Abstract
BACKGROUND
Prenatal alcohol exposure can alter physical and behavioral development, leading to a range of fetal alcohol spectrum disorders (FASD). Despite warning labels, pregnant women continue to drink alcohol, creating a need to identify effective interventions to reduce the severity of alcohol’s teratogenic effects. Choline is an essential nutrient that influences brain and behavioral development. Recent studies indicate that choline supplementation can reduce the teratogenic effects of developmental alcohol exposure. The present study examined whether choline supplementation during prenatal ethanol treatment could mitigate the adverse effects of ethanol on behavioral development.
METHODS
Pregnant Sprague-Dawley rats were intubated with 6 g/kg/day ethanol in a binge-like manner from gestational days 5–20; pair-fed and ad lib chow controls were included. During treatment, subjects from each group were intubated with either 250 mg/kg/day choline chloride or vehicle. Spontaneous alternation, parallel bar motor coordination, Morris water maze, and spatial working memory were assessed in male and female offspring.
RESULTS
Subjects prenatally exposed to alcohol exhibited delayed development of spontaneous alternation behavior and deficits on the working memory version of the Morris water maze during adulthood, effects that were mitigated with prenatal choline supplementation. Neither alcohol nor choline influenced performance on the motor coordination task.
CONCLUSIONS
These data indicate that choline supplementation during prenatal alcohol exposure may reduce the severity of fetal alcohol effects, particularly on alterations in tasks that require behavioral flexibility. These findings have important implications for children of women who drink alcohol during pregnancy.
Keywords: fetal alcohol spectrum disorders, treatment, nutrition, ethanol, fetal alcohol syndrome
Introduction
Alcohol consumption during pregnancy can disrupt development, leading to physical alterations, such as facial dysmorphology, pre- and post-natal growth deficiencies, and central nervous system (CNS) dysfunction in the offspring (Hoyme et al., 2005). Individuals that exhibit each of these features are diagnosed with fetal alcohol syndrome (FAS), which is estimated to affect 0.5–3.1 per 1000 births (Clarren et al., 2001; May and Gossage, 2001). However, the features of FAS can vary in severity and many individuals exposed to high levels of alcohol prenatally may not meet all of the criteria for a diagnosis of FAS. Thus, we now refer to the range of adverse prenatal alcohol effects as fetal alcohol spectrum disorders (FASD), which is estimated to affect 1 in 100 live births (Sampson et al., 1997). FASD is a serious global concern, particularly as women continue to drink alcohol during pregnancy despite prevention efforts (Warren et al., 2001).
Identification of effective interventions and treatments for FASD is therefore critical. Ideally, one would intervene at the time of alcohol exposure, thereby directly preventing or reducing the amount of alcohol-related damage. Based on putative mechanisms of alcohol-induced teratogenesis, a number of potential experimental therapeutics have been identified that may reduce the severity of fetal alcohol effects, including neurotrophic agents (Barclay et al., 2005; Bonthius et al., 2003; Endres et al., 2005; Heaton et al., 2000b; McGough et al., 2009), neuroactive peptides (Sari, 2009; Vink et al., 2005; Wilkemeyer et al., 2003; Wilkemeyer et al., 2002; Zhou et al., 2004), antioxidants (Antonio and Druse, 2008; Chen et al., 2004; Cohen-Kerem and Koren, 2003; Heaton et al., 2000a; Marino et al., 2004; Mitchell et al., 1999) and NMDA receptor antagonists (Lewis et al., 2007; Stepanyan et al., 2008; Thomas et al., 2001; Thomas et al., 2004a). Nutritional variables may also impact alcohol’s teratogenic effects. Nutritional supplements may compensate for changes in the bioavailability of nutrients due to alcohol metabolism (Lieber, 2000), as well as alcohol-related reductions in nutritional intake and absorption (Dreosti, 1993). However, nutritional supplements may also alter development, even if they are not targeting the same sites as alcohol-related actions (see (Summers et al., 2008; Summers et al., 2009).
Choline is recognized as an essential nutrient (Food and Nutrition Board, 1998), and is well understood to be necessary for fetal development (Zeisel, 2006a; b). A growing literature indicates that prenatal choline supplementation in typically developing (non alcohol-exposed) rats leads to long-lasting changes in CNS structure and enhancements in cognitive functioning (McCann et al., 2006; Meck and Williams, 2003). In particular, pre- and/or early postnatal choline supplementation enhances hippocampal and prefrontal cortical functioning and performance on behaviors that rely on the functional integrity of these CNS regions, including attention and spatial learning (Meck et al., 1988; 1989; Meck and Williams, 1997; 1999; 2003). In fact, prenatal choline supplementation can advance development of spatial cue learning (Meck and Williams, 2003), improve spatial learning performance (Brandner, 2002; Schenk and Brandner, 1995; Tees, 1999; Tees and Mohammadi, 1999), increase memory capacity (Meck et al., 1988; 1989; Meck and Williams, 1997) and reduce proactive interference (Meck and Williams, 1999). Moreover, these beneficial effects are evident even when rats are over 2 years old, illustrating that a prenatal nutrient can reduce cognitive decline associated with aging (Glenn et al., 2008; Meck and Williams, 2003).
Our laboratory has reported that choline supplementation during early postnatal development can likewise reduce the severity of some ethanol-induced neurobehavioral alterations. For example, choline supplementation from postnatal days (PD) 2–21, following prenatal alcohol exposure, reduced the severity of working memory deficits in rats (Thomas et al., 2000). Similarly, choline supplementation from PD 4–30 significantly reduced the severity of spatial reversal learning deficits (Thomas et al., 2004b), hyperactivity (Thomas et al., 2004b), and trace fear conditioning deficits (Wagner and Hunt, 2006), but not motor coordination deficits (Thomas et al., 2004c) associated with alcohol exposure during the 3rd trimester-equivalent brain growth spurt. The severity of learning deficits and hyperactivity associated with 3rd trimester alcohol exposure was also reduced by choline administered during the period equivalent to postnatal development in humans (PD10–30) (Ryan et al., 2008; Thomas et al., 2007). Collectively, these data suggest that choline supplementation during the early postnatal period is particularly effective in attenuating ethanol’s adverse effects on behavioral development. More recently, we have shown that choline supplementation can reduce some of ethanol’s teratogenic effects when administered during prenatal alcohol exposure. Specifically, we have shown that choline supplementation reduces the severity of alcohol-related birth weight reductions, physical anomalies, and alterations in early reflex development (Thomas et al., 2009).
The present study further examines whether choline supplementation is effective in reducing fetal alcohol effects when administered during prenatal alcohol exposure. Of particular concern is mitigation of behavioral alterations associated with alcohol-induced neuropathology. Prenatal alcohol exposure affects behavioral development in a number of domains, leading to hyperactivity, motor incoordination, alterations in social processing, and deficits in cognitive functioning (Mattson et al., 1998; Riley and McGee, 2005). Thus, the present study examines the effects of choline supplementation during prenatal alcohol exposure on a number of behavioral domains, including motor coordination, spatial learning, and working memory.
Materials and Methods
All procedures used in this study were approved by the SDSU IACUC and are in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Treatment
Timed pregnant Sprague-Dawley dams were obtained from the Center for Behavioral Teratology, San Diego State University Animal Care facilities. Gestational day (GD) 0 was designated as the day when a seminal plug was detected. Dams were housed individually in plastic cages, exposed to a 12:12 hour cycle of light and dark in a temperature- and humidity-controlled room, and received food (LabDiet® 5001, Richmond, IN, containing 2.25 g choline chloride/kg diet) and water ad libitum.
