Abstract
Affective disorders are believed to involve dysfunction within the amygdala, a key structure for processing emotional information. Chronic stress may contribute to affective disorders such as depression and anxiety via its effects on the amygdala. Previous research has shown that chronic stress increases amygdala neuronal activity in an age-dependent manner. However, whether these distinct changes in amgydala neuronal activity are accompanied by age-dependent changes in amygdala-dependent affective behavior is unclear. In this study, we investigated how chronic stress impacts amgydala-dependent auditory fear conditioning in adolescent and adult rats in a repeated restraint model. We found that repeated restraint enhanced conditioned freezing in both adolescent and adult rats. But repeated restraint led to impaired acquisition of fear extinction only in adolescent rats. Along with previous findings, these results suggest that chronic stress may precipitate affective disorders via differential mechanisms, with different outcomes at different ages.
Keywords: fear conditioning, fear extinction, chronic stress, repeated restraint, adolescent, amygdala
1. Introduction
Changes in mood and impaired cognition are characteristic of affective disorders. Chronic stress contributes to the recurrence and exacerbation of affective disorders such as depression and post-traumatic stress disorder (PTSD) [1–3]. The amygdala plays an important role in interpretation and expression of affect, especially those related to fear [4–7]. The amygdala is particularly vulnerable to the effects of stress [8,9] and contributes to stress-related affective disorders. Animal studies shown that chronic stress results in amygdala hypertrophy [10,11] and hyperactivity [12,13], which are accompanied by enhanced anxiety state measured in the elevated plus maze (EPM) [13–15] and fear behavior measured by conditioned freezing [16,17]. Human studies reported similar amygdala hypertrophy and hyperactivity in patients with affective disorders [18–21]. These findings all suggest that chronic stress precipitates affective disorders via modification of amygdala function. Therefore, understanding how chronic stress impacts amygdala physiology and amygdala-dependent behaviors is important for uncovering the pathophysiology of stress-related affective disorders. However, stress during adolescence may exert unique and particularly disruptive effects.
Adolescence is characterized by emergence of specific social and cognitive behaviors [22–24], and is therefore difficult to pinpoint. However, puberty occurs during adolescence, and is associated with distinct measurable biological changes. Puberty in male Sprague-Dawley rats is typically near postnatal day (PND) 42, and this was used as a conservative point to limit the study of adolescence in our study. The emergence of adolescence has been estimated as early as weaning (typically PND 20–21). Here, we used a conservative point of PND 28 [23] when elevation of gonadal hormones begins [25]. Adulthood is defined as sexual maturity, and typically occurs by PND 60 in male rats [23,26]. Similar to these studies, adult rats were PND 65 at the beginning of experiments, several days after sexual maturity in our study.
Our previous study has shown that repeated restraint stress leads to increased number of spontaneously active BLA neurons in adolescent rats but results in increased firing rate of individual BLA neurons in adult rats [27]. It is unclear if this age-dependency of the effects of repeated restraint on BLA neuronal activity occurs in parallel with differences in BLA-dependent behaviors. One classic behavior that reflects BLA function is cued fear conditioning [7,28,29]. A short (3 day) course of stress during adolescence had no effect on acquisition of conditioned fear, but increased conditioned freezing during testing [30]. However, the purpose of that study did not include comparison between adolescent and adult rats, nor to examine extinction of conditioned freezing. To test whether repeated stress exerts different effects on fear conditioning in different age groups, we examined the impact of repeated restraint on amygdala-dependent auditory fear conditioning in adolescent and adult rats. Freezing during acquisition was measured, as well as conditioned freezing and acquisition of extinction one day after acquisition to determine if repeated restraint stress exerts age-dependent effects on fear conditioning.
2. Material and methods
2.1 Animals
All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Rosalind Franklin University of Medicine and Science. Adolescent and adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were used in this study. They were housed 2 or 3 per cage in the Rosalind Franklin University animal facility with free access to food and water, and maintained on a 12 hr light/dark cycle (light cycle from 7:00 am to 7:00 pm). Adolescent rats arrived at the animal facility at PND 25 with an approximate body weight of 60 – 70 g. They were habituated in the facility before starting the restraint or control handling protocol which began at PND 29 (approximate body weight 70 – 80 g), and included the subsequent 9 days. Adolescent rats were PND 39 for fear conditioning with an approximate body weight of 130 – 160 g. Adult rats arrived at PND 58 with an approximate body weight of 260 – 280 g. They were PND 65 at the initiation of restraint or control procedures. Adult rats were PND 76 with an approximate body weight of 320 – 350 g on the day of fear conditioning.
