Abstract
Background
Morphogenetic modeling of tissues requires coordinated regulation of adhesion. For its correct patterning, the Drosophila pupal eye requires several Immunoglobulin superfamily cell adhesion molecules (IgCAMs) and the adaptor protein Cindr. Orthologs of these proteins are essential components of specialized junctions of the vertebrate kidney; the Cindr ortholog Cd2ap is essential for the integrity of this structure.
Results
Reducing Cindr during fly eye development led to incorrect distribution of the IgCAMs Roughest (Rst) and Hibris (Hbs). Both bound Cindr. Disrupting endocytosis similarly led to Rst and Hbs mis-localization; our data suggests an additional early requirement for endocytosis in regulating Hbs localization or stability. Finally, Rst and Hbs localized correctly only when in stable membrane complexes and we propose that Cindr anchors these to the cytoskeleton. This regulation likely does not extend to IgCAMs Kin of irre (Kirre) and Sticks and stones (Sns) in the pupal eye; neither interacted with Cindr in in vitro assays. Nonetheless, Kirre and Sns partially mis-localized when Cindr was reduced, possibly due to interactions with Rst/Hbs.
Conclusions
Our data suggests Cindr recapitulates both proposed functions of its mammalian orthologs Cd2ap and Cin85: targeting the IgCAMs Rst and Hbs for endocytosis and stabilizing these heterophilic IgCAM complexes.
Keywords: Cindr, Cd2ap, Cin85, Nephrin, Neph1, slit diaphragm, Roughest, Hibris, Sticks and Stones, Kin of irre
INTRODUCTION
Many cells retain the ability to initiate programs that remodel an organ or tissue to maintain its function in the face of changing physiological demands or damage. Such morphogenetic remodeling requires vigilant regulation of basal and lateral adhesions to achieve a level of tissue plasticity that facilitates or instructs remodeling. Regulation may include modifying adhesion protein interactions as well as regulating junction protein localization and transcription. Employing several of these mechanisms simultaneously is an effective strategy to control morphogenesis. Here we describe how a single regulating adaptor protein, Cindr, functions in two seemingly opposing mechanisms to regulate the dynamic localization of transmembrane proteins.
The Drosophila eye progresses from a dynamic to highly ordered and stable epithelium during pupal development (Cagan and Ready, 1993). This morphogenesis requires the cytoplasmic adaptor protein Cindr (Johnson et al., 2008, 2011). When cindr expression is severely reduced retinal epithelial cells fail to achieve correct niches and order is disrupted; disruption is due in part to aberrant localization of several membrane-associated or integral membrane proteins (Johnson et al., 2008, 2011). These include transmembrane Immunoglobulin Superfamily Cell Adhesion Molecules (IgCAMs) Hibris (Hbs) and Roughest (Rst), which interact in trans to dominantly instruct patterning (Bao and Cagan, 2005). Also active are Sticks and Stones (Sns) and Kin-of-Irre (Kirre), which function analogously though in a less prominent and partially-redundant manner (Bao et al., 2010). In this work, we explore the mechanisms by which Cindr regulates Rst and Hbs localization.
The importance of understanding IgCAM localization extends beyond Drosophila eye morphogenesis. The IgCAMs mediate axon projection in the developing nervous system (Boschert et al., 1990; Ramos et al., 1993; Schneider et al., 1995; Bazigou et al., 2007; Sugie et al., 2010), control muscle precursor cell fusion (reviewed in (Haralalka and Abmayr, 2010; Rochlin et al., 2010), and are components of the nephrocyte diaphragm in Drosophila and the analogous mammalian slit-diaphragm (SD) that connects podocyte foot processes (Weavers et al., 2009; Zhuang et al., 2009). Regarding the latter, SDs help filter large volumes of plasma daily and are likely subject to wear as well as dynamic changes. IgCAMs and the associated Cindr ortholog Cd2ap are candidates to regulate these aspects.
In the absence of mammalian Cd2ap, the IgCAM Nephrin failed to correctly localize within kidney glomeruli, leading to disruption of the SD and kidney dysfunction (Shih et al., 1999). Here we demonstrate a similar role for Cindr. Reducing its expression led to incorrect distribution of the IgCAMs Hbs and Rst. In addition, we identify a role for Cindr in regulating their turnover. These results have important implications for understanding the relationship between Cd2ap and IgCAM proteins during dynamic tissue remodeling and stability.
RESULTS
Ig-CAMs Fail to Localize Correctly When Cindr Is Reduced
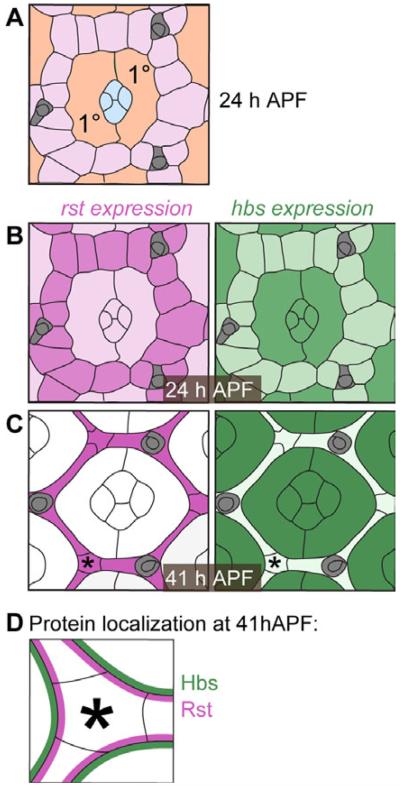
Interactions between Drosophila IgCAMs are required for correct pupal eye morphogenesis (Bao and Cagan, 2005; Bao et al., 2010). Their expression and localization are dynamic, resolving over several hours of pupal development. At approximately 24 hr after puparium formation (APF), both Rst and Hbs proteins were found in the central cone cells, primary pigment cells (1°s), and the surrounding pool of interommatidial precursor cells (IPC) (Figs. 1A,B, 2A) (Reiter et al., 1996). By 28 hr APF, (1) Hbs was lost in IPCs while (2) Rst was reduced in cone and 1°s. The result is complimentary localization of Rst and Hbs (Fig. 1C) (Bao and Cagan, 2005). A closer examination shows that Hbs and Rst proteins, which interact in trans, preferentially and then exclusively localize to adherens junctions of adjoining IPC and 1°s, respectively (Figs. 1D, 2B). This emergent, complementary expression pattern of Hbs and Rst is retained through 41 hr APF (Fig. 2C).
Fig. 1.
Illustrations of rst and hbs expression and protein localization. A: Illustration of a single ommatidium at 24 hr APF. The accessory cells include four central cone (blue) and two 1° cells (orange). A hexagonal lattice of interweaving IPCs (pink) separates neighboring ommatidia. Bristle groups (brown) occupy alternative vertices about each ommatidium. B,C: Illustrations of single ommatidia in pupal retinas at 24 and 41 hr APF, respectively. Cells expressing rst and hbs are colored pink or green, respectively, and expression level is reflected by color intensity. D: Illustration of Rst and Hbs protein localization, exclusively at 1°:IPC membranes at 41 hr APF. Asterisks in C and D mark a corresponding corner IPC.
