Abstract
Glioblastoma growth potential and resistance to therapy is currently largely attributed to a subset of tumor cells with stem-like properties. If correct, this means that cure will not be possible without eradication of the stem cell fraction and abrogation of those mechanisms through which stem cell activity is induced and maintained. Glioblastoma stem cell functions appear to be non-cell autonomous and the consequence of tumor cell residence within specialized domains such as the perivascular stem cell niche. In this review we consider the multiple cellular constituents of the perivascular niche, the molecular mechanisms that support niche structure and function and the implications of the perivascular localization of stem cells for anti-angiogenic approaches to cure.
Keywords: Glioblastoma, Stem cell, Perivascular niche, PVN, Brain tumor, Endothelial cells, Pericytes, Astrocytes, Macrophage/microglia, Extra-cellular matrix, Integrins, Cadherins, Anti-angiogenic therapy, Cancer, Pathobiology
Introduction
As initially conceived by Judah Folkman, tumor growth is indeed “angiogenesis dependent” (1). While visionary, this revolutionary statement was limited by knowledge current in 1971 to a declaration regarding the necessity of a blood supply for nutrients and oxygen. Dr. Folkman and his contemporaries could not have imagined that angiogenesis was also a process that creates a specialized domain for the support, expansion and spread of a subpopulation of tumor cells with stem cell like properties (cancer stem cells (CSCs)). This specialized space, the perivascular domain or niche (PVN) is an exquisite collaboration between tumor cells, endothelial cells, pericytes and tissue specific components, for the maintenance of the tumor stem cell population.
In light of this greater appreciation for the importance of angiogenesis to tumor persistence and progression, targeting angiogenesis for cancer therapy would seem to have even greater potential than originally conceived. Not only can it disrupt blood supply and oxygen delivery, it can abrogate the formation of niche space and thereby terminate the potential for tumor growth. However, clinical experience with anti-angiogenic therapy that targets the single most potent angiogenic factor, vascular endothelial cell growth factor (VEGF), or its receptors has taught us that there are multiple mechanisms by which tumors stimulate angiogenesis and resist anti-VEGF therapies. These mechanisms are diverse and involve additional soluble angiogenic factors, changes in the cellular constituents of the vascular unit, and even transdifferentiation of tumor cells into endothelial cells. This experience suggests that targeting the structure of the niche by simply trying to block its formation may not be practical. Instead, alternatively targeting niche function may have superior therapeutic effect without stimulating resistance mechanisms. To succeed in this endeavor it is imperative to understand the mechanisms and functions of the niche. In this review we will examine the cellular components of the brain tumor stem cell niche and core modes of intercellular communication that support its coordinated activities.
Functions of the Perivascular Niche
Experience with culturing brain tumor stem cells suggests that the stem cell state is an unstable one and that in the absence of appropriate signals these cells will undergo spontaneous differentiation. Thus we can conclude that the functions of the niche include blocking differentiation in order to maintain the stem cell phenotype. Consistent with this, when brain tumor stem cells are grown in vitro in the presence of endothelial cells, there is a measureable increase in self-renewal capacity and quaternary tumor sphere formation (2–5). Moreover, treatment of xenograft brain tumor models with anti-angiogenic agents alone or in combination with cytoxic chemotherapy results in decreases in the population of self-renewing CD133+, Nestin+ cancer stem cells (3, 6).
In addition to maintaining the cancer stem cell population, the PVN also promotes tumor cell proliferation (2, 3). Primary glioblastoma (GBM) cells grown in the presence of human brain microvascular endothelial cells (HBMECs) exhibit increased growth in vivo and in vitro compared to GBM cells alone, and like the normal neural stem cell niche this is due at least in part to the actions of endothelial cell-derived CXCL12 (7, 8). In addition, GBM-associated endothelial cells express the mitogen sonic hedgehog (SHH, (9), (10).
Importantly, the PVN can provide sanctuary and protect GBM from the actions of both radiation and chemotherapy. The backbone of malignant brain tumor treatment is DNA damaging agents like radiation therapy and alkylator chemotherapy. The efficacy of these regimens is highly dependent upon mitotic activity in target cells and a fraction of the CSCs are found in a slow-cycling or quiescent state, which would render them resistant to DNA damaging agents (11, 12). In addition, the efficacy of DNA damaging agents is sensitive to changes in DNA repair capacity. Within the PVN there is a measureable increase in DNA repair capacity, possibly through the actions of microenvironment-derived TGF-β (13). This would also mitigate against the impact of DNA damaging agents (14, 15). Moreover, CSCs exhibit increased expression of multidrug resistance transporters (such as ABC and MDR transporters), which are responsible for the efflux of chemotherapeutics out of cells and thus limit the exposure of tumor cells within the PVN to DNA damaging agents (16, 17). This property has been used to identify GBM stem cells as the Hoechst stain negative side-population of tumor cells on FACS analysis (18). Finally, GBM stem cells avoid immune detection and suppress immune activity through diminished expression of MHC (19) and secretion of immunosuppressive cytokines that block T cell proliferation and activation (20), an effect that is augmented by hypoxia (21).
The peri-endothelial space also provides an important conduit for infiltrative spread of GBM. In 1938, Scherer described the movement of GBM cells away from the primary tumor mass along the perivascular space (22), and dispersal of GBM through this space may be a critical component of tumor recurrence after gross total resections and tumor bed irradiation. The basis for this pattern of GBM cell movement may be due to chemotactic effects of high levels of CXCL12 found within the PVN (7, 23) and CXCL12’s effects on expression of cathepsins and matrix metalloproteinases (MMP) (24).