2.2 Repeated restraint protocol
To model the effects of chronic stress, a 7-day repeated restraint protocol was used [13]. Age-matched animals were randomly assigned into non-restraint and repeated restraint groups. After habituating to the animal facility for at least 4 days, rats were subjected to restraint or control handling. Rats in the repeated restraint group were placed into a hemi-cylinder restraint tube 20 min/session, 1 session/day for 7 out of 9 days in the procedure room [13]. This specific design (Fig. 1) reduces habituation to restraint, which would otherwise be significant [31,32]. The restraint tube was an acrylic cylinder with flattened bottom (3 different sizes of restraint tubes were used, depending on the size of the rat). All rats that were placed into restraint cylinders were securely immobilized, as determined by inability to turn around, but retaining restricted movement of head and limbs. If any rat displayed evidence of being too loosely secured (as demonstrated by ability to turn lower or upper body) or too tightly secured (as demonstrated by inability to move head), the position of the securing door was changed. Rats in the non-restraint group were placed into a clear Plexiglas transportation cage 20 min/session, 1 session/day for 7 out of 9 days (Fig. 1). All the procedures were performed between 8:00 am to 3:00 pm, during the light phase of the light/dark cycle. To assess the additive nature of repeated restraint, two control 1-Day restraint groups were added. Rats in 1-Day restraint B group (B ~ 1 day Before the behavior test) were handled the same way as non-restraint rats except they were subjected to restraint on the last day of this procedure. Rats in 1-Day restraint F group (F ~ First day of the restraint protocol) were subjected to restraint on the first day of the procedure and then handled identically to non-restraint rats during the remaining 8 days. Rats were run in a manner that counterbalanced age and stress groups over the course of the study.
Figure 1. Schematic of experimental design.
Rats were exposed to control handling (or restraint stress) for 5 days. This was followed by 2 days with no manipulation, and another 2 days of handling (or restraint stress). This design decreases habituation to restraint stress.
2.3 Elevated plus maze
To verify the effectiveness of our repeated restraint protocol, we tested animals in the EPM one day after the final restraint/control handling session. Two sets of EPMs designed specifically for animals of different ages were used in this study. The EPM used for adolescent rats was a scaled-down version of the EPM used for adults. The EPM was scaled down based on average crown-to-rump body length. This approach was used by other labs, and confirmed in those labs by measurement of gait width [e.g. 33]. The EPM (Scientific Designs, Pittsburgh, PA) consisted of four arms: two open arms (width x length: small maze 3.25″ × 14.75″; big maze 4.25″ × 19.75″) and two closed arms (width x length x wall height: small maze 3.25″ × 14.75″ × 14″; big maze 4.25″ × 19.75″ × 18″). Each arm was attached to a sturdy leg, elevated 32″ from the ground. The EPM test was conducted as described previously [13,27]. Animals were placed at the junction of four arms, facing the open arm opposite the experimenter. Animal behavior was recorded for 5 min and analyzed by a personal computer (Dell E6500) running video-tracking software (Any-Maze, Stoelting, Wood Dale, IL). The time spent on open arms was measured and used as an index of anxiety-like behavior. In addition, the total number of arm entries was measured and used as an indicator of locomotor activity
2.4 Fear conditioning
Fear conditioning in this study was a two-day procedure. Conditioning and testing were performed in different plexiglass chambers with distinct contexts (wall pattern and color, odors, and flooring) to minimize contextual freezing. Each chamber was enclosed by a sound-attenuating cabinet (UGO Basile, VA, Italy). Two sound attenuated cabinets were used, one for conditioning and one for testing (same dimensions: 21″ × 17.5″ × 21.3″ height). The two cabinets were in the same room. The conditioning chamber measured 10.6″ × 10.6″ × 14.1″ height. The testing chamber measured 13.5.″ × 10″ × 12″ height. Mounted inside each cabinet were an audio speaker (UGO Basile, VA, Italy), a house light, an infrared LED light and a ceiling mounted digital camera that was sensitive to light in the IR range (Fire-i, Unibrain, San Ramon, CA) which was connected to a personal computer (Dell E6500) running video-tracking software (Any-Maze, Stoelting, WI) that detects and records behavior. Conditioning consisted of 2 min habituation followed by 5 pairings of a neutral tone (10 sec, 1500 Hz, 85 dB) with a footshock (1 sec, threshold intensity; see below) that co-terminated with the tone. Conditioning trials were presented at 60 sec intertrial intervals. Rats remained in the chamber for 1 min after the end of last conditioning trial, and were then returned to their home cage. The next day, conditioned freezing and its within session extinction were tested in a contextually distinct chamber. The testing consisted of a 2 min habituation followed by 15 trials of tone presentation (20 sec, 1500 Hz, 85 dB) at a 60 sec intertrial interval. No footshock was presented during testing trials. After testing, animals were returned to their home cage.
