Abstract
Objectives
To examine the interaction between maternal pre-pregnancy body mass index (BMI) and gestational weight gain (GWG) and their association with birthweight, with a focus on racial differences.
Methods
We used birth certificate data from live singleton births of South Carolina resident mothers, who self-reported their race as non-Hispanic white (NHW, n=140,128) or non-Hispanic black (NHB, n=82,492) and who delivered at 34–44 weeks of gestation between 2004–2008 to conduct a cross-sectional study. Linear regression was used to examine the relationship between our exposures (i.e., race, BMI and GWG) and our outcome birthweight.
Results
Based on 2009 Institute of Medicine guidelines, the prevalence of adequate, inadequate and excessive GWG was 27.1%, 24.2% and 48.7%, respectively, in NHW women and 24.2%, 34.8% and 41.0%, respectively, in NHB women. Adjusting for infant sex, gestational age, maternal age, tobacco use, education, prenatal care, and Medicaid, the difference in birthweight between excessive and adequate GWG at a maternal BMI of 30 kg/m2 was 118g (95% CI: 109, 127) in NHW women and 101g (95% CI: 91, 111) in NHB women. Moreover, excessive versus adequate GWG conveyed similar protection from having a small for gestational age infant in NHW [OR=0.64 (95% CI 0.61, 0.67)] and NHB women [OR=0.68 (95% CI: 0.65, 0.72)].
Conclusions
We report a strong association between excessive GWG and higher infant birthweight across maternal BMI classes in NHW and NHB women. Given the high prevalence of excessive GWG even a small increase in birthweight may have considerable implications at the population level.
Keywords: Gestational weight gain, racial/ethnic health differences, obesity, birthweight
INTRODUCTION
Based on National Health and Nutrition Examination Survey (NHANES) data from 2007–2008, 34.0% of women of childbearing age (defined as 20 to 39 years old) were obese (body mass index (BMI) of 30 or higher), and 7.6% were extremely obese (BMI of 40 or higher) (1). Moreover, the prevalence of obesity and extreme obesity is much higher in non-Hispanic black (NHB) compared to non-Hispanic white (NHW) women of childbearing age 20 to 39 years at 47.2% and 31.3%, respectively, for obesity, and 15.0% and 6.8%, respectively, for extreme obesity (1).
Maternal pre-pregnancy obesity and excessive gestational weight gain (GWG) during pregnancy are independently associated with increased birthweight, macrosomia and giving birth to an infant who is large for gestational age (LGA) (2,3). New guidelines proposed by the Institute of Medicine (IOM) stratify recommended GWG based on pre-pregnancy BMI and, for the first time, have an upper limit of weight gain recommended for obese women (2). Racial differences in maternal and neonatal outcomes between NHB and NHW women have been examined at the low end of the birthweight distribution (4–6); however, many of these studies fail to account for major differences in pre-pregnancy BMI between NHB and NHW women. Moreover, despite the fact that maternal obesity is much higher in NHB compared to NHW women, few studies have focused on racial differences in neonatal outcomes at the high end of the birthweight distribution. Moreover, large population-based studies have not concurrently examined the impact of maternal obesity and GWG on birthweight while focusing on potential racial differences. Therefore, our objective was to examine the interaction between maternal pre-pregnancy BMI and GWG and their association with birthweight, with a focus on racial differences at the population level in South Carolina from 2004 through 2008. Our hypothesis was that racial differences exist in the association of maternal BMI and GWG with birthweight.
METHODS
Study Design and Population
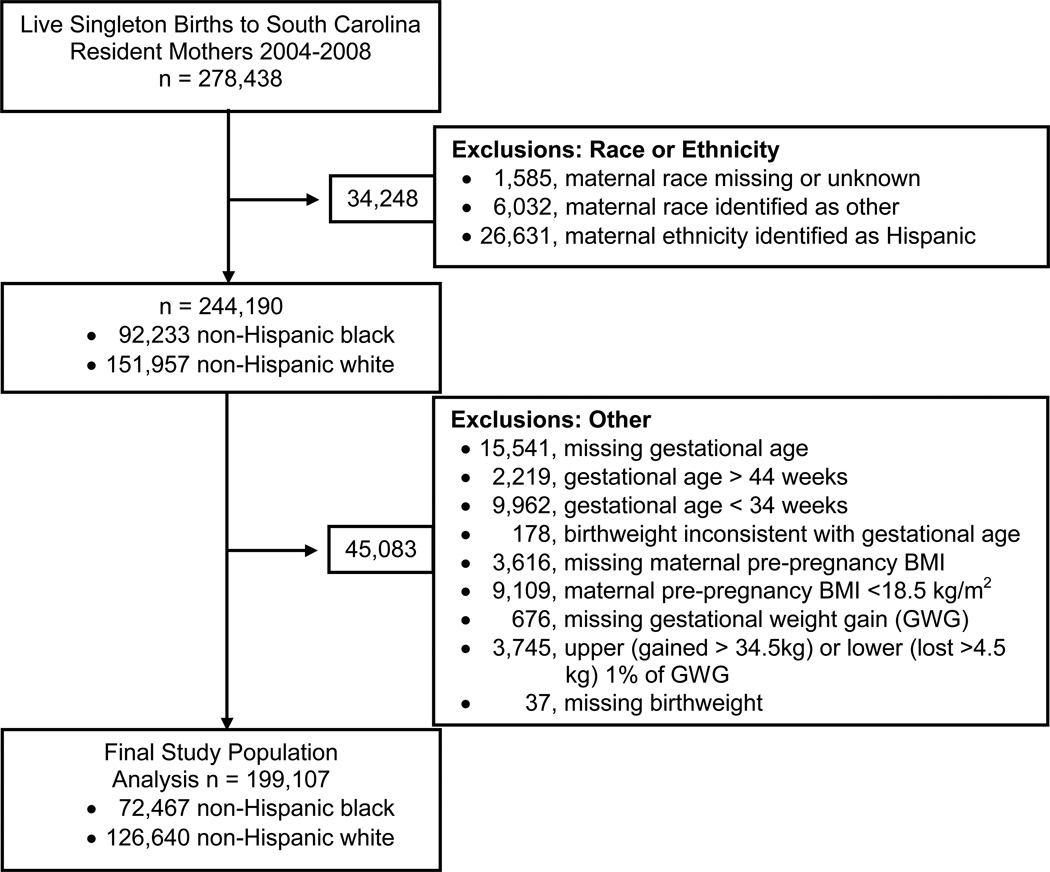
Live singleton births of South Carolina resident mothers, who self-reported their race as either NHW or NHB, who had a pre-pregnancy BMI of at least 18.5 kg/m2, and who delivered at a gestational age between 34 and 44 weeks of gestation between January 2004 and December 2008 comprise the study population (Figure 1). Information routinely collected on the birth certificate was obtained from the SC Department of Health and Environmental Control. The Institutional Review Board of the Medical University of South Carolina approved the study.
