Abstract
Objective
To compare the response in quality of life (QoL) to growth hormone (GH) replacement in women with GH deficiency (GHD) and a history of acromegaly with that in women with GHD of other causes.
Methods
Fifty-five women with GHD were studied: 17 with prior acromegaly and 38 with other causes of GHD. We compared two 6-month, randomized, placebo-controlled studies of GH therapy in women with hypopituitarism conducted with use of the same design—one in women with a history of acromegaly and one in women with no prior acromegaly. QoL was assessed with the following questionnaires: the QoL-Assessment of Growth Hormone deficiency in Adults (AGHDA), the Symptom Questionnaire, and the 36-Item Short-Form Health Survey (SF-36).
Results
The 2 groups had comparable mean pretreatment age, body mass index, and QoL scores and comparable mean GH dose at 6 months (0.61 ± 0.30 versus 0.67 ± 0.27 mg daily). After 6 months of GH replacement therapy, women with GHD and prior acromegaly demonstrated a greater improvement in AGHDA score, four SF-36 subscales (Role Limitations due to Physical Health, Energy or Fatigue, Emotional Well-Being, and Social Functioning), and the Somatic Symptoms subscale of the Symptom Questionnaire than did women with GHD of other causes. Poorer pretreatment QoL was associated with a greater improvement in QoL after administration of GH.
Conclusion
In this study, GH replacement therapy improved QoL in women with GHD and a history of acromegaly but not in women with GHD due to other hypothalamic and pituitary disorders. Further studies are needed to determine the long-term risks versus benefits of GH replacement in patients who develop GHD after definitive treatment for acromegaly.
INTRODUCTION
Acromegaly is associated with an impaired quality of life (QoL), which persists after cure (1,2). Moreover, QoL is more impaired in patients in whom growth hormone deficiency (GHD) develops after cure of acromegaly than in those with normal growth hormone (GH) reserve (3). In a 6-month, randomized, placebo-controlled study, we recently demonstrated that treatment with GH significantly improves QoL in patients who have developed GHD after treatment of acromegaly (4). These data are consistent with studies that have shown that GHD in men and women with hypothalamic and pituitary diseases other than acromegaly is associated with a notable decrease in QoL, which is substantially ameliorated by GH replacement therapy (5–8). It is not known, however, whether patients with a history of acromegaly are more likely or less likely to respond to GH replacement therapy than are patients with other hypothalamic and pituitary diseases.
We compared the effect of 6 months of GH replacement therapy on the QoL in women with GHD cured of acromegaly versus the QoL of women who developed GHD after treatment of other hypothalamic and pituitary disorders. We chose to study women because QoL has been reported to be more impaired in women with GHD than in their male counterparts (9). Evaluation of QoL was carefully performed by using 3 validated questionnaires, which have been used in patients with a wide range of diseases to assess psychologic and physical well-being (10–13). We sought to determine whether QoL responds more robustly to GH replacement therapy in women who develop GHD after definitive treatment of acromegaly than in women with GHD related to other hypothalamic and pituitary diseases.
PATIENTS AND METHODS
Study Subjects
Fifty-five women with GHD were included in the study. Thirty-eight patients had been diagnosed with GHD attributable to nonsomatotroph disease or its treatment (Cushing disease in 9, nonfunctioning adenoma in 8, prolactinoma in 7, craniopharyngioma in 7, empty sella in 3, Rathke cleft cyst in 1, pituitary stalk lesion in 1, thyroid-stimulating hormone-secreting adenoma in 1, and Sheehan syndrome in 1). The clinical characteristics of this group and the response of body composition and cardiac risk markers to GH replacement therapy were previously reported (14), but no QoL evaluation before or after administration of GH has been published previously. Seventeen patients had been diagnosed with GHD after defnitive treatment of acromegaly. These patients are a subset of a cohort of 30 subjects with GHD and prior acromegaly (17 women and 13 men) in whom the effect of GH replacement therapy on body composition, cardiovascular risk markers, and QoL variables was reported for men and women combined (4), but not for the female subset independently. The QoL for a subset of these patients at baseline (pretreatment) has been reported by Wexler et al (3). One participant in the first-mentioned study (14) dropped out before the 6-month scheduled visit, data from 1 female subject in the study by Wexler et al (3) were excluded because of the initiation of appetite suppressants during the study, and 5 women with partial GHD were excluded from the current analysis.
The cohort was classified on the basis of diagnosis (history of acromegaly versus no history of acromegaly) and randomization assignment (GH versus placebo), as follows:
Group A: Women with GHD and prior acromegaly receiving placebo (n = 8)
Group B: Women with GHD and prior acromegaly receiving GH therapy (n = 9)
Group C: Women with GHD and no history of acromegaly receiving placebo (n = 19)
Group D: Women with GHD and no history of acromegaly receiving GH therapy (n = 19)
GHD was defined by using the following criteria: peak GH level of <5 ng/mL on an insulin tolerance test (5 patients in group C and 2 in group D) or a growth hormone-releasing hormone-arginine test (6 patients in group A, 7 in group B, 9 in group C, and 8 in group D) or an insulinlike growth factor-I (IGF-I) level more than 2 standard deviations (SD) below the age-specific reference range and at least 3 concomitant anterior pituitary deficiencies (15) (2 patients in group A, 2 in group B, 5 in group C, and 9 in group D). For the insulin tolerance test and the growth hormone-releasing hormone-arginine test, standard testing protocols, as previously reported (16), were used.