To determine threshold intensity of footshock for fear conditioning, footshock was delivered in 0.1 mA increments from 0.2 mA (0.2 mA, 0.3 mA, 0.4 mA) to each animal immediately before the fear conditioning procedure. The same individual animal experienced this threshold procedure, followed by fear conditioning using its threshold footshock intensity. In this study, threshold intensity was defined as the footshock intensity which leads to forepaw withdrawal (adolescent rats typically 0.4 mA; adult rats typically 0.3 mA; see Results for further detail). A previous study demonstrated that most rats had no obvious response to 0.1 mA footshock on our apparatus [17]. Therefore in the current study, we tested intensities starting at 0.2 mA, and increased the intensity in 0.1 mA increments until forepaw withdrawal was observed. Thus, each rat received only one footshock at/near the forepaw withdrawal threshold, and a total of 1–3 footshocks at subthreshold intensities. Freezing was quantified by the software based on a threshold of change in video image pixels. A freezing episode had to last a minimum of 2 sec to be included in the software analysis. These criteria were compared against visually-confirmed freezing (behavioral immobility except for movement associated with respiration). Total freezing during each trial (entire 60 sec) was used as an index of conditioned fear and converted to a percentage ([time of freezing/60 sec] × 100) for analysis. The first trial was planned for comparison. However, in these experiments the freezing during the first trial was sub-maximal. There was no significant difference in the freezing response in the first trial in both age groups (adolescent: non-restraint 55.73 ± 6.42 %, n = 14 rats; repeated restraint 62.22 ± 6.49 %, n = 15 rats; t = 0.71, df = 27, p = 0.48; adult: non-restraint 31.89 ± 4.48 %, n = 15 rats; repeated restraint 38.57 ± 5.72 %, n = 14 rats; t = 0.93, df = 27, p = 0.36, unpaired t test). In addition to the first trial, the majority of rats in all groups still displayed significant amount of freezing response (more than 30 %) in trial 2 to 5. Therefore, the initial 5 trials were used to confirm the initial conditioned freezing during extinction. The last 5 trials were used to assess the later freezing during the extinction phase.
To examine whether increased footshock intensity could lead to resistance to acquisition of fear extinction in adult rats, a separate non-restraint and repeated restraint group were included. In this set of experiment, animals underwent the exact same experimental procedure as previously described except a suprathreshold footshock intensity was used. We defined the suprathreshold footshock intensity as 0.1 mA more than threshold footshock intensity
2.5 Statistical analysis
The percentage of time spent freezing during each trial was compared across groups using two-way repeated analysis of variance (ANOVA) with trial and treatment or age and treatment as factors (Prism, GraphPad software, La Jolla, CA). If a significant difference was detected, groups were further compared with unpaired t tests, or one-way ANOVAs if 3 groups were compared. Planned comparisons were performed between groups to compare the initial five trials and last five trials during retrieval. A p value < 0.05 was considered statistically significant. All data were presented as mean ± SEM, unless otherwise specified.
3 Results
3.1 Repeated restraint decreases EPM exploration in adolescent and adult rats
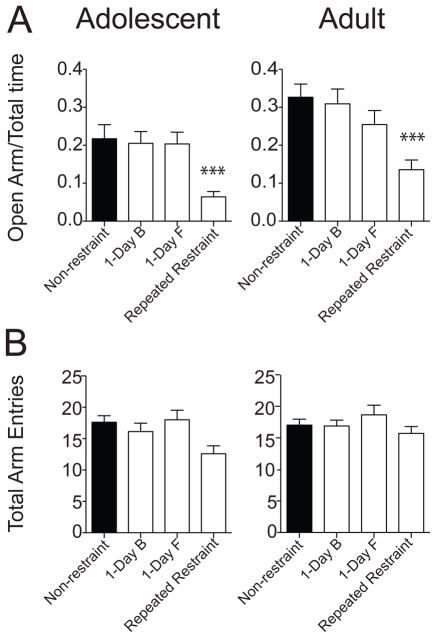
A previously established consequence of effective repeated stressors is increased anxiety-like behavior in the EPM. To gain an independent behavioral confirmation of the general effectiveness of the repeated restraint as a stressor in these rats, EPM was performed. Adult rats displayed a greater proportion of time on the open arms (percentage of time on open arm: adolescent non-restraint 21.72 ± 3.70 %, n = 21 rats; adult non-restraint 32.66 ± 3.47 %, n = 35 rats; t = 2.06, df = 54, p = 0.04, unpaired t test). Similar results have also been reported in other studies [34,35]. Consistent with previous findings [13,14,27], repeated restraint resulted in reduction of the time spent exploring the open arm of EPM in both adolescent (Fig. 2A; adolescent percentage of time on open arm: non-restraint 21.72 ± 3.70 %, n = 21 rats; 1-Day B 20.52 ± 3.09 %, n = 21 rats; 1-Day F 20.35 ± 3.10 %, n = 17 rats; repeated restraint 6.40 ± 1.40 %, n = 25 rats; F(3,80) = 7.30, p = 0.0002, one-way ANOVA) and adult rats (Fig. 2A; adult percentage of time on open arm: non-restraint 32.66 ± 3.47 %, n = 35 rats; 1-Day B 30.92 ± 3.90 %, n = 25 rats; 1-Day F 25.45 ± 3.68 %, n = 17 rats; repeated restraint 13.54 ± 2.58 %, n = 35 rats; F(3,108) = 7.72, p = 0.0001, one-way ANOVA). However, there was no significant difference in the total number of arm entries among the 4 treatment groups, indicating little effect of restraint on overall locomotion in adolescent rats (Fig. 2B; adolescent total arm entries: non-restraint 17.62 ± 1.04 entries, n = 21 rats; 1-Day B 16.14 ± 1.33 entries, n = 21 rats; 1-Day F 18.00 ± 1.53 entries, n = 17 rats; repeated restraint 12.60 ± 1.27 entries, n = 25 rats; F(3,80) = 0.58, p = 0.56, one-way ANOVA) and in adult rats (Fig. 2B; adult total arm entries: non-restraint 17.03 ± 0.93 entries, n = 35 rats; 1-Day B 16.88 ± 0.94 entries, n = 25; 1-Day F 18.67 ± 1.54 entries, n = 17; repeated restraint 15.71 ± 1.09 entries, n = 35 rats; F(3,108) = 1.01, p = 0.39, one-way ANOVA). Therefore, consistent with effectiveness as a repeated stressor, repeated restraint caused increased anxiety-like behavior, but did not impair locomotor activity. In addition, single restraint did not significantly impact exploration of EPM in adolescent or adult rats.