Figure 1.
Flow chart defining study population and exclusions.
Data Collection
Information obtained from the birth certificate included birthweight, gestational age based on reported date of last menstrual period (LMP); infant sex; maternal age, height, pre-pregnancy weight, weight at delivery and educational level; month when prenatal care was first received; number of prenatal visits; whether tobacco was used during pregnancy; Medicaid payor status during the pregnancy (yes/no); and parity. In 2004 the South Carolina birth certificate was revised to improve the quality of data collected related to maternal obesity. Specifically, information on maternal height, pre-pregnancy weight and weight at delivery was added.
Mate BMI was calculated as mother’s pre-pregnancy weight in kilograms divided by height in meters squared. Based on BMI, women were classified as normal (18.5–24.9 kg/m2), overweight (25.0 to 29.9 kg/m2) or obese (≥30 kg/m2). GWG was calculated as weight at delivery (which included the weight of the infant and products of conception) minus mother’s pre-pregnancy weight and was categorized into adequate, inadequate and excessive based on 2009 IOM guidelines (2). Specifically, adequate GWG was defined as 25 to 35 pounds for women of normal BMI, 15 to 25 pounds for overweight women and 11 to 20 pounds for obese women. Inadequate and excessive GWG were defined as less and more than adequate GWG, respectively (2).
Maternal race, education (dichotomized based on high school graduate or GED completion), first born (based on report of previous live births), and tobacco use (yes/no) were defined as reported on the birth certificate. Whether the mother received Medicaid during pregnancy was based on Medicaid payor status during the pregnancy. Adequacy of prenatal care was defined based on the revised graduated index (GINDEX), which combines information from the birth certificate on the trimester when prenatal care was first received and the total number of prenatal visits (7). Birthweights inconsistent with gestational age were identified based on a modified version of the criteria published by Alexander et al. (8) with the modification allowing for birthweight up to 6500 grams at 39 weeks of gestation and 7000 grams at 40 weeks or more of gestation.
We conducted analyses of birthweight adjusted for gestational age, representing a measure of fetal growth. Large for gestational age (LGA) and small for gestational age (SGA) were based on the 90th and 10th percentiles of weight for gestational age, respectively, based on sex specific fetal growth curves derived from a United States national reference based on births in 1999–2000 (9). The same standard growth curve was used for NHW and NHB infants to define LGA and SGA (9).
Statistical Analysis
We conducted a cross-sectional study with maternal race, maternal BMI, and GWG being the exposures of interest and birthweight being the primary outcome. Dichotomous outcomes included having a LGA or SGA infant.
Unadjusted means and proportions were determined for maternal characteristics and infant outcomes stratified by maternal BMI category (i.e., normal, overweight or obese) and GWG category (i.e., adequate, inadequate or excessive). Covariates adjusted for in all regression analysis included maternal age, infant sex, first live born infant, maternal tobacco use, maternal high school education, prenatal care as defined by the revised GINDEX, Medicaid payor status, and four terms for gestational age (i.e., linear, quadratic, cubic and 4th order polynomial term). These covariates were determined a priori and were variables we considered basic factors known to impact birthweight, or factors such as prenatal care that may impact the quality of available data. Linear regression was used to examine the relationship between maternal BMI category, GWG as a continuous variable, race, and our outcome, birthweight (i.e., Figure 3; Table 2). Three terms were used to model GWG as a continuous variable (i.e., linear, quadratic and cubic). Appropriate interaction terms were used to determine whether maternal BMI category had a similar association with birthweight in NHW and NHB women across the distribution of GWG (i.e., three way interaction). Similarly, linear regression was used to examine the relationship between GWG category, maternal BMI as a continuous variable, race, and our outcome, birthweight (i.e., Figure 4, Table 3). Two terms were used to model maternal BMI as a continuous variable (i.e., linear and quadratic). Appropriate interaction terms were used to determine whether GWG category had a similar association with birthweight in NHW and NHB women across the distribution of maternal BMI (i.e., three way interaction). A p-value of 0.05 was used as a nominal value for statistically significant interactions. Sequentially built models were compared using the Akaike Information Criterion and Bayesian Information Criterion to determine how many terms to use to model continuous variables (i.e., gestational age, maternal BMI, and GWG). Additionally, logistic regression was used to examine the association between (1) maternal BMI category and (2) GWG category and having either an LGA or SGA infant (i.e., Table 4). All data analyses were conducted using SAS 9.3. (Cary, North Carolina) and Microsoft Office Excel 2007 was used to create figures.
