Abstract
A functional adaptive immune system depends on a diverse and self-tolerant population of T lymphocytes that are generated in the thymus and maintained in the peripheral lymphoid organs. Recent studies have defined the cytokine transforming growth factor-β (TGF-β) as a critical regulator of thymic T cell development as well as a crucial player in peripheral T cell homeostasis, tolerance to self antigens, and T cell differentiation during the immune response. The unique mechanism of TGF-β activation and the plasticity of TGF-β signaling create a stage for TGF-β to integrate signals from multiple cell types and environmental cues to regulate T cells.
Introduction
The defining feature of a functional immune system is the ability to correctly discern foreign pathogenic antigens from self- or other innocuous antigens and to mount an effective immune response. T lymphocytes are the key components of the cellular arm of the adaptive immune system. Elucidation of the mechanisms underlying T cell regulation during the immune system steady state and pathogen invasion is fundamental to the understanding of immune system function. Studies of T cell regulation have begun to shed light on the pervasive and essential role that the regulatory cytokine transforming growth factor-β (TGF-β) plays in T cell development, homeostasis, tolerance, and the immune response, thereby providing a means to grasp the underlying principles of T cell biology. This review will discuss recent progress in understanding the regulation of T cells by TGF-β.
T lymphocytes express T cell receptors (TCRs) that recognize antigens in association with molecules of the major histocompatibility complex (MHC) (Box 1). The stochastic process by which a pool of TCRs with different antigen-binding specificities is generated creates the inherent problem that some receptors have a high affinity for self-antigens or for innocuous environmental antigens such as those from commensal organisms. Thus, to ensure immune system homeostasis and to prevent an autoimmune response provoked by T cell recognition of self-antigens, T cells are subjected to selection in the thymus before they migrate to the peripheral lymphoid organs. T cells bearing TCRs with low affinity for MHC–self-antigen complexes are favored in this thymic selection, whereas T cells expressing TCRs with high affinity for MHC–self-antigen are eliminated through apoptosis (Starr et al., 2003). This purge of high-affinity T cell clones from the T cell repertoire is not an infallible process. As a consequence of incomplete presentation of self-antigens in the thymus and the plasticity of TCR recognition of antigens, autoreactive T cells are present in the peripheral lymphoid organs of healthy individuals (Danke et al., 2004). Therefore, other regulatory mechanisms in addition to thymic selection must hold these autoreactive T cells in check and keep them from triggering autoimmune disease. Once low-affinity T cell clones have undergone the maturation process in the thymus, they relocate to the peripheral lymphoid organs, where they are maintained as a nonproliferating, diverse population of naive T cells with a half-life of at least 6 months in mice (Jameson, 2005). Should infection occur, naive T cells that recognize foreign antigens derived from the invading pathogen are preferentially activated and then differentiate into effector T cells (CD4+ helper T cells or CD8+ cytotoxic T cells) to combat the invading pathogen. These crucial processes of T cell development, tolerance, homeostasis, and differentiation are highly dependent on a regulatory network that is modulated by TGF-β.
Box 1. Abbreviations.
- CIA
collagen-induced arthritis
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DNRII
dominant-negative mutant of TGF-βRII
- EAE
experimental autoimmune encephalomyelitis
- ECM
extracellular matrix
- eTh17
effector Th17
- GALT
gut-associated lymphoid tissue
- IL
interleukin
- iTreg
induced CD4+Foxp3+ regulatory T
- LAP
latency-associated protein
- LTBP
latent TGF-β-binding proteinp
- MHC
major histocompatibility complex
- NKT
natural killer T
- nTreg
natural CD4+Foxp3+ regulatory T
- RA
retinoic acid
- RAG
recombination activating gene
- rTh17
regulatory Th17
- TA
TGF-β activator
- TCR
T cell receptor
- TF
transcription factor
- TGF-β
transforming growth factor-β
- TGF-βRI
TGF-β type I receptor
- TGF-βRII
TGF-β type II receptor
- Th
helper T lymphocyte
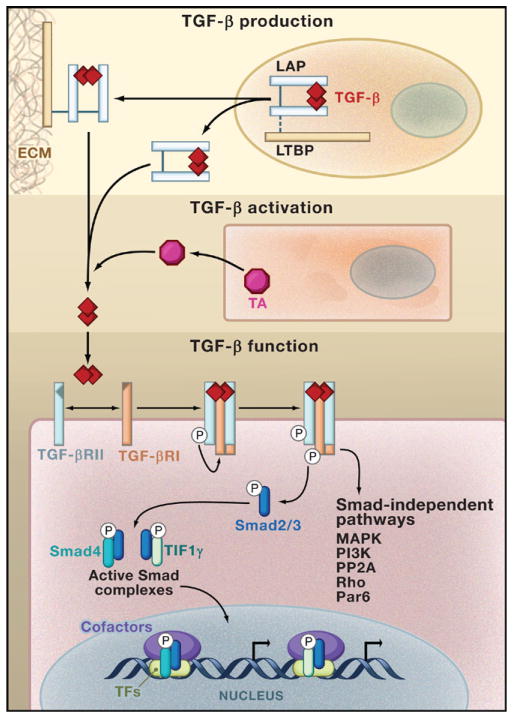
TGF-β belongs to a family of regulatory cytokines that have pleiotropic functions in a broad range of cell lineages involved in numerous physiological and pathological processes such as embryogenesis, carcinogenesis, and the immune response (Blobe et al., 2000). In mammals, three members of the TGF-β family (TGF-β1, TGF-β2, and TGF-β3) have been identified, with TGF-β1 being the predominant form expressed in the immune system (Li et al., 2006b). TGF-β is synthesized as a precursor: the pre region contains a signal peptide, and pro-TGF-β is processed in the Golgi by a furin-like peptidase that removes the N terminus of the immature protein. A homodimer of this new protein, called the latency-associated protein (LAP), is noncovalently associated with a homodimer of mature TGF-β (Figure 1). This latent complex can be secreted, or may associate with latent-TGF-β-binding protein (LTBP), which plays an important role in targeting TGF-β to the extracellular matrix. TGF-β cannot bind to its receptors in its latent form—it needs to be liberated from the constraints of LAP and LTBP by a TGF-β activator (TA) through LAP proteolysis or a conformational change (Annes et al., 2003) (Figure 1). Notably, the cells that produce TA can be different from those that secrete TGF-β. This unique activation step for TGF-β provides a means for this secreted molecule to integrate signals from multiple cell types to regulate cellular responses.
Figure 1. The TGF-β Module of Cellular Regulation.
TGF-β is synthesized in an inactive form composed of a TGF-β dimer in association with the latency-associated protein (LAP). This latent TGF-β molecule can be secreted as such, or can form a complex with latent-TGF-β-binding protein (LTBP) that mediates its deposition to the extracellular matrix (ECM). TGF-β becomes activated after the engagement of a TGF-β activator (TA) that triggers LAP degradation or alters LAP’s conformation in response to environmental cues. Active TGF-β binds to a tetrameric complex composed of TGF-β receptor II (TGF-βRII) and TGF-β receptor I (TGF-βRI) and initiates signaling pathways that are dependent on the kinase activity of the receptors. Activated TGF-βRI phosphorylates the transcription factors Smad2 and Smad3, triggering their translocation into the nucleus in complex with the proteins Smad4 or TIF1γ. Smad complexes in association with additional transcription factors (TFs) bind to the regulatory sequences in target genes and regulate gene expression by recruiting transcription cofactors. In addition, TGF-β activates Smad-independent pathways such as those mediated by mitogen-activated protein kinase (MAPK), PI3K kinase, PP2A phosphatase, Rho family proteins, and the epithelial polarity protein Par6, which trigger different cell type-specific responses.