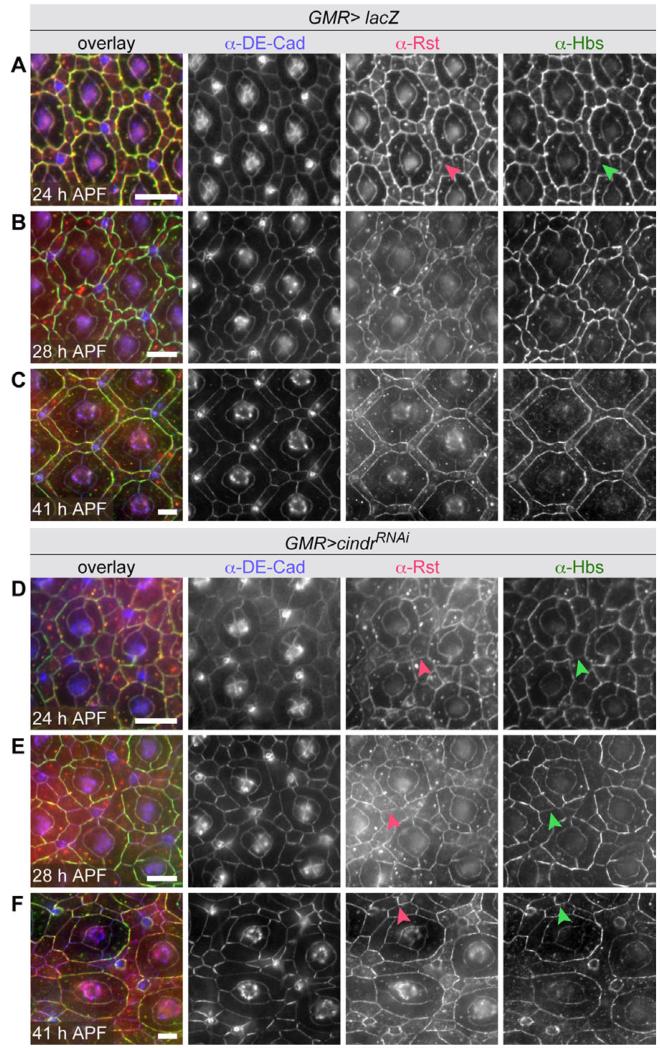
Fig. 2.
. Correct localization of Drosophila Rst and Hbs depends on Cindr. A–C: Detection of DE-Cad, Rst, and Hbs in GMR-GAL4>lacZ retina (wild type protein localization) and (D–F) retina expressing cindrRNAi transgenes. Arrowheads in A and D–F indicate Rst and Hbs localized to IPC:IPC borders. Eyes were dissected at 24, 28, and 41 hr APF as indicated. Scale bars = 10 μm.
Localization of Hbs and Rst were markedly altered when RNAi transgenes targeting cindr were expressed (Fig. 2D–F). At 24 hr APF, Rst and Hbs were not preferentially localized to 1°:IPC interfaces (Fig. 2D, compare to 2A). At 28 hr APF, stronger accumulation of Hbs at 1°:IPC interfaces was observed, but (1) Hbs was still detected inappropriately at IPC:IPC junctions while (2) Rst remained equally distributed to most IPC:IPC and 1°:IPC interfaces (Fig. 2E) (Johnson et al., 2008). By 41 hr, APF Hbs was no longer detected at some IPC:IPC interfaces (Fig. 2F) and less was detected at 1°:IPC boundaries in comparison to wild type retinas. Rst remained at most IPC:IPC boundaries (Fig. 2E). Curiously both IgCAMs accumulated at somewhat higher levels around bristle groups (Fig. 2E). In summary, loss of Cindr led to a failure of Rst and Hbs to resolve properly into complementary patterns.
Cindr Is Unlikely to Directly Affect rst and hbs Transcription
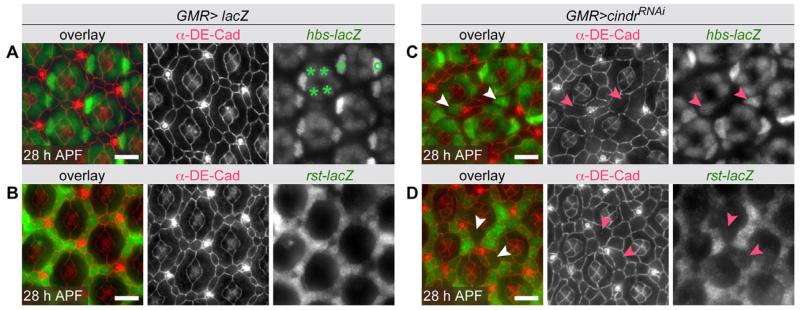
To test whether Cindr regulated rst or hbs at the level of transcription, we assayed the expression of rst and hbs lacZ reporter lines in cindrRNAi retina. In wild type ommatidia, hbs-lacZ expression was restricted to the four cone cells and two adjacent 1°s by 28 hr APF (Fig. 3A). A rst-lacZ reporter line was expressed at variable levels in the cytoplasm of all IPCs at 28 hr APF (Fig. 3B). cindr is expressed in all eye epithelial cells (Johnson et al., 2008). Reducing Cindr generated hyper-mobile cells that were commonly displaced from a 1° or IPC niche (Johnson et al., 2008, 2011). These displaced 1°s were readily distinguished from neighboring IPCs by their large block-like appearance, hbs-lacZ expression, and lack of rst-lacZ expression (Fig. 3C,D). Cells displaced from IPC into 1° niches were small and retained low levels of rst-lacZ expression (Fig. 3D). Notably, reducing cindr did not consistently modify hbs-lacZ or rst-lacZ expression in most retinal cells (Fig. 3C,D), suggesting that Cindr did not regulate gene expression per se. Instead our data may reflect a requirement for time- and position-sensitive signals to direct rst and hbs expression. Accordingly, displacement of cells in cindrRNAi eyes was sufficient to disrupt IgCAM expression and the level of lac-Z expression may in fact reflect time since cell displacement.
Fig. 3.
rst and hbs expression is not directly dependent on Cindr. A: Wild-type expression of a hbs-lacZ transcriptional reporter (green) in cone (asterisk) and 1° cell nuclei (°), detected with an antibody to β-galactosidase. B: Expression of a rst-lacZ transcriptional reporter (green) is detected in the cytoplasm of IPCs in a wild type retina. C: In retinas expressing a cindrRNAi transgene, hbs-lacZ expression is detected in cells likely to have been displaced from former 1° cell niches (examples highlighted with arrowheads). Note that in this confocal image, the adherens junction (DE-Cadherin) and nuclear planes do not lie immediately perpendicular. D: rst-lacZ expression is absent or reduced in large cells likely displaced from former 1° cell niches (examples indicated with arrowheads). Scale bars = 10 μm.
These observations demonstrate that expression of rst and hbs is regulated by position-dependent signals and underscores an important role for Cindr, regulating a cell’s positional stability, allowing it to establish appropriate niche-specific developmental programs. Altering cell positions contributes to erroneous Rst and Hbs localization, while correctly positioned cindrRNAi retinal cells express hbs-lacZ and rst-lacZ correctly. We conclude that Cindr has no direct role in regulating transcription of these IgCAMs, leading us to explore physical interactions between these proteins.