Pregnant dams were randomly assigned to one of three treatment groups: ethanol-exposed (EtOH), yoked pair-fed (PF), or ad libitum control (LC). Ethanol-exposed dams received 6.0 g/kg/day (28.5% v/v) ethanol, PF dams received an isocaloric maltose dextrin solution to control for the calories from alcohol, and LC dams received vehicle (saline), via daily oral gavage from GD 5–20. Daily food intake was measured for the EtOH dams; each PF dam was matched to an EtOH dam of similar weight and food intake was correspondingly yoked. Within each of the 3 treatment groups, dams were randomly assigned to receive either a choline supplementation (choline chloride, Blachem, New Hampton, NY; 250 mg/kg/day) or a vehicle control (saline, Sigma, Milwaukee, WI), added to the daily intubation formula. This administration increases daily choline intake by 2–3 times that of controls.
Animals were monitored until the expected day of delivery GD 22 (PD 0) and the day of birth was recorded. On PD 1, litters were culled to 10 pups (5 males and 5 females when possible). Data on litter characteristics and birth weights have been previously reported (Thomas et al., 2009). Blood alcohol levels over a 24-hour period were obtained from a separate group of pregnant rats on gestational days 5 and 20. Importantly, choline supplementation did not influence blood alcohol concentrations, which peaked at 345 mg/dL (Thomas et al., 2009), indicating that choline does not alter the amount of fetal alcohol exposure.
Behavioral Testing
Different sex pairs within each litter were tested on each behavioral task, reducing the possibility of carryover and excessive handling effects. To control for litter effects, no more than one sex pair per litter was tested on a particular task (one sex pair for spontaneous alternation, a different sex pair for parallel bar testing, and a third sex pair for spatial and working memory). For each behavioral task, the n of litters is as follows spontaneous alternation (EtOH + Saline n=11 litters; EtOH + Choline n=12; PF + Saline n=14; PF + Choline n=10; LC + Saline n=11; LC + Choline n=13); parallel bar (EtOH + Saline n=9 litters; EtOH + Choline n=12; PF+ Saline n=11; PF + Choline n=10; LC + Saline n=8; LC + Choline n=10); morris water maze spatial and working memory (EtOH + Saline n=11 litters; EtOH + Choline n=12; PF + Saline n=14; PF + Choline n=10; LC + Saline n=11; LC + Choline n=13). All testing was conducted by experimenters blind to treatment group. Body weights for each subject were taken prior to testing and are described in Table 1.
Table 1.
Mean (± SEM) Body Weights
| Prenatal Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Postnatal Day | Sex | EtOH Vehicle | EtOH Choline | PF Vehicle | PF Choline | LC Vehicle | LC Choline |
| PD 28 (Spontaneous Alternation) | Male | 88.9 ± 3.2 | 96.1 ± 3.2 | 94.0 ± 3.1 | 100.4 ± 3.5 | 94.2 ± 2.9 | 97.4 ± 2.0 |
| Female | 78.0 ± 4.3 | 88.0 ± 4.3 | 83.4 ± 2.3 | 93.1 ± 2.3 | 85.8 ± 2.5 | 90.1 ± 2.1 | |
| PD 30 (Parallel Bars) | Male | 101.7 ± 1.6 | 106.6 ± 4.0 | 110.0 ± 2.3 | 112.3 ± 3.1 | 113.6 ± 2.8 | 112.7 ± 2.8 |
| Female | 90.4 ± 2.9 | 95.1 ± 2.7 | 98.5 ± 2.4 | 99.8 ± 2.8 | 102.1 ± 2.0 | 104.1 ± 2.5 | |
| PD 45 (Morris Water Maze) | Male | 216.8 ± 7.1 | 255.4 ± 15.0 | 258.4 ± 10.2 | 261.0 ± 13.9 | 268.8 ± 10.3 | 261.2 ± 16.4 |
| Female | 177.5 ± 7.1 | 184.1 ± 4.9 | 180.0 ± 4.7 | 187.8 ± 6.4 | 186.8 ± 4.4 | 206.0 ± 8.9 | |
Spontaneous Alternation
Subjects were tested during the light cycle on PD 15–17, PD 28–30, and PD 39–41 for spontaneous alternation – a measure of natural exploratory and foraging behavior that depends on hippocampal cholinergic functioning (Lalonde, 2002; Messier et al., 1990). The subject was initially placed at the bottom of the stem (start box) of a T-maze made of black Plexiglas that had a start box and two goal boxes, found at the end of the two maze arms (stem length = 60 cm, width = 13 cm; arm length = 35 cm, width = 13 cm). After 10 seconds, a guillotine door to the start box was opened and the subject was free to explore the maze until the subject had placed four paws in one of the goal boxes or 5 mins had elapsed. After 30 seconds in the goal box, the subject was removed and placed back in the starting box. After ten seconds, the start box door was opened again and the procedure repeated. Arm choices and response latencies were recorded. The percent of subjects that alternated on 2 of the 3 consecutive days at each age served as the performance measure.
Parallel Bar Motor Coordination
On PD 30–32, motor coordination was measured on a parallel bar task. Subjects were tested for 3 consecutive days during the light cycle. The parallel bar apparatus consisted of two parallel steel rods (0.5 cm diameter, 91 cm long) held between end platforms (15.5 × 17.8 cm), all of which stood 63 cm above wood chip bedding.
Subjects were initially acclimated on each platform and then situated on the rods halfway between the platforms, so that the subject had its left paws placed on one rod and right paws placed on the other. Four successive alternating steps with the hind paws on the rods constituted a successful traversal. The rods were initially spaced 3.5 cm apart; the space widened at 0.5-cm increments following each successful traversal. The trial was considered unsuccessful if the subject placed both hind paws on one rod, fell, dragged a paw, or swung under the bars. Subjects were allowed up to five trials at a given width, being tested up to a maximum of 15 trials per day. The maximum width between rods successfully traversed and the ratio of successful traversal to total trials was recorded.
Morris Water Maze
Beginning on PD 45, subjects were tested for 7 consecutive days on the Morris maze – a spatial learning task (D’Hooge and De Deyn, 2001). The task used a circular tank (175 cm diameter) filled with water (26°C) made opaque with the addition of powdered milk and a platform (10 cm diameter) submerged 1 to 1.5 cm beneath the water surface. The tank was placed in a room rich with extramaze spatial cues.
Subjects first underwent 4 days of acquisition. During acquisition, the subject was placed in the tank and allowed to swim for up to 60 seconds, until the platform was found. Subjects were tested for 6 trials/day with an ITI of 4 mins. When the platform was located, the subject was allowed 10 seconds to observe the various visual cues of the room (e.g. posters, curtains, lights) and was then removed from the platform and placed into a drying cage during its inter-trial interval. The platform’s position was randomly selected from one of four possible positions, and remained stationary for each subject during acquisition trials. Subjects were placed in the maze, facing the outer rim; the starting position was different during each trial to prevent the learning of motor strategies. The swimming activity (e.g. path latency and distance to the platform) of each subject was monitored via a video camera mounted overhead (HVS Image, 2020 Software). The acquisition phase was followed by a 60-second probe trial on the 5th day to test for spatial memory. During the probe trial, the platform was removed and the subject allowed to swim freely. Measures such as the percent of time spent near the target position and the number of passes through the previous target’s position were recorded.
Finally, to determine if there were group differences in performance measures such as swimming ability or motivation, subjects were tested with a visible platform for 2 days, 4 trials/day. The platform sat above the water’s surface and was marked for visibility, changing position from quadrant to quadrant between trials. All previous spatial cues were eliminated by a white curtain surrounding the tank.
Working Memory
On PD 65–66, subjects were tested on a working memory version of the Morris water maze. Each day comprised two sessions (beginning at 9:00 and 15:00), each of which consisted of 2 trials (training and test). During each session, the platform was placed in one of 12 randomly chosen spatial locations; the platform’s position remaining static during the training and test trials within a session. Subjects were allowed up to 60 seconds to find the escape platform hidden below the water’s surface. Once the subject found the platform during the training trial, they remained on the platform for 10 seconds and then were immediately placed at a new starting position for the test trial. Thus, the subject had to remember the platform’s position from the training trial to the test trial using spatial cues. The platform’s spatial position changed between the sessions, requiring working memory as the subject had to disregard previous sessions and remember the location of the escape platform during that session only. Path lengths and latencies to find the hidden platform were measured using the HVS Image system. Savings was determined by subtracting the path length to find the platform during the test trial from the path length during each training trial.