Active TGF-β mediates its biological functions by binding to TGF-β type I (TGF-βRI) and type II (TGF-βRII) receptors, both of which are serine/threonine kinases. TGF-β engagement with a tetrameric receptor complex consisting of two TGF-βRI molecules and two TGF-βRII molecules activates these receptor kinases, allowing them to phosphorylate downstream targets and to activate different signaling pathways (Figure 1). The signaling output of TGF-β elicits diverse cellular responses that are primarily mediated through the actions of Smad transcription factors (Massague and Gomis, 2006; Shi and Massague, 2003). Active Smad protein complexes bind to DNA weakly; high-affinity DNA binding is achieved by the association of Smad proteins with a large number of transcription factor partners (Massague and Gomis, 2006). In addition, TGF-β activates various cell type-specific Smad-independent signaling pathways, including those mediated by mitogen-activated protein kinase (MAPK), PI3K kinase, PP2A phosphatase, Rho family proteins, and the epithelial polarity protein Par6 (Derynck and Zhang, 2003; Ozdamar et al., 2005). The plasticity of Smad proteins in transcriptional regulation and the diversity of Smad-independent pathways enable TGF-β to exert its pleiotropic actions.
TGF-β Regulates T Cell Development
Thymic T precursor cells undergo a series of molecular and phenotypic changes before they differentiate into mature T cells. One critical event in T cell differentiation occurs at the stage when immature T cells expressing both CD4 and CD8 cell surface markers (CD4+CD8+) as well as αβ TCRs are subjected to selection and lineage diversification.
CD8+ T Cell Differentiation
TGF-β may play a role at the CD4+CD8+ stage in T cell development to promote thymic CD8+ T cell differentiation, but this remains unresolved. The initial implication of TGF-β in this process came from a study that found a 2-fold reduction in conventional mature CD8+ T cells (CD8+TCRhigh) in mice lacking the Tgfbr2 gene in CD4+CD8+ T cells (Li et al., 2006a). However, a related study using the same mouse model system (Marie et al., 2006) reported normal thymic CD4+ and CD8+ T cell differentiation in these mutant mice. Another study of mice reconstituted with TGF-βRII-deficient bone marrow cells demonstrated the opposite effect, that is, increased proliferation of CD8+ thymocytes in the absence of TGF-β signaling with no effect on overall CD8+ T cell number (Leveen et al., 2005). The results of this study, however, may have been confounded by the fact that CD8+ thymocytes were not separated into mature CD8+TCRhigh T cells and immature CD8+TCRlow single positives. Because CD8+TCRlow single-positives cycle more actively than mature T cells, defects in CD8+ T cell maturation could lead to increased presentation of immature CD8+TCRlow single positives among CD8+ thymocytes and the hyperproliferation phenotype of CD8+ T cells. Furthermore, all TGF-βRII-deficient mice eventually developed severe inflammatory disorders. Although the time points chosen for the analysis of thymic phenotypes precede the appearance of severe immunopathology, systemic inflammation may affect thymic T cell development. Indeed, preliminary work in a mouse strain that circumvents this inflammation problem again suggests a role for TGF-β signaling in CD8+ T cell differentiation. These T cell-specific TGF-βRII-deficient H-Y TCR-transgenic mice harbor H-Y CD8+ T cells that exclusively recognize a male mouse-specific Y chromosome-associated antigen with high affinity but are positively selected by low-affinity self-antigens in female mice. In female H-Y TCR-transgenic mice where a deletion of the Rag2 gene (recombination activating gene 2) limits the T cell population to a single TCR specificity, no inflammation was observed in the absence of Tgfbr2 expression in T cells. In these mice, thymic maturation of TGF-βRII-deficient CD8+ H-Y T cells showed the same attenuation (M.O.L., unpublished data) as was observed for TGF-βRII-deficient CD8+ T cells in mice with normal polyclonal T cells (Li et al., 2006a). These new findings suggest that at least for some TCR specificities, TGF-β signaling in T cells promotes thymic CD8+ T cell differentiation.
nTreg and NKT Cell Differentiation
In addition to conventional CD4+ and CD8+ T lymphocytes, CD4+CD8+ T cells also give rise to the regulatory T cell lineages that include natural CD4- and Foxp3-expressing (CD4+Foxp3+) regulatory T (nTreg) cells and CD1d-dependent natural killer T (NKT) cells (Kronenberg and Rudensky, 2005). nTreg cells are essential regulators of peripheral T cell tolerance, the development of which is controlled by the affinity of TCRs for MHC II–self-peptide complexes and by costimulatory and cytokine signaling pathways (Liston and Rudensky, 2007). Experiments determining the function of TGF-β signaling in nTreg cell development were performed in the TGF-βRII T cell-specific deletion mouse models. In two studies, TGF-β signaling in T cells appeared to be dispensable for the development of nTreg cells in 12- to 16-day-old mice (Li et al., 2006a; Marie et al., 2006). However, a recent report suggests an earlier requirement for TGF-β signaling (Liu et al., 2008). Using a conditional deletion of the Tgfr1 gene in T cells, Liu et al. showed that this loss of TGF-βRI blocked nTreg cell differentiation in 3- to 5-day-old mice but triggered nTreg cell expansion in mice older than 1 week (Liu et al., 2008). The later expansion, a phenomenon also observed in mice deficient in TGF-βRII (Li et al., 2006a), was associated with enhanced nTreg cell proliferation in response to the cytokine interleukin-2 (IL-2). Indeed, genetic ablation of IL-2 in TGF-βRI-deficient mice resulted in a diminished number of thymic nTreg cells (Liu et al., 2008). These findings reveal opposing functions for TGF-β signaling in nTreg cell development that are dependent on mouse age, although the molecular mechanism by which TGF-β promotes early nTreg cell differentiation remains to be determined.
The development of NKT cells is dependent on the presentation of MHC I-like CD1d-self-glycolipid antigens by CD4+CD8+ T cells; NKT cells express TCRs that recognize glycolipid antigens in association with the CD1d molecule (Bendelac et al., 1997). In contrast to the early developmental defect seen in nTreg cell differentiation in the absence of TGF-β signaling, thymic and peripheral NKT cell numbers were sharply reduced in T cell-specific TGF-βRII-deficient mice analyzed at all ages (Li et al., 2006a; Marie et al., 2006). Taken together, these recent studies have uncovered positive regulatory roles for TGF-β signaling in the development of multiple T cell lineages (Figure 2). T cell development in general is controlled by the TCR, costimulatory receptor, and cytokine (e.g., common-γ chain cytokine) signaling pathways. How TGF-β signaling crosstalk with these pathways regulates CD8+ T cell, nTreg cell, and NKT cell differentiation will be an interesting topic for future investigation.
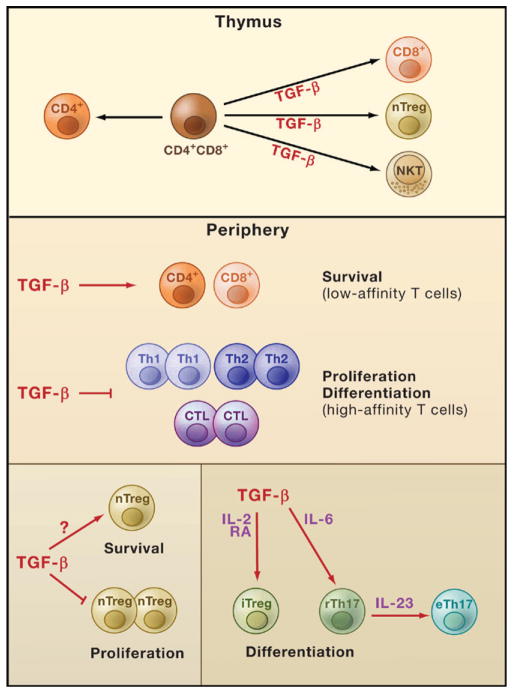
Figure 2. TGF-β Regulates T Cells.