Figure 2. Repeated restraint led to reduced open arm exploration of EPM.
(A) Repeated restraint resulted in reduced time spent on open arm of EPM in both adolescent (right) and adult (left) rats, indicating increased anxiety state after repeated restraint. (B) There was no significant difference in the total number of arm entries in both adolescent (right) and adult (left) rats, indicating no changes in locomotor activity. Black represents non-restraint controls, white represents restraint groups. ***p < 0.001 compared between non-restraint and repeated restraint groups.
3.2 Repeated restraint enhances conditioned freezing and impairs acquisition of extinction in adolescent rats
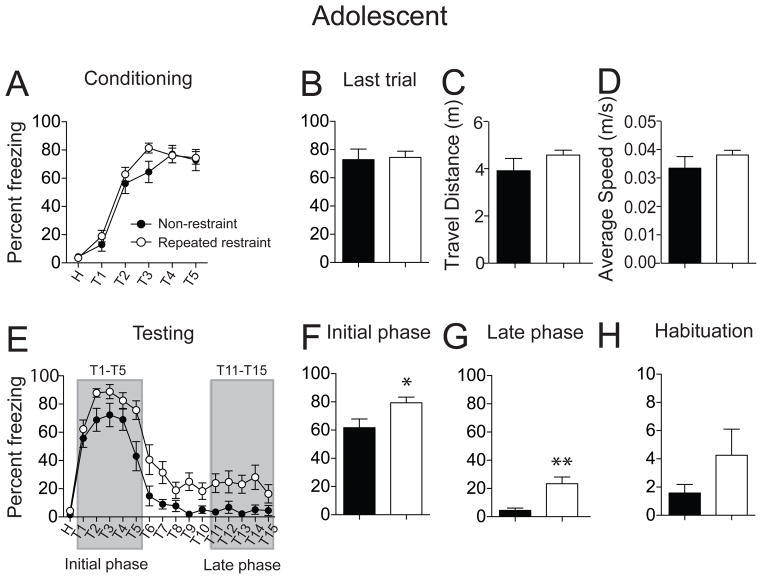
Fear conditioning was performed one day after the EPM behavior test. In this study, the mean footshock intensity that induced forepaw withdrawal in control rats was not significantly different compared to stressed rats (adolescent non-restraint 0.42 ± 0.02 Hz, n = 14 rats; repeated restraint 0.45 ± 0.03 Hz, n = 15 rats; t = 0.87, df = 27, p = 0.39, unpaired t test). Freezing was measured as an index of conditioned fear. All rats displayed increased freezing over the progression of 5 conditioning trials, consistent with acquisition of fear conditioning (Fig. 3A; F(5,162) = 77.55, p < 0.0001, non-restraint n = 14 rats, repeated restraint n = 15 rats, significant main effect of trials, two-way repeated measures ANOVA). Repeated restraint did not significantly impact this measure of acquisition of fear conditioning (Fig. 3A; F(1,162) = 2.68, p = 0.10, no significant effect of treatment, two-way repeated measures ANOVA). At the last trial rats from both treatment groups exhibited similar freezing (Fig. 3B; non-restraint 72.87 ± 7.47 %; repeated restraint 74.44 ± 4.46 %; t = 0.18, df = 27, p = 0.86, unpaired t test). In addition, there was no significant difference in the total travel distance (Fig. 3C; non-restraint 3.91 ± 0.52 m, n = 14 rats; repeated restraint 4.58 ± 0.20 m, n = 15 rats; t = 1.22, df = 27, p = 0.23, unpaired t test) and average speed (Fig. 3D; non-restraint 0.03 ± 0.004 m/s; repeated restraint 0.04 ± 0.002 m/s; t = 1.07, df = 27, p = 0.29, unpaired t test) during habituation between non-restraint and repeated restraint groups, indicating that repeated restraint did not significantly impact locomotor activity of adolescent rats.