Data Analyses
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS Science, Chicago, IL). All data were analyzed with analyses of variance (ANOVAs) with a 3 (Ethanol, PF and LC) x 2 (Choline, Control) x 2 (Female, Male) design. Repeated within-subject factors of time were included in appropriate tasks. Newman-Keuls post-hoc comparisons were conducted when a significant P-value of <0.05 was found. Non-parametric Fisher’s exact probability analyses were conducted for spontaneous alternation data.
Results
Body Weights
As reported in Thomas et al, 2009, the prenatal ethanol-exposed and pair-fed dams gained less weight during pregnancy compared to lab chow controls, but choline had no effect on maternal weight gain. There were no treatment effects on litter size, gestational length or sex ratio of pups. At birth, ethanol-exposed and PF pups weighed less than LC pups and choline transiently increased the body weights of the ethanol-exposed subjects only. Over time, PF subjects caught up in weight with LC controls, but the EtOH-exposed subjects did not.
Body weights for subjects during behavioral testing is shown in Table 1. Body weight measures for 44 subjects were not acquired on PD 28. A significant sex effect was found for all groups’ body weights taken prior to spontaneous alternation, parallel bars and Morris water maze (F(1,90)=25.3, p<0.0001; F(1,105)=50.8, p<0.0001; F(1,131)=137.8, p<0.0001, respectively), as males weighed more than females. Ethanol-exposed subjects weighed less than PF and LC controls, producing significant effects of ethanol at all ages (F(2,90)=3.1, p=0.05; F(2,105)=13.4, p<0.0001; and F(2,131)=5.3, p<0.01, for PD 28, 30, and 45, respectively). When collapsed across all treatment groups, choline significantly increased body weight at PD 28 and 45, but not PD 30 (F(1,90)=15.2, p<0.0001; F(1,105)=2.3, p=0.13; and F(1,131)=3.9, p=0.05, respectively), although choline supplementation did not significantly increase body weights within each treatment group. Importantly, no interaction between sex, ethanol or choline was found.
Spontaneous Alternation
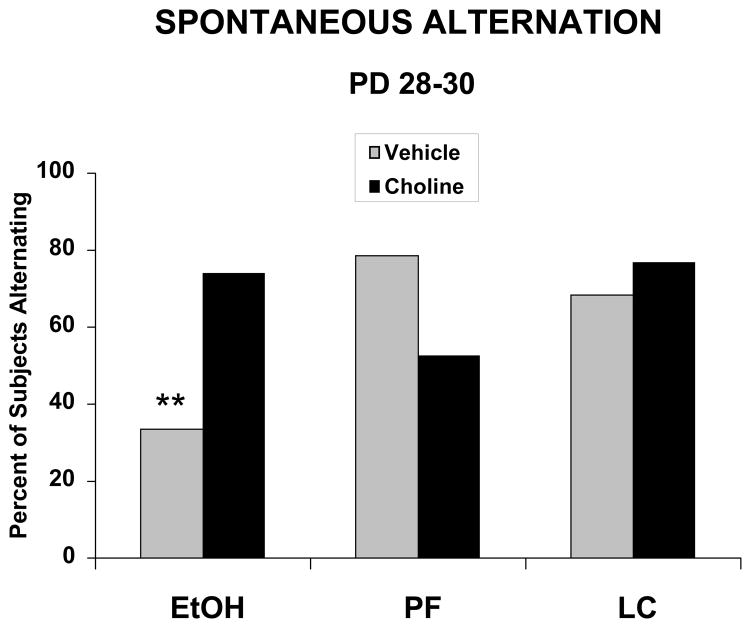
The percent of subjects in each treatment group that alternated at each age was not significantly different on PD 15–17 or on PD 39–41. However, on PD 28–30, ethanol-exposed subjects alternated significantly less than controls, an effect that was mitigated with prenatal choline supplementation (see Figure 1). Fisher’s exact probabilities confirmed that fewer ethanol-exposed subjects alternated compared to all groups, except the PF + Choline group, which did not differ from any other group. In contrast, the EtOH + Choline group performed at levels similar to that of controls.
Figure 1.
Percent of subjects that spontaneously alternated on PD 28–30. Ethanol-exposed subjects alternated significantly less than controls, an effect that was significantly mitigated with prenatal choline supplementation.
EtOH = ethanol-exposed; PF = pair fed controls; LC = lab chow controls
** = significantly different from all other groups except the PF + Choline group
Parallel Bars
Gestational exposure to alcohol did not significantly impair motor coordination of the offspring, as assessed on the parallel bar motor task. In addition, choline supplementation also did not affect the motor performance on any of the outcome measures, including success ratio and maximum width traversed (all p’s >0.1). Success ratios for each treatment group were as follows: EtOH + Saline = 0.337 ± 0.011; EtOH + Choline = 0.342 ± 0.010; PF + Saline = 0.336 ± 0.011; PF + Choline = 0.317 ± 0.025; LC + Saline = 0.344 ± 0.012; LC + Choline = 0.335 ± 0.010; (values collapsed by sex as there were no significant sex interactions).
Morris Water Maze
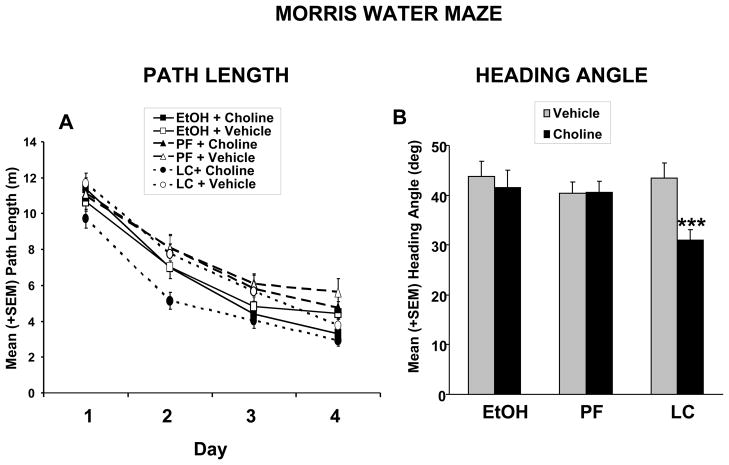
There were no significant effects of prenatal ethanol exposure on Morris maze acquisition; however, choline supplementation improved performance among LC controls. Path length to find the hidden platform is shown in Figure 2. Analyses of path length revealed significant main effects of Prenatal Exposure [F(2,129)=4.3; p<0.05] and Choline [F(1,129)=4.4; p<0.05]. The Prenatal Exposure effect was produced by subtle impairments in performance of the PF groups, which took significantly longer paths to reach the platform compared to both the EtOH and LC groups. Although the interaction of Choline with prenatal exposure did not reach statistical significance, the choline effect was the result of improved performance of the LC + Choline group, which traveled significantly shorter distances to the hidden platform (Figure 2B). Learning across days and within session resulted in significant Day [F(3,387)=240.7; p<0.001], Trial [F(5,645)=92.5; p<0.001] and Day x Trial [F(15,1935)=4.7; p<0.001] interactions (Figure 2A). A similar effect was seen with latency to find the platform (data not shown). Interestingly, the LC + Choline subjects swam slower than other groups, producing a significant effect of Prenatal Exposure x Choline [F(2,129)=4.8, p<0.05] (data not shown).
Figure 2.