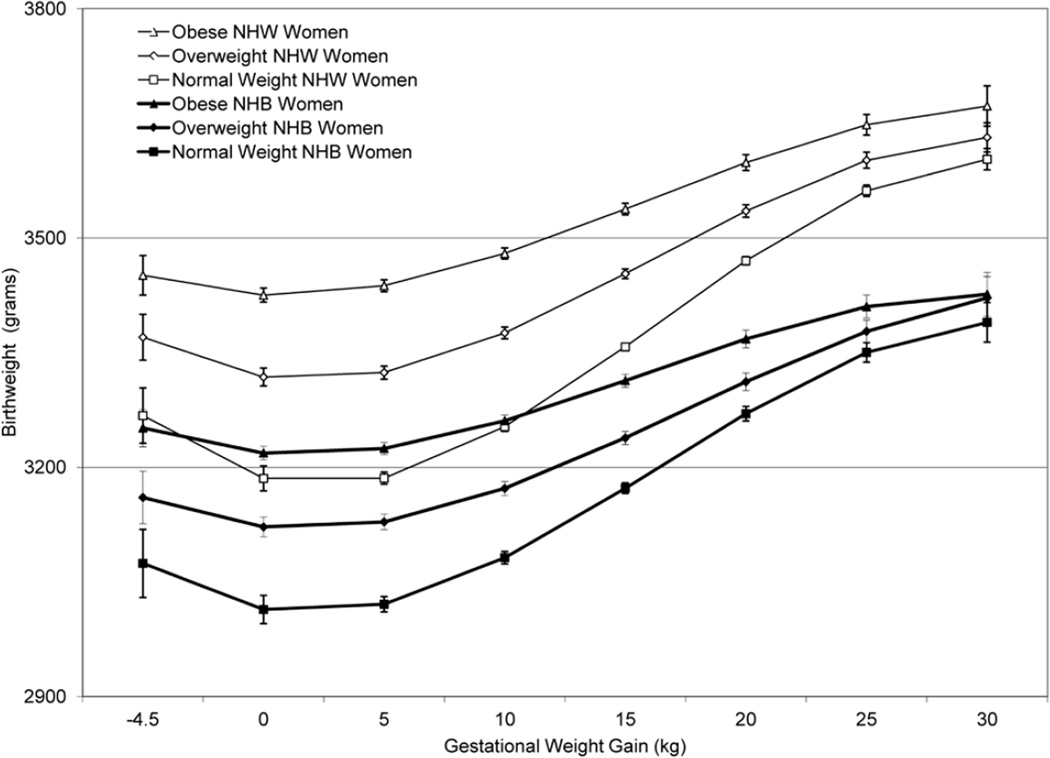
Figure 3.
Birthweight curves specific to gestational weight gain (GWG) stratified by maternal race and maternal pre-pregnancy BMI category. The linear regression model used included maternal age, infant sex, race, maternal tobacco use, maternal high school education, being first born, prenatal care as defined by the revised GINDEX, Medicaid status, four terms for gestational age (i.e., linear, quadratic, cubic and 4th order polynomial term), three terms for GWG (i.e., linear, quadratic and cubic), a categorical variable for maternal pre-pregnancy BMI category (i.e., normal weight, overweight and obese) and appropriate interaction terms between race, GWG and maternal pre-pregnancy BMI categories. Birthweight was the outcome of interest.
Table 2.
Birthweight (gm) and differences in birthweight (with 95% CI) for point estimates depicted in Figure 3 which contains birthweight curves across the continuum of gestational weight gain stratified by maternal race and maternal pre-pregnancy BMI category.
| Non-Hispanic white | Non-Hispanic-black | Interaction | |||
|---|---|---|---|---|---|
| Modela | Birthweight | Differenceb | Birthweight | Differenceb | ±Race Differencec |
| Maternal BMI Category | |||||
| Normal, 0 kg GWG | 3185 | ---- | 3014 | ---- | ---- |
| Overweight, 0 kg GWG | 3318 | 133 (113, 153) | 3122 | 108 (85, 130) | 25 (−5, 55) |
| Obese, 0 kg GWG | 3425 | 240 (221, 258) | 3219 | 205 (184, 225) | 35 (8, 63) |
| Maternal BMI Category | |||||
| Normal, 10 kg GWG | 3253 | ---- | 3082 | ---- | ---- |
| Overweight, 10 kg GWG | 3376 | 123 (114, 132) | 3172 | 91 (79, 102) | 32 (17, 47) |
| Obese, 10 kg GWG | 3480 | 227 (218, 236) | 3261 | 179 (168, 190) | 48 (34, 62) |
| Maternal BMI Category | |||||
| Normal, 20 kg GWG | 3470 | ---- | 3270 | ---- | ---- |
| Overweight, 20 kg GWG | 3535 | 65 (56, 75) | 3312 | 42 (27, 56) | 23 (6, 41) |
| Obese, 20 kg gain | 3599 | 128 (117, 140) | 3368 | 98 (83, 112) | 31 (12, 49) |
| Maternal BMI Category | |||||
| Normal, 30 kg GWG | 3603 | ---- | 3390 | ---- | ---- |
| Overweight, 30 kg GWG | 3631 | 28 (5, 52) | 3422 | 32 (−6, 70) | −3 (−48, 41) |
| Obese, 30 kg GWG | 3673 | 70 (40, 99) | 3426 | 37 (−2, 75) | 33 (−16, 81) |
BMI, body mass index;
The regression model used for results found in Table 2 is the model specified in Figure 3;
Difference in birthweights attributed to maternal pre-pregnancy BMI category [i.e., overweight (or obese) minus normal BMI]
Racial difference in difference of birthweights attributed to maternal pre-pregnancy BMI category.