Figure 3. Repeated restraint impaired acquisition of fear extinction in adolescent rats.
(A) Repeated restraint did not significantly impact acquisition of fear conditioning. In all plots, “percent freezing” is the percent of time the rat displayed freezing behavior in a trial (60 sec). (B) There was no significant difference between non-restraint rats and repeated restraint rats in freezing at the last conditioning trial. (C) There was no significant difference between groups in total travel distance or (D) average speed. (E) When tested on the next day in a novel chamber, non-restraint and repeated restraint rats exhibited initial robust freezing responses followed by gradual reduction of conditioned freezing. (F) Repeated restraint rats displayed significantly higher conditioned freezing responses compared to non-restraint rats over the initial 5 trials of testing. (G) Repeated restraint rats also displayed higher freezing responses compared to non-restraint rats when the last 5 trials were examined. (H) Rats from both groups displayed similar amounts of activity during habituation. Black represents non-restraint controls, white represents repeated restraint groups. *p < 0.05, **p < 0.01 compared between non-restraint and repeated restraint groups.
Conditioned freezing was tested 24 hr after fear conditioning. Rats displayed initial robust freezing in response to tones, followed by a gradual reduction of freezing over 15 repeated tone presentation trials (Fig. 3E; F(15,432) = 41.94, p < 0.0001, non-restraint n = 14 rats, repeated restraint n = 15 rats, significant main effect of trials, two-way repeated measures ANOVA). Repeated restraint significantly increased conditioned freezing compared to control handled rats (Fig. 3E; F(1,432) = 66.32, p < 0.0001, significant main effect of treatment, two-way repeated measures ANOVA). To further study the effect of repeated restraint, freezing during the initial 5 trials of extinction (early phase of extinction testing; corresponding to initial conditioned freezing) and the last 5 trials (late phase of extinction testing; corresponding to after significant acquisition of extinction has occurred) were separately compared between the two treatment groups. The repeated restraint group displayed higher freezing during the initial testing phase (Fig. 3F; non-restraint 61.75 ± 3.81 %, n = 14 rats; repeated restraint 79.41 ± 2.64 %, n = 15 rats; t = 3.85, df = 143, p = 0.0002, unpaired t test). Furthermore, while control adolescent rats displayed minimal conditioned freezing during the late phases of extinction testing, freezing persisted in the repeated stress adolescent group. There was a significant difference in the conditioned freezing between control and stress groups during the late extinction phase (Fig. 3G; non-restraint 4.43 ± 1.35 %; repeated restraint 23.27 ± 3.18 %; t = 5.33, df = 143, p < 0.0001, unpaired t test). Therefore, repeated restraint not only facilitated fear conditioning but also disrupted acquisition of fear extinction in adolescent rats.
This effect of repeated stress on conditioned fear does not appear to be due to general effects of repeated restraint on baseline freezing, as there was no significant difference in freezing during habituation (Fig. 3H; non-restraint 1.58 ± 0.60 %, n = 14 rats; repeated restraint 4.26 ± 1.84 %, n = 15 rats; t = 1.34, df = 27, p = 0.19, unpaired t test). Furthermore, there appeared to be minimal contribution of contextual associations to the freezing measure, as there was no significant difference in freezing during habituation between the conditioning and testing days in the non-restraint group (conditioning 4.41 ± 1.52 %; testing 1.58 ± 0.60 %, n = 14 rats; t = 1.69, df = 13, p = 0.11, paired t test) or repeated restraint group (conditioning 3.50 ± 1.53 %; testing 4.26 ± 1.84 %, n = 15 rats; t = 0.40, df = 14, p = 0.70, paired t test).
