Abstract
Stress urinary incontinence is a condition that affects mainly women and is characterized by the involuntary loss of urine in conjunction with an increase in abdominal pressure but in the absence of a bladder contraction. In spite of the large number of women affected by this condition, little is known regarding the mechanics associated with the maintenance of continence in women. Urodynamic measurements of the pressure acting on the bladder and the pressures developed within the bladder and the urethra offer a potential starting point for constructing computational models of the bladder and urethra during stress events. The measured pressures can be utilized in these models to provide information to specify loads and validate the models. The main goals of this study were to investigate the feasibility of incorporating human urodynamic pressure data into a computational model of the bladder and the urethra during a cough and determine if the resulting model could be validated through comparison of predicted and measured vesical pressure. The results of this study indicated that simplified models can predict vesical pressures that differ by less than 5 cmH2O (<10%) compared to urodynamic pressure measurements. In addition, varying material properties had a minimal impact on the vesical pressure and displacements predicted by the model. The latter finding limits the use of vesical pressure as a validation criterion since different parameters can yield similar results in the same model. However, the insensitivity of vesical pressure predictions to material properties ensures that the outcome of our models is not highly sensitive to tissue material properties, which are not well characterized.
Keywords: Stress Urinary Incontinence, Finite element method, modeling, urodynamics
Introduction
Stress urinary incontinence (SUI) is characterized by the involuntary loss of urine from an increase in abdominal pressure in the absence of a bladder contraction that raises the vesical pressure to a level that exceeds urethral pressure1. In women, injuries to the pelvic floor musculature sustained during vaginal childbirth contribute to SUI. While not a life threatening condition, SUI can detrimentally impact quality of life as this condition is frequently manifested during such every day activities as coughing, laughing, sneezing or straining1. In spite of the large number of women affected by SUI, little is known about the mechanics associated with the maintenance of continence in women2.
Three theories dominate conceptualization of the mechanics of female continence: the pressure transmission theory proposed by Enhorning, the hammock theory proposed by Delancy, and the integral theory proposed by Petros3–6. However, the mechanics behind these theories have never been validated. Several modeling issues including the appropriate assignment of loads and the effect of material properties on model behavior must be addressed in order to construct such a model.
The main goals of this study were to investigate the feasibility of incorporating human urodynamic pressure data into a computational model of the bladder and the urethra during a cough and determine if the resulting model could be validated through the comparison of the predicted and measured vesical pressure. In addition, due to the lack of sufficiently detailed material properties of the relevant tissues resulting from the difficulty of acquiring in vivo values, this work also investigated the impact of material properties assigned to the modeled structures on the mechanics predicted by the model7,8.
Materials and Methods
In-Vivo Measurements
Following IRB approval, urodynamic data during coughs at maximum cystometric capacity was obtained from six continent women between the ages of 28 and 79 chosen at random from a larger data set. To qualify for this study, women were required to have no history of incontinence, pelvic floor surgery, or neuromuscular disease. Women were also excluded from the study if they had given birth vaginally within six months prior to urodynamic testing.
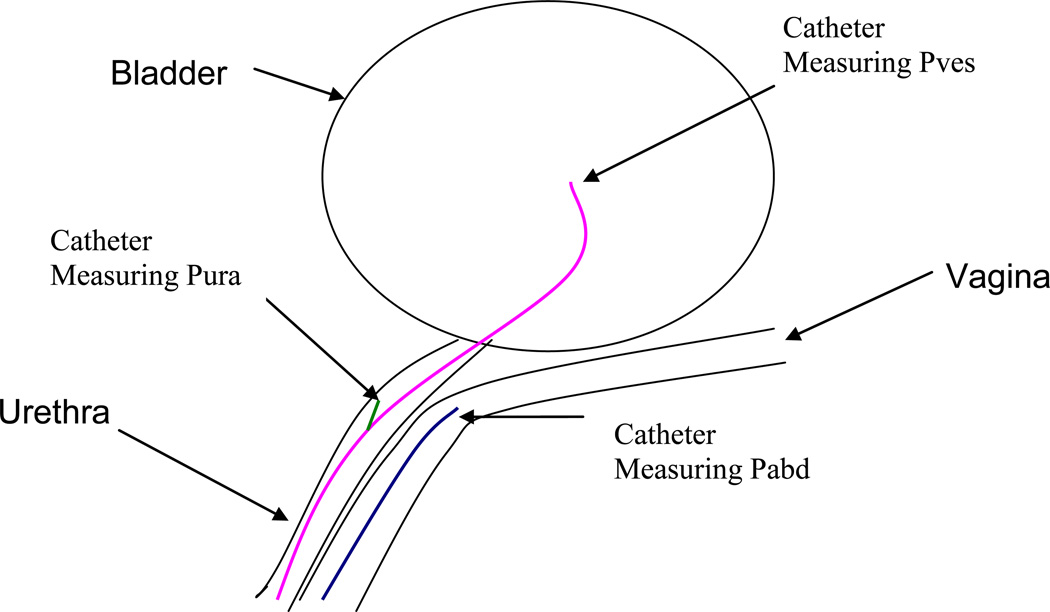
Cystometry was performed using sterilized water at a fill rate of 80 mL/min with the subject in a birthing chair reclined at 45 degrees until maximum cystometric capacity (MCC) was reached. To estimate abdominal pressure (Pabd), an 8 French micro tipped catheter (Millar Instruments, Houston, Texas) was placed in the vagina. Urethral pressure profilemetry (UPP) was performed through the use of an 8 French dual-micro tipped catheter with an infusion port (Millar Instruments, Houston, Texas). UPPs were acquired by initially placing the catheter so that both tips were located within the bladder. The catheter is then withdrawn at a rate of 1 mm per second while pressure is measured every 0.2s. The catheter was then reinserted so that the distal tip was located within the bladder to measure vesical pressure (Pves) and the proximal tip was located within the urethra at the position corresponding to the maximum urethral pressure measured during UPP, facing the 9 o’clock position to measure urethral pressure (Pura). Abdominal, vesical and urethral pressure data were acquired every 0.2s both with the participant at rest and during cough events (Figure 1)9, 10. Although Pura was not incorporated in the models created for this study, a complete description of the process by which the urodynamic data was obtained is provided for the sake of completeness and clarity.
Figure 1.
Schematic illustrating the positioning of the catheters for the acquisition of abdominal (blue), vesical (pink) and urethral (green) pressures during urodynamic testing.