All study subjects were required to have been taking stable doses of all hormone replacement therapies for at least 3 months before entering the studies. Exclusion criteria included pharmacologic therapy for acromegaly, history of acromegaly for patients in groups C and D, untreated adrenal, thyroid, or gonadal steroid hormone deficiency (except for women ≥50 years of age), GH therapy within the prior year (10 patients without a history of acromegaly and none with a history of acromegaly had received GH therapy in the past), unstable cardiovascular disease, congestive heart failure (New York Heart Association Class II, III, or IV), uncontrolled hypertension or diabetes mellitus, pregnancy or breastfeeding within 1 year before study enrollment, history of malignant disease (except for nonmelanoma skin cancer), hemoglobin <11.0 g/dL, alanine aminotransferase or aspartate aminotransferase >3 times the upper limit of normal or serum creatinine level >2.5 mg/dL, and active carpal tunnel syndrome. All study subjects with prior acromegaly were required to have undergone a colonoscopy, and women 40 years of age or older were required to have had screening mammograms within 1 year before the baseline visit. The study was approved by the Partners Healthcare, Inc. and Massachusetts Institute of Technology Institutional Review Boards, and written informed consent was obtained from all study participants.
Study Protocol
The recruitment procedures and the design of the studies have been reported elsewhere (4,14). The studies were conducted by the same investigators in the same facilities and used identical methods except for the following: (1) one protocol recruited subjects with a history of acromegaly, and the other specifically excluded such patients; (2) one protocol was single-blinded, and the other was double-blinded; (3) one protocol included men and women, and the other included women only—in the former case, the randomization was stratified by sex; and (4) one protocol allowed patients with partial GHD to participate; however, we excluded all subjects with partial GHD for this analysis. Study subjects participated in 6-month, randomized, placebo-controlled protocols performed at the Massachusetts Institute of Technology and Massachusetts General Hospital General Clinical Research Centers and the Harvard Medical School Clinical Translational Science Center; overall, the same dose titration strategy was used. After measurement of IGF-I levels and QoL at baseline, subjects were randomly assigned to receive daily subcutaneously administered recombinant human GH (Genotropin, Pfizer Inc.) or placebo (Pfizer Inc.), which was identical in appearance to the GH, for 6 months. Randomization was stratified for use of orally administered estrogen (in both protocols). The starting dosage was 3 µg/kg daily in women older than 50 years who were not receiving estrogen orally. The starting dosages were 5 µg/kg daily and 6 µg/kg daily in women younger than 50 years who were not receiving orally administered estrogen and in women younger than 50 years who were receiving estrogen orally, respectively. Follow-up visits were scheduled for 1, 3, 4, 5, and 6 months after baseline testing.
QoL was assessed as previously described (3,4) at baseline and after 6 months of treatment in all study subjects. Specifically, the following 3 self-administered questionnaires were used. The QoL-Assessment of Growth Hormone Deficiency in Adults (AGHDA) is a disease-generated questionnaire (12), specifically designed to evaluate QoL in adult patients with GHD. Higher scores are indicative of a poorer QoL. The Symptom Questionnaire is a validated 92-item questionnaire including 4 subscales: (1) Anxiety, (2) Depression, (3) Somatic Symptoms, and (4) Anger or Hostility (10). Higher scores on this questionnaire are associated with greater severity of symptoms. Scores between 1 and 2 SD above the mean for healthy control subjects suggest moderate distress, whereas scores greater than 2 SD above the mean are indicative of severe distress or a psychopathologic condition (10). The 36-Item Short-Form Health Survey (SF-36) is a validated questionnaire that addresses short-term well-being during the prior month. The following subscales are specifically evaluated: (1) Physical Functioning, (2) Role Limitations due to Physical Health (RLPH), (3) General Health Perception, (4) Bodily Pain, (5) Energy or Fatigue, (6) Emotional Well-Being (EWB), (7) Role Limitations due to Emotional Health (RLEH), and (8) Social Functioning (11,13). Higher scores on this survey reflect a better QoL.
Assays
Serum samples were stored at −80°C. Serum IGF-I levels were measured with the Immulite 2000 automated immunoanalyzer (Siemens Healthcare Diagnostics, Deerfield, Illinois), a solid-phase, enzyme-labeled, chemiluminescent immunometric assay, with an interassay coefficient of variation (CV) of 3.7% to 4.2%. Before July 2005, serum GH levels were measured with a chemiluminescent immunometric method (Nichols Institute Diagnostics, San Juan Capistrano, California), with an intra-assay CV of ≤5.4% and a sensitivity of 0.02 ng/mL. After July 2005, serum GH levels were measured by using an immunoradiometric assay kit (Diagnostic Systems Laboratories, Inc., Webster, Texas), with a detection limit of 0.01 ng/mL, an intra-assay CV of 3.1% to 5.4%, and an interassay CV of 5.9% to 11.5% (R2 = 0.98; slope = 1.083; intercept = 0.4457). The average bias for comparison between the 2 assays was 8.4% (unpublished data).
Statistical Analysis
JMP Statistical Discovery (version 4.0.2; SAS Institute Inc., Cary, North Carolina) software was used for statistical analysis. Statistical significance was defined as a 2-tailed P value ≤.05. Baseline clinical characteristics were examined with use of overall analysis of variance. Categorical variables were compared by using the Fisher exact test. Because we were primarily interested in whether QoL responds more robustly to GH replacement therapy in women who develop GHD after definitive treatment of acromegaly than in women with GHD due to other hypothalamic or pituitary disorders, we used the Dunnett test, with the patients who had prior acromegaly and received GH therapy as the control group, to correct for multiple comparisons for QoL variables. The Tukey-Kramer test was used to correct for multiple comparisons for other continuous variables, and the Bonferroni correction was used for categorical variables. Multivariate least squares models were constructed to determine whether the following variables were predictors of response in women who received GH therapy, with and without prior acromegaly (groups B and D combined): history of acromegaly, pretreatment QoL score, age, body mass index (BMI), baseline IGF-I level, history of radiation therapy, history of hypothyroidism, hypoadrenalism, hypogonadism, or diabetes insipidus, number of pituitary hormone deficiencies, prior GH treatment, history of a transsphenoidal surgical procedure, alcohol consumption, and history of smoking.