Figure 4.
Birthweight curves specific to maternal pre-pregnancy BMI stratified by maternal race and gestational weight gain (GWG) category. The linear regression model used included maternal age, infant sex, race, maternal tobacco use, maternal high school education, being first born, prenatal care as defined by the revised GINDEX, Medicaid status, four terms for gestational age (i.e., linear, quadratic, cubic and 4th order polynomial term), two terms for maternal pre-pregnancy BMI (i.e., linear and quadratic) and a categorical variable for GWG (i.e., adequate, inadequate and excessive) and appropriate interaction terms between race, maternal pre-pregnancy BMI and GWG categories. Birthweight was the outcome of interest.
Table 3.
Birthweight (gm) and differences in birthweight (with 95% CI) for point estimates depicted in Figure 4 which contains birthweight curves across the continuum of maternal pre-pregnancy-BMI stratified by maternal race and maternal gestational weight gain category.
| Non-Hispanic white | Non-Hispanic black | Interaction | |||
|---|---|---|---|---|---|
| Modela | Birthweight | Differenceb | Birthweight | Differenceb | ±Race Differencec |
| Gestational Weight Gain | |||||
| Adequate; 20 kg/m2 BMI | 3311 | ---- | 3120 | ---- | ---- |
| Inadequate; 20 kg/m2 BMI | 3172 | −139 (−150,−128) | 3009 | −111 (−127, −96) | −27 (−47, −8) |
| Excessive; 20 kg/m2 BMI | 3460 | 149 (140, 158) | 3255 | 135 (120, 151) | 14 (−5, 32) |
| Gestational Weight Gain | |||||
| Adequate; 30 kg/m2 BMI | 3408 | ---- | 3200 | ---- | ---- |
| Inadequate; 30 kg/m2 BMI | 3354 | −54 (−64, −44) | 3151 | −49 (−59, −38) | −6 (−20, 9) |
| Excessive; 30 kg/m2 BMI | 3526 | 118 (109, 127) | 3301 | 101 (91, 111) | 17 (3, 31) |
| Gestational Weight Gain | |||||
| Adequate; 40 kg/m2 BMI | 3497 | ---- | 3264 | ---- | ---- |
| Inadequate; 40 kg/m2 BMI | 3473 | −24 (−40, −8) | 3248 | −16 (−31, −1) | −8 (−30, 14) |
| Excessive; 40 kg/m2 BMI | 3591 | 94 (78, 109) | 3343 | 79 (63, 94) | 15 (−7, 37) |
BMI, body mass index;
The regression model used for results found in Table 3 is the model specified in Figure 4;
Difference in birthweights attributed to GWG [i.e., inadequate (or excessive) minus adequaqte weight gain];
Racial difference in difference of birthweights attributed to GWG category.
Table 4.
Adjusted odds ratios (and 95% confidence intervals) for LGA and SGA outcomes in singleton live births in South Carolina 2004–2008.
| Model | LGA | SGA |
|---|---|---|
| Maternal BMI Categorya | ||
| Normal BMI | 1.00 | 1.00 |
| Overweight | 1.63 (1.56, 1.70) | 0.58 (0.56, 0.60) |
| Obese | 2.79 (2.67, 2.91) | 0.75 (0.72, 0.77) |
| Gestational Weight Gainb | ||
| Adequate | 1.00 | 1.00 |
| Inadequate | 0.83 (0.79, 0.88) | 1.38 (1.33, 1.43) |
| Excessive | 2.01 (1.92, 2.10) | 0.66 (0.64, 0.68) |
LGA and SGA models are adjusted for maternal age, infant sex, maternal tobacco use, high school education, being first born, prenatal care, Medicaid status and GWG (i.e., with a linear, quadratic and cubic term);
Adjusted for maternal age, infant sex, maternal tobacco use, high school education, being first born, prenatal care, Medicaid status and maternal BMI (i.e., with a linear term).