Interactions Between Cindr and Ig-CAM Proteins
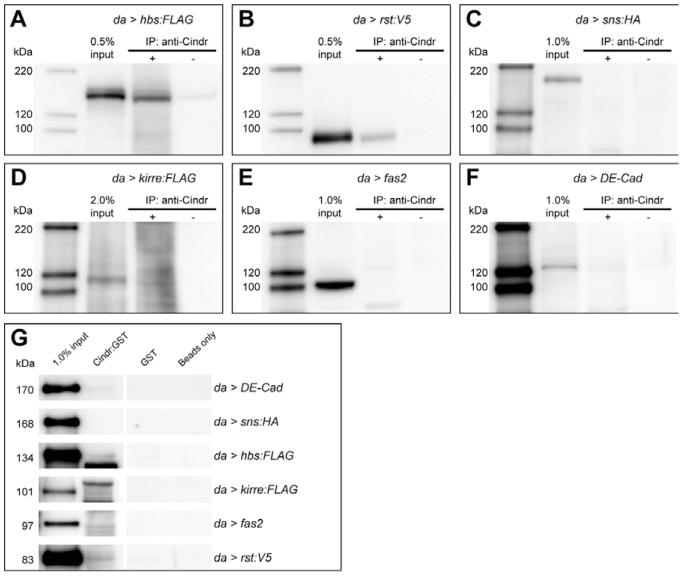
Mammalian Cd2ap and Cin85 interact with Nephrin, the single vertebrate ortholog of Hbs and Sns (Schwarz et al., 2001; Shih et al., 2001; Tossidou et al., 2010). We, therefore, used immunoprecipitation studies to determine whether Cindr associated with tagged versions of Drosophila IgCAM proteins. Ectopic Hbs:FLAG and Rst:V5 co-immunoprecipitated with endogenous Cindr from embryos (Fig. 4A,B). Embryos have low levels of endogenous Rst or Hbs, indicating that Cindr may bind them directly. In contrast, we failed to detectably isolate Sns:HA together with Cindr (Fig. 4C). Interactions between Cindr and Kirre:FLAG were not convincing despite chemical cross-linking to preserve complexes prior to embryo lysis (Fig. 4D). cindr is also highly expressed in the embryonic nervous system (data not shown) but Fasciclin 2 (Fas2), an Ig-like adhesion molecule richly expressed in neurons, did not co-immunoprecipitate with Cindr (Fig. 4E). Though interactions between mammalian Cd2ap and Cadherins have been reported (Lehtonen et al., 2004; Mustonen et al., 2005), DE-Cadherin did not consistently immunoprecipitate with Cindr (Fig. 4F).
Fig. 4.
Cindr interacts with specific adhesion receptors. Tagged (A) hbs, (B) rst, (C) sns, or (D) kirre were expressed in embryos using the da-GAL4 driver and precipitated with (+) or without (−) the anti-Cindr antibody. Western blot analyses were performed with antibodies to the protein tags. (E) fas2 or (F) DE-Cad were expressed in embryos and similarly precipitated with anti-Cindr; blots were probed with antibodies to Fas2 and DE-Cad, respectively. For C and D, 80% of the eluant of each co-immunoprecipitation reaction was analysed; 50% of each reaction was loaded in all other Western analyses shown.
To confirm these data, we tested whether Rst, Hbs, Sns, Kirre, Fas2, and DE-Cad isolated from embryo lysates interacted with Cindr:GST fusion proteins. Both Rst and Hbs bound Cindr:GST (Fig. 4G). As before Fas2, Sns:HA, and Kirre:FLAG did not interact with Cindr:GST and binding of DE-Cad to Cindr was inconsistent (Fig. 4G).
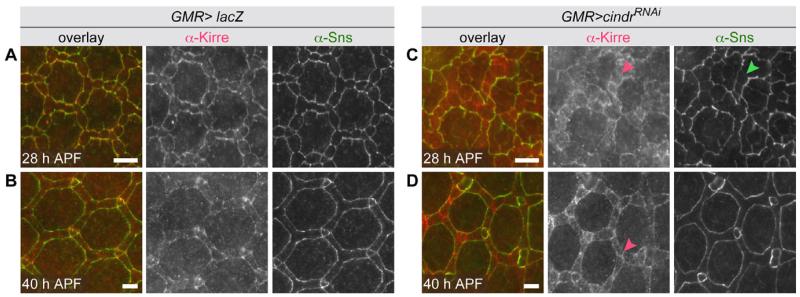
Our failure to observe convincing interactions between Cindr and Sns or Kirre confirms an earlier study (Weavers et al., 2009) and underscores differences between the functions of Sns/Kirre and Hbs/Rst complexes. However, Sns and Kirre localization was mildly disrupted in cindrRNAi eye tissue (Fig. 5). In wild type tissue, Sns/Kirre complexes were observed exclusively at IPC:1° boundaries by 28 hr APF (Fig. 5A) (Bao et al., 2010) but when Cindr was reduced, Sns and Kirre remained at some IPC:IPC boundaries and more cytoplasmic Kirre was detected (Fig. 5C). By 40 hr APF, Sns mis-localization was resolved, though Kirre remained at many IPC:IPC borders suggesting that these complexes were disrupted (Fig. 5B and D). These defects were similar, though far milder, than disruptions to Hbs/Rst complex localization.
Fig. 5.
cindrRNAi expression mildly disrupts Kirre and Sns. A,B: Detection of Kirre and Sns in GMR-GAL4>lacZ retina (wild type protein localization) and (C) and (D) retina expressing cindrRNAi transgenes. Arrowheads in C and D indicate Kirre and Sns mis-localized to IPC:IPC borders. Eyes were dissected at 28 and 41 hr APF as indicated. Scale bars = 10 μm.
Are Rst and Hbs Localization Regulated by Endocytosis?
Cin85 binds Casitas B-lineage lymphoma (Cbl) to mediate endocytosis of a variety of cell surface proteins including growth factor receptors (Petrelli et al., 2002; Soubeyran et al., 2002; Kobayashi et al., 2004), immunoglobulin receptors (Molfetta et al., 2005; Marois et al., 2011), the dopamine receptor (Shimokawa et al., 2010), and Nephrin (Tossidou et al., 2010). To test whether the IgCAMs are conserved targets of Cbl:Cin85, we reduced Drosophila cbl expression. Rst, and to a milder extent Hbs, remained at most IPC:IPC boundaries (Fig. 6A,B). Reducing cbl also enhanced cindrRNAi mis-patterning (Fig. 7A–C). We conclude that Cindr:Cbl complexes are likely functionally conserved and target IgCAMs for endocytosis.