The cytokine TGF-β regulates T cell development, homeostasis, tolerance, and immunity. TGF-β signaling in T cells promotes differentiation of thymic T cells into natural killer T (NKT) cells, natural regulatory T (nTreg) cells, and CD8+ T cells. In peripheral tissues, TGF-β signaling in T cells is likely to be essential for the survival of low-affinity CD4+ and CD8+ T cells. It also modulates immune tolerance by inhibiting high-affinity CD4+ and CD8+ T cell proliferation and differentiation into T helper 1 (Th1), Th2, and cytotoxic T lymphocytes (CTL). In addition, TGF-β signaling in nTreg cells inhibits their proliferation but supports their maintenance in peripheral lymphoid organs, a process likely mediated by TGF-β control of nTreg cell survival. TGF-β also positively regulates differentiation of certain peripheral T cells in conjunction with other factors. TGF-β promotes the differentiation of induced Treg (iTreg) cells that is potentiated by the cytokine interleukin-2 (IL-2) and retinoic acid (RA). In the presence of IL-6, TGF-β drives the differentiation of Th17 cells and maintains them in a regulatory state (rTh17). Stimulation of rTh17 cells by IL-23 in the absence of TGF-β enables these cells to acquire effector functions and promotes their differentiation into effector Th17 cells (eTh17).
TGF-β Regulates Naive T Cell Homeostasis
T cells are maintained at a constant number in adult animals under steady-state conditions, even after thymic atropy that results in diminished T cell production (Jameson, 2005). This maintenance of a diverse and long-lived naive T cell repertoire is fundamental to the function of the adaptive immune system because these T cells will be called upon to defend the host when infection occurs. Naive T cell homeostasis is regulated by cytokine signaling pathways such as that of IL-7 and also possibly by the engagement of MHC–self-antigen complexes in peripheral tissues (Surh and Sprent, 2005). TGF-β signaling in T cells is essential for the maintenance of peripheral T cell tolerance (see below), and T cells from TGF-β1-deficient mice or mice with T cell-specific inactivation of TGF-β receptors exhibit hyperactivation of their T cell populations. The loss of naive T cells in these mice could be caused by a lack of thymic production of naive T cells, activation and differentiation of naive T cells by low-affinity MHC–self-antigen complexes, the ability of activated T cells to out-compete naive T cells, compromised survival or migration of naive T cells to peripheral lymphoid organs, or a combination of these possibilities.
Recent studies with TCR-transgenic mouse models have begun to address how TCR specificity modulates T cell responses under conditions of compromised TGF-β regulation and to decipher the reason for naive T cell loss when TGF-β signaling is disrupted (Bommireddy et al., 2006; Li et al., 2006a; Marie et al., 2006; Robinson et al., 2006). In one of these studies, the effects of defective TGF-β signaling in T cells was examined in mice engineered such that T cell TCRs had a high binding affinity only for a nonself–MHC II antigen (OT-II T cells). This enabled the complicating factors of T cell competition and host systemic inflammation to be circumvented (Li et al., 2006a). In these mice, ablation of the Tgfbr2 gene in T cells did not affect T cell selection, suggesting that thymic production under these conditions was unaffected by the lack of TGF-β signaling. In contrast to the hyperactivated phenotype of TGF-βRII-deficient CD4+ T cells in mice harboring a normal polyclonal T cell population with mixed TCR affinities, most peripheral TGF-βRII-deficient OT-II T cells maintained a naive T cell phenotype and did not differentiate into T helper 1 (Th1) or Th2 effector T cells (Li et al., 2006a). Similar observations were also made with TGF-βRII-deficient T cells or T cells from TGF-β1-deficient mice with other single nonself TCR affinities (TEα transgenic T cells and DO11.10 T cells) (Bommireddy et al., 2006; Marie et al., 2006; Robinson et al., 2006). These findings suggest that the loss of naive CD4+ T cells observed when TGF-β signaling is lacking is not likely due to activation and differentiation of naive T cells. Instead, a striking observation suggests another explanation. TGF-βRII-deficient OT-II T cells were seen to undergo a high rate of cell death and were largely depleted in the peripheral lymphoid organs (Li et al., 2006a). These findings demonstrate an essential role for TGF-β signaling in promoting naive OT-II T cell survival and also imply that cell death at least partly accounts for the loss of CD4+ naive T cells in T cell-specific TGF-βRII-deficient mice. This conclusion is supported by a recent study that determined the TCR diversity of CD4+ T cells in TGF-β1-deficient mice (Robinson and Gorham, 2007). Peripheral but not thymic TGF-β1-deficient CD4+ T cells exhibited a nonpolyclonal distribution of the third complementarity-determining region of TCR Vβ chains, which could be explained by the depletion of peripheral naive CD4+ T cells in these mice. Future studies with additional TCR transgenic models are needed to corroborate these findings. Potential crosstalk between the TGF-β pathway and other signaling pathways involved in naive T cell homeostasis also warrants investigation. The role of TGF-β signaling in regulating naive CD8+ T cells also remains to be determined. Preliminary results from the study of peripheral T cells in the aforementioned H-Y TCR-transgenic mouse strain showed that the lack of TGF-β signaling in CD8+ H-Y T cells also led to diminished mature T cell numbers in the peripheral lymphoid organs of female mice (M.O.L., unpublished data). These findings suggest that TGF-β may have an essential role in promoting the survival of both CD4+ and CD8+ naive T cells that interact with low affinity to self-antigens (Figure 2) to maintain a diverse repertoire of T cells.
TGF-β Regulates Peripheral T Cell Tolerance
Multiple mechanisms have evolved to control T cell-mediated autoimmunity. These include the deletion of high-affinity self-reactive T cell clones in the thymus (Mathis and Benoist, 2004) and various peripheral mechanisms that keep escaped auto-reactive T cells in check (Walker and Abbas, 2002). Active immune suppression by cytokine TGF-β1 or CD4+Foxp3+ Treg cells is a pivotal mechanism of peripheral T cell tolerance (Li et al., 2006b; Sakaguchi et al., 2008). Indeed, mice lacking either TGF-β1 or Foxp3, the transcription factor required for Treg cell function, develop early fatal multifocal inflammatory diseases.
T Cells as a Direct TGF-β Target
The inflammatory disorder developed in TGF-β1-deficient mice is characterized by the hyperactivation of T cells and the progressive infiltration of leukocytes into multiple organs (Kulkarni et al., 1993; Shull et al., 1992). Depletion of either CD4+ or CD8+ T cells alleviates this immunopathology (Kobayashi et al., 1999; Letterio et al., 1996), thereby demonstrating that T cells are essential mediators of the disease. Because disease development is not prevented by the absence of foreign antigens when TGF-β1-deficient mice are raised under germ-free conditions (Boivin et al., 1997), the T cell activation observed seemed likely to be driven by self-antigens. However, because TGF-β1 can modulate the activity of multiple leukocyte lineages, it was not clear from these studies whether TGF-β1 directly controlled T cell tolerance. In early 2000, work by several groups, using transgenic approaches to inhibit TGF-β signaling in T cells, demonstrated a specific disturbance of T cell homeostasis or tolerance in the absence of TGF-β signaling (Gorelik and Flavell, 2000; Lucas et al., 2000). In one study, the expression of a dominant-negative mutant of TGF-βRII (DNRII) from the CD4 promoter that lacks the CD8 silencer inhibited TGF-β signaling in CD4+ and CD8+ T cells, leading to the activation and differentiation of CD4+ and CD8+ T cells and the development of inflammatory disease (Gorelik and Flavell, 2000). In another report, expression of DNRII from the CD2 promoter resulted in a CD8+ T cell lymphoproliferative disorder with little inflammation (Lucas et al., 2000). Later studies demonstrating that T cell-specific TGF-βRII- or TGF-βRI-deficient mice developed a neonatal lethal inflammatory disease similar to that of TGFβ1-deficient mice (Li et al., 2006a; Liu et al., 2008; Marie et al., 2006) further corroborate the earlier findings and establish T cells as the central direct target of TGF-β1-regulated immune tolerance in vivo.