RESULTS
Population Characteristics
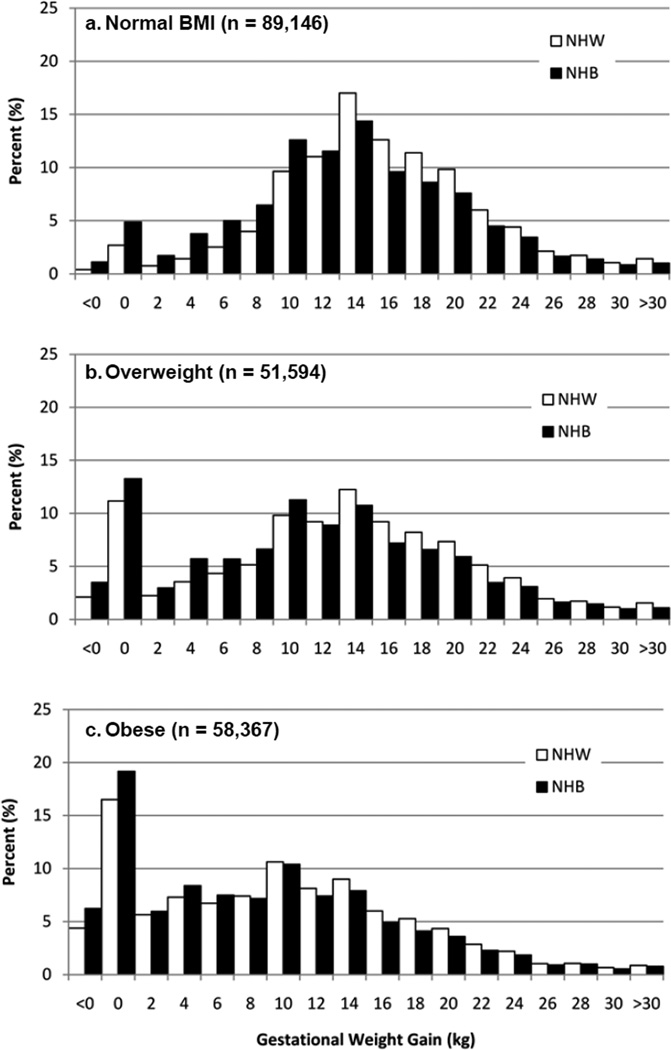
Of 278,438 live singleton births to South Carolina resident mothers from 2004 through 2008, 72,467 births to NHB mothers and 126,640 births to NHW mothers were included in the analysis. Exclusions are detailed in Figure 1. Comparing the final study population (n=199,107) to those excluded for reasons other than race or ethnicity (n=45,083), those excluded had a slightly lower maternal age (25.2 versus 26.4 years) and were more likely to be NHB (43.8% versus 36.4%) than those included in the final study population. During the 5-year period 168,948 mothers had 199,107 singleton births included in the analysis. The prevalence of adequate, inadequate and excessive GWG was 27.1%, 24.2% and 48.7%, respectively, in NHW women and 24.2%, 34.8% and 41.0%, respectively, in NHB women. The prevalence of overweight and obesity was 25.2% and 24.0%, respectively, in NHW women and 27.1% and 38.6%, respectively, in NHB women. Maternal and infant characteristics are found in Table 1. Figure 2 is a histogram of GWG in NHW and NHB women stratified by maternal BMI category.
Table 1.
Population characteristicsa (percent or mean) stratified by maternal gestational weight gain and pre-pregnancy obesity status for singleton live births in South Carolina 2004–2008.
| Gestational Weight Gain Category | Pre-pregnancy BMI Category | |||||
|---|---|---|---|---|---|---|
| Adequate n=51837 |
Inadequate n=55848 |
Excessive n=91422 |
Normal n=89146 |
Overweight n=51594 |
Obese n=58367 |
|
| Maternal Characteristics | ||||||
| Age (years) | 26.8 | 25.8 | 26.5 | 26.1 | 26.4 | 26.7 |
| Age < 18 years | 3.8 | 5.2 | 3.7 | 5.4 | 3.9 | 2.4 |
| Age ≥ 35 years | 12.0 | 10.0 | 10.3 | 10.2 | 11.0 | 11.0 |
| African American (%) | 33.9 | 45.1 | 32.5 | 27.9 | 38.0 | 47.9 |
| High School Education (%) | 83.3 | 75.9 | 83.6 | 81.4 | 82.0 | 80.8 |
| Medicaid (%) | 47.6 | 59.1 | 50.0 | 46.9 | 51.6 | 59.8 |
| BMIb (kg/m2) | 26.0 | 28.9 | 27.5 | 21.9 | 27.3 | 36.2 |
| Gestational Weight Gain (kg) | 11.4 | 3.4 | 18.7 | 14.9 | 12.3 | 9.1 |
| First Live Born Infant (%) | 38.8 | 37.5 | 46.1 | 46.6 | 40.3 | 35.7 |
| Normal BMI, 18.5–25 kg/m2 (%) | 58.5 | 40.5 | 31.6 | ---- | ---- | ---- |
| Overweight, 25–30 kg/m2 (%) | 20.2 | 22.8 | 31.0 | ---- | ---- | ---- |
| Obese, ≥ 30 kg/m2 (%) | 21.3 | 36.7 | 29.4 | ---- | ---- | ---- |
| Adequate Weight Gain (%) | ---- | ---- | ---- | 34.0 | 20.3 | 18.9 |
| Inadequate Weight Gain (%) | ---- | ---- | ---- | 25.4 | 24.7 | 35.1 |
| Excessive Weight Gain (%) | ---- | ---- | ---- | 40.6 | 55.0 | 46.0 |
| Tobacco Use (%) | 12.8 | 15.3 | 13.8 | 14.4 | 13.9 | 13.3 |
| Prenatal Careb (%) | ||||||
| Adequate Utilization | 38.2 | 33.8 | 39.5 | 38.1 | 37.9 | 36.5 |
| Intensive Utilization | 12.9 | 13.5 | 14.8 | 11.3 | 13.7 | 18.2 |
| Intermediate Utilization | 38.6 | 37.2 | 36.7 | 39.5 | 37.1 | 34.1 |
| Inadequate Utilization | 9.2 | 14.0 | 8.1 | 10.0 | 10.2 | 10.1 |
| No Care | 0.6 | 0.7 | 0.6 | 0.6 | 0.6 | 0.6 |
| Missing Required Data | 0.6 | 0.9 | 0.3 | 0.5 | 0.6 | 0.6 |
| Infant Characteristics | ||||||
| Female Infant (%) | 49.4 | 50.4 | 47.9 | 49.0 | 49.2 | 48.7 |
| Gestational Age (weeks) | 38.7 | 38.6 | 38.9 | 38.7 | 38.7 | 38.7 |
| Gestational Age ≥ 37 weeks | 90.2 | 87.6 | 91.7 | 90.2 | 90.2 | 89.9 |
| LGAb, > 90th percentile | 5.6 | 4.7 | 10.8 | 5.9 | 8.0 | 10.2 |
| SGAb, ≤ 10th percentile | 12.9 | 18.2 | 8.9 | 13.4 | 12.1 | 11.7 |
BMI, Body Mass Index;
Due to the large sample size and the implied tight confidence intervals, confidence intervals are not presented;
Large for gestational age (LGA) was based on the 90th percentile of weight for gestational age and small for gestational age (SGA) was based on the 10th percentile of weight for gestational age according to fetal growth curves derived using a United States national reference based on births in1999–2000(9); Prenatal care was defined based on the revised GINDEX as reported by Alexander et al. which combines information from the birth certificate on the trimester when prenatal care was first received and the total number of prenatal visits(7).