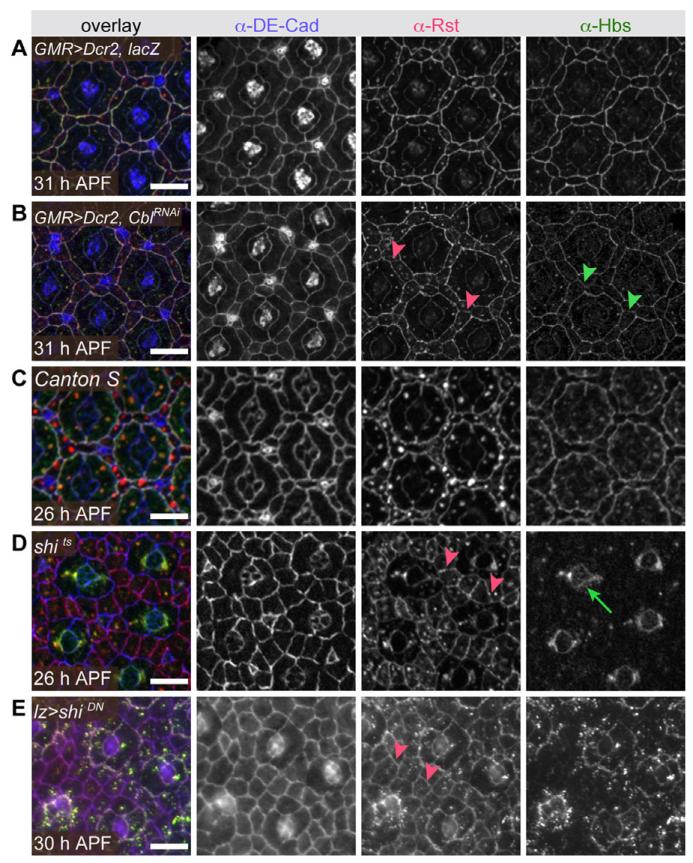
Fig. 6.
Localization of Rst and Hbs is endocytically regulated. DE-Cad, Rst, and Hbs are distributed normally in a retina expressing (A) Dicer2 and lacZ but when (B) an RNAi transgene targeting Cbl is expressed Rst and Hbs remain at most IPC:IPC boundaries (arrowheads point to examples). Similarly IgCAM localization was wild type in (C) a Canton S retina despite a 4-hr temperature shift to 31°C, but abberant in (D) a shi2 retina following 4 hr at 31°C to inhibit endocytosis and (E) in a retina expressing moderate levels of dominant-negative shibire (using a lozenge-GAL4 driver line). Red arrowheads highlight Rst detected at IPC:IPC membranes; green arrow in D marks Hbs localized around the cone cell group. Tissue was dissected at times indicated. Scale bars = 10 μm.
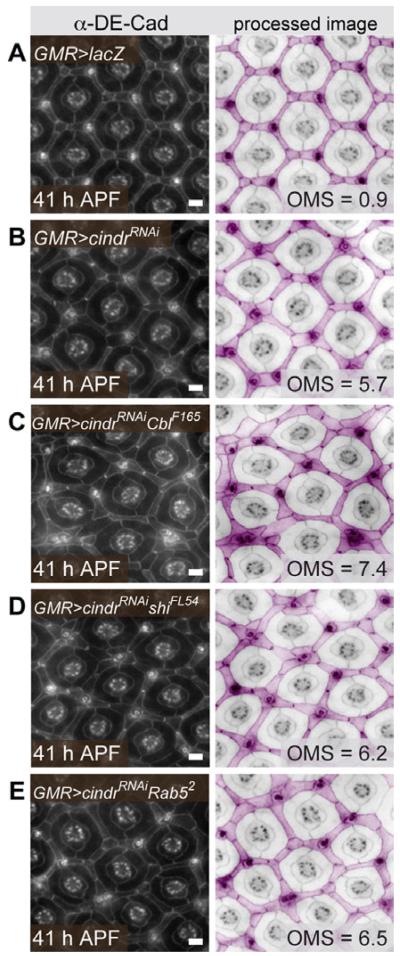
Fig. 7.
cindrRNAi mis-patterning is modified by endocytic loci. A: The correct position and shape of IPCs, 1°, and cone cells is disrupted when (B) cindr is mildly targeted by RNAi. C: This mis-patterning is enhanced when tissue is also heterozygous for Cbl and mildly but not significantly enhanced in (D) shi and (E) Rab5 heterozygotes. Tissue was dissected at 41 hr APF. Images on left were modified (right) and pseudo-colored to highlight IPCs (pink) and central cone and 1° cells (not colored). The mean number of patterning errors observed (OMS) in each genotype is indicated. See Supp. Table S1 for further information and statistical test values. Scale bars = 10 μm.
To validate that endocytosis per se mediated redistribution of Rst-Hbs complexes away from IPC:IPC junctions, we temporally restricted endocytosis during pupal development by reducing activity of the Drosophila Dynamin ortholog Shibire. Shifting the temperature-sensitive allele shibirets2 to the non-permissive temperature or expressing a dominant-negative shibire transgene at low levels (lozenge>shiDN) delayed patterning (Fig. 6C–E); developmental delay may reflect suppressed endocytosis-mediated signal transduction. However, even in regions where development was least affected, Rst remained abnormally localized to IPC:IPC boundaries and was evenly distributed about IPC circumferences (Fig. 6D,E). These observations are consistent with the view that endocytosis normally removes Rst from IPC:IPC boundaries; we cannot rule out the possibility that rst transcription was also affected indirectly by other endocytosis-mediated processes. The number of large cytoplasmic Rst puncta was noticeably reduced in both shibirets2 and shiDN IPCs, suggesting that trafficking was indeed disrupted in these genotypes. Small cytoplasmic Rst puncta were still observed.
In contrast, Hbs remained largely absent from IPC:IPC junctions when shibire activity was reduced, suggesting that clearance from these membranes was not dependent on endocytosis (Fig. 6D,E). Interestingly, in shibirets2 retinas Hbs localized to 1°:cone cell boundaries but was not detected at 1°:IPC borders, its normal place of accumulation by 28 hr APF (Fig. 6D). This effect was less marked in lozenge>shiDN retinas (Fig. 6E). This intriguing phenotype may reflect a requirement for an initial step of recycling endocytosis to generate mature adhesive Hbs proteins that are stabilized when returned to the cell surface.
Overall, our shibire results demonstrate distinct roles for endocytosis in regulating Rst and Hbs localization. However, reducing cindr had similar effects on the distribution of both IgCAMs. Our data suggest that Cindr targets both IgCAMs for endocytosis but employs additional strategies to regulate IgCAM localization. Previous work has implicated Cindr in border cell endocytosis (Quinones et al., 2010; see below). To better assess Cindr’s role in endocytosis, we tested whether reducing the genetic complement of shi or Rab5 modified GMR>cindrRNAi patterning defects. Null alleles of both loci mildly modified cindr but enhancement did not rise to statistical significance (Fig. 7D and E; see Supp. Table S1, which is available online). In contrast, Cbl demonstrated significant enhancement of cindr mis-patterning (Fig. 7C, Supp. Table S1). Heterozygous shi, Rab5, and Cbl retinas showed only occasional patterning errors (data not shown, Supp. Table S1). These data suggest that Cindr plays a minor role in regulating endocytic trafficking per se during pupal eye development.