TGF-β Control of Effector T Cells and nTregs
So what is the mechanism underlying TGF-β regulation of T cell tolerance? In peripheral T cell tolerance, extrathymic immune regulation by Treg cells plays an essential role. Indeed, as mentioned above, mice devoid of the Treg cell-specific transcription factor Foxp3 develop a similarly severe autoimmune phenotype as T cell-specific TGF-βRII-deficient mice (Brunkow et al., 2001; Fontenot et al., 2003). Intriguingly, it has been observed that despite uncompromised thymic production of nTreg cells in 12- to 16-day-old T cell-specific TGF-β receptor-deficient mice, Treg cell number is reduced in the spleens of these mice (Li et al., 2006a; Liu et al., 2008; Marie et al., 2006). These findings are consistent with similar observations in TGF-β1-deficient mice (Marie et al., 2005), suggesting that a loss in TGF-β signaling somehow affects Treg cells. Because TGF-βRII-deficient Treg cells proliferate at a higher rate than wild-type Treg cells (Li et al., 2006a), the compromised maintenance of Treg cells is likely due to their failed survival. The observation that neonatal thymectomy of mice on day 3 substantially diminishes peripheral Treg cells (Sakaguchi et al., 2008), taken in the context of the finding that thymic Treg cell differentiation is kinetically slower than that of nonregulatory CD4+ T cells (Fontenot et al., 2005), suggests that thymus-derived nTreg cells make up the majority, if not all, of the peripheral Treg cells in young mice. Together, these studies suggest that the failed maintenance of nTreg cells likely accounts for most of the Treg cell reduction in mice lacking TGF-β signaling. But is the reduced Treg cell number the cause of cell tolerance loss in TGF-βRII-deficient mice? Or is a lack of cell-intrinsic control of T cell activation by TGF-β a contributing factor? Alternatively, is this failed maintenance of TGF-βRII-deficient Treg cells a direct consequence of a lack of TGF-β signaling in nTreg cells or of environmental factors such as severe systemic inflammation?
These questions were addressed in a series of T cell transfer and bone marrow chimera experiments. Although the transfer of wild-type Treg cells to neonatal TGF-βRII-deficient mice restores Treg cell number to the level of wild-type mice, it is unable to correct the phenotypes of hyper-T cell activation and autoimmune disease development (Li et al., 2006a). Therefore, depletion of Treg cells does not solely account for the loss of T cell tolerance in TGF-βRII-deficient mice. These findings are consistent with earlier reports using CD4-DNRII transgenic T cells. In one of these studies, Treg cells were incapable of suppressing the inflammatory bowel disease induced by naive CD4-DNRII CD4+ T cells in a mouse model of colitis, a condition associated with T cell activation and differentiation (Fahlen et al., 2005). Intact TGF-β signaling was also required for Treg cell-mediated suppression of CD8+ T cell activation in mouse models of diabetes and tumor immunity (Chen et al., 2005; Green et al., 2003; Mempel et al., 2006).
The precise functions of TGF-β signaling in the control of effector T cells and Treg cells have been further studied in mixed bone marrow chimera experiments. By creating chimeric mice that harbor both wild-type and TGF-βRII-deficient T cells, cell-intrinsic effects can be differentiated from the effects of cell-extrinsic environmental changes. Strikingly, TGF-βRII-deficient T cell populations exhibited a more activated and differentiated phenotype than wild-type T cell populations in the chimeric mouse. Furthermore, chimeric mice had a lower number of TGF-βRII-deficient Treg cells relative to wild-type Treg cells (Li et al., 2006a; Marie et al., 2006). These findings demonstrate that TGF-β promotes T cell self-tolerance through direct regulation of both effector T cells and nTreg cells (Figure 2).
TGF-β Control of iTreg Cells
In addition to nTreg cells that are produced in the thymus, naive T cells can acquire Foxp3 expression and differentiate into induced Treg (iTreg) cells in peripheral tissues (Bluestone and Abbas, 2003). In vitro activation of CD4+CD25− or CD4+Foxp3− T cells in the presence of TGF-β leads to their acquisition of T cell suppressive activity, which is associated with the induction of Foxp3 expression (Chen et al., 2003b; Fantini et al., 2004; Wan and Flavell, 2005; Zheng et al., 2002). TGF-β induction of Foxp3 expression appears to be mediated by the recruitment of its downstream transcription factor Smad3 to a Foxp3 enhancer element (Tone et al., 2008). TGF-β-induced iTreg cell differentiation was augmented by the T cell-produced cytokine IL-2 (Davidson et al., 2007; Zheng et al., 2007), which activates the transcription factor STAT5 for binding to the Foxp3 promoter (Burchill et al., 2007). These findings suggest that Smad3 may synergize with STAT5 to induce Foxp3 gene transcription (Figure 3). Overexpression of TGF-β1 in the pancreatic islets also resulted in expansion of CD4+Foxp3+ Treg cells that protected nonobese diabetic (NOD) mice from developing diabetes (Peng et al., 2004). Taken together, these observations suggest that when TGF-β is present in excess, it can induce Foxp3 expression and the differentiation of iTreg cells.
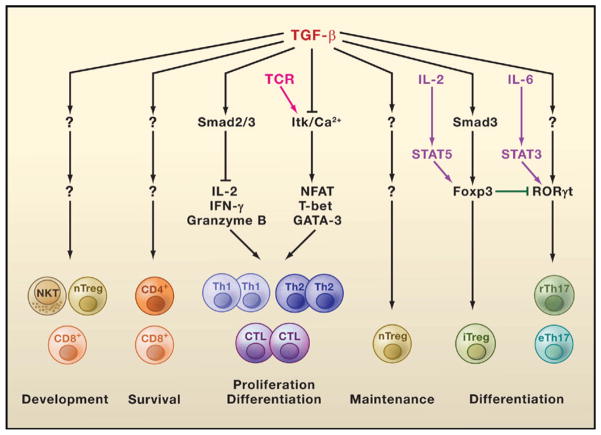
Figure 3. TGF-β Engages Multiple Signaling Pathways to Control T Cell Development.
The Smad2/3 complexes are involved in TGF-β inhibition of interleukin-2 (IL-2), interferon-γ (IFN-γ), and granzyme B transcription required for effector T helper 1 (Th1), Th2, and cytotoxic T lymphocyte (CTL) proliferation and differentiation. In addition, TGF-β blocks T cell receptor (TCR)-induced Tec kinase Itk activation and Ca2+ responses that are required for the expression of other transcription factors such as NFAT, T-bet, and GATA-3, which are also involved in T cell activation and differentiation. Induced regulatory T (iTreg) cell differentiation triggered by TGF-β occurs via Smad3-dependent induction of Foxp3 expression, which is enhanced by activation of the transcription factor STAT5 (induced by the cytokine IL-2). TGF-β induction of Th17 cell differentiation is associated with its induction of RORγt expression, which is potentiated by the IL-6-activated transcription factor STAT3. The transcription factor Foxp3 interacts with the transcription factor RORγt and suppresses its function, providing a mechanism for the reciprocal differentiation of iTreg and Th17 cells. The mechanisms of TGF-β-induced development of natural regulatory T (nTreg) cells, natural killer T (NKT) cells, and CD8+ T cells are unknown. How TGF-β regulates the survival of low-affinity CD4+ and CD8+ T cells and maintains nTreg cells is also unknown.