RESULTS
Baseline Clinical Characteristics
Baseline clinical characteristics for patients with prior acromegaly versus patients without prior acromegaly are presented in Table 1. No statistically significant differences in age, BMI, and GH peak were observed among the groups. The age range of the study subjects was 23 to 74 years (median, 45), and the range of BMIs was 19.2 to 45.6 kg/m2 (median, 29.0).
Table 1.
Baseline Clinical Characteristics of Study Patients, Stratifed by Presence or Absence of Prior Acromegalya
| Prior acromegaly |
No prior acromegaly |
||||
|---|---|---|---|---|---|
| Factor | Placebo (n = 8) |
Growth hormone (n = 9) |
Placebo (n = 19) |
Growth hormone (n = 19 ) |
P value |
| Age (y) | 41 ± 7.5 | 44 ± 15 | 47 ± 7.9 | 44 ± 10 | .44 |
| Body mass index (kg/m2) | 29.9 ± 4.8 | 30.4 ± 2.9 | 29.0 ± 6.5 | 30.1 ± 7.7 | .96 |
| Growth hormone peak (ng/mL) | 3.6 ± 0.7 | 3.3 ± 1.2 | 2.3 ± 1.3 | 1.8 ± 1.9 | .05 |
| Insulinlike growth factor-I SDS | −2.1 ± 0.7 | −2.3 ± 0.5 | −2.3 ± 0.4 | −2.5 ± 0.3 | .24 |
| Growth hormone mean dose at 6 mo (mg/d) | … | 0.61 ± 0.3 | … | 0.67 ± 0.3 | .66 |
| History of irradiation, no. (%) | 6 (75) | 7 (78) | 10 (53) | 10 (53) | .40 |
| Time since irradiation (mo) | 141.4 ± 91 | 132.5 ± 118 | 87.2 ± 104 | 62.6 ± 101 | .24 |
| Hypoadrenalism, no. (%) | 3 (38) | 4 (44) | 7 (37) | 14 (74) | .11 |
| Hypothyroidism, no. (%) | 7 (88) | 8 (89) | 13 (68) | 16 (84) | .62 |
| Hypogonadism, no. (%) | 7 (88) | 8 (100)b | 12 (63) | 12 (63) | .16 |
| Diabetes insipidus, no. (%) | 4 (50) | 1 (11) | 5 (26) | 6 (32) | .41 |
| Pituitary hormone defciencies, no. (%)c | |||||
| 0 | 1 (12) | 0 (0) | 4 (21) | 1 (5) | .29 |
| 1 | 0 (0) | 1 (11) | 4 (21) | 3 (16) | .72 |
| 2 | 3 (38) | 3 (33) | 2 (11) | 5 (26) | .33 |
| 3 | 1 (12) | 4 (44) | 7 (37) | 5 (26) | .50 |
| 4 | 3 (38) | 4 (44) | 2 (11) | 5 (26) | .18 |
| Current tobacco use, no. (%) | 2 (25) | 0 (0) | 1 (5) | 4 (21) | .23 |
| Estrogen use, no. (%) | 5 (62) | 5 (56) | 8 (42) | 9 (47) | .81 |
| Antidepressant use, no. (%) | 2 (25) | 1 (11) | 5 (26) | 8 (42) | .42 |
| Previous transsphenoidal surgery, no. (%) | 8 (100) | 8 (89) | 15 (79) | 17 (89) | .73 |
| Time since surgery (mo) | 148 ± 83 | 104 ± 80 | 126 ± 103 | 125 ± 117 | .87 |
| Time since cure of acromegaly (mo) | 95 ± 97 | 56 ± 53 | … | … | .34 |
| Quality of Life-AGHDAd | 11.5 ± 5.7 | 10.0 ± 6.8 | 8.21 ± 6.2 | 9.2 ± 7.6 | .70 |
| Symptom Questionnaired | |||||
| Anxiety | 11.1 ± 5.4e | 6.4 ± 4.7 | 6.4 ± 5.3 | 2.5 ± 2.8e | .0037 |
| Depression | 9.9 ± 6.1 | 5.6 ± 6.4 | 4.2 ± 5.4 | 2.8 ± 3.4 | .053 |
| Anger or Hostility | 5.9 ± 4.6 | 3.8 ± 6.4 | 2.6 ± 4.3 | 1.8 ± 2.4 | .26 |
| Somatic Symptoms | 10.4 ± 6.9 | 8.8 ± 4.0 | 8.2 ± 5.7 | 6.3 ± 4.7 | .45 |
| 36-Item Short-Form Health Surveyf | |||||
| Physical Functioning | 63.8 ± 21.3 | 80.6 ± 15.8 | 79.4 ± 19.3 | 68.1 ± 29.7 | .24 |
| Role Limitations due to Physical Health | 68.8 ± 39.5 | 58.3 ± 41.5 | 77.8 ± 34.2 | 56.3 ± 41.3 | .39 |
| Bodily Pain | 63.1 ± 38.1 | 76.4 ± 20.2 | 71.0 ± 26.5 | 75.2 ± 21.9 | .70 |
| General Health Perception | 38.8 ± 22.8 | 61.7 ± 16.6 | 59.4 ± 23.4 | 50.9 ± 24.0 | .12 |
| Energy or Fatigue | 26.9 ± 19.8 | 28.9 ± 20.3 | 45.6 ± 21.1 | 38.8 ± 24.7 | .14 |
| Social Functioning | 65.6 ± 12.9 | 70.8 ± 26.5 | 81.3 ± 24.7 | 75.8 ± 26.8 | .46 |
| Role Limitations due to Emotional Health | 62.5 ± 37.5 | 77.8 ± 37.3 | 88.9 ± 16.2 | 81.2 ± 27.1 | .18 |
| Emotional Well-Being | 55.0 ± 23.5e | 65.3 ± 20.0 | 74.4 ± 11.1 | 75.5 ± 13.0e | .02 |
Abbreviations: AGHDA = Adult Growth Hormone deficiency Assessment; SDS = standard deviation score.