(A) Mean (+ SEM) path length to find the platform in the Morris Water Maze task over testing days. Prenatal alcohol exposure did not significantly affect acquisition, although PF subjects were significantly impaired compared to both EtOH and LC subjects. Choline supplementation improved spatial learning performance among LC controls, producing a significant effect of choline. (B) Mean (+ SEM) heading angle (chosen path compared to direct path to platform) during Morris water maze acquisition. Choline supplementation significantly improved performance accuracy in LC, but not EtOH or PF subjects, producing a significant of choline. No significant effect of ethanol was found.
***= significantly different from all other groups
Heading angle is indicative of the relationship of the subject’s initial chosen path compared to the path that would lead directly to the hidden target platform. Performance improved over the course of the testing days, producing significant effects of Day [F(3,387)=53.5; p<0.001] and Trial [F(5,645)=13.0; p<0.001]. There was no effect of prenatal ethanol exposure. Although the Prenatal Exposure x Choline interaction failed to reach statistical significance (p<0.07), choline supplementation significantly improved accuracy among LC controls, but not EtOH or PF controls, producing a main effect of choline [F(1,129)=9.0; p<0.01].
Significant improvements in spatial memory induced by choline supplementation were also evident during the probe trial. Analysis of the number of passes through the platform position (target) revealed significant main effects of Prenatal Exposure [F(2,131)=3.7; p<0.05] and Choline [F(1,131)=4.6; p<0.05]. Similar to acquisition, the PF groups were significantly impaired compared to the EtOH and LC groups, exhibiting fewer passes through the platform area. Overall, choline-treated animals performed better than their vehicle counterparts on this measure.
No significant effects were observed when analyzing the percentage of time swimming in the target quadrant, the heading angle, or the swimming speed during the probe trial. Similarly, there were no significant effects of ethanol or choline on the visible platform version of the Morris water maze, suggesting that factors other than spatial memory did not influence performance.
Working Memory
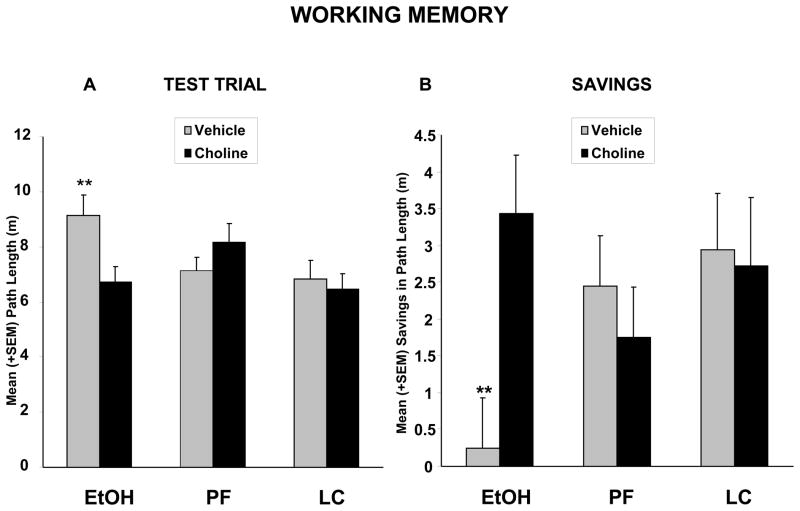
In contrast to simple Morris maze acquisition, prenatal ethanol did impair spatial working memory, an effect that was significantly mitigated with prenatal choline supplementation. First, no significant differences in path length to find the platform were observed among the groups during the training trial (p’s >0.05). However, during the test trial, ethanol-exposed subjects that were not choline supplemented took longer path lengths to find the platform, compared to all other groups except the PF + Choline group, producing a significant interaction of Prenatal EtOH Exposure x Choline [F(2,129)=4.1; p<0.05] (see Figure 3A). Figure 3B depicts the savings in path length during the test trial relative to the initial training trial. The EtOH group showed no evidence of learning, as the path length to find the hidden platform during the test trial was not significantly better than the training trial. In contrast, all other groups, including the EtOH + Choline group, took shorter path lengths during the test trial than during the training trial, suggesting that the memory of the immediately previous experience facilitated finding the platform (which was located in the same position during that test trial). These effects produced a significant interaction of Prenatal EtOH Exposure x Choline [F(2,129)=3.7; p<0.05]. No significant differences were evident among the Choline-treated groups in this test (p’s >0.05).
Figure 3.
Mean (+ SEM) path length during the test trials (A) of the spatial working memory task. During the test trials, ethanol-exposed subjects that were not choline supplemented took longer path lengths to find the platform, compared to all other group, except the PF + Choline group. Thus, choline supplementation mitigated ethanol-related deficits. Panel B shows the savings from training to test trial, which serves as a measure of memory retention of the platform’s position. Ethanol-exposed subjects that did not receive choline supplementation showed no evidence of savings, whereas all other groups did. Indeed, the ethanol-exposed subjects treated with choline performed at levels similar to those of the control subjects.
** = significantly different from all other groups except the PF + Choline group
Discussion
Prenatal alcohol exposure altered development of spontaneous alternation and produced deficits in spatial working memory, effects that were significantly mitigated with prenatal choline supplementation. These data suggest that choline administration during prenatal alcohol exposure can reduce the severity of neurobehavioral alterations and, more generally, that nutritional variables may modify the long-lasting expression of fetal alcohol effects.
First, ethanol-exposed subjects alternated less frequently than their control counterparts on the spontaneous alternation task. This task measures a rodent’s natural foraging and exploratory tendencies to shift between spatial locations. This behavior develops around PD 25–30 (Egger et al., 1973); adult rats normally alternate 60–80% of the time (Lalonde, 2002), as was observed in our control subjects. However, ethanol-treated subjects alternated at a rate less than chance (50%). This result is consistent with earlier studies that likewise found reduced spontaneous alternation following prenatal ethanol exposure (Abel, 1982; Stone et al., 1996) and is consistent with studies that have shown that developmental alcohol exposure leads to perseverative-type behaviors (Thomas et al., 2001; Thomas et al., 2004a). However, since alternation rates did not differ significantly among treatment groups on PD 39–41, alcohol may simply delay the expression of this behavior. Abel (1982) reported that prenatally alcohol-exposed animals exhibit less spontaneous alternation on PD16, 63 and 112 (Abel, 1982), but did not differ significantly from controls at the older ages. Other reports also demonstrate that alcohol-induced memory retention deficits can decrease in severity with age, being more pronounced in the juvenile rat (Nagahara and Handa, 1997; 1999). Thus, it is possible that prenatal alcohol exposure either delays the maturation of certain neurological systems, or that neuroadaptive changes in the CNS may eventually compensate for alcohol-related deficits.
Ethanol also impaired spatial working memory, consistent with previous clinical (Green et al., 2009; Spadoni et al., 2009) and animal model (Popovic et al., 2006) studies. In fact, ethanol-exposed subjects not treated with choline failed to show any evidence of savings across trials. This finding further illustrates that prenatal alcohol exposure disrupts working memory and behavioral flexibility. It is important to note that since the same subjects were tested on both the spatial memory and spatial working memory versions of the Morris maze, there could be some carryover effects. However, these carryover effects would also reflect impairments in behavioral flexibility. Moreover, in contrast to working memory, prenatal ethanol exposure did not significantly impair performance on the spatial memory version of the Morris water maze, although the pair-fed controls were impaired. These results were surprising, given previous clinical (Hamilton et al., 2003) and animal model studies (Toso et al., 2006; Zimmerberg and Weston, 2002) that have shown that developmental alcohol exposure disrupts performance on this spatial learning task. However, many of the spatial learning deficits associated with developmental ethanol have been the result of exposure during, or at least including, the third trimester equivalent (Goodlett and Johnson, 1997; McAdam et al., 2008; Ryan et al., 2008), a period of rapid brain development that occurs postnatally in rats. Thus, differences in results may be related to the developmental timing of alcohol exposure.