Endogenous TGF-β may also be involved in the induction of iTreg cells in peripheral tissues. The delivery of low doses of agonistic peptide ligands to steady-state dendritic cells can induce de novo generation of Treg cells from naive T cells (Kretschmer et al., 2005). The efficiency of this conversion appeared to be inversely correlated with the rate of cell proliferation and was inhibited by high-dose antigens or costimulation with a CD40 antibody agonist. Although proliferation was detrimental to the induction of iTreg cells during the time window of conversion, converted iTreg cells maintained a stable phenotype and could be expanded upon immunization with antigens in the presence of adjuvants, nonantigenic substances that are needed to fully activate immune cells in the absence of an infection. Inhibition of TGF-β signaling in naive T cells by the expression of DNRII reduced conversion of iTreg cells in this mouse model that typically exhibited T cell hyperproliferation. It remains to be determined whether TGF-β inhibition of T cell proliferation provides the mechanism for its role in promoting iTreg cell differentiation. The contribution of iTreg cells generated via this pathway to the peripheral repertoire of Treg cells also remains to be investigated.
iTreg cells can also be generated in the gut-associated lymphoid tissue (GALT). In a T cell transfer mouse model, a subset of polyclonal CD4+Foxp3− T cells were converted to iTreg cells and accumulated in the GALT (Sun et al., 2007). Naive transgenic T cells could also differentiate into iTreg cells in response to orally administered antigens (Coombes et al., 2007). The preferential induction of iTreg cells at the mucosal site has been attributed to GALT dendritic cells, a major subset of which expresses the surface marker protein CD103. GALT dendritic cells have the unique ability to imprint T cells for migration back to the gut by inducing the expression on T cells of homing receptors such as the α4β7 integrin and cc chemokine receptor 9 (Mora et al., 2003). Notably, mucosal dendritic cells, especially the CD103+ subset, expressed high amounts of retinal dehydrogenase, the enzyme that converts vitamin A to its metabolite retinoic acid (RA), the molecule responsible for the upregulation of gut-homing receptors on T cells (Iwata et al., 2004). Interestingly, administration of neutralizing TGF-β antibody or RA receptor inhibitors attenuated the generation of iTreg cells, whereas addition of RA potentiated TGF-β-induced iTreg cell differentiation (Benson et al., 2007; Coombes et al., 2007; Mucida et al., 2007; Sun et al., 2007). Thus, CD103+ dendritic cell-induced conversion of iTreg cells is dependent on both TGF-β and RA. These studies reveal a new pathway for the generation and homing of iTreg cells to the GALT (Figure 2), which may provide an important mechanism for the induction of tolerance to innocuous mucosal antigens such as those derived from commensal flora or those of dietary origins.
TGF-β Control of Effector T Cell Differentiation
Activation of naive T cells during immune responses leads to their differentiation into various effector T cell subsets that are capable of killing target cells, in the case of CD8+ T cells, or capable of providing helper function, in the case of CD4+ T cells. These armed effector T cells play important roles in host defense against pathogen invasion. When dysregulated, however, effector T cells can also induce the development of autoimmune diseases or atopic syndromes such as asthma and allergy. Effector T cell differentiation is orchestrated by signals from the TCR, costimulatory molecules, and cytokine signaling pathways. This combinatorial input, often imprinted by the nature of infectious agents, culminates in the expression of key transcription factors that specify the T cell phenotypes. For example, the expression of the transcription factor T-bet specifies Th1 cells, whereas the expression of GATA-3 specifies Th2 cells. RORγt expression generates the newly identified Th17 cells, and a combination of T-bet and eomesodermin expression specifies cytotoxic T lymphocytes development.
Studies of T cell-specific TGF-βRII-deficient mice have revealed a cell-intrinsic role for TGF-β signaling in the prevention of T cell proliferation and activation, as well as in the inhibition of effector T cell differentiation (Li et al., 2006a; Marie et al., 2006). TGF-β inhibited the expression of the T cell mitogenic cytokine IL-2 in vitro in a Smad3-dependent manner (McKarns et al., 2004) (Figure 3). TGF-β also potently inhibited Th1 and Th2 cell differentiation in vitro through the downregulation of T-bet and GATA-3 expression, respectively (Gorelik et al., 2000, 2002; Heath et al., 2000; Neurath et al., 2002). This inhibition has been associated with the TGF-β blockade of TCR-induced activation of the Tec kinase Itk, calcium ion influx in T cells, and the activation of the transcription factor NFAT (Chen et al., 2003a) (Figure 3). Consistent with these findings, CD4+ T cells from CD4-DNRII mice, which have attenuated TGF-β signaling, spontaneously differentiated into Th1 and Th2 cells (Gore-lik and Flavell, 2000). Interestingly, CD4+ T cells from T cell-specific TGF-βRII-deficient mice, which have more complete elimination of TGF-β signaling than those from CD4-DNRII mice, produced copious amounts of the cytokine interferon-γ (IFN-γ) and differentiated almost exclusively into Th1 cells (Li et al., 2006a; Marie et al., 2006). These findings suggest that Th1 cell differentiation likely represents a default effector pathway for CD4+ T cells in the absence of TGF-β signaling. However, it remains to be determined whether the difference of CD4+ effector T cell differentiation observed in CD4-DNRII mice and T cell-specific TGF-βRII-deficient mice is a consequence of varied inhibition of TGF-β signaling or is due to distinct antigen stimulation of CD4+ T cells.
Associated with the dominant Th1 cell differentiation phenotype, peripheral CD4+ T cells from T cell-specific TGF-βRII-deficient mice expressed high amounts of T-bet (Li et al., 2006a; Marie et al., 2006). Reduced IFN-γ-producing Th1 cells and increased IL-4-producing Th2 cells were observed in T cell-specific TGF-βRII-deficient mice also lacking T-bet (Li et al., 2006a), consistent with a function of T-bet in promoting Th1 and suppressing Th2 cell differentiation (Finotto et al., 2002). Intriguingly, deficiency of T-bet also resulted in extensive depletion of TGF-βRII-deficient CD4+ T cells (Li et al., 2006a). This prosurvival function of T-bet is associated with T-bet regulation of CD122 expression in CD4+ T cells. CD122 is the shared receptor β chain for cytokines IL-15 and IL-2. Indeed, IL-15-triggered CD122 signaling enhanced the survival of TGF-βRII-deficient CD4+ T cells (Li et al., 2006a). These findings, together with the observation that TGF-βRII-deficient naive CD4+ T cells underwent high rates of cell death, suggest that lack of TGF-β signaling in T cells results in cell death of CD4+ T cells unless they are activated and differentiate into T-bet-expressing Th1 cells. Therefore, the prosurvival function of T-bet likely accounts for the prominent Th1 cell phenotype observed in T cell-specific TGF-βRII-deficient mice.
In addition to limiting CD4+ T cell differentiation, TGF-β is also a potent inhibitor of CD8+ T cell activation and differentiation (Figure 2). A subset of TGF-βRII-deficient CD8+ T cells, but not wild-type CD8+ T cells, expressed cell surface markers that are typically found on NK cells such as NK1.1 and NKG2D (Marie et al., 2006). NK1.1+ T cells induced more severe immunopathology than NK1.1− T cells upon adoptive-transfer to RAG1-deficient mice. The differentiation of NK1.1+ T cells is due to the activation of TGF-βRII-deficient T cells in peripheral tissues, because the transfer of cultured NK1.1− T cells lacking the Tgfbr2 gene to recipients with abnormally low blood lymphocyte levels (lymphopenic) allowed the cells to acquire NK cell markers and the ability to induce disease pathology (Marie et al., 2006). TGF-βRII-deficient CD8+ T cells also produced high amounts of IFN-γ and cytolytic molecules, including FasL, perforin, and granzymes (Li et al., 2006a; Marie et al., 2006). These finding are consistent with a study of TGF-β regulation of CD8+ T cell responses in the context of tumor immunity (Thomas and Massague, 2005). Neutralization of TGF-β by the administration of soluble TGF-βRII potentiated immune responses to thymic tumor cells injected into mice. This response was associated with the expansion of tumor-specific cytotoxic T lymphocytes that expressed high levels of cytolytic molecules (Thomas and Massague, 2005). Gene expression profiling experiments demonstrated that TGF-β strongly inhibited expression of the cytolytic genes in CD8+ T cells. TGF-β inhibition of granzyme B expression was associated with the recruitment of its downstream transcription factors Smad2 and Smad3 to the Gzmb promoter where they synergize with CREB and ATF1 transcription factors to block transcription (Thomas and Massague, 2005). siRNA knockdown of Smad2 and Smad3, but not Smad3 alone, alleviates TGF-β inhibition of granzyme B expression (Thomas and Massague, 2005). These observations suggest that Smad2 and Smad3 are redundant in the control of granzyme B expression (Figure 3). Future studies with mice bearing T cell-specific deletion of Smad2 and/or Smad3 genes can be used to test this hypothesis.