Computational Model
The initial model for this study incorporated urodynamic data, including Pabd and Pves, obtained from a 28 year old continent female participant during a 1.8 second cough. This model was then used to determine which set of material properties (linear or nonlinear) when incorporated in the model would result in the most accurate prediction of Pves as well as the sensitivity of Pves to variations in material properties. Once determined, an additional 5 models were constructed utilizing the urodynamic data from 5 additional continent control participants selected at random from a larger data set to test whether the results obtained in the original model were reproducible in other similarly constructed models.
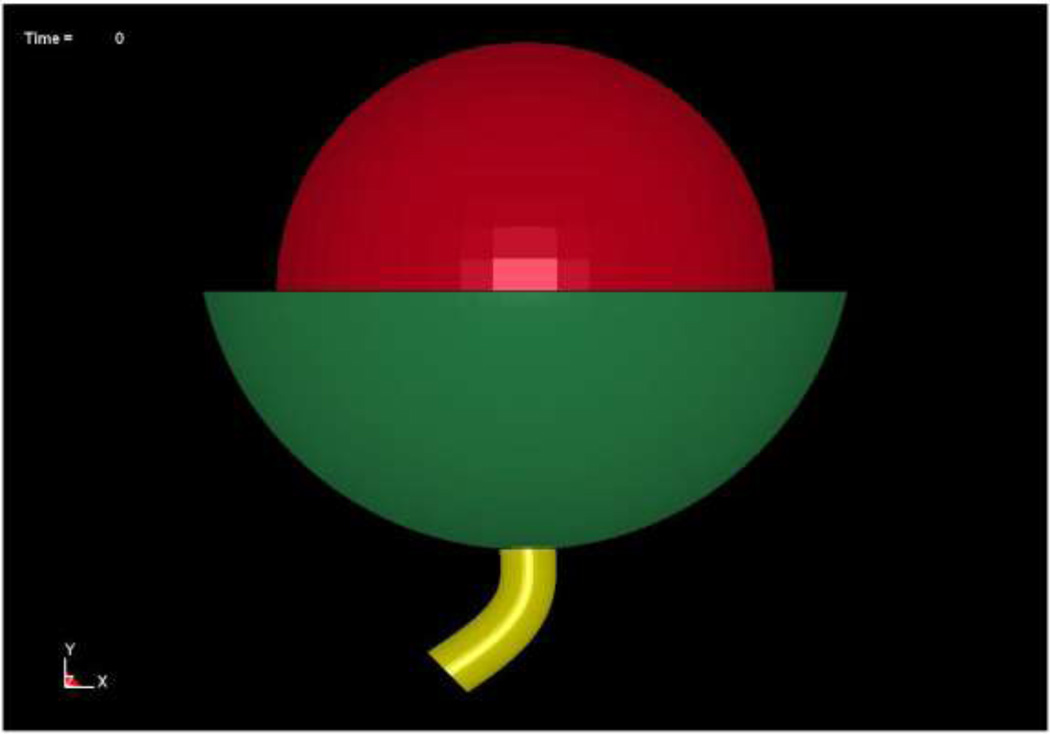
Several simplifying assumptions were made to create the geometry of the models as medical images were not available for this group of patients. These assumptions included modeling the bladder as a sphere, the urethra as a cylinder with no lumen and the support structures as a bowl shaped structure with a hole in the base through which the urethra could pass (Figure 2). The decision to model the bladder as a sphere was based on the work of Damaser and Lehman who determined that the material properties assigned to the bladder are more important in predicting the mechanics of this structure than the shape of the bladder11. The inner diameter of the bladder was set based on the MCC recorded for the subject. The thickness of the bladder wall was calculated based on the findings of Chan et al, who reported that average bladder wall thickness was 1.7mm when the bladder contained 200 cc of fluid in a cohort of normal women12. By assuming that bladder wall material is incompressible, as has been done previously, the wall thickness of the bladder could be calculated based on the subject’s MCC11,13.
Figure 2.
Finite element model based on urodynamic data acquired from a 28 year old continent female. Subject specific dimensions: bladder inner diameter = 100.8mm; bladder outer diameter = 102.6mm; urethral length = 34mm.
In the current model, the geometry of the urethra was simplified by modeling it as a cylindrical tube as has been done in several other studies utilizing computational models of the urethra14–17. The urethral length was set based on the duration of the subject’s UPP and the withdrawal rate of the catheter (1mm/s). The outer diameter of the urethra was set to 11.5 mm based on measurements published by Umek et al18,19. Since the participants utilized for this study were continent, we assumed that the urine remains in the bladder at all times, allowing the geometry to be simplified by not including a urethral lumen.
The support structure through which the urethra passes and on which the bladder rests was assumed to be bowl-shaped, based on drawings observed in anatomy text books20,21. The dimensions of the bowl were based on the dimensions of a pelvic inlet determined by Janda et al who reported the pelvic inlet to be elliptical in shape with a long axis dimension of 140 mm and a short axis dimension of 122 mm22. To further simplify the geometry, a circular shape was adopted by taking the average of the two axis values and setting the diameter of the top rim of the support structure to 131mm. The outlet of the bowl was set to be equal to the diameter of the urethra to prevent excessive movement of the urethra. As an initial approximation, the thickness of the bowl was set to 2 mm to equal the thickness of the levator ani muscle reported by D’Aulignac et al23. These dimensions were used in all models in this study.
All structures were represented in the models as a mesh of solid eight node hexahedral elements with the exception of the fluid which was represented using eight node hexahedral elastic fluid elements, allowing the fluid to be treated as a quasi-solid24,25. All meshes were created using the commercially available software package TrueGrid (XYZ Corporation Livermore, CA).
To prevent rigid body translation, the top rim of the support structure was completely constrained to fix this portion of the structure in space and prevent the rigid body motion of this structure. In addition, to prevent non physiologic movement of the urethra the walls of the urethral orifice were constrained to prevent motion along the x axis (right to left) and z axis (front to back) of the structure while allowing for the motion along the Y axis (top to bottom) of the structure in this region. Vaginal support was simulated by constraining the distal two-thirds of the dorsal wall of the urethra to prevent translation of this region of the urethra in all directions. In addition, this region was also constrained to prevent rotation about the z-axis Contact occurring between the structures was modeled using a surface to surface contact algorithm provided with the LS Dyna software package (LSTC Corporation, Livermore California).