Origins of the Perivascular Brain Tumor Stem Cell Niche
Multiple mechanisms have been proposed through which brain tumor cells might forge stem cell supportive interactions with endothelial cells, including: co-opting existing blood vessels and stimulating angiogenesis. Surprisingly however, in three recent papers (25–27) it was shown that GBM stem cells themselves can transdifferentiate into endothelial cells. Up to 60% of tumor-associated endothelial cells shared genetic background with tumor cells, and a subset of the CD133 positive brain tumor stem cell fraction were also positive for vascular endothelial-cadherin (CD144). Similar transdifferentiation of normal neural stem cells into endothelial cells has also been described (28) and may represent a broadly important phenomenon. The frequency of GBM-derived endothelial cells in patient specimens remains to be fully determined and the potential for these GBM-derived endothelial cells to provide structural niche space and regulatory control of niche function remains to be defined.
Components of the brain tumor stem cell niche
Development of the tumor PVN involves recruitment of a multiple cell types to the niche. We are only starting to understand the complex cellular architecture of the niche and the significance of each cell type to the functions of this microdomain. Similar to the adult neurogenic niche in the subventricular (SVZ) or the subgranular (SGZ) zones, the brain tumor PVN includes endothelial cells, pericytes, astrocytes as well as immune cells such as macrophages/microglia. Understanding the molecular mechanisms by which the niche cells interact with each other and with the CSCs will help us therapeutically target those interactions within the PVN and block tumor progression.
Endothelial cells
In adult neurogenic niche, CSCs are often localized along the tumor vasculature (29). Glioma stem cells, which are frequently identified by their expression of surface markers such as CD133 (30), constitute a small fraction of the total tumor population. They appear to preferentially align themselves in the peri-endothelial space, compared to their non-stem cell counterparts, and their fractional abundance within total tumor cell numbers is strongly and positively correlated with tumor grade (31, 32). A repertoire of soluble and cell-surface molecules have been identified, which through paracrine and/or autocrine mechanisms mediate reciprocal cross talk between the endothelium and tumor cells in GBM. We recently reported that brain endothelial cell derived CXCL12 chemoattracts and supports proliferation of primary human GBM cells (7). Signaling pathways such as Notch, sonic hedgehog (SHH), VEGF, hepatocyte growth factor (HGF), pigment epithelium-derived factor (PEDF) and nitric oxide (NO), many of which are also important for neural stem cell proliferation, have been implicated in the inter-cellular communication between endothelial and tumor stem cells within the PVN (2, 4, 15, 33–37). It is interesting to note that a major distinction between tumor cells and normal neural cells, is that the tumor stem cell population can be replenished from the non-stem cell fraction, a phenomenon that is not observed for normal neural cells (38). Based on the frequent localization of tumor stem cells to the PVN, as well as the observation that pathways critical for stem cell survival are active within this niche, the PVN may function to chemoattract tumor cells, promote their transition to a “stem” like phenotype and support their maintenance and proliferation.
Pericytes
Pericytes are mesenchymal cells that are usually embedded in the vascular basement membrane where they surround and stabilize the newly formed vasculature. Several reports have indicated that pericytes are an integral part of the tumor PVN and regulate proliferation, invasion and angiogenesis through their interactions with endothelial cells. Studies in a variety of cancers including melanomas, pancreatic cancer, lung adenocarcinoma and GBM have identified different signaling pathways such as platelet-derived growth factor-β) (PDGF-β), epidermal growth factor (EGF), hypoxia-inducible factor- 1α (HIF-1α) and CXCL12 that are involved in the recruitment of pericytes to the tumor vessels (39). Reciprocal signaling between endothelial cells and adjacent pericytes through soluble, as well as membrane bound, factors such as PDGF, angiopoetin-Tie2, and angiotensin can actively regulate angiogenesis (40). In contrast to normal pericytes, tumor pericytes are loosely associated with the endothelial cells leading to leaky vasculature suggesting that normalization of the tumor vessels may have therapeutic relevance. The limited success of anti-VEGF therapy in GBM and other tumors has led to the proposal that double targeting of pericytes and endothelial cells might be productive of greater therapeutic effect (41). However, the failure of endothelial targeting in the absence of pericytes in certain tumor models suggests that the role of pericytes in the PVN needs further investigation (42).
Astrocytes
In the normal brain, astrocytes provide structural support to the brain vasculature and maintain blood brain barrier (BBB) integrity through end processes that interact with the vascular endothelial cells (43). In the normal adult neurogenic niche, astrocytes induce stem cell proliferation through the activation of purinegic receptors on stem cells while negatively regulating neurogenesis through the Notch pathway (29, 44). Gliomas induce changes in proteins expressed in astrocytic endfeet leading to a loss of astrocytic regulation of endothelial functions and dysregulation of the BBB (45). Gliomas often contain pathology-associated or reactive astrocytes, which may mediate tumor cell invasion via activation of MMPs. Astrocyte elevated gene (AEG-1), initially isolated in fetal astrocytes is often implicated in metastatic progression and invasion of gliomas (40). In a PDGF-induced glioma model, SHH expressing reactive astrocytes were identified in close association with nestin expressing tumor cells (9). Glioma stem cells have been shown to express the SHH receptor patched (PTC) and inhibition of the pathway leads to the disruption of stem-like and tumorigenic properties suggesting that SHH producing microenvironment may act as a stem cell niche.