Data are presented as number (%) or mean ± standard deviation.
Data on hypogonadism were available for 8 subjects in this group.
Other than growth hormone deficiency.
Tests in which lower scores refect better quality of life.
These 2 groups in this subscale were signifcantly different.
Test in which higher scores refect better quality of life.
GH Dosing and IGF-I Concentrations
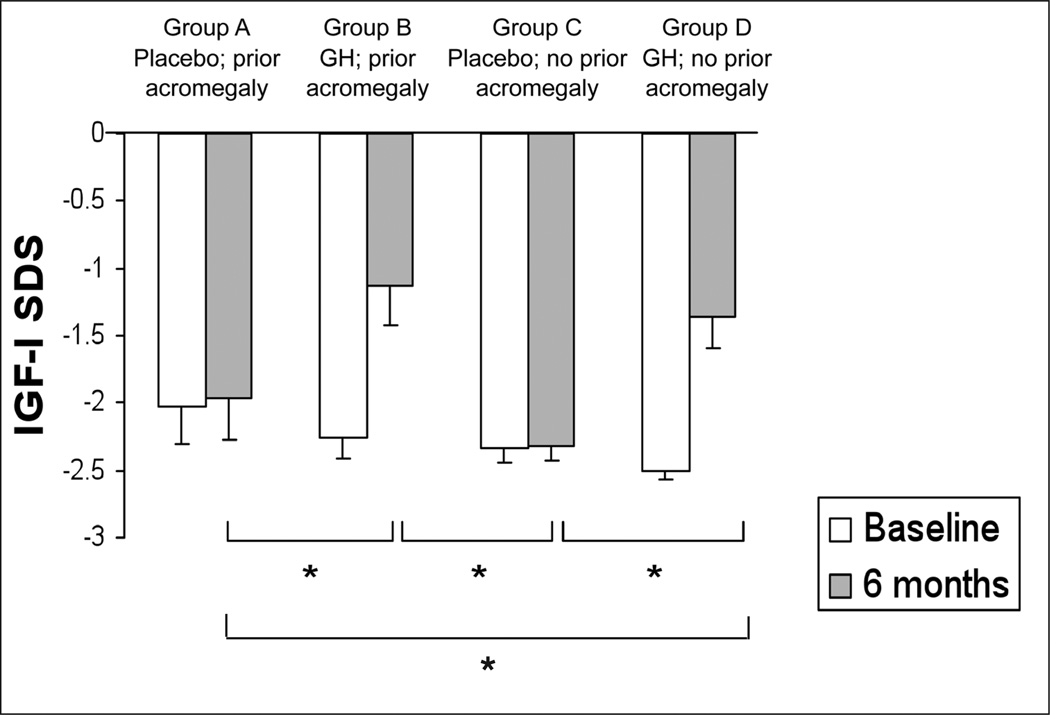
There was no difference in mean GH dose, IGF-I levels, or IGF-I standard deviation score (SDS) at 6 months between women with and those without prior acromegaly who received GH therapy. The mean GH dose at 6 months in patients with prior acromegaly (group B) was 0.61 ± 0.30 (SD) mg daily. The mean IGF-I SDS in this group rose from −2.3 ± 0.5 at baseline to −1.1 ± 0.8 at 6 months (Fig. 1). Patients without a history of acromegaly receiving GH treatment (group D) received a mean GH dose at 6 months of 0.67 ± 0.27 (SD) mg daily, which resulted in a mean IGF-I SDS increase from −2.5 ± 0.3 to −1.4 ± 0.9 after 6 months (Fig. 1). IGF-I SDS values at 6 months were significantly higher in patients in both groups B and D than those in group C (Fig. 1).
Fig. 1.
Mean (± standard error of the mean) pretreatment and 6-month insulinlike growth factor-I (IGF-I) standard deviation score (SDS) values. *P<.05.
Quality of Life Variables
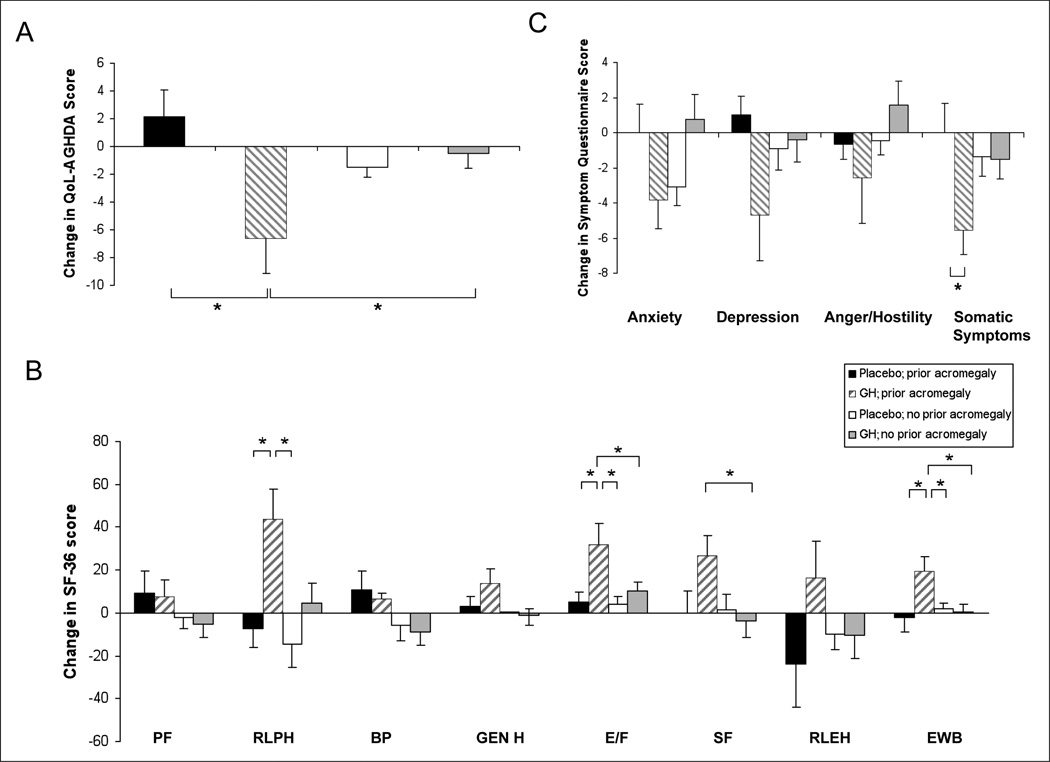
QoL, as measured by the AGHDA, significantly improved in group B (women with a history of acromegaly receiving GH treatment) in comparison with groups A (women with a history of acromegaly receiving placebo) and D (women without a history of acromegaly receiving GH therapy) (Fig. 2 A).