Plan of Simulations
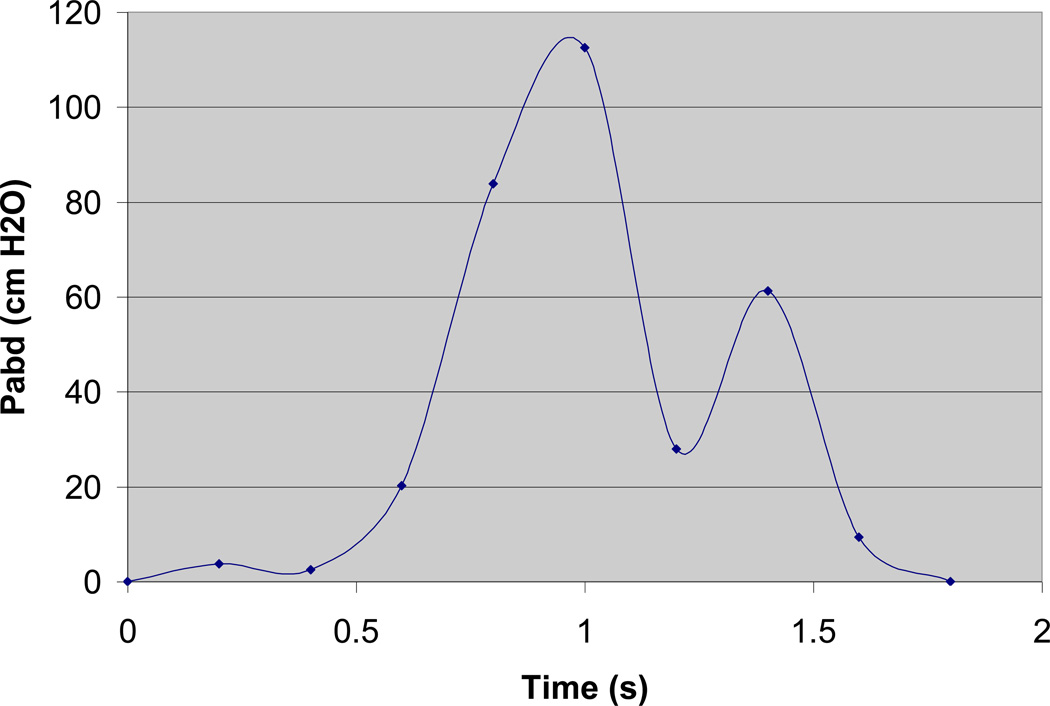
In each model a cough was simulated as a dynamic event with the magnitude of the pressure loads based on urodynamic measurements of Pabd during the cough event. Pabd acting on the bladder during a cough event was modeled as the change in Pabd relative to its baseline value with time (Figure 3). The time dependent pressure loads were applied to the top hemisphere of the bladder in such a manner that the magnitude of the pressure is equal at all nodes to which the pressure load was applied. Each of the structures incorporated in the model was defined utilizing the Lagrangian kinematic description. The time dependent equations relating force and displacement were solved with the explicit finite element solver LS Dyna. Each of the 6 models was loaded based on a unique cough event that was recorded for each participant. In modeling each cough event it was assumed that the rise in bladder pressure observed during a cough resulted solely from increased abdominal pressure that occurred in the absence of bladder contractions.
Figure 3.
Pressure loads acquired from the same 28 year old continent subject as modeled in Figure 1, during a 1.8 second cough event and normalized to baseline.
A sensitivity analysis was conducted to evaluate the effects of material properties on Pves predicted by the model. For the original models of the sensitivity analysis, two versions of the model based on the urodynamics of a continent 28 year old were constructed. The models were identical in every way except for the material properties assigned to the bladder and urethra (Table 1). In the first version of the model, the material comprising the bladder was defined as a Mooney Rivlin hyperelastic material, while the material comprising the bladder base and urethra was described as a Blatz Ko hyperelastic material based on the work of Haridas et al8. In the second version of the model the material comprising the bladder and urethra were defined as linearly elastic material based on the work of Yamada7,26. The support structure in both models were described as linearly elastic while the fluid was described as an elastic fluid whose deformation was determined based on the bulk modulus of the fluid24,25. These two original versions of the model were evaluated by comparing Pves predicted by each model to Pves measured during urodynamics. The effect of varying the material properties was evaluated by running the simulations detailed in Table 2 in both versions of the model. Each model was evaluated by comparing Pves predicted in each of the simulations to Pves predicted by the original linear or nonlinear version of the model, respectively.
Table 1.
Material Properties Used in Non-Linear and Linear Models (A and B = Experimentally determined constants for a Mooney Rivlin Hyperelastic material, E = Young’s Modulus, μ = Shear Modulus, K = Bulk Modulus, Poisson’s Ratio = 0.45 for all solid structures)
| Structure | Density (kg/m3) |
Material Type (Non-Linear Models) |
Constants(Non- Linear Models) |
Constants (Linear Models) |
|---|---|---|---|---|
| Bladder (Published) |
1030 | Mooney Rivlin | A=7500 B=2500 |
E = 5e4 Pa |
| Bladder (Stiff) |
1030 | Mooney Rivlin | A=15000 B=5000 |
E = 1e5 Pa |
| Bladder (Compliant) |
1030 | Mooney Rivlin | A=3750 B=1250 |
E = 3.75e4 Pa |
| Support Structure (Published) |
1040 | Linear Elastic | E = 5e6 Pa | E = 5e6 Pa |
| Support Structure (Stiff) |
1040 | Linear Elastic | E = 1e7 Pa | E = 1e7 Pa |
| Support Structure (Compliant) |
1040 | Linear Elastic | E = 3.75e6 Pa | E = 3.75e6 Pa |
| Urethra & Bladder Neck (Published) |
1030 | Blatz Ko | μ = 1e5 Pa | E = 5e6 Pa |
| Urethra & Bladder Neck (Stiff) |
1030 | Blatz Ko | μ = 2e5 Pa | E = 1e7 Pa |
| Urethra & Bladder Neck (Compliant) |
1030 | Blatz Ko | μ = 7.5e4 Pa | E = 3.75e6 Pa |
| Urine | 1000 | Elastic Fluid | K = 2.2e9 Pa | K = 2.2 e9 Pa |
Table 2.
Details of simulations used to test model sensitivity to material properties
| Simulation | Description |
|---|---|
| A | Published Values for all Structures |
| B | Stiff Bladder Wall |
| C | Stiff Support Structure |
| D | Stiff Urethra & Bladder Neck |
| E | Compliant Bladder Wall |
| F | Compliant Support Structure |
| G | Compliant Urethra & Bladder Neck |
| H | Stiff values for all structures |
| I | Compliant values for all structures |
To test the repeatability of the model, 5 additional models were constructed utilizing the urodynamic data from 5 additional continent control subjects as described above. In each case, the model was evaluated by comparing Pves predicted by the model to Pves measured during urodynamic testing. Nonlinear material properties were used in these models since our initial results demonstrated that Pves predicted by models incorporating nonlinear material properties more closely replicated measured Pves.