Macrophage/microglia
Tumor associated macrophages/microglia (TAM/Ms) may constitute up to 5–30% of the tumor cell population. They are frequently localized adjacent to tumor stem cells in the PVN (46). Chemokines such as macrophage chemotactic protein (MCP)-1 and 3 as well as cytokines including colony stimulating factor (CSF)-1, granulocyte colony stimulating factor (G-CSF), and HGF have been implicated in the chemo-attraction of the macrophages to the PVN and CSCs (40). Reciprocal interactions between the glioma cells and macrophages facilitate an immune suppressive but tumor supportive phenotype for macrophages that promote tumor growth and invasion through activation of MMPs. Glioma CSCs have been shown to inhibit macrophage/microglia phagocytosis, induce secretion of immune-suppressive cytokines such as IL-10 and transforming growth factor (TGF)-β1 and enhance macrophage/microglia induced T-cell proliferation via STAT-3 pathway (47, 48). Recent studies have demonstrated that TAM/Ms can enhance angiogenesis, as well as the proliferation and invasiveness of glioma CSCs via release of TGF-β1, which induces expression of MMP-9 by glioma CSCs (49).
Extra-cellular matrix
In addition to the cellular milieu cancer stem cells like neural stem cells also interact with the extracellular matrix components within the PVN (50), especially laminin (51). The composition of laminins has been correlated with tumor grade and patient survival in gliomas (52). Furthermore, the laminin receptor integrin α6 β1 has been shown to promote endothelial cell growth in GBM, which may indirectly modulate tumor stem cell survival (53). The role of other ECM components in modulating CSCs and tumorigenesis needs further investigation.
Ependymal Cells
While the tumor PVN and normal neural stem cell niche share many features, there are also distinct differences on both the cellular and molecular levels. For example, ependymal cells are a critical component of the SVZ stem cell niche, and their cell number within the neurogenic zone correlates with stem cells number and neurogenesis (54–56). Among the identified mechanisms by which ependymal cells regulate stem cell function is the negative regulation of BMP signaling through expression of LRP2 (55). Recently, molecular profiles of the cellular constituents of the niche have been published and provide several additional intriguing candidate mediators of ependymal effects on stem cell function (56). Whether ependymal cells are similarly involved in the brain tumor PVN is unknown at this time, though the deeper parenchymal location of most GBM-associated niches would suggest that ependymal cell involvement is unlikely. This raises the interesting question of what, if any, impact this has on the regulation of stem cell activity within the tumor PVN.
Cell adhesion signaling in the PVN
Many important pathways serve the functions of the niche, and most of these have been expertly reviewed elsewhere. Therefore, we will focus on a less frequently discussed aspect of the PVN for which potential therapeutics exist, cellular adhesion signaling including: integrins and cadherins, and how these molecules influence both cell to cell and cell to ECM interactions within the PVN.
Integrins in the niche
Integrins are essential transmembrane proteins that both anchor cells to the extracellular matrix and transmit extracellular signals across the cell membrane in response to ligation by extracellular matrix components like laminin, fibronectin vitronectin, collagen, thrombospondin and osteopotin as well as other factors such as FGF. There are currently 24 known heterodimeric integrins, comprised of one of 18 alpha subunits and 8 beta subunits. While integrins lack intrinsic kinase activity they transmit signals by forming multimeric complexes called focal adhesions with other signaling proteins such as focal adhesion kinase (FAK) (57) and adaptor proteins like p130CAS (58). Unbound integrins can transmit pro-apoptotic signals (59) while complexed integrins activate core growth and migratory pathways such as the MAPK, PI3K, NF-kB and Src pathways (60). These activities regulate cell:cell interactions between tumor cells and endothelial cells as well as between non-tumor stromal elements of the PVN such as perictytes and endothelial cells. In this fashion, integrins regulate the three-dimensional structure and function of the stem cell niche.
Importantly, the only gene in common between expression profiling analyses of multiple stem and progenitor cell populations is the laminin receptor integrin α6 (61–63). Integrin α6 is also highly expressed by GBM stem cells where it appears to be required for self-renewal activity (64). Consistent with the importance of laminin and laminin receptors to the functions of the neural stem cell niche, expression of integrin β1, one of two dimerization partners for integrin α6, exhibits restricted expression to proliferative cells within the normal subepenbdymal neural stem cell niche (65). Moreover, surface localization of integrin β1 is enhanced by Galectin 1 (66), an adhesion molecule that is expressed in normal neural stem cells where it is known to regulate proliferation (67, 68), as well as in GBM where it additionally promotes invasion (69).
Integrin β1 can function in a signaling axis together with the chemokine receptor CXCR4 (70). As both Integrin β1 and CXCR4 are highly expressed within the PVN, their crosstalk might regulate GBM stem cell functions. The impact of integrins on stem cell biology may relate to their modulation of key stem cell pathways like the Wnt (71), SHH (10) and Notch (72) pathways.