Fig. 2.
Mean (± standard error of the mean) change during a 6-month period in quality of life (QoL) as assessed by the QoL-Assessment of Growth Hormone Deficiency in Adults (AGHDA) (panel A), 36-Item Short-Form Health Survey (SF-36) (panel B), and Symptom Questionnaire (panel C). Higher scores are indicative of a more impaired QoL on the QoL-AGHDA and Symptom Questionnaire and a better QoL on the SF-36. *P<.05. Subscales on the SF-36: BP = Bodily Pain; E/F = Energy or Fatigue; EWB = Emotional Weil-Being; Gen H = General Health Perception; PF = Physical Functioning; RLEH = Role Limitations due to Emotional Health; RLPH = Role Limitations due to Physical Health; SF = Social Functioning.
Among the SF-36 subscales (Fig. 2 B), Role Limitations due to Physical Health (RLPH) scores improved significantly during a period of 6 months in group B (women with a history of acromegaly receiving GH therapy) in comparison with groups A (women with a history of acromegaly receiving placebo) and C (women without a history of acromegaly receiving placebo). There was a significantly greater improvement in the Energy or Fatigue subscale scores in group B (women with a history of acromegaly receiving GH therapy) in comparison with each of the other study groups. Social Functioning scores were significantly higher in group B (women with a history of acromegaly receiving GH treatment) in comparison with group D (women without a history of acromegaly receiving GH therapy). Emotional Well-Being subscale scores also improved significantly more during a 6-month period in group B in comparison with the other study groups.
The Somatic Symptoms subscale of the Symptom Questionnaire significantly improved in group B (women with a history of acromegaly receiving GH therapy) in comparison with group A (women with a history of acromegaly receiving placebo) (Fig. 2 C).
Predictors of Response
Predictors of response were determined in women who received GH therapy, with and without prior acromegaly (groups B and D combined).
History of acromegaly (P = .01) and poorer QoL at baseline (P = .002) were significant predictors of improvement in AGHDA score during the 6-month period. When history of acromegaly and pretreatment AGHDA scores were included in the model, both remained signifcant predictors of improvement in QoL (history of acromegaly, P = .01; pretreatment AGHDA score, P = .001).
History of acromegaly (P = .03) and poorer RLPH score predicted a greater improvement in RLPH after 6 months (P = .0003). Both remained significant predictors of response when controlling for each other (history of acromegaly, P = .006; pretreatment RLPH score, P<.0001). Previous history of acromegaly (P = .01) and poorer pretreatment EWB score (P = .004) were associated with a greater amelioration of EWB at 6 months. Both remained significant predictors after controlling for each other (history of acromegaly, P = .05; pretreatment EWB score, P = .01). History of acromegaly (P = .02) and poorer Social Functioning score at baseline (P = .0002) predicted a greater improvement in Social Functioning score after GH replacement for 6 months. Both remained independent predictors after controlling for each other (history of acromegaly, P = .02; pretreatment Social Functioning score, P = .0002). History of acromegaly (P = .02) predicted a greater improvement in Energy or Fatigue score after 6 months of GH treatment. Poorer pretreatment RLEH score predicted a greater improvement in RLEH score after the 6 months of GH treatment (P = .0007), but history of acromegaly did not. Pretreatment RLEH score remained a predictor of improvement in RLEH score after controlling for history of acromegaly (P = .0009). History of acromegaly was not a significant predictor of treatment response for any of the other SF-36 subscales.
A poorer baseline Depression symptom score predicted a greater improvement in the Depression score after 6 months (P<.0001). After controlling for history of acromegaly, a poorer Depression symptom score remained a significant predictor of improvement in this subscale score (P<.0001). A similar pattern was observed for the Anxiety subscale. A poorer pretreatment Anxiety symptom score predicted a greater improvement in the Anxiety score after 6 months (P = .003). After controlling for a history of acromegaly, a poorer Anxiety symptom score remained a signifcant predictor of improvement in this subscale score (P = .019). A history of acromegaly and a higher pretreatment score for Somatic Symptoms (indicating poorer QoL) were significant predictors of improvement in the Somatic Symptoms subscale after 6 months of treatment (P = .04 and P = .002, respectively). A history of acromegaly was no longer a predictor of improvement after adjusting for baseline Somatic Symptoms (P = .10). A poorer pretreatment Anger subscale score was a predictor of response to GH (P = .0002). After controlling for a history of acromegaly, a poorer pretreatment Anger subscale score was no longer a signifcant predictor of Anger response, but a history of acromegaly became a significant predictor (P = .0002).
A history of hypothyroidism and a higher pretreatment Anxiety symptom score were significant predictors of improvement in the Anxiety symptom subscale (P = .01 and P = .003, respectively). Both remained significant predictors of response when controlling for each other (hypothyroidism, P = .006; pretreatment Anxiety score, P = .002).