Results
The initial model used to test if the incorporation of non-linear and linear material properties would have an effect on the Pves predicted by the model was based on a 28 year old continent female. The inner diameter of the bladder which was set based on the MCC for this subject (535.7 mL) was set to 100.8 mm and the outer diameter was determined by the method described earlier was set to 102.6 mm. (Bladder wall thickness = 1.8 mm). The length of the urethra as determined from the UPP was 34 mm. In the five additional models used to validate the findings of the original model, the range of bladder inner diameter was 92.2–108 mm (mean 98.6 mm), corresponding to MCCs of 410–660mL, while the range of bladder wall thickness was 1.6–2.0 mm (mean = 1.9 mm). While the urethral length varied between 15.8–39.8 mm (mean 27.9 mm). The urodynamic measurements acquired during a 1.8 second cough were used to define the Pabd loads acting on the bladder in the initial model while the Pves measured during the event served to evaluate the Pves predicted by the model. In cough events utilized in constructing and validating the additional models varied between 1.4 and 2.0 seconds in duration.
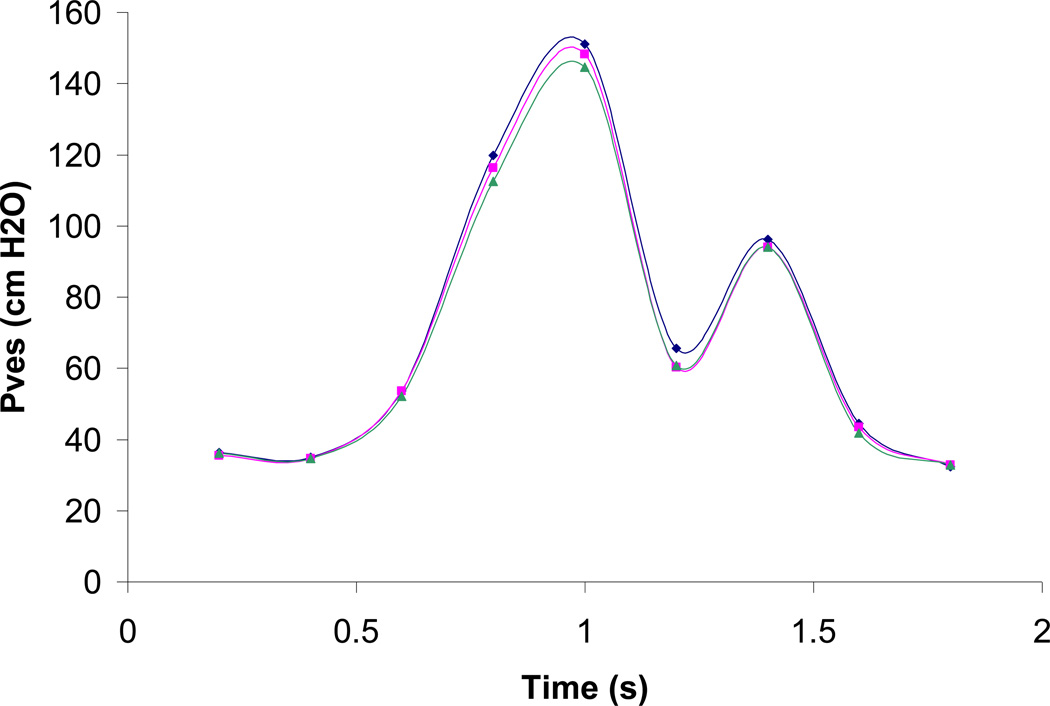
The original version of the model generally underestimated Pves compared to the measured values of Pves when either linear or non-linear published material properties were utilized. In both the original versions of the non-linear and the linear model, Pves was overestimated in only one instance and in both cases this overestimation was less than 1 cmH2O (3.1%). Pves predicted by the original model incorporating published nonlinear material properties was in closer agreement with measured Pves than those predicted by the original model incorporating published linear material properties (Figure 4). The least difference between measured and predicted values in the nonlinear model was −0.1cmH2O (0.2%), which occurred at 0.6s; whereas the greatest difference was −5.2 cmH2O (8.6%), which occurred 0.2 s after peak abdominal pressure. In contrast, in the model incorporating linear material properties the least difference between predicted and measured Pves was −0.28 cmH2O (0.8%), which occurred at 0.2s; while the greatest difference was −7.2 cmH2O (6.1%), which occurred 0.2s prior to peak abdominal pressure.
Figure 4.
Example Pves predicted by a model incorporating non-linear material properties (pink) and by a model incorporating all linear material properties (green) compared to Pves measured during urodynamics (blue).