Malignant transformation is associated with changes in integrin expression in a tumor specific fashion. Increased αvβ3 and αvβ5 is found in glioblastoma and associated with increased invasion, especially at the margins of the tumor (73). Interestingly, αvβ8 is also expressed in GBM and levels of αvβ8 expression correlate with two important growth phenotypes of GBM: angiogenic and infiltrative. GBM cells with high levels of αvβ8 expression exhibit correspondingly high levels of TGF-β pathway activation and an invasive pattern of growth (74). In contrast, GBM cells with low levels of αvβ8 expression exhibit correspondingly low levels of TGF-β pathway activation and an angiogenic pattern of growth. This is relevant to the present discussion, as a shift from an angiogenic to infiltrative pattern of growth has been observed in GBM treated with anti-angiogenic therapies, which alter PVN structure and function (75–77).
While the molecular basis for changes in integrin expression remains to be fully defined, components of the PVN including TGF-β and CXCL12/CXCR4 regulate integrin expression in various tumor types. In GBM, both TGF-β1 and TGF-β2 can increase expression of αvβ3 in tumor cells and increase their migratory activity (78). The chemokine CXCL12 and its receptor CXCR4 are important components of the PVN where they recruit brain tumor cells and stimulate brain tumor cell proliferation (7). Recently it was shown that CXCL12 signaling in prostate cancer affects the expression of two different integrins; αvβ3 (79) and α5β3 (80) both of which are correlated with tumor progression (73).
Cadherins in the niche
Cadherins are calcium-dependent cell adhesion molecules that mediate cell:cell interactions critical for the maintenance of normal tissue structure including the neural stem cell niche (81). The cadherin superfamily contains multiple members within several subfamiles in which individual members mediate primarily homotypic interactions to form adherens junctions that serve to segregate different cells into homogeneous populations or functional units within tissues. A number of regulators of fate and function are concentrated in adherens junctions in the central nervous system including: β-catenin (82), protein kinase C (83), cdc42 (84) and Numb (85). Consequently, dynamic regulation of cadherin expression or cadherin-switching controls cell migration, fate and function during normal development and oncogenesis. In the normal neural stem cell niche, N-cadherin expression is required to maintain the progenitor state while loss leads to delamination and differentiation of newly generated neurons (86). Much attention has been focused on the regulation of cadherin expression in cancer as dramatic changes in cadherins accompany Epithelial-Mesenchymal Transition (EMT), a critical step in malignant progression.
Alterations in GBM cadherin expression are also documented to accompany alterations in growth. The switch from angiogenic to infiltrative pattern of growth seen with VEGF pathway antagonism is accompanied not only by changes in integrin expression but also by a T to N cadherin switch (87). Similarly, Cadherin 11, a marker of mesenchymal subtype of GBM, enhances GBM cell migration and appears to be required for tumor growth in vivo (88). Possibly most exciting with regard to cadherins and GBM stem cell activity and the PVN is the observation that expression of E-Cadherin in GBM patient specimens is associated with poor prognosis (89) and that a subset of E-cadherin expressing CD133 positive GBM stem cells appears to have the capacity for transdifferentiation into endothelial cells (25, 27).
Cadherin expression is regulated by several transcription factors including FoxP2 and 4 (86), Twist (90) and Snail (91). In cancer, it appears that cadherin expression is also regulated by cytokines like IL-8 (92). Increased IL-8 expression is associated with EMT in breast cancer (93) and positively correlated with astrocytoma grade (94). Importantly, IL8 is expressed at high levels by tumor associated endothelial cells (95) and thus is likely to be active within the PVN.
Finally, interactions between cadherins and integrins have been recently observed in GBM stem cells (96). These interactions appear to regulate intracellular signaling and migration. Moreover, co-regulation of N-cadherin and integrin β1 by the receptor tyrosine kinase Tie2 is required for the adhesion of GBM cells to the endothelium as occurs within the PVN (97).
Targeting the Niche
The identification of brain tumor stem cells and their perivascular niche has energized efforts to develop stem cell directed therapies. Targeting stem cell activity can theoretically be achieved by: 1) targeting the stem cells themselves, 2) by targeting PVN formation or, 3) by targeting PVN function.
Abrogation of PVN formation through anti-angiogenic therapy is a potentially powerful approach to stopping tumor progression. VEGF antagonism is well tolerated and has efficacy but alone, or in combination with irinotecan, it does not have a lasting effect on survival. Multiple mechanisms can drive tumor progression in the setting of VEGF antagonism (98). The mechanisms of resistance to VEGF antagonism are diverse and instructive when considering how to block PVN formation. In response to bevacizumab there are increases in expression of pro-angiogenic factors like FGFs 1 and 2 and CXCL12 (99, 100), as well as increased recruitment of pro-angiogenic bone-marrow-derived cells (101–103). In addition, transdifferentiation of glioma stem cells into endothelial-like cells may also contribute to VEGF-independent angiogenesis (26, 104, 105). Finally, avastin therapy may produce a shift in the growth pattern of GBM from angiogenic to infiltrative in which new niche formation may be induced in co-opted existing blood vessels (75–77). Thus resistance to VEGF antagonism involves a complex mix of responses that suggests it may be difficult to completely block the formation of new GBM stem cell niches.
While the logic of targeting stem cells themselves is robust, recent work has demonstrated that the stem cell population is heterogeneous and may not be a discrete subpopulation. Instead, stem cells may exist as a component of a dynamic steady state involving a number of tumor cell phenotypes in which transitions occur between stem cell and non-tumor cell states, including into endothelial cells (38, 106). Therefore targeting the stem cell state may also prove to have limitations with regard to abrogating stem cell activity.