TGF-β Regulates Th17 Cells
By secreting cytokines and other effector molecules, CD4+ helper T cells mobilize various host cell types to tailor the immune response to the specific invading pathogen. It is generally thought that Th1 cells secrete IFN-γ and lymphotoxin to activate the cellular arm of the adaptive immune system to combat intracellular pathogens, whereas Th2 cells produce the cytokines IL-4, IL-5, and IL-13, which direct antibody production to control extracellular parasites. Recent studies have expanded the Th1-Th2 cell paradigm with the addition of a newer helper T cell type, the Th17 cell (McGeachy and Cua, 2008). Although the physiological role of Th17 cells remain to be fully explored, it is known that they secrete a distinct set of cytokines, including IL-17A, IL-17F, and IL-22, that act on a broad range of innate immune and nonhematopoietic cells to protect the host from a subset of extracellular pathogens.
A New Type of T Helper Cell
As with Th1 and Th2 cells, dysregulated Th17 cells can trigger immunopathology. In fact, one of the original clues to the existence of Th17 cells came from the studies of adjuvant-induced autoimmune diseases such as experimental allergic encephalomyelitis (EAE) and collagen-induced arthritis (CIA). These autoimmune diseases were thought to be triggered by dysfunctional Th1 cells whose differentiation is regulated by the innate immune cytokine IL-12 (Murphy and Reiner, 2002) until the startling finding that the cytokine IL-23 and not IL-12 was essential for the induction of EAE or CIA (Cua et al., 2003; Langrish et al., 2005; Murphy et al., 2003). This seminal discovery challenged the belief that Th1 cells were responsible for these diseases. Recent studies have further established the IL-17A-producing T cells as a distinct lineage of helper T cells whose differentiation is controlled by the transcription factor RORγt (Ivanov et al., 2006) but inhibited by Th1 or Th2 cytokines (Harrington et al., 2005; Park et al., 2005).
Th17 Cell Differentiation
Although IL-23 is indispensable for the development of pathological Th17 cell responses in vivo, it does not induce Th17 cell differentiation from naive CD4+ T cells. In contrast to the inhibitory effects of TGF-β on Th1 and Th2 cell differentiation, recent studies have established TGF-β as an essential regulator of Th17 cell differentiation from naive T cells. One of the reports found that naive T cells activated in the presence of Treg cells produced low amounts of IFN-γ but expressed high levels of IL-17A (Veldhoen et al., 2006a). However, neutralization of TGF-β in an in vitro culture environment containing naive T cells, Treg cells, and lipopolysaccharide (LPS)-activated dendritic cells inhibited Th17 cell differentiation. Interestingly, the study also found that in addition to TGF-β, IL-6 produced by LPS-stimulated dendritic cells was also required for the differentiation of Th17 cells. This requirement for IL-6 in modulating TGF-β regulatory effects on T cell differentiation was substantiated by another study showing that whereas TGF-β alone induced Foxp3 expression and iTreg cell differentiation from activated CD4+ T cells, inclusion of IL-6 in the T cell culture inhibited iTreg cell generation and diverted T cell differentiation to the Th17 cell lineage (Bettelli et al., 2006). IL-6 induction of Th17 cells has been attributed to its activation of the transcription factor STAT3 (Yang et al., 2007a) (Figure 3). However, the molecular mechanisms of TGF-β signaling in Th17 cell differentiation remain to be determined. Consistent with these in vitro studies, overexpression of TGF-β1 in T cells enhanced Th17 cell differentiation in mice (Bettelli et al., 2006). Concordantly, Th17 cell numbers were reduced in TGF-β1-deficient or CD4-DNRII mice (Mangan et al., 2006; Veldhoen et al., 2006b).
Although TGF-β and IL-6 are essential for the initial commitment of CD4+ T cells to the Th17 cell lineage, they may not be sufficient to induce the pathological effector Th17 cell responses in vivo. In a T cell transfer model of EAE, myelin-reactive T cells expanded by IL-23, but not those expanded by TGF-β plus IL-6, induced disease in mice (McGeachy et al., 2007). Intriguingly, TGF-β- and IL-6-stimulated Th17 cells produced high amounts of IL-10 that suppressed the immunopathology induced by IL-23-stimulated Th17 cells. From this, it appears that TGF-β and IL-6 together drive the differentiation of Th17 cells to a state with regulatory activity (regulatory Th17, rTh17) associated with IL-10 production. In contrast, continued stimulation and expansion of rTh17 cells by IL-23 in the absence of TGF-β leads to the acquisition of full effector Th17 cell functions (effector Th17, eTh17; Figure 2) including the production of pathogenic chemokines and inflammatory cytokines such as IL-22.
The Th17-iTreg Dichotomy
Involvement of TGF-β in the differentiation of both Th17 cells and iTreg cells suggests a special kinship between these two lineages of CD4+ T cells. However, the induction of the two lineages appears to be mutually exclusive: conditions favoring Th17 cell differentiation (TGF-β and IL-6) inhibit iTreg cell generation, and conditions promoting iTreg cell production (TGF-β, IL-2, and retinoic acid) block Th17 cell differentiation. The molecular mechanisms underlying this reciprocal differentiation of Th17 and iTreg cells remain incompletely understood, though a recent study has uncovered some clues. The study found that although TGF-β induced the expression of both Foxp3 and RORγt in a subset of CD4+ T cells, RORγt induction of Th17 cell differentiation was inhibited by Foxp3 though a mechanism partially mediated by physical interaction between the two transcription factors (Zhou et al., 2008) (Figure 3).
Th17 cells, presumably of the regulatory form, are present constitutively in the mouse intestinal lamina propria (Ivanov et al., 2006). Gut-associated lymphoid tissue may also be a primary site for the generation of iTreg cells. Together, these observations suggest that TGF-β-induced Th17 cells and iTreg cells have important functions in the regulation of immune responses in the gut. TGF-β induction of Th17 cell and iTreg cell differentiation is reminiscent of TGF-β promotion of B cell IgA antibody class switching (Li et al., 2006b). Because IgA is also critically involved in mucosal immune defense, it is tempting to speculate that the positive effects of TGF-β on T and B lymphocyte differentiation were selected for during evolution to fulfill the special requirements of mucosal immune homeostasis.
T Cell-Derived TGF-β1 Regulates T Cells
Among the three types of TGF-β molecules found in mammals, TGF-β1 plays the most critical role in the regulation of T cell responses in vivo. TGF-β1 can be produced by multiple lineages of leukocytes and stromal cells and is secreted as a latent form in a complex with LAP and LTBP (Figure 1). TGF-β1 cannot bind to its receptors in its latent form until it is liberated from the constraints of LAP and LTBP binding. This complex mode of TGF-β1 regulation presents many new questions, including those concerning the identity of the cellular sources of TGF-β1 and the mechanisms of TGF-β1 activation from its latent form.