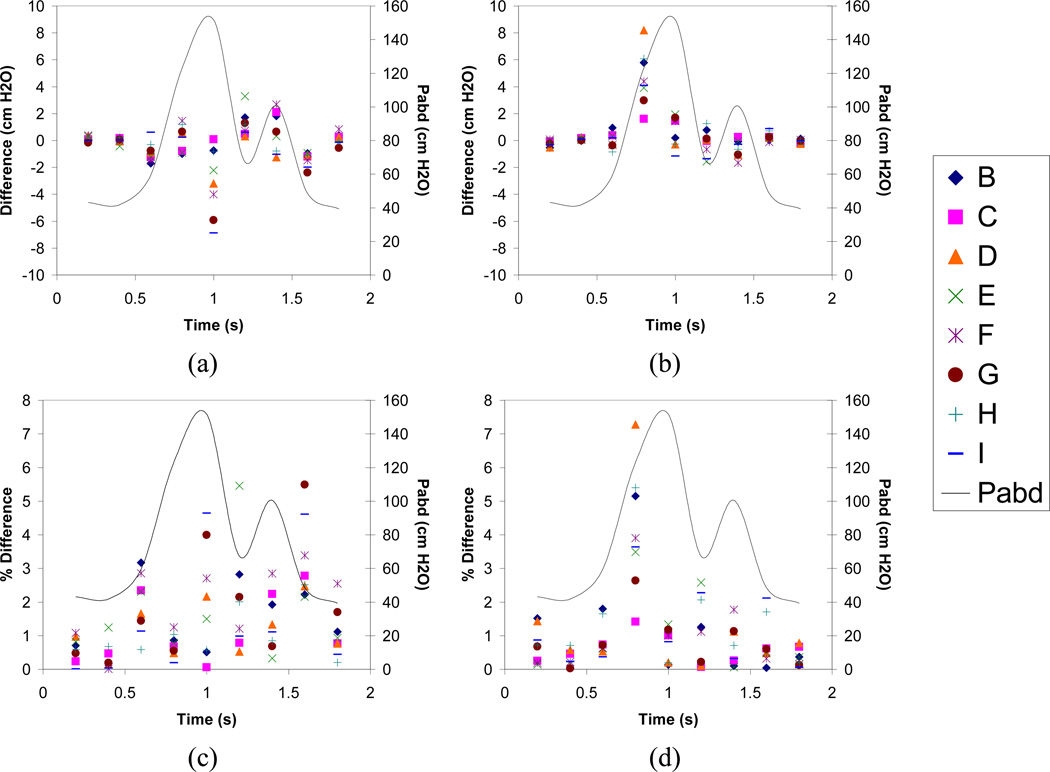
Altering material properties in the version of the model incorporating non-linear material properties produced changes of less than 7.0 cmH2O (5.4%) in predicted Pves in all simulations compared to the original version of the model (Figure 5). The greatest changes in Pves predicted by the model with nonlinear material properties resulted from increasing compliance of all modeled structures. Varying material properties in the version of the model with linear material properties resulted in increases in predicted Pves between 3.0 and 8.2 cmH2O (2.7% – 7.2%) at 0.8s compared to the original version of this model (Figure 5). However, these large increases were limited to this time, since at all other times these changes resulted in Pves changes of less than 2 cmH2O. The largest changes in Pves predicted by the version of the model with linear material properties were realized by stiffening the bladder neck and the urethra (Simulation D) and by stiffening the material properties of all modeled structures (Simulation H; Figure 5).
Figure 5.
Effect of varying material properties on Pves predicted by the models. All models were evaluated by comparing Pves predicted by each model to Pves predicted by the original model in which published values were incorporated for all structures (solid line). a. Differences vs. time in models incorporating non-linear material properties for the bladder and urethra. b. Differences vs. time in models incorporating all linear material properties. c. Percent differences vs. time for models incorporating non-linear material properties for the bladder and urethra. d. Percent differences vs. time for models incorporating linear material properties. Letters in legend denote simulation parameters identified in Table 2.
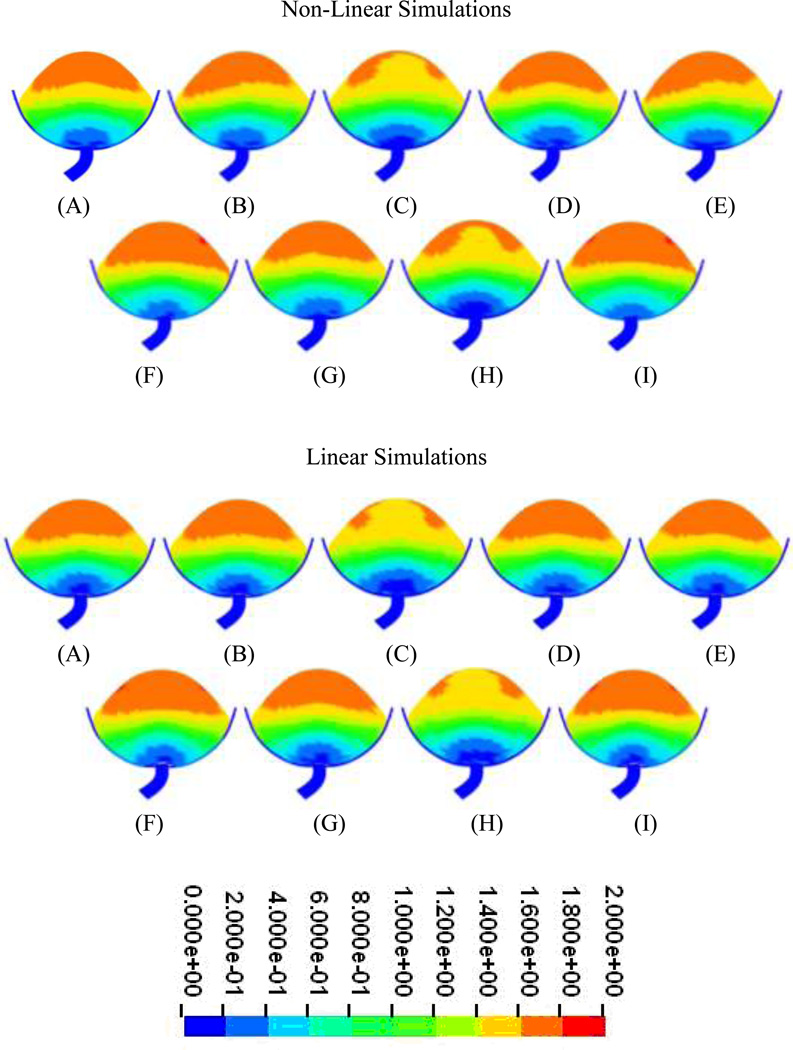
In all simulations in which nonlinear material properties were incorporated into the model, resultant displacements in all structures was less than 2cm (Figure 6). However, notable differences in displacement distribution were observed when the stiffness of the support structure was increased (Simulation C) and when the stiffness of all structures was increased (Simulation H) relative to the original version of this model. In both cases, a reduction in displacement is observed in the center top half of the bladder (Figure 6). Similar results were also noted in simulations in which the material properties of all structures were defined as being linear in that the resultant displacement in all structures was 2 cm or less (Figure 6). Reduced displacement was also observed in the simulations in which the support structure was stiffened (Simulation C) as well as in the simulation where all structures were stiffened (Simulation H). In both of these cases, displacement in the center of the top half of the bladder was also reduced.
Figure 6.
Displacements at peak pressure in models incorporating nonlinear material properties (top) and linear material properties (bottom). Letters denote simulation parameters identified in Table 2 (Displacements in cm).