Thus it may be more important to target the mechanisms that favor transitions to the stem cell state and thereby prevent the replenishment of tumor-initiating capacity from non-tumor stem cells. This may also have the added advantage of targeting functions of non-neoplastic cells within the niche, i.e., astrocytes, endothelial cells, pericytes, which may have more limited capacity for resistance.
As described above, homeostasis within the stem cell niche is maintained through the choreographed activities of a small network of cell types (Figure 1). While the cellular diversity and molecular mechanisms that serve the niche provides many opportunities for targeted approaches to GBM therapy, targeting adhesion molecules may have the potential advantage of blocking the ability of the component cell types to band together and perform their coordinated functions. Over the past several years a number agents that target cadherins and integrins have been evaluated in cancer clinical trials including for GBM (107, 108). In general these have been well tolerated. In fact, in several cases maximal tolerated doses were not defined. In addition there are early indications of efficacy that have been attributed to anti-angiogenic effects as well as to direct anti-tumor cell effects. Not fully evaluated is whether a component of the anti-tumor effects are the result of reduced stem cell activity, and whether a more complete appreciation for this potential target of adhesion molecule therapeutics might support refined efforts to abrogate stem cell niche function. Particularly important might be the combination of adhesion molecule directed therapies with cytotoxic agents and anti-angiogenics. The efficacy of these approaches is currently being evaluated for GBM.
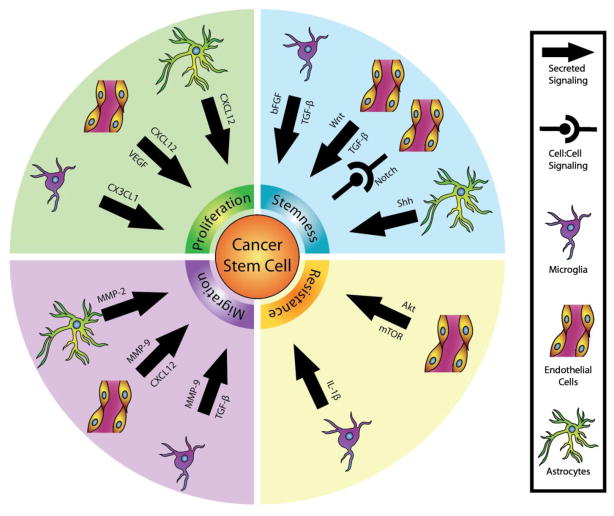
Figure 1.
The functions of the PVN to maintain the stem cell population may be divided into four categories: Induction/Maintenance of Stemness, Proliferation, Resistance, and Migration/Invasion. Shown are key pathways utilized by each cellular component of the niche to communicate with the CSCs.
While Cilengitide, an integrin αV antagonist has progressed furthest in clinical trial for GBM (Table 1), the N cadherin targeting agent ADH-1 or drugs with the potential to target integrin β1 (PF-04605412, M200) may deserve special attention for their potential to disrupt the GBM stem cell niche.
Table 1.
Clinical Trials of Cilengitide for High Grade Gliomas (HGG)
| Trial Number | Details | Status |
|---|---|---|
| NCT01165333 | Phase I evaluation of increasing doses of cilengitide with irradiation for newly diagnosed diffuse intrinsic pontine glioma in individuals 6 mos to 21 yrs of age. | R* |
|
|
||
| NCT01517776 | Phase II evaluation of cilengitide with oral metronomic temozolomide for individuals ≥ 3 yrs and < 18 yrs old with progressive or refractory HGG. | R |
|
|
||
| NCT00679354 | Phase II evaluation of cilengitide in individuals < 21 yrs old with recurrent or progressive HGG. | S |
|
|
||
| NCT00063973 | Phase I evaluation of escalating doses of cilengitide in individuals < 21 yrs old with recurrent, progressive or refractory CNS tumors including HGG. | C |
|
|
||
| NCT00813943 | Phase II evaluation of cilengitide with standard radiation and temozolomide in individuals >18 yrs old with newly diagnosed GBM and unmethylated MGMT gene promoter. | A |
|
|
||
| NCT00689221 | Phase III evaluation of cilengitide with standard radiation and temozolomide versus standard therapy alone in individuals >18 yrs old with newly diagnosed GBM and methylated MGMT gene promoter. | A |
|
|
||
| NCT01558687 | Phase I evaluation of cilengitide with standard radiation and temozolomide in individuals >18, <70 yrs old yrs old with newly diagnosed GBM. Evaluations also include measurements of vascular function. | R |
|
|
||
| NCT00979862 | Phase I evaluation of cilengitide with cediranib maleate in individuals >18 yrs old with progressive or recurrent GBM. | A |
|
|
||
| NCT01122888 | Biomarker study of cilengitide with sunitinib in individuals >18 yrs old with progressive or recurrent GBM and other solid tumors. | R |
|
|
||
| NCT01124240 | Phase II evaluation of cilengitide with standard radiation and chemotherapy followed by temozolomide and procarbazine in individuals >18 yrs old with newly diagnosed GBM and unmethylated MGMT gene promoter. | R |
|
|
||
| NCT00112866 | Phase II evaluation of cilengitide in individuals >18 yrs old with progressive or recurrent GBM undergoing surgery. Evaluations will include tissue correlates of cilengitide effects on integrin expression. | C |
|
|
||
| NCT00085254 | Phase I/II evaluation of cilengitide with standard radiation and chemotherapy in individuals >18 yrs old with newly diagnosed GBM. | C |
|
|
||
| NCT00093964 | Phase II evaluation of cilengitide in individuals >18 yrs old with progressive or recurrent GBM. | C |
|
|
||
| NCT00006093 | Phase I/II evaluation of cilengitide in individuals >18 yrs old with progressive or recurrent HGG. |
R=recruiting, S=suspended, C=completed, A=active nut not recruiting.