T Cell-Derived TGF-β1 Control of T Cell Tolerance
An early attempt to study the cellular mechanisms by which TGF-β1 regulates T cells investigated whether endocrine TGF-β1 is sufficient to control T cells in vivo. Liver-specific expression of an active form of TGF-β1 restored TGF-β1 levels in the blood of TGF-β1-deficient mice (Longenecker et al., 2002). However, this circulation of TGF-β1 at normal levels in the mutant mice still failed to ameliorate the lethal phenotype of TGF-β1 deficiency, thus suggesting that TGF-β1 control of T cell tolerance is mediated by both autocrine and paracrine sources of TGF-β1. A recent study investigating the function of T cell-produced TGF-β1 in vivo found that mice lacking the Tgfb1 gene specifically in T cells (Li et al., 2007) developed wasting colitis similarly to CD4-DNRII mice that have attenuated TGF-β signaling in T cells, suggesting that T cell-produced TGF-β1 directly regulated T cells. Indeed, in the absence of T cell-produced TGF-β1, T cells underwent hyperproliferation, activation, and effector T cell differentiation. Studies of T cell-specific TGF-βRII-deficient mice or TGF-β1-deficient mice have revealed that TGF-β1 is involved in the maintenance of peripheral Treg cells (Li et al., 2006a; Marie et al., 2005; Marie et al., 2006). In addition, despite diminished Treg cell numbers in the spleen, TGF-βRII-deficient Treg cells underwent enhanced proliferation (Li et al., 2006a). These findings demonstrate that TGF-β plays a role in both the inhibition of Treg cell proliferation and the maintenance of Treg cells. Deletion of the Tgfb1 gene in T cells led to enhanced Treg cell proliferation and expansion in peripheral lymph nodes and the spleen (Li et al., 2007). These findings suggest that Treg cell maintenance is likely dependent on other cellular sources of TGF-β1. Interestingly, colonic Treg cells from these mice lacking TGF-β1 in T cells expressed reduced levels of Foxp3 as compared to the Treg cells from peripheral lymph nodes (Li et al., 2007). Because TGF-β, in synergy with retinoic acid, is likely involved in the induction of iTreg cells in gut-associated lymphoid tissue, it is conceivable that T cell-produced TGF-β1 might be required for the optimal induction of Foxp3 expression and the de novo generation of iTreg cells in the colon.
In vitro stimulation of cultured T cells via their TCRs induced TGF-β1 production from both Treg cells and naive T cells (Li et al., 2007). To examine the function of TGF-β1 produced by these T cell subsets, Li et al. used a T cell transfer model of colitis in which the transfer of naive T cells to lymphopenic hosts induces colitis that is inhibited by the cotransfer of Treg cells. These transfer experiments showed that Treg-produced TGF-β1 was essential for the inhibition of colitis induced by naive T cells by blocking naive T cell differentiation into Th1 cells (Li et al., 2007). These findings suggest that production of TGF-β1 is a critical mechanism used by Treg cells to control immune tolerance (Figure 4).
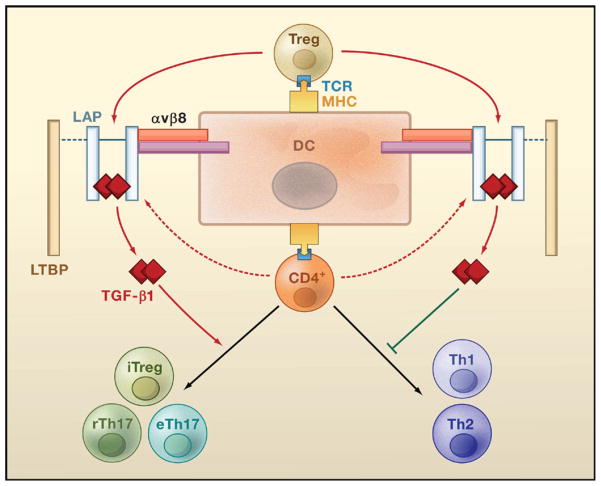
Figure 4. A Three-Cell Model for TGF-β1-Dependent Regulation of T Cells.
Upon recognition of antigens presented by dendritic cells (DCs), Treg cells are activated and secrete the latent form of TGF-β1. This latent form comprises a TGF-β1 dimer associated with the latency-associated protein (LAP), which may additionally recruit latent TGF-β-binding protein (LTBP). Interaction of LAP with αvβ8 integrin expressed by DCs triggers degradation of LAP (mediated by un-identified proteases, not shown) and the release of the active form of TGF-β1. Active TGF-β1 subsequently acts on naive CD4+ T cells via a paracrine mechanism to inhibit their differentiation into Th1 or Th2 cells and to promote their differentiation into induced regulatory T (iTreg) cells, regulatory Th17 (rTh17) cells, or effector Th17 (eTh17) cells. Activated CD4+ T cells can also produce low amounts of latent TGF-β1 that potentially regulate T cell differentiation through an autocrine route.
T Cell-Derived TGF-β1 Control of Th17 Cells
Local but not systemic administration of neutralizing TGF-β1 antibody inhibited Th17 cell differentiation and the induction of EAE (Veldhoen et al., 2006b), suggesting that Th17 cell differentiation is also mediated by autocrine and paracrine sources of TGF-β1. Th17 cell number was diminished in T cell-specific TGF-β1-deficient mice both under steady-state conditions and during EAE (Li et al., 2007). As a consequence, these mice were almost completely resistant to the induction of EAE. These findings demonstrate an essential role for T cell-produced TGF-β1 in promoting Th17 cell differentiation in vivo. It remains to be determined whether TGF-β1 produced by Treg cells, non-Treg cells, or both are involved in Th17 cell differentiation. Notably, Treg cells enhanced the Th17 cell differentiation induced by LPS-stimulated dendritic cells in vitro (Veldhoen et al., 2006a). In addition, in a model of systemic autoimmune disease, cotransfer of Treg cells with naive T cells inhibited IFN-γ expression but enhanced IL-17 production (Lohr et al., 2006). These observations suggest a proinflammatory function for Treg cells in the promotion of Th17 cell differentiation through the production of TGF-β1. Should this be proven in animal models such as Treg cell-specific TGF-β1-deficient mice, it would have ramifications for the precautions that would need to be taken in attempts to use Treg cells for treating autoimmune diseases and transplant rejection.
Integrins Mediate TGF-β1 Activation
TGF-β1 activation proceeds through the LAP degradation or altered conformation that results in the release of the mature TGF-β1 homodimer. Putative activators of TGF-β1 in this process include proteases such as plasmin and matrix metalloproteinases, reactive oxygen species, the protein thrombospondin-1, and the integrins αvβ6 or αvβ8 (Annes et al., 2003). Activation of TGF-β1 in the latent complex by the integrins αvβ6 and αvβ8 is dependent on their binding to an RGD (arginyl-glycyl-aspartic acid) motif in LAP. Upon binding, the two integrins activate TGF-β1 though different mechanisms that may allow spatial regulation of TGF-β action. αvβ6-mediated activation occurs through a conformational change in LAP and not via the release of active TGF-β1 from the latent complex (Munger et al., 1999). As a consequence, presentation of αvβ6-activated TGF-β1 requires cell-to-cell contact. On the other hand, αvβ8-induced TGF-β1 activation is dependent on metalloproteinase-mediated degradation of LAP that results in the release of active TGF-β1 into the extracellular environment (Mu et al., 2002). This mode of activation ensures that TGF-β1 action occurs at some distance away from the site of TGF-β1 activation. Interestingly, mutation of a single residue in the integrin binding motif on LAP was sufficient to abolish the integrin-dependent activation of TGF-β1, though TGF-β1 processing and secretion remained normal. Mice homozygous for this Tgfb1 mutant allele developed a lethal immune phenotype indistinguishable from that of TGF-β1-deficient mice, thus revealing an indispensable role for integrins in the activation of TGF-β1 in vivo (Yang et al., 2007b).