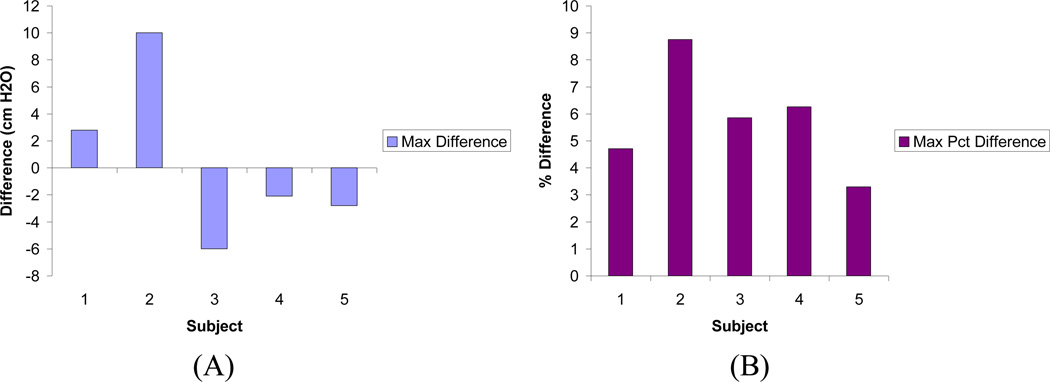
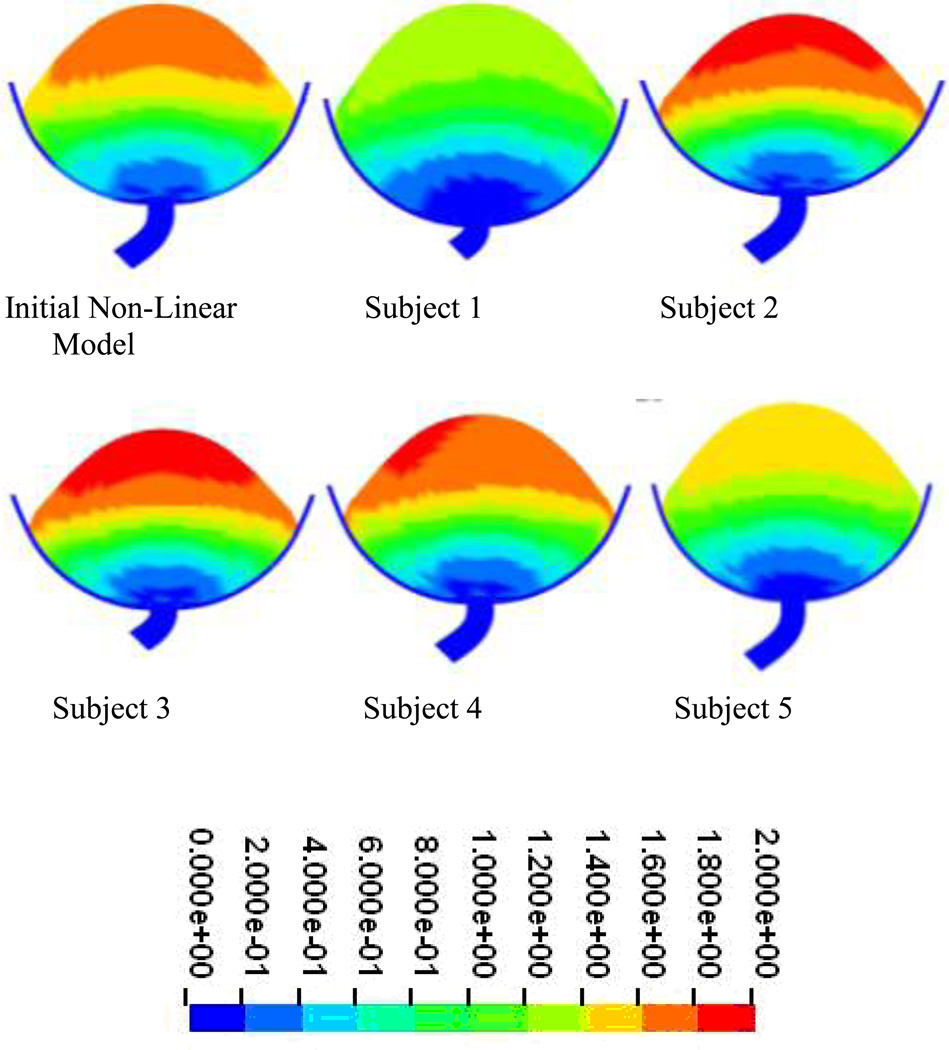
Similar results were obtained in the models generated using urodynamic data from 5 additional subjects incorporating published nonlinear material properties. In all models the maximum difference between predicted Pves and measured Pves was less than 10.0 cmH2O (8.8%), with 3 of the five producing maximum differences of 3.0 cmH2O (5%) or less (Figure 7). Deformation distributions were similar in all models with predicted maximum displacements of less than 2cm (Figure 8).
Figure 7.
Maximum differences (A) and percent differences (B) in Pves predicted by models created from urodynamic data of additional 5 subjects. Both maximum difference and maximum percent difference were determined relative to Pves measured during urodynamic testing for each subject.
Figure 8.
Displacements at peak pressure in models constructed from urodynamic data obtained from 5 additional subjects incorporating nonlinear material properties. It should be noted that peak pressure varied in each subject based on the cough event (Displacement in cm).
Discussion
In this work, a simplified computational model of the lower urinary tract was used to determine the feasibility of using urodynamics to simulate the mechanics of these structures during a cough. The female pelvic floor and lower urinary tract consist of complex structures that are not well characterized biomechanically. Most published descriptions of the geometry of these structures are qualitative in nature, focusing on the shape and location of each structure while providing minimal quantitative descriptions of geometry5,27. Therefore several simplifying assumptions were incorporated into our models.
In an ideal situation, the geometry of the modeled structures would have been obtained from medical images and the deformations of the structures also tracked and measured by medical images to allow for the validation of the model while urodynamic data would also be collected. However, this is not standard clinical practice. Even if medical images were acquired, the usefulness of these images would have been limited due to the fact that the medical images would have been acquired with the patient in the supine position whereas the urodynamic data was collected with the patient in a birthing chair reclined to 45 degrees. Furthermore, due to the transient and non-repetitive nature of coughs and the limitations of temporal resolution in MRI, soft tissue deformations could not be captured accurately for use as further validation criteria. As a result, several simplifying assumptions were made with respect to the geometry of the model. The bladder was assumed to be spherical in shape based on the work of Damaser and Lehman, who found that the shape of the bladder was not as important as the mechanical properties of the bladder wall when modeling its mechanics11. The decision to model the urethra as a tube was informed by the work of Bush et al who found that minimal differences existed in the pressure and flow data collected from a straight tube and the corresponding data collected from a patient specific model of the urethra during micturition28. The bowl shape geometry used in our model to represent the support structure was selected based on drawings of the levator ani muscle and pelvis in anatomy text books which show that these two structures form a bowl-like shape inside which the bladder rests20,21.