Conclusions
The complexity of the cancer stem cell niche creates many potential obstacles to the successful inhibition of stem cell activity. Understanding the mechanisms that support niche formation and function may expose the Achilles heel(s) of the PVN and the key to GBM cure. Particularly important will be considerations of treatment regimens that can both target niche formation and the mechanisms of stem cell induction and maintenance. In this regard, therapies that target adhesion molecules may have the advantage of dual function, with the potential to block both niche structure and function.
Acknowledgments
Work in the Rubin Lab is supported by the NIH RO1CA118389 (J.B. Rubin) and RO1CA136573 (J.B. Rubin).
Footnotes
Disclosure M.D. Brooks has received research funding from National Institutes of Health (NIH).
R. Sengupta has received research funding from NIH.
S.C. Snyder has received research funding from NIH.
J.B. Rubin has received research funding from NIH.
Contributor Information
Michael D. Brooks, Email: michaeldbrooks@gmail.com.
Rajarshi Sengupta, Email: sengupta_r@kids.wustl.edu.
Steven C. Snyder, Email: stevensnyder@wustl.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Zhu TS, Costello MA, Talsma CE, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–72. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Charles N, Ozawa T, Squatrito M, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–52. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galan-Moya EM, Le Guelte A, Lima Fernandes E, et al. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep. 2011;12:470–6. doi: 10.1038/embor.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–4. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 7.Rao S, Sengupta R, Choe EJ, et al. CXCL12 mediates trophic interactions between endothelial and tumor cells in glioblastoma. PLoS One. 2012;7:e33005. doi: 10.1371/journal.pone.0033005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Peng H, Cui M, Whitney NP, Huang Y, Zheng JC. CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem. 2009;109:1157–67. doi: 10.1111/j.1471-4159.2009.06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becher OJ, Hambardzumyan D, Fomchenko EI, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68:2241–9. doi: 10.1158/0008-5472.CAN-07-6350. [DOI] [PubMed] [Google Scholar]
- 10.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusumbe AP, Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res. 2009;69:9245–53. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 12.Quesnel B. Tumor dormancy and immunoescape. APMIS. 2008;116:685–94. doi: 10.1111/j.1600-0463.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 13.Hardee ME, Marciscano AE, Medina-Ramirez CM, et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res. 2012;72:4119–29. doi: 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 16.Nakai E, Park K, Yawata T, et al. Enhanced MDR1 expression and chemoresistance of cancer stem cells derived from glioblastoma. Cancer Invest. 2009;27:901–8. doi: 10.3109/07357900801946679. [DOI] [PubMed] [Google Scholar]
- 17.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 18.Bleau AM, Huse JT, Holland EC. The ABCG2 resistance network of glioblastoma. Cell Cycle. 2009;8:2936–44. [PubMed] [Google Scholar]
- 19.Wu A, Wiesner S, Xiao J, et al. Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–31. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
- 20.Wei J, Barr J, Kong LY, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9:67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Wu A, Kong LY, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2012;6:e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherer HJ. Structural development in gliomas. American Journal of Cancer. 1938;34:333–51. [Google Scholar]
- 23.Zagzag D, Esencay M, Mendez O, et al. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer’s structures. Am J Pathol. 2008;173:545–60. doi: 10.2353/ajpath.2008.071197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenig S, Alonso MB, Mueller MM, Lah TT. Glioblastoma and endothelial cells crosstalk, mediated by SDF-1, enhances tumour invasion and endothelial proliferation by increasing expression of cathepsins B, S, and MMP-9. Cancer Lett. 2010;289:53–61. doi: 10.1016/j.canlet.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 25*.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. Together with the Ricci-Vitiani. et al. study, this paper was the first demonstration that GBM stem cells possess the plasticity to transdifferentiate into endothelial cells. [DOI] [PubMed] [Google Scholar]
- 26*.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. Together with the Wang. et al. study, this paper was the first demonstration that GBM stem cells possess the plasticity to transdifferentiate into endothelial cells. [DOI] [PubMed] [Google Scholar]
- 27.Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A. 2011;108:4274–80. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wurmser AE, Nakashima K, Summers RG, et al. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–6. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- 29.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–9. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S, Dirks PB. Brain tumor stem cells: identification and concepts. Neurosurg Clin N Am. 2007;18:31–8. viii. doi: 10.1016/j.nec.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Kappadakunnel M, Eskin A, Dong J, et al. Stem cell associated gene expression in glioblastoma multiforme: relationship to survival and the subventricular zone. J Neurooncol. 2010;96:359–67. doi: 10.1007/s11060-009-9983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan X, Ma L, Yi D, et al. A CD133-related gene expression signature identifies an aggressive glioblastoma subtype with excessive mutations. Proc Natl Acad Sci U S A. 2011;108:1591–6. doi: 10.1073/pnas.1018696108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 34.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–68. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 36.Pumiglia K, Temple S. PEDF: bridging neurovascular interactions in the stem cell niche. Nat Neurosci. 2006;9:299–300. doi: 10.1038/nn0306-299. [DOI] [PubMed] [Google Scholar]