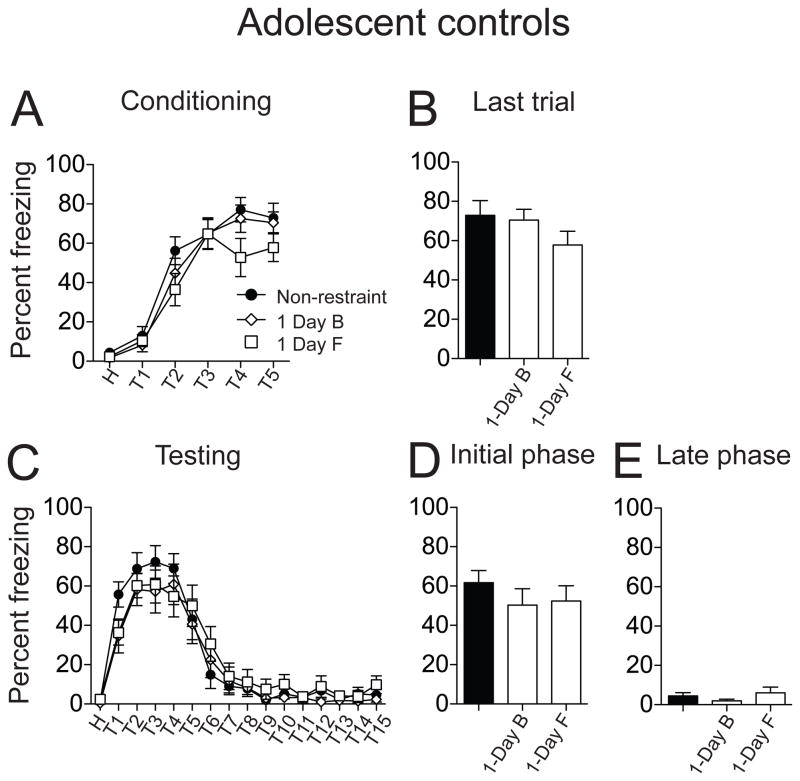
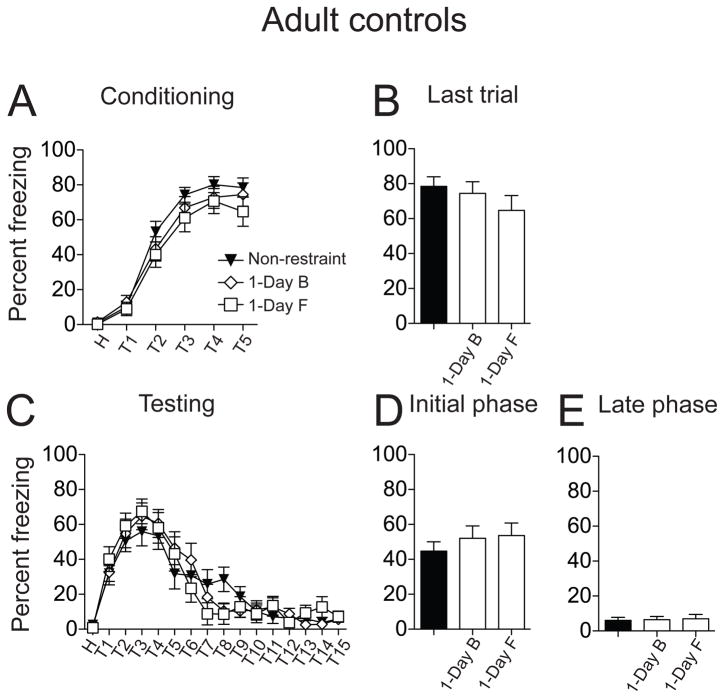
To test whether a single restraint is adequate, and could account for the effects of repeated restraint, fear conditioning was also performed in two 1-Day control groups (F: restraint on first day, handled remaining days, and B: restraint on last day, handled on preceding days, see Methods). During conditioning, there was a significant difference in the acquisition of fear conditioning among the 1-Day control and non-restraint groups (Fig. 4A; F(2,228) = 4.36, p = 0.01, non-restraint n=14 rats, 1-Day B n = 13 rats, 1-Day F n = 14 rats, significant main effect of treatment, two-way repeated measures ANOVA). However, at the last conditioning trial, animals in all three groups exhibited comparable freezing (Fig. 4B; non-restraint 72.87 ± 7.47 %; 1-Day B 70.47 ± 5.52 %; 1-Day F 57.79 ± 7.04 %; F(2,38) = 1.45, p = 0.25, one-way ANOVA). On testing day, 1-Day restraint rats displayed the same conditioned freezing pattern as non-restraint controls: robust initial conditioned freezing followed by a gradual reduction in the freezing response over the course of the 15 trials (Fig. 4C; F(15,608) = 47.01, p < 0.0001, significant main effect of trials, two-way repeated measures ANOVA). Single restraint did not significantly impact conditioned freezing during the testing day (Fig. 4C; F(2,608) = 1.95, p = 0.14, no significant effect of treatment, two-way repeated measures ANOVA). Further analysis also did not find a significant difference among non-restraint and 1-Day restraint groups in conditioned freezing during the initial testing phase (Fig. 4D; non-restraint 61.75 ± 3.81 %, n = 14 rats; 1-Day B 50.27 ± 4.36 %, n = 13 rats; 1-Day F 52.40 ± 3.97 %, n = 14 rats; F(2,202) = 2.29, p = 0.10, one-way ANOVA) or or the late phase (Fig. 4E; non-restraint 4.43 ± 1.35 %; 1-Day B 1.95 ± 0.73 %; 1-Day F 6.06 ± 1.62 %; F(2,202) = 2.44, p = 0.09, one-way ANOVA). Therefore, a single restraint did not significantly impact fear conditioning in adolescent rats, and is unlikely to be the cause of the differences between non-restraint and repeated restraint groups.
Figure 4. Single restraint did not significantly impact fear conditioning in adolescent rats.