Figure 2.
Histograms of gestational weight gain in NHW and NHB women stratified by pre-pregnancy BMI category. According to Institute of Medicine, recommended gestational weight gain (GWG) is defined as 25 to 35 pounds (11.34 to 15.88 kg) for women with normal BMI, 15 to 25 pounds (6.80 to 11.34 kg) for overweight women and 11 to 20 pounds (4.99 to 9.07 kg) for obese women
Relationship between GWG and Birthweight stratified by race and Maternal BMI category
Across the distribution of GWG, for each maternal BMI category, birthweight was considerably higher in NHW than NHB (Figure 3). We found a strong association between increasing GWG and increasing infant birthweight across maternal BMI categories in NHW and NHB women. For these analyses we used linear regression with a third order polynomial for GWG and, while the slopes of the curves were similar in NHW and NHB, there were statistically significant racial differences at the level of the intercept (Figure 3). The difference in mean infant birthweight between obese and normal BMI women decreased with increasing GWG, and the difference in mean infant birthweight between obese and normal BMI women was greater in NHW than NHB from a GWG of 0 to 25 kg (Figure 3). The average infant birthweight in NHW women of normal BMI and a 10 kg GWG was 3253 g, while that of an obese NHW woman was 3480 g, a difference of 227 g (95% CI: 218, 236) (Table 2). Similarly, the average infant birthweight in NHB women of normal BMI and a 10 kg GWG was 3082 g, while that of a NHB obese woman was 3261 g, a difference of 179 g (95% CI: 168, 190) (Table 2). Increasing the GWG to 30 kg narrowed the difference in birthweight between obese and normal BMI NHW [70 g (95% CI: 40, 99)] and NHB [37 g (95% CI: −2, 75)] women and attenuated the racial difference (Table 2).
Relationship between Maternal BMI category and Birthweight stratified by race and GWG category
We found that in both NHW and NHB women, higher maternal BMI was associated with higher infant birthweight for all categories of GWG, and that this relationship was strongest in those with inadequate GWG, resulting in differences in infant birthweight comparing inadequate to adequate GWG categories decreasing with increasing maternal BMI (Figure 4). For these analyses we used linear regression with linear and quadratic terms for maternal BMI and, while the slopes of the curves were similar in NHW and NHB, there were statistically significant racial differences for the comparison of inadequate to adequate GWG as well as for the comparison of excessive to adequate GWG (Figure 4). The difference in infant birthweight between women with inadequate and adequate GWG was greater in NHW than NHB women at maternal BMI levels from 18.5 to 25 kg (Figure 4). Additionally, the difference in birthweight between women with excessive and adequate GWG was greater in NHW than NHB women at maternal BMI levels from 22 to 34 (Figure 4). In NHW with a maternal BMI of 30 kg/m2, the average infant birthweight for women with adequate, inadequate and excessive weight gain, respectively, was 3408 g, 3354 g [i.e., difference with normal −54g (95% CI: −64, −44)] and 3526 g [i.e., difference with normal 118 g (95% CI: 109,127)] (Table 3). In NHB women with a maternal BMI of 30 kg/m2, the average infant birthweight for women with adequate, inadequate and excessive weight gain, respectively, was 3200 g, 3151 g [i.e., difference with normal −49g (95% CI: −59, −38)] and 3301 g [i.e., difference with normal 101 g (95% CI: 91, 111)] (Table 3).
LGA and SGA as Outcomes
The odds of having an LGA infant were 179% higher in obese than normal BMI women (Table 4). Conversely, the odds for having an SGA infant were 42% lower in obese than normal BMI women. The odds of having an LGA infant were 101% higher in those with excessive GWG and 17% lower in those with inadequate GWG than in those with adequate GWG (Table 4). Inadequate versus adequate GWG was associated with a larger increase in the odds of having an SGA infant in NHW women [OR=1.52 (95% CI 1.45, 1.60)] than NHB women [OR=1.28 (95% CI: 1.22, 1.34)] (p-value for corresponding interaction term <0.0001). Excessive GWG conveyed similar protection from having an SGA infant in NHW women [OR=0.64 (95% CI 0.61, 0.67)] and NHB women [OR=0.68 (95% CI: 0.65, 0.72)] when compared to their adequate GWG counterparts (p-value for corresponding interaction term 0.0697).
DISCUSSION
We report a strong association between increasing GWG and increasing infant birthweight across maternal BMI categories in NHW and NHB women. Additionally, we report a strong association between increasing maternal BMI and increasing infant birthweight across GWG categories and that this relationship was strongest in those with inadequate GWG, resulting in differences in infant birthweight due to inadequate GWG decreasing with increasing maternal BMI. Briefly, we have shown that the differences in mean birthweight (1) between obese and normal BMI women were greater in NHW than NHB women from a GWG of 0 to 25 kg, (2) between women with inadequate versus adequate GWG were greater in NHW than NHB women from a maternal BMI of 18.5 to 25.0 kg/m2, and (3) between women with excessive versus adequate GWG were greater in NHW than NHB women from a maternal BMI of 22.0 to 34.0 kg/m2.