- 37.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Gupta PB, Fillmore CM, Jiang G, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–44. doi: 10.1016/j.cell.2011.07.026. Important model for the transitions between stem and non-stem cell states in cancer. [DOI] [PubMed] [Google Scholar]
- 39.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–80. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 41.Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85:593–8. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 42.Nisancioglu MH, Betsholtz C, Genove G. The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-A blockade. Cancer Res. 2010;70:5109–15. doi: 10.1158/0008-5472.CAN-09-4245. [DOI] [PubMed] [Google Scholar]
- 43.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114:4886–96. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilhelmsson U, Faiz M, de Pablo Y, et al. Astrocytes negatively regulate neurogenesis through the Jagged1-mediated notch pathway. Stem Cells. 2012;30:2320–9. doi: 10.1002/stem.1196. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Borboa AK, Chun HB, Baird A, Eliceiri BP. Conditional deletion of the focal adhesion kinase FAK alters remodeling of the blood-brain barrier in glioma. Cancer Res. 2010;70:10131–40. doi: 10.1158/0008-5472.CAN-10-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi L, Xiao H, Xu M, et al. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011;232:75–82. doi: 10.1016/j.jneuroim.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–25. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye XZ, Xu SL, Xin YH, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol. 2012;189:444–53. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 49.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–55. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 50.Lathia JD, Patton B, Eckley DM, et al. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol. 2007;505:630–43. doi: 10.1002/cne.21520. [DOI] [PubMed] [Google Scholar]
- 51.Kawataki T, Yamane T, Naganuma H, et al. Laminin isoforms and their integrin receptors in glioma cell migration and invasiveness: Evidence for a role of alpha5-laminin(s) and alpha3beta1 integrin. Exp Cell Res. 2007;313:3819–31. doi: 10.1016/j.yexcr.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 52.Ljubimova JY, Fugita M, Khazenzon NM, et al. Association between laminin-8 and glial tumor grade, recurrence, and patient survival. Cancer. 2004;101:604–12. doi: 10.1002/cncr.20397. [DOI] [PubMed] [Google Scholar]
- 53.Huang P, Rani MR, Ahluwalia MS, et al. Endothelial expression of TNF receptor-1 generates a proapoptotic signal inhibited by integrin alpha6beta1 in glioblastoma. Cancer Res. 2012;72:1428–37. doi: 10.1158/0008-5472.CAN-11-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kazanis I, Ffrench-Constant C. The number of stem cells in the subependymal zone of the adult rodent brain is correlated with the number of ependymal cells and not with the volume of the niche. Stem Cells Dev. 2012;21:1090–6. doi: 10.1089/scd.2011.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gajera CR, Emich H, Lioubinski O, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. Journal of cell science. 2010;123:1922–30. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- 56.Lee C, Hu J, Ralls S, et al. The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS One. 2012;7:e50501. doi: 10.1371/journal.pone.0050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilmore AP, Romer LH. Inhibition of Focal Adhesion Kinase (FAK) Signaling in Focal Adhesions Decreases Cell Motility and Proliferation. Molecular Biology of the Cell. 1996;7:1209–24. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cary LA, Han DC, Polte TR, Hanks SK, Guan J-L. Identification of p130CAS as a Mediator of Focal Adhesion Kinase-promoted Cell Migration. The Journal of cell biology. 1998;140:211–21. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. The Journal of cell biology. 2001;155:459–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu P, Luo BH. Integrin bi-directional signaling across the plasma membrane. J Cell Physiol. 2013;228:306–12. doi: 10.1002/jcp.24154. [DOI] [PubMed] [Google Scholar]
- 61.Fortunel NO, Otu HH, Ng HH, et al. Comment on “ ‘Stemness’: transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature”. Science. 2003;302:393. doi: 10.1126/science.1086384. author reply. [DOI] [PubMed] [Google Scholar]
- 62.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–4. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 63.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 64.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–32. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kazanis I, Lathia JD, Vadakkan TJ, et al. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:9771–81. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fortin S, Le Mercier M, Camby I, et al. Galectin-1 is implicated in the protein kinase C epsilon/vimentin-controlled trafficking of integrin-beta1 in glioblastoma cells. Brain pathology. 2010;20:39–49. doi: 10.1111/j.1750-3639.2008.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakaguchi M, Shingo T, Shimazaki T, et al. A carbohydrate-binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7112–7. doi: 10.1073/pnas.0508793103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imaizumi Y, Sakaguchi M, Morishita T, et al. Galectin-1 is expressed in early-type neural progenitor cells and down-regulates neurogenesis in the adult hippocampus. Molecular brain. 2011;4:7. doi: 10.1186/1756-6606-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toussaint LG, 3rd, Nilson AE, Goble JM, et al. Galectin-1, a gene preferentially expressed at the tumor margin, promotes glioblastoma cell invasion. Mol Cancer. 2012;11:32. doi: 10.1186/1476-4598-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miura K, Uniyal S, Leabu M, et al. Chemokine receptor CXCR4-beta1 integrin axis mediates tumorigenesis of osteosarcoma HOS cells. Biochem Cell Biol. 2005;83:36–48. doi: 10.1139/o04-106. [DOI] [PubMed] [Google Scholar]