The behavioral deficits observed in the ethanol-treated subjects were attenuated with prenatal choline supplementation. These results extend our previous report that prenatal choline loading attenuates alcohol-related birth weight reductions, reduced brain size, delayed incisor eruption, and alterations in reflex development (Thomas et al., 2009). Furthermore, ethanol-exposed subjects treated with choline performed at levels that did not significantly differ from that of control subjects on all behavioral tasks that were examined. We have previously shown that postnatal choline administration in the rodent similarly mitigates deficits in spatial, working, and reversal learning associated with developmental alcohol exposure (Thomas et al., 2007; Thomas et al., 2004b; Thomas et al., 2000); however, this is the first study to show amelioration of ethanol-induced deficits in cognitive functioning following prenatal treatment with choline.
Alcohol-related deficits in spontaneous alternation and spatial working memory are likely to reflect dysfunction of the hippocampus and/or prefrontal cortex, areas that are known to be sensitive to developmental alcohol exposure (Berman and Hannigan, 2000; Wass et al., 2001; Whitcher and Klintsova, 2008). First, damage to the hippocampus can cause significant deficits in spatial and reversal learning (Morris et al., 1982), decreases in spontaneous alternation rates (Lalonde, 2002) and deficits in spatial working memory (Jo et al., 2007). Studies have further shown that hippocampal cholinergic activity affects development of spontaneous alternation (Roland and Savage, 2009; Savage et al., 2007) and spatial working memory performance (Khakpour-Taleghani et al., 2009). However, impairments in spontaneous alternating behavior and spatial working memory have also been associated with frontal lobe damage (Avery et al., 2000; Mao et al., 1999; Sarti et al., 2002). Moreover, lesions to the basal forebrain reduces cortical cholinergic activity, which similarly leads to preservative-type behavioral errors on a reversal learning task (Loy et al., 1991), impaired spatial working memory (Khakpour-Taleghani et al., 2009) and deficits in spontaneous alternation (Lalonde, 2002; Roland and Savage, 2009). The greater sensitivity of the working memory version of the Morris water maze compared to simple spatial learning may indicate that alcohol-related deficits are only evident when the task is more difficult, but also may suggest that prefrontal cortical areas are more sensitive to prenatal alcohol exposure.
We have yet to elucidate the CNS changes associated with choline supplementation in prenatally ethanol-exposed subjects; however, prenatal choline availability has been shown to influence morphology, neurochemical, electrophysiological, and functional activity of the hippocampus and prefrontal cortex in otherwise typically developing rats. For example, prenatal choline supplementation from GD 11–17 increases cell division within the neuroepithelial layer of the hippocampus and the septum (Albright et al., 1999a; Albright et al., 1999b), reduces apoptosis (Holmes-McNary et al., 1997), and alters differentiation (Albright et al., 1999b; Zeisel, 2006b). Changes in the structure and functioning of the hippocampus and cortex are long-lasting. For example, prenatal choline supplementation leads to increased concentrations of neurotrophic factors in the hippocampus and frontal cortex (Napoli et al., 2008; Sandstrom et al., 2002) and hippocampal neurogenesis that persists into adulthood (Glenn et al., 2007; Glenn et al., 2008). Furthermore, prenatal choline loading increases the size of cholinergic neurons in the adult basal forebrain, and alters acetylcholine turnover in the hippocampus and the forebrain (Blusztajn et al., 1998; Cermak et al., 1998; Meck and Williams, 2003), reducing acetylcholine recycling and increasing acetylcholine release in choline-supplemented animals. Cholinergic neurotransmission is therefore organized to be highly efficient in supplemented subjects, months after choline supplementation is complete. Finally, the threshold for hippocampal long-term potentiation (LTP), a putative mechanism for learning and memory, is reduced following prenatal choline supplementation (Li et al., 2004; Pyapali et al., 1998). In contrast, prenatal alcohol exposure reduces hippocampal neurogenesis (Glenn et al., 2007; Klintsova et al., 2007; Redila et al., 2006), reduces neurotrophic factors (Angelucci et al., 1999; Breese et al., 1993; Climent et al., 2002; Ghiselli et al., 2003; McAlhany et al., 1999), impairs cholinergic functioning (Janiri et al., 1994; Nagahara and Handa, 1999; VanDemark et al., 2009), and impairs LTP (Berman and Hannigan, 2000; Krahl et al., 1999; Samudio-Ruiz et al., 2009). Thus, any of these potential effects could be influencing outcome following prenatal alcohol exposure.
That being said, it is also possible that choline may have some different effects on brain development and function in the presence of an alcohol-related insult. For example, choline supplementation did not significantly alter performance of control subjects on the spontaneous alternation and spatial working memory tasks. In contrast, choline supplementation improved performance on the Morris water maze in LC controls, but not EtOH or PF controls, as seen in path length and heading angle during acquisition. Further investigation of the CNS changes would elucidate how choline may differentially affect brain development in typically developing subjects and subjects that experience adverse prenatal events.
Unlike tasks that depend on behavioral flexibility, prenatal ethanol did not impair motor coordination on the parallel bar task. Although some studies have shown that prenatal alcohol exposure can disrupt motor coordination, these results were not unexpected given that the cerebellum is most sensitive to alcohol during the third trimester equivalent brain growth spurt, a period of development that occurs postnatally in the rat (from birth to PD 10) (Dobbing and Sands, 1979). Performance on the parallel bar task is strongly correlated with alcohol-induced Purkinje cell loss within the cerebellum (Thomas et al., 1998). It is interesting to note that prenatal alcohol did delay the development of grip strength and hindlimb coordination reflexes, suggesting that it may cause some transient deficits in motor function (Thomas et al., 2009), but not long-lasting motor performance. In contrast, ethanol exposure during the 3rd trimester equivalent does impair parallel bar performance and this is not attenuated with choline supplementation from PD 4–30 (Thomas et al., 2004c).
Combined with our previous findings, the present results suggest that choline’s therapeutic window is quite large, effectively attenuating ethanol’s teratogenic effects whether administered during prenatal ethanol exposure or after the alcohol insult is complete. In control rats, choline administration can improve cognitive functioning when administered either during prenatal or early postnatal development (PD 15–30) (Meck et al., 2008). Choline is also effective in protecting against other types of CNS damage, either when administered before, during, or after the insult. For example, choline supplementation reduces the severity of emotional and cognitive deficits associated with early maternal separation and undernutrition when administered at the time of separation (Tonjes et al., 1986). Similarly, choline can protect against NMDA-induced toxicity when administered during the insult (Mulholland et al., 2004). However, prenatal choline supplementation can also protect against later CNS damage. For example, prenatal choline loading protects against MK-801-induced neurotoxicity initiated during either adolescence (Guo-Ross et al., 2002) or adulthood (Guo-Ross et al., 2003). Finally, choline supplementation can reduce hippocampal neuropathology associated with seizures whether administered during prenatal development, weeks before seizure induction (Yang et al., 2000), or when administered after postnatal seizures (Holmes et al., 2002). Thus, it is clear that choline can mitigate the adverse effects of various insults at varying developmental stages, although the mechanisms of choline’s protective effects may depend on the developmental timing of administration.
How choline may be inducing such a wide range of beneficial effects has yet to be fully elucidated in either control or prenatally alcohol-exposed subjects. What is known is that choline is essential for normal brain development and plays a number of important roles. First, choline acts as a precursor to the neurotransmitter, acetylcholine, and, in fact, can act directly as a nicotinic receptor agonist (Albright et al., 1998; Albuquerque et al., 1997; Mike et al., 2000). As mentioned above, metabolic imprinting may lead to enhanced cholinergic functioning that is observed months after choline supplementation. In addition, choline can act as a methyl donor, influencing the methionine-homocysteine cycle (Zeisel and Niculescu, 2006) and serving as an epigenetic factor. Recent studies show that prenatal choline availability affects both global and gene-specific DNA and histone methylation (Davison et al., 2009; Kovacheva et al., 2007; Niculescu et al., 2006). Finally, choline also acts as a precursor to phosphatidylcholine, phosphocholine, and other membrane constituents and can act as a precursor to intracellular signals (Zeisel, 2006b; Zeisel and Blusztajn, 1994). Thus, choline has several potential courses of action to attenuate ethanol’s teratogenic effects and lead to long-lasting changes in CNS structure and function.