Previous studies examining the association between GWG and infant outcomes have recently been reviewed (2,3), with the consensus that there is strong evidence for a positive relationship between GWG and birthweight. However, prior large population-based studies have not concurrently examined the impact of maternal pre-pregnancy BMI and GWG on birthweight while focusing on potential racial differences. Two prior studies conducted by Hickey et al. focused on racial differences in the relationship between GWG, maternal pre-pregnancy BMI and birthweight (10,11). The larger of these two studies reported on 2219 black and 3966 white infants at term from low-income women and concluded that their results did not support ethnicity-specific recommendations for GWG (10). In a cohort of 582 consecutive black, low income, term, singleton pregnancies, pre-pregnancy obesity and high GWG were associated with higher birthweight (12). In a recent large population-based study utilizing birth certificate data (n = 1,164,750 singleton offspring) and within-family comparisons, a consistent linear association was observed between GWG and birthweight (13). In contrast to our results which report similar slopes for the association between GWG and birthweight in NHW and NHB , this study reports the association between GWG and birthweight was slightly weaker for African American participants; however, they constrained the main effect of GWG to be linear and did not have information on pre-pregnancy BMI (13).
Additionally, three recent studies each using birth certificate data have examined the impact of GWG and maternal BMI on infant birthweight or being small or large for gestational age (14–16). The first study focused on ~120,000 obese women, residing in Missouri, who delivered live born, term, singleton infants between 1990 and 2001 (14). Over 75% of the women were NHW and there was a strong direct association between GWG and LGA as well as a strong inverse association between GWG and SGA, regardless of the severity of maternal pre-pregnancy obesity. Based on their findings they concluded that limited or no GWG had favorable outcomes in obese women (14). A second study, including New York City residents born outside the city but within New York State, reported among underweight, normal BMI, overweight and obese women a direct association between GWG and the odds of having an LGA infant, as well as an inverse association between GWG and the odds of having an SGA infant (16). This study also reported an increase in mean birthweight associated with increasing GWG with similar associated increases in birthweight in NHW and NHB across the spectrum of GWG (16). Finally, a study conducted using birth certificate data collected in Florida between 2004 and 2007, which included information on pre-pregnancy maternal BMI as well as GWG, reported that GWG influenced the risk of having an LGA and SGA infant in opposite directions; however, this study did not examine racial differences in the association between GWG and infant size for gestational age (15).
A strength of our study is that we focused on racial differences in the association between maternal BMI, GWG, and birthweight. A limitation of the current study is that we did not account for lack of independence among maternal siblings (i.e., 15.1% of all births are not the first live birth to a mother during the study period). Another limitation of the current study is the use of birth certificate data to determine maternal race, maternal BMI, and maternal GWG. Previous studies have validated the reliability of maternal BMI from birth certificates with mixed but overall encouraging results (17–19), with high correlation between self-report and clinically measured pre-pregnancy BMI, and these correlations do not seen to differ by race/ethnicity, gestational age, or weight itself (20). Nevertheless, the possible limitations in the quality of our birth certificate data led us to exclude women with BMI<18.5 in the current analysis. With regard to GWG, a few studies have examined data reliability with encouraging results: high concordance was found between self-reported and clinically recorded weight (19) as well as between birth certificate data and clinically recorded GWG (21). Additionally, because timing and consistency of prenatal care may be associated with the quality of data pertaining to maternal BMI, GWG and gestational age, we have controlled for prenatal care throughout all analyses using the revised GINDEX (7). We therefore believe misclassification of maternal BMI and GWG to be minimal and our analyses to be valid. However, one aspect we were not able to consider was the separate components of GWG including infant birthweight, the products of conception and actual maternal weight gain. For instance, because infant birthweight is a component of GWG as we have calculated it in this study we may have overestimated the association between increased GWG and increased birthweight. Moreover, the use of LMP to calculate gestational age has some limitations (22) which may be differential with respect to maternal BMI given the association between obesity and irregular menses (23); however, because birthweight, our outcome, may differentially impact the clinical/obstetric estimate of gestational age we relied on LMP to calculate gestational age. Finally, we note that we cannot determine in the current study whether there is a causal relationship between GWG or maternal BMI and infant birthweight.
Early life exposures are emerging as potentially important risk factors for adult diseases including obesity and diabetes. The “fetal origin of disease” hypothesis proposes that gestational programming may critically influence adult health and disease (24). Given the high prevalence of obesity in childbearing women and the frequency of excessive GWG, it is important to understand their association with tangible infant outcomes. Moreover, despite the fact that maternal obesity is much higher in NHB compared to NHW women, few studies have focused on racial differences in neonatal outcomes at the high end of the birthweight distribution. While our study found that maternal BMI, GWG and race were all strongly associated with infant birthweight, we did not find strong evidence for racial differences in the association between maternal BMI and birthweight, or in the association between GWG and birthweight. In fact, the absolute difference in increased infant birthweight associated with inadequate GWG, excessive GWG, or obesity in NHW compared to NHB women is partially explained by higher baseline birthweights in NHW as compared to NHB infants. Moreover, the increased odds of having an LGA infant associated with excessive versus adequate GWG was similar in NHW and NHB. Similarly, excessive when compared to adequate GWG conveyed similar protection from having an SGA infant in NHW and NHB women as did obese when compared to normal BMI women. In fact the only comparison in which there were racial differences associated with having an SGA or LGA infant was the comparison of inadequate versus adequate GWG which was associated with a larger increase in the odds of having an SGA infant in NHW than NHB women. In summary, our results do not support establishing race-specific recommendations for GWG or pre-pregnancy BMI.