On the other hand, our genetic interaction data underscore a significant relationship between Cindr and Cbl, most likely for targeting specific cargo for endocytosis during pupal eye patterning. Indeed, Cin85 and Cbl have been extensively implicated in RTK receptor endocytosis (Dikic, 2003) and more recent data describe a role for Cin85/Cbl in targeting Nephrin/Neph1 for endocytosis (Tossidou et al., 2010).
Are Rst and Hbs Localization Regulated by the Formation of Stable Junctions?
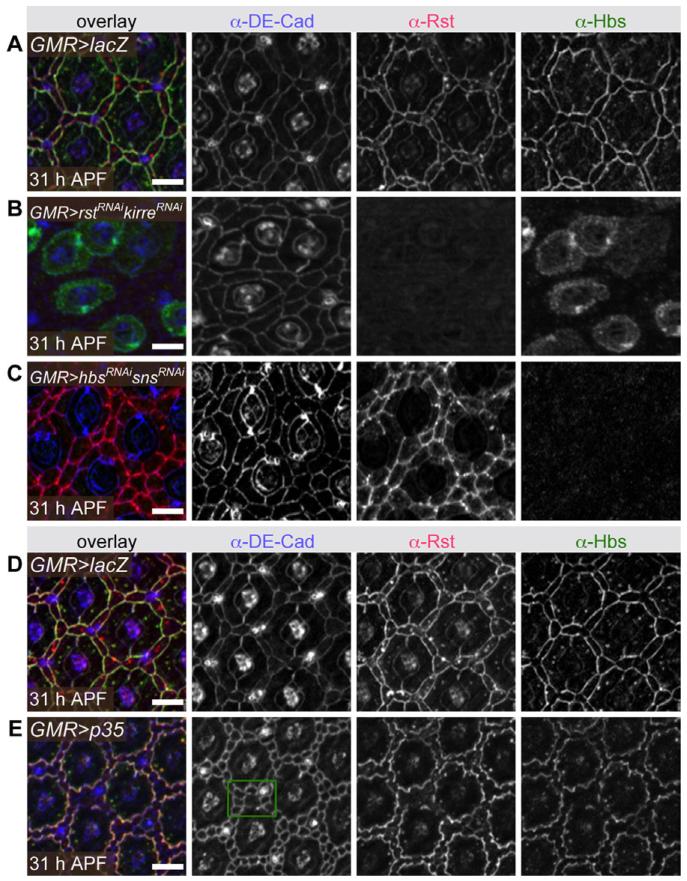
When endocytosis was disrupted, Rst protein failed to be cleared from IPC:IPC junctions even in the absence of Hbs (Fig. 6D). This suggests that stable Rst-Hbs complex formation is required for IgCAM re-localization to 1°:IPC borders. Indeed, depleting both Rst and Kirre (a Rst paralog also able to bind Hbs) (Bao et al., 2010) strongly disrupted localization of Hbs to 1°:IPC boundaries (Fig. 8A,B). In the converse experiment, reduction of both Hbs and Sns, Rst failed to localize to 1°:IPC boundaries but was still observed at IPC:IPC junctions (Fig. 8C). Interestingly, Rst remained at IPC:IPC boundaries, suggesting the protein is stabilized by the weaker homophilic interactions previously reported (Schneider et al., 1995). As both Rst and Hbs are detected at 1°:IPC borders in cindrRNAi retina, we infer that formation of Rst-Hbs complexes is independent of Cindr.
Fig. 8.
Disrupting complex formation affects Rst and Hbs localization. Detection of DE-Cad, Rst, and Hbs in (A) GMR,Dcr2>lacZ, (B) GMR,Dcr2>rstRNAi,kirreRNAi, (C) GMR,Dcr2>hbsRNAi,snshbsRNAi,snsRNAI, (D) GMR>lacZ, and (E) GMR>p35 retinas, all dissected at 31 hr APF. In E, green box highlights one region where IPCs remain in multiple rows, yet Rst and Hbs localize to only 1:IPC borders (right-hand panels). Scale bars = 10 μm.
Reducing rst/kirre or hbs/sns also severely disrupted correct IPC progression into single rows about each ommatidium (Fig. 8B and C). To confirm that these patterning defects did not contribute to IgCAM mis-localization, we generated a similar “multiple cell row” phenotype using an independent genetic manipulation. Inhibition of apoptosis left excess IPCs that occasionally failed to intercalate into one row of cells; Rst and Hbs nonetheless localized specifically to 1°:IPC junctions in these regions (Fig. 8D and E).
DISCUSSION
Our data emphasize that multiple mechanisms cooperate to generate discrete localization of the IgCAMs Rst and Hbs at 1°:IPC boundaries in wild type retina. These mechanisms include restriction of gene expression to different compartments (Bao and Cagan, 2005), removal of Rst-Hbs complexes from IPC:IPC boundaries (Fig. 6), and stabilization of Rst-Hbs complexes at 1°:IPC borders (Fig. 8). Here we identify two mechanisms that are regulated by Cindr.
With the exception of a small number of displaced cells, reducing cindr had little effect on IgCAM expression (Fig. 3). Impeding endocytosis, by reducing Cbl or Shibire, prevented removal of Rst from IPC:IPC borders (Fig. 6), phenocopying the effect of reducing cindr (Fig. 2D–F). Our suggestion that directed endocytosis of IgCAMs by Cindr has been conserved in Drosophila is further supported by a significant genetic interaction between cindr and the E3 ubiquitin ligase Cbl (Fig. 7, Supp. Table S1). shi and Rab5 also modified cindrRNAi though mildly (Fig. 7, Supp. Table S1), signifying a less likely role for Cindr in generally regulating endocytosis in the pupal eye. Surprisingly, disrupting endocytosis prevented accumulation of Hbs at 1°:cone cell adherens junctions (Fig. 6). This was not observed in cindrRNAi tissue (Fig. 2D–F) and we speculate that Rst and Hbs are subject to different endocytic regulation. Finally, we showed that interactions between Rst and Hbs stabilize the overall complex at 1°:IPC junctions (Fig. 8), indicating that Cindr was unnecessary for this interaction.
Using two assays, we found that Cindr is contained within a complex that includes Rst and Hbs (Fig. 4). Though not detected in our assays (Fig. 4), Sns and Kirre may be weakly associated with the Cindr complex, and localization of these IgCAMs was mildly disrupted by loss of cindr (Fig. 5). Cindr and Cd2ap contain consensus Actin-binding sites and associate with several actin regulators including Capping Proteins, ArfGAPs, and Synaptopodin (Bruck et al., 2006; Johnson et al., 2008, 2011; Yaddanapudi et al., 2011). As such, we previously proposed that Cindr functions to tether adhesion complexes to the cytoskeleton and limit actin remodeling (Johnson et al., 2008, 2011). Here we show that disruption of Cindr leads to phenotypes that may reflect a disruption of cytoskeleton-IgCAM interactions. A speculative model illustrating the complex relationship between Cindr and IgCAMs is presented in Figure 9. We propose that Cindr mediates endocytosis at IPC:IPC boundaries whilst stabilizing IgCAM complexes at IPC:1° borders. Cindr’s contradictory roles, stabilization plus endocytic dysregulation, presumably reflect recruitment of different sets of interacting proteins in a context- or position-dependent manner. Whether this complexity is observed in other tissues such as the mammalian slit diaphragm remains to be determined.