Given the importance of nutritional factors during brain development, it is somewhat surprising that more studies have not focused on interactions of prenatal alcohol exposure and nutrition. To date, most studies have examined the effects of undernutrition as a risk factor for FASD. Rates of FAS are higher in areas with poor nutrition (May et al., 2000) and animal studies have shown that compromised nutrition can exacerbate many of the teratogenic effects of alcohol including low birth weight (Wiener et al., 1981), physical anomalies (Weinberg et al., 1990), and brain dysfunction (Shankar et al., 2006; Wainwright and Fritz, 1985). It should be noted that blood alcohol levels are often more elevated among malnourished subjects. Conversely, nutritional supplements, such as vitamins E, C and beta carotene (a form of vitamin A) (Cohen-Kerem and Koren, 2003), zinc (Summers et al., 2008; Summers et al., 2006; 2009) and folate (Wang et al., 2009) can attenuate the effects of prenatal alcohol exposure, although the effectiveness depends on many factors, including the level of alcohol exposure and the outcome measure (Weinberg, 1985). The present study indicates that choline is among these supplements.
It is not yet known if ethanol itself leads to changes in choline levels, either by reducing nutritional intake or by altering choline absorption or metabolism. Nor is it known how prenatal alcohol affects nutritional and metabolic status in the offspring. However, since choline influences CNS development among non-alcohol exposed subjects with adequate nutrition, it would suggest that choline’s beneficial effects on CNS development are not dependent on choline deficiency.
Importantly, the effects of prenatal choline supplementation on ethanol-exposed subjects are long-lasting, choline having mitigated a variety of ethanol-related behavioral deficits that were evident into adulthood. These data suggest that prenatal dietary interventions may reduce the severity of fetal alcohol effects, including long-lasting cognitive deficits. These findings have important implications for individuals who are born to women who drink alcohol during pregnancy, suggesting that prenatal nutritional supplements may reduce the risk of some fetal alcohol spectrum disorders.
Acknowledgments
This work was supported by NIAAA grant AA12446.
Literature Cited
- Abel EL. In utero alcohol exposure and developmental delay of response inhibition. Alcoholism, clinical and experimental research. 1982;6(3):369–376. doi: 10.1111/j.1530-0277.1982.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain research. 1999a;115(2):123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain research. 1999b;113(1–2):13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- Albright CD, Tsai AY, Mar MH, Zeisel SH. Choline availability modulates the expression of TGFbeta1 and cytoskeletal proteins in the hippocampus of developing rat brain. Neurochemical research. 1998;23(5):751–758. doi: 10.1023/a:1022411510636. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. The Journal of pharmacology and experimental therapeutics. 1997;280(3):1117–1136. [PubMed] [Google Scholar]
- Angelucci F, Fiore M, Cozzari C, Aloe L. Prenatal ethanol effects on NGF level, NPY and ChAT immunoreactivity in mouse entorhinal cortex: a preliminary study. Neurotoxicology and teratology. 1999;21(4):415–425. doi: 10.1016/s0892-0362(99)00005-7. [DOI] [PubMed] [Google Scholar]
- Antonio AM, Druse MJ. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain research. 2008;1204:16–23. doi: 10.1016/j.brainres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23(3):240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Barclay DC, Hallbergson AF, Montague JR, Mudd LM. Reversal of ethanol toxicity in embryonic neurons with growth factors and estrogen. Brain research bulletin. 2005;67(6):459–465. doi: 10.1016/j.brainresbull.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Cermak JM, Holler T, Jackson DA. Imprinting of hippocampal metabolism of choline by its availability during gestation: implications for cholinergic neurotransmission. Journal of physiology, Paris. 1998;92(3–4):199–203. doi: 10.1016/s0928-4257(98)80010-7. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Karacay B, Dai D, Pantazis NJ. FGF-2, NGF and IGF-1, but not BDNF, utilize a nitric oxide pathway to signal neurotrophic and neuroprotective effects against alcohol toxicity in cerebellar granule cell cultures. Brain research. 2003;140(1):15–28. doi: 10.1016/s0165-3806(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Brandner C. Perinatal choline treatment modifies the effects of a visuo-spatial attractive cue upon spatial memory in naive adult rats. Brain Res. 2002;928(1–2):85–95. doi: 10.1016/s0006-8993(01)03363-7. [DOI] [PubMed] [Google Scholar]
- Breese CR, D’Costa A, Ingram RL, Lenham J, Sonntag WE. Long-term suppression of insulin-like growth factor-1 in rats after in utero ethanol exposure: relationship to somatic growth. The Journal of pharmacology and experimental therapeutics. 1993;264(1):448–456. [PubMed] [Google Scholar]
- Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. Faseb J. 1998;12(3):349–357. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- Chen SY, Dehart DB, Sulik KK. Protection from ethanol-induced limb malformations by the superoxide dismutase/catalase mimetic, EUK-134. Faseb J. 2004;18(11):1234–1236. doi: 10.1096/fj.03-0850fje. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Randels SP, Sanderson M, Fineman RM. Screening for fetal alcohol syndrome in primary schools: a feasibility study. Teratology. 2001;63(1):3–10. doi: 10.1002/1096-9926(200101)63:1<3::AID-TERA1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Climent E, Pascual M, Renau-Piqueras J, Guerri C. Ethanol exposure enhances cell death in the developing cerebral cortex: role of brain-derived neurotrophic factor and its signaling pathways. J Neurosci Res. 2002;68(2):213–225. doi: 10.1002/jnr.10208. [DOI] [PubMed] [Google Scholar]
- Cohen-Kerem R, Koren G. Antioxidants and fetal protection against ethanol teratogenicity. I. Review of the experimental data and implications to humans. Neurotoxicology and teratology. 2003;25(1):1–9. doi: 10.1016/s0892-0362(02)00324-0. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone h3, expression of histone methyltransferases g9a (kmt1c) and suv39h1 (kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem. 2009;284(4):1982–1989. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early human development. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dreosti IE. Nutritional factors underlying the expression of the fetal alcohol syndrome. Annals of the New York Academy of Sciences. 1993;678:193–204. doi: 10.1111/j.1749-6632.1993.tb26122.x. [DOI] [PubMed] [Google Scholar]
- Egger GJ, Livesey PJ, Dawson RG. Ontogenetic aspects of central cholinergic involvement in spontaneous alternation behavior. Developmental psychobiology. 1973;6(4):289–299. doi: 10.1002/dev.420060402. [DOI] [PubMed] [Google Scholar]
- Endres M, Toso L, Roberson R, Park J, Abebe D, Poggi S, Spong CY. Prevention of alcohol-induced developmental delays and learning abnormalities in a model of fetal alcohol syndrome. American journal of obstetrics and gynecology. 2005;193(3 Pt 2):1028–1034. doi: 10.1016/j.ajog.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board IoM. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, Biotin, and choline. Washington, D.C: National Academy Press; 1998. [PubMed] [Google Scholar]
- Ghiselli G, Chen J, Kaou M, Hallak H, Rubin R. Ethanol inhibits fibroblast growth factor-induced proliferation of aortic smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(10):1808–1813. doi: 10.1161/01.ATV.0000090140.20291.CE. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. The European journal of neuroscience. 2007;25(8):2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicology and teratology. 1997;19(6):435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, Reynolds JN. Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB) Journal of child psychology and psychiatry, and allied disciplines. 2009;50(6):688–697. doi: 10.1111/j.1469-7610.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- Guo-Ross SX, Clark S, Montoya DA, Jones KH, Obernier J, Shetty AK, White AM, Blusztajn JK, Wilson WA, Swartzwelder HS. Prenatal choline supplementation protects against postnatal neurotoxicity. J Neurosci. 2002;22(1):RC195. doi: 10.1523/JNEUROSCI.22-01-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Jones KH, Shetty AK, Wilson WA, Swartzwelder HS. Prenatal dietary choline availability alters postnatal neurotoxic vulnerability in the adult rat. Neurosci Lett. 2003;341(2):161–163. doi: 10.1016/s0304-3940(03)00119-8. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behavioural brain research. 2003;143(1):85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M. Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res. 2000a;24(4):512–518. [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M. Overexpression of NGF ameliorates ethanol neurotoxicity in the developing cerebellum. J Neurobiol. 2000b;45(2):95–104. [PubMed] [Google Scholar]