The following variables did not predict response of any QoL scales or subscales: age, BMI, baseline IGF-I level, history of radiation therapy, hypoadrenalism, hypogonadism, diabetes insipidus, number of pituitary deficiencies, prior GH treatment, history of a transsphenoidal surgical procedure, alcohol consumption, or smoking, except for the following: (1) younger age predicted a greater response of Physical Functioning score to GH therapy (P = .02), (2) higher BMI predicted greater response to GH in the Anxiety symptom subscale (P = .04), and (3) the presence of adrenal insufficiency predicted a greater response of the Bodily Pain subscale (P = .05) and a poorer response of the General Health Perception subscale (P = .0001).
DISCUSSION
Our data demonstrate that women with GHD and a history of acromegaly have a greater improvement in QoL after 6 months of GH replacement therapy than do women with GHD attributable to other causes. We assessed QoL using questionnaires that identify aspects of QoL specifically known to be impaired in patients with GHD as well as questionnaires that provide a comprehensive evaluation of important aspects of QoL in a wide range of diseases (10–13). We found that women with prior acromegaly receiving GH therapy reported an improved perception not only of physical and emotional aspects of their QoL but also of somatic, mental, and social complaints typically associated with GHD. These favorable changes were considerably more evident in women with prior acromegaly than in women receiving GH for GHD due to other hypothalamic and pituitary illnesses.
We previously reported that, among patients with prior acromegaly, those who were diagnosed with GHD had poorer QoL than those with normal peak GH levels (3). Additionally, we reported that GH replacement significantly ameliorated several aspects of QoL in patients (men and women combined) with a history of acromegaly and GHD in comparison with subjects receiving placebo (4), as has been shown in patients with GHD from other causes (5–8). These observations support the hypothesis that treatment of GHD may positively influence QoL in patients with prior acromegaly even long after remission of the disease. Two other reports have evaluated the effect of GH replacement on QoL in patients with a history of acromegaly (17,18). One of these investigations assessed the effects of GH therapy on QoL in patients with prior acromegaly in comparison with the response of patients who had other causes of hypopituitarism (17). In that study, Feldt-Rasmussen et al (17) retrospectively analyzed the AGHDA scores from a pharmacoepidemiologic survey of patients of both sexes with GHD attributable to several causes including acromegaly. They reported an improvement in mean AGHDA score after 6 months of GH treatment in patients (men and women combined) with etiologic factors other than acromegaly or Cushing disease in comparison with baseline scores. Moreover, GH treatment resulted in a nonsignificant improvement in mean AGHDA score in patients who had prior acromegaly (17). van der Klaauw et al (18) prospectively studied the effects of GH replacement in 8 female and 8 male patients with cured acromegaly and GHD and did not observe any change in QoL, including the AGHDA score, after 1 year of treatment with GH. A comparison with GH effects on QoL in patients with GHD of other causes was not performed.
We found that a greater improvement in QoL after 6 months of GH treatment was predicted by a more severe pretreatment impairment. This finding suggests that one possible explanation for the more robust treatment response in the patients with acromegaly may be their greater pretreatment impairment of QoL, perhaps because of the persistence of comorbidities even long after cure of acromegaly. Indeed, the degree of improvement in QoL with GH replacement has been reported in other studies to be proportional to the degree of impairment of QoL at baseline (19). This result is consistent with data from Feldt-Rasmussen et al (17), who reported that QoL was significantly more impaired in patients with previous acromegaly and GHD than in those with GHD related to other diseases. Therefore, it is possible that women with prior acromegaly are more likely to respond to administration of GH because they tend to have a poorer pretreatment QoL than those with GHD attributed to other causes.
Investigators have shown that acromegaly is associated with a persistent impairment of both emotional and physical aspects of QoL even long after cure (1,2). Biermasz et al (1) found that most of the SF-36 subscale scores were significantly lower (an indication of a poorer QoL) in patients with previous acromegaly who were successfully treated than in healthy control subjects and did not differ from those of patients with active acromegaly. Similarly, van der Klaauw et al (2), who studied a cohort of patients with a history of treatment of pituitary adenomas, showed that patients with cured acromegaly had worse overall QoL than patients treated for either nonfunctioning adenomas or prolactinomas. Persistence of acromegaly-related comorbidities, including hypertension, diabetes, and arthropathy, may contribute to these findings (20). In particular, persistent joint-related complaints have been reported to be independently associated with signifcant impairment in both physical and social or emotional subscales during long-term follow-up after cure of acromegaly (20). The observation that patients in remission after cure of acromegaly without joint complaints had the same mean SF-36 scores as healthy control subjects without arthropathy corroborates this hypothesis (21). Of note, van der Klaauw et al (2) reported that diminished physical functioning and increased bodily pain were the primary factors contributing to the reduced QoL reported in patients with prior acromegaly. Interestingly, we found that improvement in subjective perception of pain as assessed by the SF-36 scale was significantly greater in women with a history of acromegaly than in the group of women with GHD from other causes.
Female sex is a major determinant of QoL, having been observed to be associated with a poorer QoL in several populations, including patients with brain tumors or previous myocardial infarction (22–24). Similarly, QoL scores have been shown to be worse in female patients than in male patients after cure of acromegaly (2). In addition, although Feldt-Rasmussen et al (17) found that female patients with GHD and prior acromegaly had a significantly worse QoL, as assessed by AGHDA scores, than did women with GHD from other causes, no such differences were observed between the male groups. In that study, after GH replacement therapy, women with a history of acromegaly showed a tendency to have a greater improvement of their AGHDA scores in comparison with their male counterparts, but the difference was not signifcant, possibly because of the small sample size (17). Moreover, among patients with GHD from different causes, some studies have shown that women experience a reduced QoL in comparison with men and a greater improvement after GH replacement therapy (9). Interestingly, female sex has also been associated with a higher prevalence of joint-related complaints in patients after definitive therapy for acromegaly (21). Because we cannot extrapolate our current results to men, further studies are needed to determine whether men with GHD after definitive therapy for acromegaly are more likely to benefit from GH replacement therapy than are their counterparts without a history of acromegaly.