Our study is unique because we apply the 2009 IOM GWG Guidelines and report that less than half of NHW and NHB women in South Carolina who delivered in 2004 through 2008 met the current recommendations for GWG. Moreover, because the prevalence of obesity (i.e., 38.6% in NHB and 24.0% in NHW) and excessive GWG (i.e., 41.0% in NHB and 48.7% in NHW) are high in our study population, even a small shift in birthweight is likely to have a large impact at the population level. Hence, at the population or public health level, our findings are quite relevant. Currently, there are limited data available on racial differences in the association between either maternal BMI or GWG and infant birthweight, and our study helps to address that gap. Further research is needed to understand both (1) the association between GWG, maternal BMI and birthweight with a focus on racial and ethnic minority populations, and (2) how maternal healthcare providers should respond to women who experience difficulties in maintaining recommended weight levels before, during, and after pregnancy.
Acknowledgements
This work was supported by a grant from the National Institute on Minority Health and Health Disparities (R01-MD004251). The funding agency did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Footnotes
Conflict of Interest: None of the authors disclosed any financial or other conflicts of interest.
Reference List
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults: 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine and National Research Council 2009. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The national Academies Press; 2011. [PubMed] [Google Scholar]
- 3.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am.J.Obstet.Gynecol. 2009;201:339–314. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Alexander GR, Tompkins ME, Allen MC, Hulsey TC. Trends and racial differences in birth weight and related survival. Matern.Child Health J. 1999;3:71–79. doi: 10.1023/a:1021849209722. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GR, Wingate MS, Bader D, Kogan MD. The increasing racial disparity in infant mortality rates: composition and contributors to recent US trends. Am.J Obstet.Gynecol. 2007 doi: 10.1016/j.ajog.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Schempf A, Kroelinger C, Guyer B. Rising infant mortality in Delaware: an examination of racial differences in secular trends. Matern.Child Health J. 2007;11:475–483. doi: 10.1007/s10995-007-0198-z. [DOI] [PubMed] [Google Scholar]
- 7.Alexander GR, Kotelchuck M. Quantifying the adequacy of prenatal care: a comparison of indices. Public Health Rep. 1996;111:408–418. [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet.Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 9.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC.Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickey CA, McNeal SF, Menefee L, Ivey S. Prenatal weight gain within upper and lower recommended ranges: effect on birth weight of black and white infants. Obstet.Gynecol. 1997;90:489–494. doi: 10.1016/s0029-7844(97)00301-3. [DOI] [PubMed] [Google Scholar]
- 11.Hickey CA, Cliver SP, Goldenberg RL, Kohatsu J, Hoffman HJ. Prenatal weight gain, term birth weight, and fetal growth retardation among high-risk multiparous black and white women. Obstet.Gynecol. 1993;81:529–535. [PubMed] [Google Scholar]
- 12.Ogunyemi D, Hullett S, Leeper J, Risk A. Prepregnancy body mass index, weight gain during pregnancy, and perinatal outcome in a rural black population. J.Matern.Fetal Med. 1998;7:190–193. doi: 10.1002/(SICI)1520-6661(199807/08)7:4<190::AID-MFM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010;376:984–990. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet.Gynecol. 2007;110:752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Sappenfield WM, Bish C, Salihu H, Goodman D, Bensyl DM. Assessment of the Institute of Medicine Recommendations for Weight Gain During Pregnancy: Florida: 2004–2007. Matern.Child Health J. 2010 doi: 10.1007/s10995-010-0596-5. [DOI] [PubMed] [Google Scholar]
- 16.Savitz DA, Stein CR, Siega-Riz AM, Herring AH. Gestational weight gain and birth outcome in relation to prepregnancy body mass index and ethnicity. Ann.Epidemiol. 2011;21:78–85. doi: 10.1016/j.annepidem.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern.Child Health J. 2007;11:137–144. doi: 10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Sappenfield WM, Bish C, Bensyl DM, Goodman D, Menges J. Reliability and Validity of Birth Certificate Prepregnancy Weight and Height Among Women Enrolled in Prenatal WIC Program: Florida 2005. Matern.Child Health J. 2009 doi: 10.1007/s10995-009-0544-4. [DOI] [PubMed] [Google Scholar]
- 19.Lederman SA, Paxton A. Maternal reporting of prepregnancy weight and birth outcome: consistency and completeness compared with the clinical record. Matern.Child Health J. 1998;2:123–126. doi: 10.1023/a:1022996924094. [DOI] [PubMed] [Google Scholar]
- 20.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am.J.Obstet.Gynecol. 2007;196:322–328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buescher PA, Taylor KP, Davis MH, Bowling JM. The quality of the new birth certificate data: a validation study in North Carolina. Am.J.Public Health. 1993;83:1163–1165. doi: 10.2105/ajph.83.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer MS, McLean FH, Boyd ME, Usher RH. The validity of gestational age estimation by menstrual dating in term, preterm, and postterm gestations. JAMA. 1988;260:3306–3308. [PubMed] [Google Scholar]
- 23.Wei S, Schmidt MD, Dwyer T, Norman RJ, Venn AJ. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity.(Silver.Spring) 2009;17:1070–1076. doi: 10.1038/oby.2008.641. [DOI] [PubMed] [Google Scholar]
- 24.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]