- Holmes-McNary MQ, Loy R, Mar MH, Albright CD, Zeisel SH. Apoptosis is induced by choline deficiency in fetal brain and in PC12 cells. Brain research. 1997;101(1–2):9–16. doi: 10.1016/s0165-3806(97)00044-8. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48(1–2):3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri L, Gobbi G, Persico AM, Santarelli M, Minciacchi D, Tempesta E. Alterations of neocortical neuronal responses to acetylcholine and GABA in rats born to alcohol-dependent mothers. Alcohol and alcoholism (Oxford, Oxfordshire) 1994;29(5):611–619. [PubMed] [Google Scholar]
- Jo YS, Park EH, Kim IH, Park SK, Kim H, Kim HT, Choi JS. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J Neurosci. 2007;27(49):13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpour-Taleghani B, Lashgari R, Motamedi F, Naghdi N. Effect of reversible inactivation of locus ceruleus on spatial reference and working memory. Neuroscience. 2009;158(4):1284–1291. doi: 10.1016/j.neuroscience.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcoholism, clinical and experimental research. 2007;31(12):2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J Biol Chem. 2007;282(43):31777–31788. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Berman RF, Hannigan JH. Electrophysiology of hippocampal CA1 neurons after prenatal ethanol exposure. Alcohol (Fayetteville, NY. 1999;17(2):125–131. doi: 10.1016/s0741-8329(98)00043-3. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neuroscience and biobehavioral reviews. 2002;26(1):91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lewis B, Wellmann KA, Barron S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacology, biochemistry, and behavior. 2007;88(1):114–121. doi: 10.1016/j.pbb.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo-Ross S, Lewis DV, Turner D, White AM, Wilson WA, Swartzwelder HS. Dietary prenatal choline supplementation alters postnatal hippocampal structure and function. Journal of neurophysiology. 2004;91(4):1545–1555. doi: 10.1152/jn.00785.2003. [DOI] [PubMed] [Google Scholar]
- Lieber CS. ALCOHOL: its metabolism and interaction with nutrients. Annual review of nutrition. 2000;20:395–430. doi: 10.1146/annurev.nutr.20.1.395. [DOI] [PubMed] [Google Scholar]
- Loy R, Heyer D, Williams CL, Meck WH. Choline-induced spatial memory facilitation correlates with altered distribution and morphology of septal neurons. Advances in experimental medicine and biology. 1991;295:373–382. doi: 10.1007/978-1-4757-0145-6_21. [DOI] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AF, Li BM. Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biological psychiatry. 1999;46(9):1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int J Dev Neurosci. 2004;22(5–6):363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12(1):146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. American journal of public health. 2000;90(12):1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25(3):159–167. [PMC free article] [PubMed] [Google Scholar]
- McAdam TD, Brien JF, Reynolds JN, Dringenberg HC. Altered water-maze search behavior in adult guinea pigs following chronic prenatal ethanol exposure: lack of mitigation by postnatal fluoxetine treatment. Behavioural brain research. 2008;191(2):202–209. doi: 10.1016/j.bbr.2008.03.029. [DOI] [PubMed] [Google Scholar]
- McAlhany RE, Jr, Miranda RC, Finnell RH, West JR. Ethanol decreases Glial-Derived Neurotrophic Factor (GDNF) protein release but not mRNA expression and increases GDNF-stimulated Shc phosphorylation in the developing cerebellum. Alcoholism, clinical and experimental research. 1999;23(10):1691–1697. [PubMed] [Google Scholar]
- McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30(5):696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- McGough NN, Thomas JD, Dominguez HD, Riley EP. Insulin-like growth factor-I mitigates motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicology and teratology. 2009;31(1):40–48. doi: 10.1016/j.ntt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Developmental psychobiology. 1988;21(4):339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behavioral neuroscience. 1989;103(6):1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8(14):3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain research. 1999;118(1–2):51–59. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neuroscience and biobehavioral reviews. 2003;27(4):385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Frontiers in Integrative Neuroscience. 2008;1:1–11. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier C, Durkin T, Mrabet O, Destrade C. Memory-improving action of glucose: indirect evidence for a facilitation of hippocampal acetylcholine synthesis. Behavioural brain research. 1990;39(2):135–143. doi: 10.1016/0166-4328(90)90100-s. [DOI] [PubMed] [Google Scholar]
- Mike A, Castro NG, Albuquerque EX. Choline and acetylcholine have similar kinetic properties of activation and desensitization on the alpha7 nicotinic receptors in rat hippocampal neurons. Brain Res. 2000;882(1–2):155–168. doi: 10.1016/s0006-8993(00)02863-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Paiva M, Heaton MB. The antioxidants vitamin E and beta-carotene protect against ethanol-induced neurotoxicity in embryonic rat hippocampal cultures. Alcohol. 1999;17(2):163–168. doi: 10.1016/s0741-8329(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Self RL, Harris BR, Littleton JM, Prendergast MA. Choline exposure reduces potentiation of N-methyl-D-aspartate toxicity by corticosterone in the developing hippocampus. Brain Res Dev Brain Res. 2004;153(2):203–211. doi: 10.1016/j.devbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Handa RJ. Fetal alcohol exposure produces delay-dependent memory deficits in juvenile and adult rats. Alcoholism, clinical and experimental research. 1997;21(4):710–715. [PubMed] [Google Scholar]
- Nagahara AH, Handa RJ. Fetal alcohol-exposed rats exhibit differential response to cholinergic drugs on a delay-dependent memory task. Neurobiology of learning and memory. 1999;72(3):230–243. doi: 10.1006/nlme.1999.3909. [DOI] [PubMed] [Google Scholar]
- Napoli I, Blusztajn JK, Mellott TJ. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Res. 2008;1237:124–135. doi: 10.1016/j.brainres.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. Faseb J. 2006;20(1):43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Caballero-Bleda M, Guerri C. Adult rat’s offspring of alcoholic mothers are impaired on spatial learning and object recognition in the Can test. Behavioural brain research. 2006;174(1):101–111. doi: 10.1016/j.bbr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. Journal of neurophysiology. 1998;79(4):1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16(3):305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Experimental biology and medicine (Maywood, NJ. 2005;230(6):357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. The role of cholinergic and GABAergic medial septal/diagonal band cell populations in the emergence of diencephalic amnesia. Neuroscience. 2009;160(1):32–41. doi: 10.1016/j.neuroscience.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Samudio-Ruiz SL, Allan AM, Valenzuela CF, Perrone-Bizzozero NI, Caldwell KK. Prenatal ethanol exposure persistently impairs NMDA receptor-dependent activation of extracellular signal-regulated kinase in the mouse dentate gyrus. Journal of neurochemistry. 2009;109(5):1311–1323. doi: 10.1111/j.1471-4159.2009.06049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;947(1):9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Sari Y. Activity-dependent neuroprotective protein-derived peptide, NAP, preventing alcohol-induced apoptosis in fetal brain of C57BL/6 mouse. Neuroscience. 2009;158(4):1426–1435. doi: 10.1016/j.neuroscience.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti C, Pantoni L, Bartolini L, Inzitari D. Persistent impairment of gait performances and working memory after bilateral common carotid artery occlusion in the adult Wistar rat. Behavioural brain research. 2002;136(1):13–20. doi: 10.1016/s0166-4328(02)00090-6. [DOI] [PubMed] [Google Scholar]