Limitations of our study include the small sample size, the short duration of GH administration, the study sample inhomogeneity, and the fact that we performed a post hoc analysis combining 2 studies. Using data collected from 2 studies could introduce bias and may lead to misattribution of effects as a result of potential confounders. The studies, however, were prospectively performed, randomized, placebo-controlled, and conducted with use of very similar methods—including the same brand of GH and placebo and the same dose titration scheme—and they were conducted by the same investigators at the same facilities. In addition, the mean GH doses administered in the 2 studies were comparable, as were the IGF-I SDS values at study beginning and end among the 2 groups that received GH. The inhomogeneity of the study sample—namely, differences in the prevalence of specific anterior pituitary hormone deficiencies or history of radiation therapy—is important to consider as well. The multivariate models, however, controlled for these factors. Some (25,26), but not all (2), previous studies have suggested that a history of radiotherapy might contribute to the impairment of QoL in patients cured of acromegaly, but these studies did not assess GH status. In our study, history of radiation therapy did not predict a deterioration in QoL during the 6-month study period.
Finally, it is important to acknowledge that our findings demonstrated a lack of response to GH replacement in QoL variables in patients with nonsomatotroph tumors, a result that contrasts with most (5–8), but not all (27,28), other published randomized, placebo-controlled studies examining the effects of GH replacement in such patients. Whether this lack of response in our study is attributable to our study population being entirely female, in contrast to the other studies, or to other factors is unclear. This raises the question of whether the enhanced response we found in patients with a history of acromegaly could reflect a nonrepresentative lack of response in the comparison (nonsomatotroph tumor) group and warrants further investigation.
Our data suggest that GH replacement therapy may have beneficial effects on QoL in patients with GHD after definitive treatment of acromegaly. These positive effects need to be confirmed in larger, long-term studies before GH replacement should be recommended. Moreover, because one of the 4 previously published studies in patients with GHD demonstrated serious cardiovascular and cerebrovascular events in patients with prior acromegaly receiving GH replacement (29), further studies to determine the safety of administration of GH in this patient population are necessary before this treatment can be recommended.
CONCLUSION
We demonstrated a significantly greater amelioration of several aspects of QoL in women with GHD and prior acromegaly after 6 months of GH replacement therapy than in patients with GHD attributable to other hypothalamic and pituitary diseases. Treatment with GH may improve the poor QoL, which has been documented in patients with GHD after definitive treatment of acromegaly. Further studies are necessary to confirm these data and establish whether such treatment is safe in this particular patient population.
ACKNOWLEDGMENT
We thank the nurses of the Massachusetts General Hospital General Clinical Research Center and the Harvard Catalyst Clinical Translational Science Center as well as the patients who participated in this study. This work was supported in part by the following grants: National Institutes of Health grants MO1 RR01066 and ULI RR02578, an investigator-initiated grant from Pfizer Inc., and a grant from the Guthart Family Foundation.
Abbreviations
- AGHDA
Assessment of Growth Hormone deficiency in Adults
- BMI
body mass index
- CV
coeffcient of variation
- EWB
Emotional Well-Being
- GH
growth hormone
- GHD
growth hormone deficiency
- IGF-I
sinsulinlike growth factor-I
- QoL
quality of life
- RLEH
Role Limitations due to Emotional Health
- RLPH
Role Limitations due to Physical Health
- SD
standard deviations
- SDS
standard deviation score
- SF-36
36-Item Short-Form Health Survey
Footnotes
DISCLOSURE
Dr. Klibanski received funding for this investigator-initiated study from Pfzer Inc. Dr. Biller consults for and has received research funding from Pfizer Inc. The other authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Biermasz NR, van Thiel SW, Pereira AM, et al. Decreased quality of life in patients with acromegaly despite long-term cure of growth hormone excess. J Clin Endocrinol Metab. 2004;89:5369–5376. doi: 10.1210/jc.2004-0669. [DOI] [PubMed] [Google Scholar]
- 2.van der Klaauw AA, Kars M, Biermasz NR, et al. Disease-specific impairments in quality of life during long-term follow-up of patients with different pituitary adenomas. Clin Endocrinol (Oxf) 2008;69:775–784. doi: 10.1111/j.1365-2265.2008.03288.x. [DOI] [PubMed] [Google Scholar]
- 3.Wexler T, Gunnell L, Omer Z, et al. Growth hormone deficiency is associated with decreased quality of life in patients with prior acromegaly. J Clin Endocrinol Metab. 2009;94:2471–2477. doi: 10.1210/jc.2008-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller KK, Wexler T, Fazeli P, et al. Growth hormone deficiency after treatment of acromegaly: a randomized, placebo-controlled study of growth hormone replacement. J Clin Endocrinol Metab. 2010;95:567–577. doi: 10.1210/jc.2009-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attanasio AF, Lamberts SW, Matranga AM, et al. Adult growth hormone (GH)-deficient patients demonstrate heterogeneity between childhood onset and adult onset before and during human GH treatment. J Clin Endocrinol Metab. 1997;82:82–88. doi: 10.1210/jcem.82.1.3643. Adult Growth Hormone deficiency Study Group. [DOI] [PubMed] [Google Scholar]
