP, Granholm A, Jacocks HM, Gannon D, Henry M, Pierce R, Baden DG, Wolny J, Landsberg JH. Brevetoxins, like ciguatoxins, are potent ichthyotoxic neurotoxins that accumulate in fish. Toxicon. 2007;50:707–723. doi: 10.1016/j.toxicon.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin D. Estimation of growth rate, production and age of the marine angiosperm Thalssia testudinum Koenig. Caribb J Sci. 1973;13:111–123. [Google Scholar]
- Pierce RH, Henry MS, Blum PC, Hamel SL, Kirkpatrick B, Cheng YS, Zhou Y, Irvin CM, Naar J, Weidner A, Fleming LE, Backer LC, Baden DG. Brevetoxin composition in water and marine aerosol along a Florida beach: assessing potential human exposure to marine biotoxins. Harmful Algae. 2005;4:965–972. [Google Scholar]
- Pierce RH, Henry MS, Blum PC, Plakas SM, Granade HR, Jester ELE, Said KRE, Dickey RW, Steidinger KA, Scott PS, Flewelling LJ, Wright JLC. In: Henshilwood K, Deegan B, Mc-Mahon T, Cusack C, Keaveney S, Silke J, O’Cinneide M, Lyons D, Hess P, editors. Comparison of methods for determination of brevetoxin and their metabolites in NSP-toxic bivalvedmolluscs; Proceedings of the Fifth International Conference on Molluscan Shellfish Safety; Galway Ireland. June 14–18, 2004; Rinville, Oranmore, Galway, Ireland: The Marine Institute; 2006. pp. 37–42. [Google Scholar]
- Plakas SM, Wang Z, Said KRE, Jester ELE, Granade HR, Flewelling L, Scott P, Dickey RW. Brevetoxin metabolism and elimination in the Eastern oyster (Crassostrea virginica) after controlled exposures to Karenia brevis. Toxicon. 2004;44:677–685. doi: 10.1016/j.toxicon.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Roberts BS. Occurrence of Gymnodinium breve red tides along the west and east coasts of Florida during 1976 and 1977. In: Taylor FJR, Seliger H, editors. Toxic Dinoflagellate Blooms; Proceedings of Second International Conference; New York: Elsevier; 1979. pp. 199–202. [Google Scholar]
- Sinclair GA, Kamykowski D. Benthic–pelagic coupling in sediment associated populations of Karenia brevis. J Plankton Res. 2006;30:829–838. [Google Scholar]
- Sotka EE, McCarty A, Monroe EA, Oakman N, Van Dolah FM. Benthic herbivores are not deterred by brevetoxins produced by the red tide dinoflagellate Karenia brevis. J Chem Ecol. 2009;35:851–859. doi: 10.1007/s10886-009-9658-9. [DOI] [PubMed] [Google Scholar]
- Steidinger KA, Vargo GA, Tester PA, Tomas CR. Bloom dynamics and physiology of Gymnodinium breve with emphasis on the Gulf of Mexico. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological Ecology of Harmful Algal Blooms. Springer Verlag; Berlin: 1998. pp. 134–153. [Google Scholar]
- Steidinger KA, Wolny JL, Haywood AJ. Identification of Kareniaceae (Dinophyceae) in the Gulf of Mexico. Nova Hedwigia Beiheft. 2008;133:269–284. [Google Scholar]
- Tester PA, Geesey ME, Vukovich FM. Gymnodinium breve and global warming: what are the possibilities? In: Smayda TJ, Shimizu Y, editors. Toxic Phytoplankton Blooms in the Sea. Elsevier; New York: 1993. pp. 67–72. [Google Scholar]
- Tester PA, Stump RP, Vukovich FM, Fowler PK, Turner JT. An expatriated tide bloom: transport, distribution, and persistence. Limnol Oceanogr. 1991;6:1053–1061. [Google Scholar]
- Tester PA, Turner JT, Shea D. Vectorial transport of toxins from the dinoflagellate Gymnodimun breve through copepods to fish. J Plankton Res. 2000;22:47–61. [Google Scholar]
- Tomasko DA, Dawes CJ, Hall MO. The effects of anthropogenic nutrient enrichment on turtle grass (Thalassiates tudinum) in Sarasota Bay, Florida. Estuaries. 1996;19:448–456. [Google Scholar]
- Trainer VL, Baden DG. An enzyme immunoassay for the detection of Florida red tide brevetoxins. Toxicon. 1991;29:1387–1394. doi: 10.1016/0041-0101(91)90126-c. [DOI] [PubMed] [Google Scholar]
- Turner JT, Tester PA. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol Oceanogr. 1997;42:1203–1214. [Google Scholar]
- Walker LM. Evidence for a sexual cycle in the Florida red tide dinoflagellate Ptychodiscus brevis (=Gymnodinmium breve) Trans Am Microsc Soc. 1982;101:287–293. [Google Scholar]
- Walsh JJ, Weisberg RH, Lenes JM, Chen FR, Dieterle DA, Zheng L, Carder KL, Vargo GA, Havens JA, Peebles E, Hollander DJ, He R, Heil CA, Mahmoudi B, Landsberg JH. Isotopic evidence for dead fish maintenance of Florida red tides, with implications for coastal fisheries over both source regions of the West Florida shelf and within downstream waters of the South Atlantic Bight. Prog Oceanogr. 2009;80:51–73. [Google Scholar]
- Weisberg RH, Barth A, Alvera-Azcarate A, Zheng L. A coordinated coastal ocean observing and modeling system for the West Florida continental shelf. Harmful Algae. 2009;7:585–597. [Google Scholar]
- Woofter RT, Brendtro K, Ramsdell JS. Uptake and elimination of brevetoxin in blood of striped mullet (Mugil cephalus) after aqueous exposure to Karenia brevis. Environ Health Perspect. 2005;113:11–16. doi: 10.1289/ehp.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman JC, Thayer GW, Robblee MB, Zieman RT. Production and export of seagrasses from a tropical bay. In: Livingston RJ, editor. Ecological Processes in Coastal and Marine Systems. Plenum Press; New York: 1979. pp. 21–33. [Google Scholar]