- Savage LM, Roland J, Klintsova A. Selective septohippocampal - but not forebrain amygdalar - cholinergic dysfunction in diencephalic amnesia. Brain Res. 2007;1139:210–219. doi: 10.1016/j.brainres.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk F, Brandner C. Indirect effects of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiology. 1995;23(4):302–313. [Google Scholar]
- Shankar K, Hidestrand M, Liu X, Xiao R, Skinner CM, Simmen FA, Badger TM, Ronis MJ. Physiologic and genomic analyses of nutrition-ethanol interactions during gestation: Implications for fetal ethanol toxicity. Experimental biology and medicine (Maywood, NJ. 2006;231(8):1379–1397. doi: 10.1177/153537020623100812. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Bazinet AD, Fryer SL, Tapert SF, Mattson SN, Riley EP. BOLD response during spatial working memory in youth with heavy prenatal alcohol exposure. Alcoholism, clinical and experimental research. 2009;33(12):2067–2076. doi: 10.1111/j.1530-0277.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyan TD, Farook JM, Kowalski A, Kaplan E, Barron S, Littleton JM. Alcohol withdrawal-induced hippocampal neurotoxicity in vitro and seizures in vivo are both reduced by memantine. Alcoholism, clinical and experimental research. 2008;32(12):2128–2135. doi: 10.1111/j.1530-0277.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- Stone WS, Altman HJ, Hall J, Arankowsky-Sandoval G, Parekh P, Gold PE. Prenatal exposure to alcohol in adult rats: relationships between sleep and memory deficits, and effects of glucose administration on memory. Brain Res. 1996;742(1–2):98–106. doi: 10.1016/s0006-8993(96)00976-6. [DOI] [PubMed] [Google Scholar]
- Summers BL, Henry CM, Rofe AM, Coyle P. Dietary zinc supplementation during pregnancy prevents spatial and object recognition memory impairments caused by early prenatal ethanol exposure. Behavioural brain research. 2008;186(2):230–238. doi: 10.1016/j.bbr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Summers BL, Rofe AM, Coyle P. Prenatal zinc treatment at the time of acute ethanol exposure limits spatial memory impairments in mouse offspring. Pediatric research. 2006;59(1):66–71. doi: 10.1203/01.pdr.0000190573.23893.13. [DOI] [PubMed] [Google Scholar]
- Summers BL, Rofe AM, Coyle P. Dietary zinc supplementation throughout pregnancy protects against fetal dysmorphology and improves postnatal survival after prenatal ethanol exposure in mice. Alcoholism, clinical and experimental research. 2009;33(4):591–600. doi: 10.1111/j.1530-0277.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behavioural brain research. 1999;105(2):173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Developmental psychobiology. 1999;35(3):226–240. [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31(5):303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behavioral neuroscience. 2007;121(1):120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Fleming SL, Riley EP. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcoholism, clinical and experimental research. 2001;25(5):764–773. [PubMed] [Google Scholar]
- Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004a;175(2):189–195. doi: 10.1007/s00213-004-1806-x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004b;26(1):35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain research. 1998;105(2):159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22(5):703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, O’Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004c;26(2):223–229. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Tonjes R, Hecht K, Brautzsch M, Lucius R, Dorner G. Behavioural changes in adult rats produced by early postnatal maternal deprivation and treatment with choline chloride. Experimental and clinical endocrinology. 1986;88(2):151–157. doi: 10.1055/s-0029-1210590. [DOI] [PubMed] [Google Scholar]
- Toso L, Roberson R, Woodard J, Abebe D, Spong CY. Prenatal alcohol exposure alters GABA(A)alpha5 expression: a mechanism of alcohol-induced learning dysfunction. American journal of obstetrics and gynecology. 2006;195(2):522–527. doi: 10.1016/j.ajog.2006.01.098. [DOI] [PubMed] [Google Scholar]
- VanDemark KL, Guizzetti M, Giordano G, Costa LG. Ethanol inhibits muscarinic receptor-induced axonal growth in rat hippocampal neurons. Alcoholism, clinical and experimental research. 2009;33(11):1945–1955. doi: 10.1111/j.1530-0277.2009.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink J, Auth J, Abebe DT, Brenneman DE, Spong CY. Novel peptides prevent alcohol-induced spatial learning deficits and proinflammatory cytokine release in a mouse model of fetal alcohol syndrome. American journal of obstetrics and gynecology. 2005;193(3 Pt 1):825–829. doi: 10.1016/j.ajog.2005.02.101. [DOI] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behavioral neuroscience. 2006;120(2):482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Wainwright P, Fritz G. Effect of moderate prenatal ethanol exposure on postnatal brain and behavioral development in BALB/c mice. Experimental neurology. 1985;89(1):237–249. doi: 10.1016/0014-4886(85)90279-1. [DOI] [PubMed] [Google Scholar]
- Wang LL, Zhang Z, Li Q, Yang R, Pei X, Xu Y, Wang J, Zhou SF, Li Y. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Human reproduction (Oxford, England) 2009;24(3):562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- Warren KR, Calhoun FJ, May PA, Viljoen DL, Li TK, Tanaka H, Marinicheva GS, Robinson LK, Mundle G. Fetal alcohol syndrome: an international perspective. Alcoholism, clinical and experimental research. 2001;25(5 Suppl ISBRA):202S–206S. doi: 10.1097/00000374-200105051-00033. [DOI] [PubMed] [Google Scholar]
- Wass TS, Persutte WH, Hobbins JC. The impact of prenatal alcohol exposure on frontal cortex development in utero. American journal of obstetrics and gynecology. 2001;185(3):737–742. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Effects of ethanol and maternal nutritional status on fetal development. Alcoholism, clinical and experimental research. 1985;9(1):49–55. doi: 10.1111/j.1530-0277.1985.tb05049.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J, D’Alquen G, Bezio S. Interactive effects of ethanol intake and maternal nutritional status on skeletal development of fetal rats. Alcohol (Fayetteville, NY. 1990;7(5):383–388. doi: 10.1016/0741-8329(90)90020-d. [DOI] [PubMed] [Google Scholar]
- Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse (New York, NY. 2008;62(8):566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener SG, Shoemaker WJ, Koda LY, Bloom FE. Interaction of ethanol and nutrition during gestation: influence on maternal and offspring development in the rat. The Journal of pharmacology and experimental therapeutics. 1981;216(3):572–579. [PubMed] [Google Scholar]
- Wilkemeyer MF, Chen SY, Menkari CE, Brenneman DE, Sulik KK, Charness ME. Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. Proc Natl Acad Sci U S A. 2003;100(14):8543–8548. doi: 10.1073/pnas.1331636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkemeyer MF, Menkari CE, Spong CY, Charness ME. Peptide antagonists of ethanol inhibition of l1-mediated cell-cell adhesion. The Journal of pharmacology and experimental therapeutics. 2002;303(1):110–116. doi: 10.1124/jpet.102.036277. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu Z, Cermak JM, Tandon P, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK, Holmes GL. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. J Neurosci. 2000;20(22):RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annual review of nutrition. 2006a;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. The fetal origins of memory: the role of dietary choline in optimal brain development. The Journal of pediatrics. 2006b;149(5 Suppl):S131–136. doi: 10.1016/j.jpeds.2006.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Annual review of nutrition. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutrition reviews. 2006;64(4):197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek TA, Spong CY. A neuroprotective peptide antagonizes fetal alcohol exposure-compromised brain growth. J Mol Neurosci. 2004;24(2):189–199. doi: 10.1385/JMN:24:2:189. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Weston HE. Postnatal stress of early weaning exacerbates behavioral outcome in prenatal alcohol-exposed juvenile rats. Pharmacology, biochemistry, and behavior. 2002;73(1):45–52. doi: 10.1016/s0091-3057(02)00797-9. [DOI] [PubMed] [Google Scholar]