Two single restraint control groups were examined, a restraint on the first day followed by handling on the remaining days (1-Day F), and handling on the first days followed by restraint on the last day (1-Day B). (A) There was significant difference in acquisition of fear conditioning between non-restraint and 1-Day control groups. (B) There was no significant difference in the freezing response between non-restraint and two 1-Day control groups on the last conditioning trial. (C) There was no significant effect of a single restraint on conditioned freezing during the testing day when compared to the non-restraint group. (D) Single restraint did not significant impact conditioned freezing during the initial testing phase compared to non-restraint group. (E) Single restraint did not significantly impact freezing during the late testing phase compared to non-restraint group. Black represents non-restraint controls, white represents single restraint groups.
3.3 Repeated restraint enhances conditioned freezing in adult rats
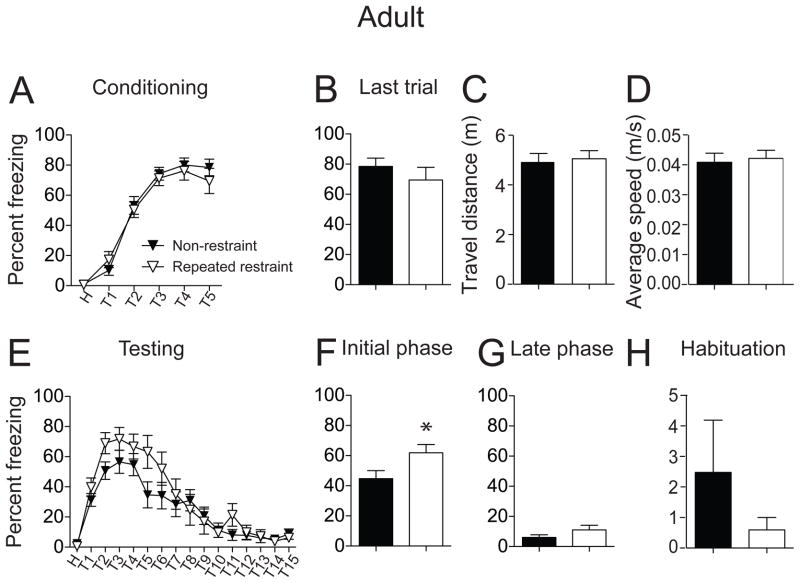
In this study, the mean footshock intensity that induced forepaw withdrawal in control rats was not significantly different compared to stressed rats (adult non-restraint 0.35 ± 0.02 Hz, n = 15 rats; repeated restraint 0.36 ± 0.03 Hz, n = 14 rats; t = 0.31, df = 27, p = 0.76, unpaired t test). Similar to adolescents, all adult rats acquired conditioned freezing, displayed as an increase of freezing over the progression of 5 conditioning trials (Fig. 5A; F(5,162) = 90.01, p < 0.0001, non-restraint n = 15 rats, repeated restraint n = 14 rats, significant main effect of trials, two-way repeated measures ANOVA). Repeated restraint did not significantly impact acquisition of fear conditioning in adult rats (Fig. 5A; F(1,162) = 0.48, p = 0.49, no significant effect of treatment, two-way repeated measures ANOVA). At the last conditioning trial, rats displayed a similar freezing response (Fig. 5B; non-restraint 78.44 ± 5.52 %; repeated restraint 69.48 ± 8.37 %; t = 0.91, df = 27, p = 0.37, unpaired t test). In addition, there was no significant difference in the total travel distance (Fig. 5C; non-restraint 4.90 ± 0.37 m, n = 15 rats; repeated restraint 5.05 ± 0.33 m, n = 14 rats; t = 0.31, df = 27, p = 0.76, unpaired t test) or average speed (Fig. 5D; non-restraint 0.04 ± 0.003 m/s; repeated restraint 0.04 ± 0.003 m/s; t = 0.31, df = 27, p = 0.76, unpaired t test) during habituation between non-restraint and repeated restraint groups, indicating that repeated restraint did not significantly impact locomotion.
Figure 5. Repeated restraint enhanced conditioned freezing in adult rats with no effect on extinction acquisition.
(A) Repeated restraint did not significantly impact the acquisition of fear conditioning compared to non-restraint controls. (B) There was no significant difference in the freezing response between non-restraint rats and repeated restraint rats during the last conditioning trial. (C) There was no significant difference between non-restraint rats and repeated restraint rats in total travel distance as well as average speed (D), indicating no impairment of locomotion after repeated restraint. (E) When tested on the next day in a novel chamber, repeated restraint and non-restraint groups exhibited initial robust freezing responses followed by gradual reduction of freezing. (F) Repeated restraint rats displayed significantly higher freezing during the initial testing phase compared to non-restraint rats. (G) No significant difference was observed in the late phase of testing between non-restraint and repeated restraint groups. (H) Rats from both groups displayed similar amounts of activity during habituation. Black represents non-restraint controls, white represents repeated restraint groups. *p < 0.05 compared between non-restraint and repeated restraint groups.