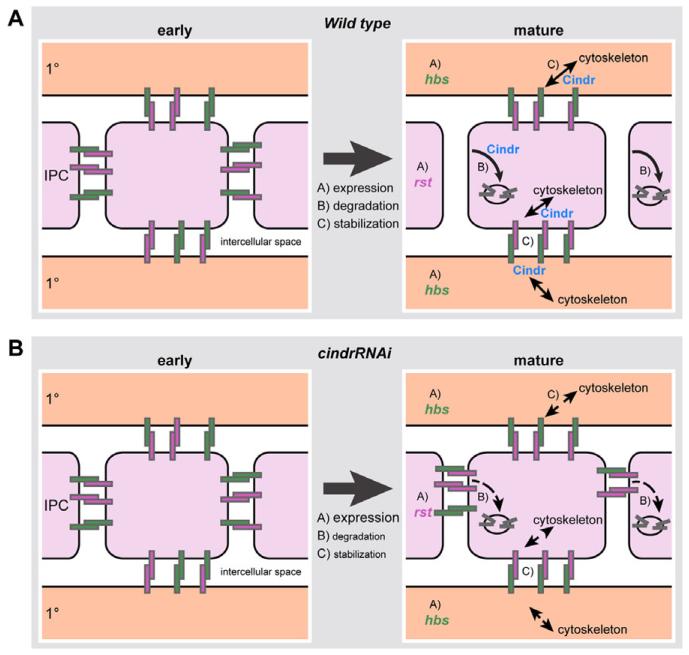
Fig. 9.
A model of the processes that regulate correct localization of Rst and Hbs in mature Drosophila eye epithelia. A: At early stages, Hbs (green rectangles) and Rst (pink rectangles) are localized to adherens junctions of all epithelial cells where they form homo- and heterophilic complexes (left; IPCs are colored light pink, I°s are colored light orange). The proteins have a greater affinity to form heterophilic complexes. As the eye matures (A) hbs and rst expression is differentially restricted, (B) complexes are internalized and degraded, and (C) Hbs-Rst complexes are anchored to the cytoskeleton. Localization is consequently restricted to I°:IPC boundaries. B: Cindr plays a role in the latter two processes. Hence in cindrRNAi retina, internalization is disrupted or delayed and Hbs-Rst complexes are not securely tethered to the cytoskeleton. Hbs and Rst complexes, therefore, remain at all IPC membranes.
We have previously considered the role of Cindr in pupal eye patterning from the perspective of actin regulation (Johnson et al., 2008, 2011). However, Cindr has been implicated in regulating endocytosis during Drosophila border cell migration (Quinones et al., 2010) and here we describe a role for Cindr in endocytosis of receptors instrumental in eye patterning. An Endophilin/Cd2ap/Cortactin complex is believed to induce local actin polymerization required for scission of endocytic vesicles and, indeed, Quinones et al. also observed a reduction in endocytosis in cortactin mutant embryos (Quinones et al., 2010). However, our laboratory observed no phenotypic defects in null cortactinM7 clones generated in the eye (S. Warner, unpublished observations) and cortactin did not significantly modify cindrRNAi-induced pupal eye mis-patterning (R.I.J., unpublished observations). Further verification will determine whether a functional Endophilin/Cindr/Cortactin complex is less relevant in tissues such as the pupal eye than in border cells within the oocyte.
We have not observed defects in IgCAM localization when mildly perturbing core actin regulators, Arfs, or ArfGAPs that interact (directly or indirectly) with Cindr (our unpublished observations). Additionally, GMR>cindrRNAi mis-patterning was greatly enhanced by actin regulatory loci (Johnson et al., 2008, 2011) but comparatively mildly enhanced by Cbl, shi, and Rab5 (Fig. 7). Finally, ectopic expression of the Arp2/3 complex component Arp66B partially rescued GMR>cindrRNAi mis-patterning without restoring Rst localization to only IPC:1°cell borders (Johnson et al., 2008). Together these data suggest that actin rather than endocytic regulation may be the dominant role of Cindr during eye patterning but a toolbox of cindr mutations perturbing select functions will be required to confirm this suggestion. Together, data on Cindr indicate that it recapitulates the function of both mammalian orthologs Cd2ap and Cin85.
EXPERIMENTAL PROCEDURES
Drosophila Genetics and Dissection
Unless stated, all crosses were cultured at 25°C until dissection between 24 and 41 hr APF, as previously described (Bao and Cagan, 2005).
RNA interference
cindr was targeted by expression of UAS-cindrRNAi2.21+23, 3.63+76 (Johnson et al., 2008) in the pupal eye, driven by GMR-GAL4 (Figs. 2E,F, 3C,D, 5C,D). and UAS-lacZ was expressed in control eye epithelia (Figs. 2A–C, 3A,B, 5A,B). To reduce kirre and rst a UAS-kirreRNAi D201A1; UAS-rstRNAi E101B1 line was crossed to GMR-GAL4, UAS-Dcr2 (Fig. 8B) (Bao and Cagan, 2005; Bao et al., 2010). To target hbs and sns, we crossed a UAS-hbsRNAi B207A2; UAS-snsRNAi B103A1 line (Bao and Cagan, 2005; Bao et al., 2010) to GMR-GAL4, UAS-Dcr2 (Fig. 8C). For control tissue, UAS-lacZ was crossed to GMR-GAL4, UAS-Dcr2 (Fig. 8A). UAS-CblRNAi 14.A was similarly used to disrupt Cbl (Fig. 6B, see below).
Transcriptional reporters
hbs expression was assayed in a P[w+]36.1 background (Artero et al., 2001) and rst expression was assessed using rstF6-lacZ (gift of Karl Fischbach) (Fig. 3).
Endocytosis
To disrupt Dynamin temperature-sensitive shi2, pupae were shifted to 31°C for 4 hr immediately prior to dissection (Fig. 6B) along with control CantonS pupae (Fig. 6A). To mildly disrupt Dynamin activity, dominant-negative UAS-shiK44A (Moline et al., 1999) was expressed using lz-GAL4 (gift from G. Struhl) (Flores et al., 1998).
Genetic interactions
We tested whether Rab52 (Wucherpfennig et al., 2003), shiFL54 (Yan et al., 2009) (gifts from D. Bilder), and CblF165 (Pai et al., 2000) modified cindr by generating heterozygous tissue in a GMR-GAL4/+; UAS-cindrR-NAi2.21/+ background (Fig. 7). Tissue dissected at 41 hr APF was analyzed to determine the mean number of patterning errors (ommatidial mis-patterning score, OMS) for each genotype as previously described (Johnson and Cagan, 2009). Statistical significance was determined using Student’s t-tests.
Patterning disruption
Expression of UAS-p35 (driven by GMR-GAL4) inhibited apoptosis of IPCs; ectopic cells occasionally failed to intercalate into singe rows (Fig. 8E).