In this model two sets of material data were tested based on the work of Haridas et al and Zhang et al7,8. These sets were selected since the structures of interest in both cases were modeled as part of a larger model of the lower urinary tract and pelvic floor and not as individual structures. It is anticipated that any future model focusing on the mechanics underlying stress urinary incontinence will not be able to focus on individual structures but will need utilize a model of the entire pelvic floor and lower urinary tract. In addition, future models will need to account for fluid-structure interactions occurring during stress events. The complexity of these models will require simplifying assumptions of the material properties to generate solvable models. Therefore our model sought to determine if either material data set would impact Pves predicted by the model.
Pves predicted by the nonlinear model more closely matched Pves measured during urodynamic testing than Pves predicted by the linear model; however, the difference between predicted and measured Pves never exceeded 8 cmH2O (8%) in either model. Biological tissues are known to exhibit nonlinear behavior when subjected to outside forces. As a result, this outcome was not unexpected as one would assume that the nonlinear material properties would more closely replicate the in vivo behavior of the tissues manifested in the urodynamic measurements29,30.
To further investigate the effects of nonlinear and linear material properties on predicted Pves, a sensitivity analysis was performed in which the material properties of the two original models were stiffened or made more compliant in subsequent simulations. Varying material properties in this manner resulted in mean changes to predicted Pves of less than 3 cmH2O, and an average percent difference of less than 3%, indicating that material properties have minimal impact on Pves predicted by the model. As a result, the models cannot be described as singular when predicting Pves, since similar results can be obtained with different parameters defining the model. When coupled with the finding that varying the material properties of the bladder and urethra resulted in different deformation patterns, we conclude that Pves cannot be used as the sole validation criteria for the model. However, the insensitivity of predicted Pves to changes in material properties may prove beneficial in evaluating more complex models of the pelvic floor and lower urinary tract since this parameter could be utilized in the early stages of model construction to evaluate whether the model is appropriately constructed and loaded, as demonstrated by matching predicted Pves to measured Pves.
Mathematical biomechanical models have been shown to be useful for gaining insight into the underlying mechanics of various physiological phenomenon that cannot be acquired by any other means31. This study focused on determining if the increase in Pves resulting from an increase in Pabd recorded during clinical urodynamics could be replicated in such a model and how varying that models parameters would affect the Pves prediction of the model. One of the most difficult tasks encountered when constructing a biomechanical model is validating that the model is an accurate representation of the physiological phenomenon that it is being used to study. Frequently clinically obtained data is utilized for this purpose with the model considered to be accurate if the clinical data is replicated by the model31. Pves measured during urodynamics offers a potential method for validating future models of the lower urinary tract being used to study stress urinary incontinence as by definition in this condition the rise in Pves is the direct result of forces acting on the bladder in this case Pabd. Therefore in order to be considered accurate this increase must be accurately replicated. This study showed that this could be achieved in this simple model to within 5 cm of H2O. which indicates that the model can accurately reproduce this increase in Pves. However, it was also noted that this increase was not sensitive to other modeling parameters such as material properties.
In an ideal situation, medical images would be acquired during the stress event and the displacement of the modeled structures would be used as validation criteria. Unfortunately the highly transient nature of the events modeled and the temporal resolution required, coupled with the need to acquire urodynamic data simultaneously, makes this approach unfeasible at this time. The ultimate goal of modeling in stress urinary incontinence is to study the mechanics behind which leakage occur. In such a model a potential validation criteria would be if the model could accurately predict whether a leak would result during a given stress event, along with accurate prediction of vesical pressure. However, this would require the incorporation of a collapsible tube representing the urethra and the forces that act on that tube to maintain it in its collapsed orientation, which are poorly understood. Accurate prediction of the vesical pressure in such a model will still be critical to determine if the pressure upstream of the urethra in the bladder is accurate and sufficient to generate flow under the appropriate circumstances.
The finding that varying material properties of the model did not appreciably affect quantitative displacements agrees with the findings of Chi et al32. Using image guided radiotherapy, they found that increasing or decreasing the linear material properties of the bladder wall did not significantly affect its deformation32. Displacements predicted by this model are also qualitatively in agreement with those published by Zhang et al., who found in their model of a subject landing a jump, that the largest displacements in the bladder appear to be in the top portion of the bladder dome with only minimal displacements occurring in the bladder neck and urethra7. It should be noted that their model did not incorporate urodynamic data either as loads or as validation criteria7. Our results also meet one of the validation criteria proposed by Kim in his two dimensional model of the bladder and urethra during increased abdominal pressure: that the inferior displacement of the pelvic floor was less than 2cm13.
Although varying material properties resulted in little quantitative difference in the deformations predicted by the model, it produced qualitative asymmetric differences in the deformation patterns predicted by the model. The greatest differences in the deformations predicted by the model were realized by stiffening the support structure and stiffening all structures in both the linear and the non-linear models, which resulted in a decrease in deformation as was expected.
Conclusions
We found that simplified models can replicate Pves measured during urodynamics within 5 cmH2O and that varying material properties had minimal impact on Pves and displacements predicted by the model. The finding that varying the material properties had minimal impact on Pves predicted by the model limits the use of Pves as a validation criterion. Pves cannot be considered singular since similar results can be obtained when different parameters are used to define the model. However, the insensitivity of Pves ensures that the outcome of our models is not highly sensitive to tissue material properties, which are not well characterized.
Acknowledgements
This study was funded in part through the following grants: K24 DK064044-03 and K23 HD047325-010. Additional support was provided by the Ohio Supercomputer Center (Columbus OH), the Cleveland Clinic, and the Rehabilitation Research and Development Service of the Department of Veterans Affairs.