- 71.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Bolós V, Blanco M, Medina V, Aparicio G, Díaz-Prado S, Grande E. Notch signalling in cancer stem cells. Clinical and Translational Oncology. 2009;11:11–9. doi: 10.1007/s12094-009-0305-2. [DOI] [PubMed] [Google Scholar]
- 73.Bello L, Francolini M, Marthyn P, et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–9. doi: 10.1097/00006123-200108000-00022. discussion 90. [DOI] [PubMed] [Google Scholar]
- 74.Tchaicha JH, Reyes SB, Shin J, Hossain MG, Lang FF, McCarty JH. Glioblastoma angiogenesis and tumor cell invasiveness are differentially regulated by beta8 integrin. Cancer Res. 2011;71:6371–81. doi: 10.1158/0008-5472.CAN-11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233–42. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108:3749–54. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wick W, Platten M, Weller M. Glioma Cell Invasion: Regulation of Metalloproteinase Activity by TGF-β. Journal of Neuro-Oncology. 2001;53:177–85. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- 79.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 80.Engl T, Relja B, Marian D, et al. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 2006;8:290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karpowicz P, Willaime-Morawek S, Balenci L, DeVeale B, Inoue T, van der Kooy D. E-Cadherin regulates neural stem cell self-renewal. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:3885–96. doi: 10.1523/JNEUROSCI.0037-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. The Journal of cell biology. 1994;127:2061–9. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewis JE, Jensen PJ, Johnson KR, Wheelock MJ. E-cadherin mediates adherens junction organization through protein kinase C. Journal of cell science. 1994;107 ( Pt 12):3615–21. doi: 10.1242/jcs.107.12.3615. [DOI] [PubMed] [Google Scholar]
- 84.Broman MT, Kouklis P, Gao X, et al. Cdc42 regulates adherens junction stability and endothelial permeability by inducing alpha-catenin interaction with the vascular endothelial cadherin complex. Circ Res. 2006;98:73–80. doi: 10.1161/01.RES.0000198387.44395.e9. [DOI] [PubMed] [Google Scholar]
- 85.Rasin MR, Gazula VR, Breunig JJ, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–27. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 86.Rousso DL, Pearson CA, Gaber ZB, et al. Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron. 2012;74:314–30. doi: 10.1016/j.neuron.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu KV, Chang JP, Parachoniak CA, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaur H, Phillips-Mason PJ, Burden-Gulley SM, et al. Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo. Mol Cancer Res. 2012;10:293–304. doi: 10.1158/1541-7786.MCR-11-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis-Tuffin LJ, Rodriguez F, Giannini C, et al. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One. 2010;5:e13665. doi: 10.1371/journal.pone.0013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochemical and biophysical research communications. 2008;367:235–41. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature cell biology. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 92.Ju D, Sun D, Xiu L, Meng X, Zhang C, Wei P. Interleukin-8 is associated with adhesion, migration and invasion in human gastric cancer SCG-7901 cells. Med Oncol. 2011;29:91–9. doi: 10.1007/s12032-010-9780-0. [DOI] [PubMed] [Google Scholar]
- 93.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samaras V, Piperi C, Levidou G, et al. Analysis of interleukin (IL)-8 expression in human astrocytomas: associations with IL-6, cyclooxygenase-2, vascular endothelial growth factor, and microvessel morphometry. Human immunology. 2009;70:391–7. doi: 10.1016/j.humimm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 95.Charalambous C, Pen LB, Su YS, Milan J, Chen TC, Hofman FM. Interleukin-8 differentially regulates migration of tumor-associated and normal human brain endothelial cells. Cancer Res. 2005;65:10347–54. doi: 10.1158/0008-5472.CAN-05-0949. [DOI] [PubMed] [Google Scholar]
- 96.Velpula KK, Rehman AA, Chelluboina B, et al. Glioma stem cell invasion through regulation of the interconnected ERK, integrin alpha6 and N-cadherin signaling pathway. Cell Signal. 2012;24:2076–84. doi: 10.1016/j.cellsig.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Liu D, Martin V, Fueyo J, et al. Tie2/TEK modulates the interaction of glioma and brain tumor stem cells with endothelial cells and promotes an invasive phenotype. Oncotarget. 2010;1:700–9. doi: 10.18632/oncotarget.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98*.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. An excellent review of angiogenic pathways and the varied mechanisms for resistance to anti-angiogenic therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 100.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin DK, Shido K, Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–67. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaked Y, Tang T, Woloszynek J, et al. Contribution of granulocyte colony-stimulating factor to the acute mobilization of endothelial precursor cells by vascular disrupting agents. Cancer Res. 2009;69:7524–8. doi: 10.1158/0008-5472.CAN-09-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong J, Zhao Y, Huang Q, et al. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem Cell Rev. 2011;7:141–52. doi: 10.1007/s12015-010-9169-7. [DOI] [PubMed] [Google Scholar]
- 105.Scully S, Francescone R, Faibish M, et al. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:12950–60. doi: 10.1523/JNEUROSCI.2017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106*.Chen R, Nishimura MC, Bumbaca SM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–75. doi: 10.1016/j.ccr.2009.12.049. An important demonstration that tumor-initiating activity can be replenished from the non-stem cell fraction of tumor cells. [DOI] [PubMed] [Google Scholar]
- 107.Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33:405–12. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 108.Blaschuk OW, Devemy E. Cadherins as novel targets for anti-cancer therapy. Eur J Pharmacol. 2009;625:195–8. doi: 10.1016/j.ejphar.2009.05.033. [DOI] [PubMed] [Google Scholar]