Immunofluorescence and Microscopy
Primary antibodies were rat anti-DE-Cad2 (1:20, DSHB), mouse anti-Rst (Mab24A5.1; 1:40) (Schneider et al., 1995), rabbit anti-Hbs (AS14; 1:2,500) (Bao et al., 2010), rat anti-kirre (1:5,000) (Bao et al., 2010), rabbit anti-Sns (1:300) (Bour et al., 2000), and rabbit anti-βGal (40-1-a, 1:20, DSHB). Images were gathered using Leica Microsystems (Exton, PA) TCS SP5 DM or DMI microscopes (Figs. 2 and 3), a Zeiss (Thornwood, NY) Axio-plan2 and Apotome (Figs. 5, 6, and 8), or Leica DM5500 (Fig. 7). Maximal projection images were minimally processed in Photoshop (Adobe, San Jose, CA).
Transgenic Fly Lines
To generate pUAST-Rst:V5, a rst:V5 fragment was released with BglII/NotI cleavage from hs-rst-V5 (Bao and Cagan, 2005) and inserted into pUAST.
To generate pUASh-hbs:3×FLAG, hbs, cDNA was first sub-cloned into pGEM-S1 (Bao and Cagan, 2006) to yield pGEM-S1-hbs. A small 3′ fragment of hbs and the 3×FLAG sequence were joined and amplified using a linking PCR technique. 3′-hbs:3×FLAG was released with EcoNI/SpeI to replace the corresponding fragment of hbs (3′-hbs) in pGEM-S1-hbs. The entire hbs:3×FLAG was inserted into the XbaI site of pUASh (derived from pUAST by replacing the 3′-UTR of SV40 with that from hbs using XbaI/StuI), giving rise to pUASh-hbs:3×FLAG.
We generated pUAST-CblRNAi to contain inverted 461-bp repeats (beginning 276 bp downstream from the Cbl translation start) as described previously (Bao and Cagan, 2006). The PCR template was clone LD46082 (Drosophila Genomics Resource Center). Quantitative PCR confirmed targeted reduction of Cbl transcripts to less than 30% when this transgene was expressed in embryos (data not shown, primer information available on request).
The completed vectors were used to generate transgenic UAS-Rst:V5, UAS-hbs:FLAG and UAS-CblRNAi fly lines using standard techniques (BestGene, Chino Hills, CA).
Binding Assays
Three independent replicates of binding assays were performed.
Drosophila crosses
UAS-Rst:V5, UAS-hbs:FLAG (both described above), UAS-sns:HA (Zhuang et al., 2009), UAS-kirre:FLAG (Menon et al., 2005), fas2EP1462 (UAS-fas2, P.Rorth), or UAS-DE-Cadherin:GFP (Oda and Tsukita, 2001) were crossed to da-GAL4. Embryos were gathered 2–24 hr after egg laying.
Co-immunoprecipitation
Embryos were dechorionated, washed in distilled H2O, and resuspended in 20 mM HEPES (pH 7.5) with 5% glycerol and 0.2% BSA. Dimethyl 3,3′-dithiobis-proppionimidate-2HCl) (DTBP) was added to a final concentration of 3 mM and the embryos incubated at room temperature for 30 min with gentle rocking. The cross-linking reaction was terminated by addition of 30 mM Tris and a further 15-min incubation. Embryos were washed three times, lysed in a modified Tris buffer (Veraksa et al., 2005) with 1% Triton-X, and incubated on ice for 20 min. The lysate was centrifuged (5 min at 5,500 rpm), the supernatant first pre-cleared with 20-μL beads (Protein A/G UltraLink® Resin, Thermo Scientific, Waltham, MA), and then incubated with 60 μL of rabbit anti-Cindr (Johnson et al., 2008) and 40-μL beads overnight at 4°C.
GST pull-down
Embryo lysate was prepared as described above though the cross-linking incubation in DTBP was omitted. After centrifugation, the lysate was divided into equal parts and incubated overnight at 4°C with 100 μL glutathione-agarose beads (Sigma, St. Louis, MO) and GST:Cindr, (Johnson et al., 2011), GST, or beads only.
Western analysis
Following multiple washes, coimmunoprecipitation or GST-pulldown samples were separated by SDS-PAGE. Blots were probed with mouse anti-FLAG (1:6,000, M2, Sigma), mouse anti-V5 (1:3,000, V8012, Sigma), mouse anti-HA (1:2,000, 3F10, Roche, Indianapolis, IN), mouse anti-Fas2 (1:500, 1DE, DSHB, Iowa City, IA), or rat anti-ECad1 (1:500; Oda et al., 1994).
Supplementary Material
Key Findings.
In the Drosophila eye, distribution of the IgCAMs Rst and Hbs is regulated by the adaptor protein Cindr.
Cindr functions in two converse processes, targeting Rst and Hbs for endocytic regulation and stabilizing Rst-Hbs complexes at cell junctions.
Hence, Cindr recapitulates both proposed functions of its mammalian orthologs Cd2ap and Cin85.
Regulation of Cindr is a key step for facilitating tissue plasticity during morphogenesis and maintaining functional structures in mature tissues.
ACKNOWLEDGMENTS
This work was funded by RO1 EY11495 awarded to R.L.C. and a grant from the KMD Foundation awarded to S.B. R.I.J. was supported by a Robin Chemers Neustein Post-doctoral Fellowship award. Imaging was performed in the laboratory of RLC or at the MSSM Shared Resource Facility (supported by NIH-NCI shared resources grant 5R24 CA095823-04, NSF Major Research Instrumentation grant DBI-9724504, and NIH shared instrumentation grant 1 S10 RR0 9145-01).