Effects of αvβ6 and αvβ8 Integrin Loss
The αvβ6 integrin is expressed in a subset of epithelial cells. Mice deficient in αvβ6 showed a defect in Langerhans cell differentiation (Yang et al., 2007b), a process recently found to require Langerhans cell-produced TGF-β1 (Kaplan et al., 2007). However, the inflammatory phenotype developed in αvβ6-deficient mice is much milder than that of TGF-β1-deficient mice, suggesting that αvβ6 is not essential for the activation of TGF-β1 involved in the regulation of T cells. αvβ8 integrin is expressed in multiple immune cell types including T cells and the myeloid lineage dendritic cells. Interestingly, deletion of the gene encoding integrin β8 in dendritic cells, but not in T cells, in mice led to the development of colitis and a reduction in colonic Treg cells (Travis et al., 2007). In addition, compared to wild-type dendritic cells, β8-deficient dendritic cells produced lower levels of active TGF-β1 and were incapable of supporting iTreg cell differentiation in vitro. Similar colitis and T cell defects were also observed in mice with the ablation of αv integrins in the myeloid cell lineage (Lacy-Hulbert et al., 2007).
A Three-Cell Model of TGF-β1 Control of T Cells
The inflammatory and T cell phenotypes developed by the dendritic cell-specific β8-deficient mice are remarkably similar to those observed in mice with a T cell-specific deletion of the Tgfb1 gene (Li et al., 2007; Travis et al., 2007). On the basis of these findings, we propose a “three-cell” model for TGF-β1-dependent regulation of T cell responses involving Treg cells, dendritic cells, and naive T cells (Figure 4). In this model, Treg cells produce latent TGF-β1 upon TCR stimulation by dendritic cell-presented antigens. This latent TGF-β1 is activated via dendritic cell αvβ8-dependent mechanisms to create an active TGF-β1 milieu around the dendritic cells that regulate the activation and differentiation of naive T cells. Active TGF-β1 inhibits naive T cell differentiation into effector Th1 or Th2 cells and promotes their differentiation into iTreg or Th17 cells. The involvement of dendritic cells in the activation of Treg cell-produced TGF-β1 is consistent with the finding that Treg cells formed long-lasting interactions with dendritic cells, which impaired the subsequent dendritic cell induction of effector T cell differentiation (Tang et al., 2006). Given that dendritic cells are likely the only antigen-presenting cells capable of priming naive T cells, the engagement of dendritic cells in TGF-β1 activation ensures the availability of active TGF-β1 at sites of naive T cell antigen recognition and subsequent T cell regulation by TGF-β1. On the other hand, the engagement of a different cell type (Treg cells) from dendritic cells as the source of TGF-β1 warrants its conditional availability, which may be important for dendritic cells to induce potent Th1 and Th2 cell responses during infection, when Treg cell production of TGF-β1 might be inhibited. However, it is important to note that the inflammatory and T cell phenotypes observed in T cell-specific TGF-β1-deficient mice or dendritic cell-specific αvβ8 integrin-deficient mice are less severe than those of mice completely lacking TGF-β1 or mice expressing the mutant allele of Tgfb1 defective for integrin interaction. These observations suggest that TGF-β1 produced by cell types other than T cells and integrins other than dendritic cell-expressed αvβ8 are also involved in the regulation of T cells. The identities of these alternative cellular sources of T cell regulation remain to be determined.
Future Perspectives
By regulating thymic T cell development and peripheral T cell survival, proliferation, and differentiation, TGF-β ensures the maintenance of a diverse and self-tolerant T cell repertoire and the initiation of appropriate T cell responses essential for an effective adaptive immune system. The versatile functions of TGF-β in T cell control are likely due to the amendable nature of TGF-β signaling, which can be integrated with signals from various environmental stimuli. The unique mechanism of extracellular TGF-β activation also creates a platform for TGF-β to integrate signals from multiple cell types to regulate T cells. The TGF-β pathway originated about one billion years ago (Newfeld et al., 1999), well before the inception of the lymphocyte-based adaptive immune system. The remarkably potent and pleiotropic functions of TGF-β in regulating T cell responses are reminiscent of its roles in more ancient biological processes such as embryonic development and carcinogenesis. It remains incompletely understood how the TGF-β system has been rewired at the molecular and cellular levels to regulate T cells. Additional insights into the control of T cell responses by TGF-β will not only help to illuminate the fundamental principles of T cell regulation but also should facilitate the harnessing of TGF-β to treat a variety of immune-related disorders.
Acknowledgments
M.O.L. thanks members of his laboratory for discussion, J. Allison, E. Pamer, and S. Sanjabi for reading the manuscript, and NIAMS-NIH, Arthritis Foundation, and the Rita Allen Foundation for support. R.A.F. is supported by the American Diabetes Association and NIH DK51665. M.O.L. is a Rita Allen Foundation Scholar. R.A.F. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: Development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Boivin GP, Ormsby I, Jones-Carson J, O’Toole BA, Doetschman T. Germ-free and barrier-raised TGF beta 1-deficient mice have similar inflammatory lesions. Transgenic Res. 1997;6:197–202. doi: 10.1023/a:1018490007745. [DOI] [PubMed] [Google Scholar]
- Bommireddy R, Pathak LJ, Martin J, Ormsby I, Engle SJ, Boivin GP, Babcock GF, Eriksson AU, Singh RR, Doetschman T. Self-antigen recognition by TGF beta1-deficient T cells causes their activation and systemic inflammation. Lab Invest. 2006;86:1008–1019. doi: 10.1038/labinvest.3700460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- Chen CH, Seguin-Devaux C, Burke NA, Oriss TB, Warkins SC, Clipstone N, Ray A. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med. 2003a;197:1689–1699. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003b;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-{beta} escape control by CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Heath VL, Murphy EE, Crain C, Tomlinson MG, O’Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jameson SC. T cell homeostasis: Keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17:231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Yoshida K, Ward JM, Letterio JJ, Longenecker G, Yaswen L, Mittleman B, Mozes E, Roberts AB, Karlsson S, et al. Beta 2-microglobulin-deficient background ameliorates lethal phenotype of the TGF-beta 1 null mouse. J Immunol. 1999;163:4013–4019. [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ, Geiser AG, Kulkarni AB, Dang H, Kong L, Nakabayashi T, Mackall CL, Gress RE, Roberts AB. Autoimmunity associated with TGF-beta1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Invest. 1996;98:2109–2119. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveen P, Carlsen M, Makowska A, Oddsson S, Larsson J, Goumans MJ, Cilio CM, Karlsson S. TGF-beta type II receptor-deficient thymocytes develop normally but demonstrate increased CD8+ proliferation in vivo. Blood. 2005;106:4234–4240. doi: 10.1182/blood-2005-05-1871. [DOI] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006a;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006b;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker G, Thyagarajan T, Nagineni CN, Flanders KC, Factor V, Miller G, Ward JM, Nalca A, Rangnekar VM, Thorgeirsson S, et al. Endocrine expression of the active form of TGF-beta1 in the TGF-beta1 null mice fails to ameliorate lethal phenotype. Cytokine. 2002;18:43–50. doi: 10.1006/cyto.2002.1025. [DOI] [PubMed] [Google Scholar]
- Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor. J Exp Med. 2000;191:1187–1196. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-{beta}1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Massague J, Gomis RR. The logic of TGF-beta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newfeld SJ, Wisotzkey RG, Kumar S. Molecular evolution of a developmental pathway: phylogenetic analyses of transforming growth factor-beta family ligands, receptors and Smad signal transducers. Genetics. 1999;152:783–795. doi: 10.1093/genetics/152.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGF-beta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RT, Gorham JD. TGF-beta 1 regulates antigen-specific CD4+ T cell responses in the periphery. J Immunol. 2007;179:71–79. doi: 10.4049/jimmunol.179.1.71. [DOI] [PubMed] [Google Scholar]
- Robinson RT, French MA, Kitzmiller TJ, Gorham JD. Restriction of the CD4+ T-cell receptor repertoire prevents immune pathology in TGF-beta1 knockout mice. Lab Invest. 2006;86:815–828. doi: 10.1038/labinvest.3700439. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006a;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006b;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- Walker LS, Abbas AK. The enemy within: Keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007a;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007b;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing ROR-gammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]