Reference List
- 1.Norton P, Brubaker L. Urinary Incontinence in Women. Lancet. 2006:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Women's Health Research: Progress, Pitfalls and Promise. Washington DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 3.Enhorning G, Miller ER, Hinman F. Simultaneous recording of intravesical and intraurethral pressure. Acta Chirurgica Scandinavica. 1961:1–68. [PubMed] [Google Scholar]
- 4.Delancey JOL. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. American Journal of Obstetrics and Gynecology. 1994:1713–1720. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 5.Ashton-Miller JA, Delancey JOL. Functional Anatomy of the Female Pelvic Floor. Annals of the New York Academy of Sciences. 2007:266–296. doi: 10.1196/annals.1389.034. [DOI] [PubMed] [Google Scholar]
- 6.Petros PE, Ulmsten UI. An Integral Theory of Female Urinary Incontinence. Acta Obstetricia et Gynecologica Scandinavica. 1990:7–31. doi: 10.1111/j.1600-0412.1990.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Kim S, Erdman AG, Roberts KP, Timm GW. Feasibility of using a computer modeling approach to study sui induced by landing a jump. Annals of Biomedical Engineering. 2009:1425–1433. doi: 10.1007/s10439-009-9705-2. [DOI] [PubMed] [Google Scholar]
- 8.Haridas B, Hong H, Minoguchi R, Owens S, Osborn T. PelvicSim - A Computational Experimental System for Biomechanical Evaluation of Female Pelvic Floor Organ Disorders and Associated Minimally Invasive Interventions. Studies in Health Technology and Informatics. 2006:182–187. [PubMed] [Google Scholar]
- 9.Abrams P, Blaivas JG, Stanton SL, Andersen JT. The Standardization of Terminology of Lower Urinary Tract Function Recommended by the International Continence Society. Intenational Urogynecology Journal. 1990:45–58. [PubMed] [Google Scholar]
- 10.Lose G, Griffiths D, Hosker G, Kulseng-Hanssen S, Perucchini D, Schafer W, Thind P, Versi E. Standardization of Urethral Pressure Measurement: Report from the Standardisation Sub-Committee of the International Continece Society. Neurourology and Urodynamics. 2002:258–260. doi: 10.1002/nau.10051. [DOI] [PubMed] [Google Scholar]
- 11.Damaser MS, Lehman SL. The Effect of Urinary Bladder Shape on its Mechanics During Filling. Journal of Biomechanics. 1995:725–732. doi: 10.1016/0021-9290(94)00169-5. [DOI] [PubMed] [Google Scholar]
- 12.Chan L, The S, Titus J, Tse V. The value of bladder wall thickness measurement in the assessment of overactive bladder syndrome. Ultrasound in Obstetrics and Gynecology. 2005:460. [Google Scholar]
- 13.Kim KJ. Biomechanical Analyses of Female Stress Urinary Incontinence. Doctor of Philosophy (Mechanical Engineering) Dissertation. University of Michigan; 1994. [Google Scholar]
- 14.Spangberg A, Terio H, Engberg A, Ask P. Quantification of urethral function based on Griffiths model of flow through elastic tubes. Neurourology and Urodynamics. 1989:29–52. [Google Scholar]
- 15.Bastiaanssen EHC, van Leeuwen JL, Vanderschoot J, Redert PA. A myocybernetic model of the lower urinary tract. Journal of Theoretical Biology. 1996a:113–133. doi: 10.1006/jtbi.1996.0011. [DOI] [PubMed] [Google Scholar]
- 16.Bastiaanssen EHC, Vanderschoot J, van Leeuwen JL. State-space analysis of a myocybernetic model of the lower urinary tract. Journal of Theoretical Biology. 1996b:215–227. doi: 10.1006/jtbi.1996.0098. [DOI] [PubMed] [Google Scholar]
- 17.Hosein RA, Griffiths DJ. Computer simulation of the neural control of the bladder and urethra. Neurourology and Urodynamics. 1990:601–618. [Google Scholar]
- 18.Umek WH, Laml T, Stutterecker D, Obermair A, Leodolter S, Hanzal E. The urethra during pelvic floor contraction observations on three-dimensional ultrasound. Ultrasound in Obstetrics and Gynecology. 2002:796–800. doi: 10.1016/s0029-7844(02)02146-4. [DOI] [PubMed] [Google Scholar]
- 19.Umek WH, Obermair A, Stutterecker D, Hausler G, Leodolter S, Hanzal E. Three-dimensional ultrasound of the female urethra: comparing transvaginal and transrectal scanning. Obstetrics and Gynecology. 2001:425–430. doi: 10.1046/j.1469-0705.2001.00416.x. [DOI] [PubMed] [Google Scholar]
- 20.Drake RL, Vogl W, Mitchell AWM. Gray's Anatomy for Students. New York, NY: Elsevier Churchill Livingstone; 2005. [Google Scholar]
- 21.Netter FH. Atlas of Human Anatomy. Philadelphia: Saunders Elsevier; 2006. [Google Scholar]
- 22.Janda S, van der Helm FCT, de Blok SB. Measuring Morphological Parameters of the Pelvic Floor for Finite Element Modeling Purposes. Journal of Biomechanics. 2003:749–757. doi: 10.1016/s0021-9290(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 23.D'Aulignac D, Martins JAC, Pires EB, Mascarenhas T, Natal Jorge RM. A shell finite element model of the pelvic floor muscles. Computer Methods in Biomechanics and Biomedical Engineering. 2005:339–347. doi: 10.1080/10255840500405378. [DOI] [PubMed] [Google Scholar]
- 24.Hallquist JO. LS Dyna Keyword Manual. Livermore Software Technology Co.; 2008a. [Google Scholar]
- 25.Hallquist JO. LS Dyna Theory Manual. Livermore Software Technology Co.; 2008b. [Google Scholar]
- 26.Yamada H. Strength of Biological Materials. Baltimore, MD: Williams and Wilkins; 1970. [Google Scholar]
- 27.Haderer JM, Pannu HK, Genadry R, Hutchins GM. Controversies in Female Urethral Anatomy and their Significance for Understanding Urinary Continence: Observations and Literature Review. International Urogynecology Journal. 2002:236–252. doi: 10.1007/s001920200051. [DOI] [PubMed] [Google Scholar]
- 28.Bush MB, Petros PE, Barrett-Lennard BR. On the flow through the human female urethra. Journal of Biomechanics. 1997:967–969. doi: 10.1016/s0021-9290(97)00050-x. [DOI] [PubMed] [Google Scholar]
- 29.Fung YC. Biomechanics Mechanical Properties of Living Tissues. New York: Springer-Verlag; 1993. [Google Scholar]
- 30.Taber L. Nonlinear Theory of Elasticity: Applications in Biomechanics. River Edge, NJ: World Scientific Publishing Co.; 2004. [Google Scholar]
- 31.Anderson AE, Ellis BJ, Weiss JA. Verification, validation and sensitivity studies in computational biomechanics. Computer Methods in Biomechanics and Biomedical Engineering. 2007:171–184. doi: 10.1080/10255840601160484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi Y, J Liang J, Yan D. A material sensitivity study on the accuracy of deformable organ registration using linear biomechanical models. Mecical Physics. 2006:421–433. doi: 10.1118/1.2163838. [DOI] [PubMed] [Google Scholar]