Grant sponsor: NIH; Grant number: RO1EY11495; Grant sponsor: The KMD Foundation.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- Artero RD, Castanon I, Baylies MK. The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development. 2001;128:4251–4264. doi: 10.1242/dev.128.21.4251. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Fast cloning inverted repeats for RNA interference. Rna. 2006;12:2020–2024. doi: 10.1261/rna.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Fischbach KF, Corbin V, Cagan RL. Preferential adhesion maintains separation of ommatidia in the Drosophila eye. Dev Biol. 2010;344:948–956. doi: 10.1016/j.ydbio.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–975. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Boschert U, Ramos RG, Tix S, Technau GM, Fischbach KF. Genetic and developmental analysis of irreC, a genetic function required for optic chiasm formation in Drosophila. J Neurogenet. 1990;6:153–171. doi: 10.3109/01677069009107107. [DOI] [PubMed] [Google Scholar]
- Bour BA, Chakravarti M, West JM, Abmayr SM. Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 2000;14:1498–1511. [PMC free article] [PubMed] [Google Scholar]
- Bruck S, Huber TB, Ingham RJ, Kim K, Niederstrasser H, Allen PM, Pawson T, Cooper JA, Shaw AS. Identification of a novel inhibitory actin-capping protein binding motif in CD2-associated protein. J Biol Chem. 2006;281:19196–19203. doi: 10.1074/jbc.M600166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagan R, Ready D. Cell fate specification in the developing Drosophila retina. Dev Suppl. 1993:19–28. [PubMed] [Google Scholar]
- Dikic I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem Soc Trans. 2003;31:1178–1181. doi: 10.1042/bst0311178. [DOI] [PubMed] [Google Scholar]
- Flores GV, Daga A, Kalhor HR, Banerjee U. Lozenge is expressed in pluripotent precursor cells and patterns multiple cell types in the Drosophila eye through the control of cell-specific transcription factors. Development. 1998;125:3681–3687. doi: 10.1242/dev.125.18.3681. [DOI] [PubMed] [Google Scholar]
- Haralalka S, Abmayr SM. Myoblast fusion in Drosophila. Exp Cell Res. 2010;316:3007–3013. doi: 10.1016/j.yexcr.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RI, Cagan RL. A quantitative method to analyze Drosophila pupal eye patterning. PLoS One. 2009;4:e7008. doi: 10.1371/journal.pone.0007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RI, Seppa MJ, Cagan RL. The Drosophila CD2AP/CIN85 orthologue Cindr regulates junctions and cytoskeleton dynamics during tissue patterning. J Cell Biol. 2008;180:1191–1204. doi: 10.1083/jcb.200706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RI, Sedgwick A, D’Souza-Schorey C, Cagan RL. Role for a Cindr-Arf6 axis in patterning emerging epithelia. Mol Biol Cell. 2011;22:4513–4526. doi: 10.1091/mbc.E11-04-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Sawano A, Nojima Y, Shibuya M, Maru Y. The c-Cbl/CD2AP complex regulates VEGF-induced endocytosis and degradation of Flt-1 (VEGFR-1) Faseb J. 2004;18:929–931. doi: 10.1096/fj.03-0767fje. [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar MG. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol. 2004;165:923–936. doi: 10.1016/S0002-9440(10)63354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois L, Vaillancourt M, Pare G, Gagne V, Fernandes MJ, Rollet-Labelle E, Naccache PH. CIN85 modulates the down-regulation of Fc gammaRIIa expression and function by c-Cbl in a PKC-dependent manner in human neutrophils. J Biol Chem. 2011;286:15073–15084. doi: 10.1074/jbc.M110.213660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon SD, Osman Z, Chenchill K, Chia W. A positive feedback loop between Dumbfounded and Rolling pebbles leads to myotube enlargement in Drosophila. J Cell Biol. 2005;169:909–920. doi: 10.1083/jcb.200501126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfetta R, Belleudi F, Peruzzi G, Morrone S, Leone L, Dikic I, Piccoli M, Frati L, Torrisi MR, Santoni A, Paolini R. CIN85 regulates the ligand-dependent endocytosis of the IgE receptor: a new molecular mechanism to dampen mast cell function. J Immunol. 2005;175:4208–4216. doi: 10.4049/jimmunol.175.7.4208. [DOI] [PubMed] [Google Scholar]
- Moline MM, Southern C, Bejsovec A. Directionality of wingless protein transport influences epidermal patterning in the Drosophila embryo. Development. 1999;126:4375–4384. doi: 10.1242/dev.126.19.4375. [DOI] [PubMed] [Google Scholar]
- Mustonen H, Lepisto A, Lehtonen S, Lehtonen E, Puolakkainen P, Kivilaakso E. CD2AP contributes to cell migration and adhesion in cultured gastric epithelium. Biochem Biophys Res Commun. 2005;332:426–432. doi: 10.1016/j.bbrc.2005.04.140. [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci. 2001;114:493–501. doi: 10.1242/jcs.114.3.493. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- Pai LM, Barcelo G, Schupbach T. D-cbl, a negative regulator of the Egfr pathway, is required for dorsoventral patterning of Drosophila oogenesis. Cell. 2000;103:51–61. doi: 10.1016/s0092-8674(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent down-regulation of c-Met. Nature. 2002;416:187–190. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- Quinones GA, Jin J, Oro AE. I-BAR protein antagonism of endocytosis mediates directional sensing during guided cell migration. J Cell Biol. 2010;189:353–367. doi: 10.1083/jcb.200910136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RG, Igloi GL, Lichte B, Baumann U, Maier D, Schneider T, Brandstatter JH, Frohlich A, Fischbach KF. The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes Dev. 1993;7:2533–2547. doi: 10.1101/gad.7.12b.2533. [DOI] [PubMed] [Google Scholar]
- Reiter C, Schimansky T, Nie Z, Fischbach KF. Reorganization of membrane contacts prior to apoptosis in the Drosophila retina: the role of the IrreC-rst protein. Development. 1996;122:1931–1940. doi: 10.1242/dev.122.6.1931. [DOI] [PubMed] [Google Scholar]
- Rochlin K, Yu S, Roy S, Baylies MK. Myoblast fusion: when it takes more to make one. Dev Biol. 2010;341:66–83. doi: 10.1016/j.ydbio.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Reiter C, Eule E, Bader B, Lichte B, Nie Z, Schimansky T, Ramos RG, Fischbach KF. Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron. 1995;15:259–271. doi: 10.1016/0896-6273(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa N, Haglund K, Holter SM, Grabbe C, Kirkin V, Koibuchi N, Schultz C, Rozman J, Hoeller D, Qiu CH, Londono MB, Ikezawa J, Jedlicka P, Stein B, Schwarzacher SW, Wolfer DP, Ehrhardt N, Heuchel R, Nezis I, Brech A, Schmidt MH, Fuchs H, Gailus-Durner V, Klingenspor M, Bogler O, Wurst W, Deller T, de Angelis MH, Dikic I. CIN85 regulates dopamine receptor endocytosis and governs behaviour in mice. EMBO J. 2010;29:2421–2432. doi: 10.1038/emboj.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- Sugie A, Umetsu D, Yasugi T, Fischbach KF, Tabata T. Recognition of pre- and postsynaptic neurons via nephrin/NEPH1 homologs is a basis for the formation of the Drosophila retinotopic map. Development. 2010;137:3303–3313. doi: 10.1242/dev.047332. [DOI] [PubMed] [Google Scholar]
- Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, Schiffer M. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J Biol Chem. 2010;285:25285–25295. doi: 10.1074/jbc.M109.087239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraksa A, Bauer A, Artavanis-Tsakonas S. Analyzing protein complexes in Drosophila with tandem affinity purification-mass spectrometry. Dev Dyn. 2005;232:827–834. doi: 10.1002/dvdy.20272. [DOI] [PubMed] [Google Scholar]
- Weavers H, Prieto-Sanchez S, Grawe F, Garcia-Lopez A, Artero R, Wilsch-Brauninger M, Ruiz-Gomez M, Skaer H, Denholm B. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. 2009;457:322–326. doi: 10.1038/nature07526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Moller CC, Wei C, Peev V, Flesche JB, Forst AL, Li J, Patrakka J, Xiao Z, Grahammer F, Schiffer M, Lohmuller T, Reinheckel T, Gu C, Huber TB, Ju W, Bitzer M, Rastaldi MP, Ruiz P, Tryggvason K, Shaw AS, Faul C, Sever S, Reiser J. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J Clin Invest. 2011;121:3965–3980. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Denef N, Schupbach T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell. 2009;17:387–402. doi: 10.1016/j.devcel.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development. 2009;136:2335–2344. doi: 10.1242/dev.031609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.