- 6.Burman P, Broman JE, Hetta J, et al. Quality of life in adults with growth hormone (GH) deficiency: response to treatment with recombinant human GH in a placebo-controlled 21-month trial. J Clin Endocrinol Metab. 1995;80:3585–3590. doi: 10.1210/jcem.80.12.8530603. [DOI] [PubMed] [Google Scholar]
- 7.Cuneo RC, Judd S, Wallace JD, et al. The Australian Multicenter Trial of Growth Hormone (GH) Treatment in GH-Deficient Adults. J Clin Endocrinol Metab. 1998;83:107–116. doi: 10.1210/jcem.83.1.4482. [DOI] [PubMed] [Google Scholar]
- 8.McGauley GA, Cuneo RC, Salomon F, Sönksen PH. Psychological well-being before and after growth hormone treatment in adults with growth hormone deficiency. Horm Res. 1990;33(suppl 4):52–54. doi: 10.1159/000181584. [DOI] [PubMed] [Google Scholar]
- 9.Koltowska-Häggström M, Mattsson AF, Shalet SM. Assessment of quality of life in adult patients with GH deficiency: KIMS contribution to clinical practice and pharma-coeconomic evaluations. Eur J Endocrinol. 2009;161(suppl 1):S51–S64. doi: 10.1530/EJE-09-0266. [DOI] [PubMed] [Google Scholar]
- 10.Kellner R. A symptom questionnaire. J Clin Psychiatry. 1987;48:268–274. [PubMed] [Google Scholar]
- 11.McHorney CA, Ware JE, Jr, Raczek AE. The MOS36-Item Short-Form Health Survey (SF-36): II Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 12.McKenna SP, Doward LC, Alonso J, et al. The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Qual Life Res. 1999;8:373–383. doi: 10.1023/a:1008987922774. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 14.Beauregard C, Utz AL, Schaub AE, et al. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2063–2071. doi: 10.1210/jc.2007-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartman ML, Crowe BJ, Biller BM, Ho KK, Clemmons DR, Chipman JJ. Which patients do not require a GH stimulation test for the diagnosis of adult GH deficiency? J Clin Endocrinol Metab. 2002;87:477–485. doi: 10.1210/jcem.87.2.8216. (HypoCCS Advisory Board; U.SHypoCCS Study Group) [DOI] [PubMed] [Google Scholar]
- 16.Biller BM, Samuels MH, Zagar A, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2002;87:2067–2079. doi: 10.1210/jcem.87.5.8509. [DOI] [PubMed] [Google Scholar]
- 17.Feldt-Rasmussen U, Abs R, Bengtsson BA, et al. Growth hormone deficiency and replacement in hypopituitary patients previously treated for acromegaly or Cushing’s disease. Eur J Endocrinol. 2002;146:67–74. doi: 10.1530/eje.0.1460067. (KIMS International Study Board of KIMS Study Group) [DOI] [PubMed] [Google Scholar]
- 18.van der Klaauw AA, Bax JJ, Roelfsema F, et al. Limited effects of growth hormone replacement in patients with GH deficiency during long-term cure of acromegaly. Pituitary. 2009;12:339–346. doi: 10.1007/s11102-009-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray RD, Shalet SM. Adult growth hormone replacement: lessons learned and future direction. J Clin Endocrinol Metab. 2002;87:4427–4428. doi: 10.1210/jc.2002-020430. [DOI] [PubMed] [Google Scholar]
- 20.Woodhouse LJ, Mukherjee A, Shalet SM, Ezzat S. The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr Rev. 2006;27:287–317. doi: 10.1210/er.2004-0022. [DOI] [PubMed] [Google Scholar]
- 21.Biermasz NR, Pereira AM, Smit JW, Romijn JA, Roelfsema F. Morbidity after long-term remission for acromegaly: persisting joint-related complaints cause reduced quality of life. J Clin Endocrinol Metab. 2005;90:2731–2739. doi: 10.1210/jc.2004-2297. [DOI] [PubMed] [Google Scholar]
- 22.Agewall S, Berglund M, Henareh L. Reduced quality of life after myocardial infarction in women compared with men. Clin Cardiol. 2004;27:271–274. doi: 10.1002/clc.4960270506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mainio A, Tuunanen S, Hakko H, Niemelä A, Koivukangas J, Räsänen P. Decreased quality of life and depression as predictors for shorter survival among patients with low-grade gliomas: a follow-up from 1990 to 2003. Eur Arch Psychiatry Clin Neurosci. 2006;256:516–521. doi: 10.1007/s00406-006-0674-2. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen OS, Bjorner JB, Newman B, et al. Gender differences in health-related quality of life following ST-elevation myocardial infarction: women and men do not benefit from primary percutaneous coronary intervention to the same degree. Eur J Cardiovasc Prev Rehabil. 2007;14:37–43. doi: 10.1097/HJR.0b013e3280114f00. DANAMI-2 Study. [DOI] [PubMed] [Google Scholar]
- 25.Kauppinen-Mäkelin R, Sane T, Sintonen H, et al. Quality of life in treated patients with acromegaly. J Clin Endocrinol Metab. 2006;91:3891–3896. doi: 10.1210/jc.2006-0676. [DOI] [PubMed] [Google Scholar]
- 26.Rowles SV, Prieto L, Badia X, Shalet SM, Webb SM, Trainer PJ. Quality of life (QOL) in patients with acro-megaly is severely impaired: use of a novel measure of QOL; acromegaly quality of life questionnaire. J Clin Endocrinol Metab. 2005;90:3337–3341. doi: 10.1210/jc.2004-1565. [DOI] [PubMed] [Google Scholar]
- 27.Florkowski CM, Stevens I, Joyce P, Espiner EA, Donald RA. Growth hormone replacement does not improve psychological well-being in adult hypopituitarism: a randomized crossover trial. Psychoneuroendocrinology. 1998;23:57–63. doi: 10.1016/s0306-4530(97)00093-0. [DOI] [PubMed] [Google Scholar]
- 28.Baum HB, Katznelson L, Sherman JC, et al. Effects of physiological growth hormone (GH) therapy on cognition and quality of life in patients with adult-onset GH deficiency. J Clin Endocrinol Metab. 1998;83:3184–3189. doi: 10.1210/jcem.83.9.5112. [DOI] [PubMed] [Google Scholar]
- 29.Norrman LL, Johannsson G, Sunnerhagen KS, Svensson J. Baseline characteristics and the effects of two years of growth hormone (GH) replacement therapy in adults with GH defiiciency previously treated for acromegaly. J Clin Endocrinol Metab. 2008;93:2531–2538. doi: 10.1210/jc.2007-2673. [DOI] [PubMed] [Google Scholar]