Conditioned freezing measured after 24 hr displayed an overall similar pattern in adult non-restraint and repeated restraint groups, with an initial robust freezing in response to the tone followed by a gradual reduction of freezing over the course of 15 repeated tone presentation trials (Fig. 5E; F(15,432) = 23.01, p < 0.0001, non-restraint n = 15 rats, repeated restraint n = 14 rats, significant main effect of trials, two-way repeated measures ANOVA). However, repeated restraint rats exhibited higher conditioned freezing compared to non-restraint rats (Fig. 5E; F(1,432) = 16.44, p < 0.0001, significant main effect of treatment, two-way repeated measures ANOVA). Upon further analysis, it was found that repeated restraint rats displayed greater conditioned freezing during the initial testing phase (Fig. 5F; non-restraint 44.70 ± 3.37 %, n = 15 rats; repeated restraint 61.88 ± 3.59 %, n = 14 rats; t = 3.49, df = 143, p = 0.0006, unpaired t test). However, unlike adolescents, adult repeated restraint rats did not display greater conditioned freezing during the late phase of extinction testing (Fig. 5G; non-restraint 5.81 ± 1.18 %; repeated restraint 10.96 ± 2.07%; t = 1.93, df = 143, p = 0.06, unpaired t test). Therefore, repeated restraint facilitated conditioned freezing but did not significantly impact acquisition of fear extinction in adult rats.
This effect of repeated restraint on conditioned freezing does not appear to be due to general effects of repeated restraint on baseline freezing, as there was no significant difference between groups in freezing during habituation (Fig. 5H; non-restraint 2.48 ± 1.71 %, n = 15 rats; repeated restraint 0.60 ± 0.41 %, n = 14 rats; t = 1.04, df = 27, p = 0.31, unpaired t test). In addition, there was no significant difference in freezing during habituation when the same treatment group was compared between conditioning day and testing day (non-restraint conditioning: 1.03 ± 0.57 %, testing: 2.48 ± 1.71 %, n = 15 rats; t = 0.76, df = 14, p = 0.46; repeated restraint conditioning: 0.63 ± 0.43 %, testing: 0.60 ± 0.41 %, n = 14 rats; t = 0.06, df = 13, p = 0.97, paired t test), consistent with no contribution of context to the freezing measure.
To examine whether a single restraint could account for the effects of repeated restraint in adult rats, fear conditioning was also performed with two 1-Day control groups (group B and group F, as above). There was a significant difference in the acquisition of fear conditioning among the 1-Day control and non-restraint groups (Fig. 6A; F(2,252) = 3.56, p = 0.03, significant main effect of treatment; F(5,252) = 101.71, p < 0.0001, non-restraint n = 15 rats, 1-Day B n = 15 rats, 1-Day F n = 15 rats, significant main effect of trials, two-way repeated measures ANOVA). However, on the last conditioning trial, there was no significant difference in the freezing response among these 3 groups (Fig. 6B non-restraint 78.44 ± 5.52 %; 1-Day B 74.44 ± 6.68 %; 1-Day F 64.74 ± 8.44 %; F(2,42) = 1.02, p = 0.37, one-way ANOVA). On the testing day there was also no significant difference between these groups in conditioned freezing measured 24 hr after acquisition (Fig. 6C; F(2,672) = 0.09, p = 0.92, no significant effect of treatment, two-way repeated measures ANOVA). Furthermore, there were no significant differences between these groups when examining only the initial conditioned freezing during testing (Fig. 6D; non-restraint 44.70 ± 3.37 %, n = 15 rats; 1-Day B 52.08 ± 3.74 %, n = 15 rats; 1-Day F 53.60 ± 3.71 %, n = 15 rats; F(2,222) = 1.74, p = 0.18, one-way ANOVA) or late phase of extinction testing (Fig. 6E; non-restraint 5.81 ± 1.18 %; 1-Day B 6.47 ± 1.36 %; 1-Day F 9.29 ± 1.95 %; F(2,222) = 1.46, p = 0.24, one-way ANOVA). Therefore, a single restraint did not significantly impact fear conditioning in adolescent rats, and is unlikely to account for the effects of repeated restraint.
Figure 6. Single restraint did not significantly impact fear conditioning in adult rats.
Two single restraint groups (1-Day B and 1-Day F, as above) were compared to non-restraint controls. (A) There was significant difference in acquisition of fear conditioning among the 3 groups. (B) There was no significant difference in the freezing response on the last conditioning trial among 3 groups. (C) There was no significant effect of a single restraint on conditioned freezing tested after 24 hr, when compared to the non-restraint group. (D) There was no significant difference in conditioned freezing response during the initial testing phase on the following day among 3 groups. E) There was no significant difference in freezing during the late phase of testing when the 1-Day restraint groups and the non-restraint group were compared. Black represents non-restraint controls, white represents single restraint groups.
3.4 Adult non-restraint rats display less conditioned freezing compared to adolescent rats
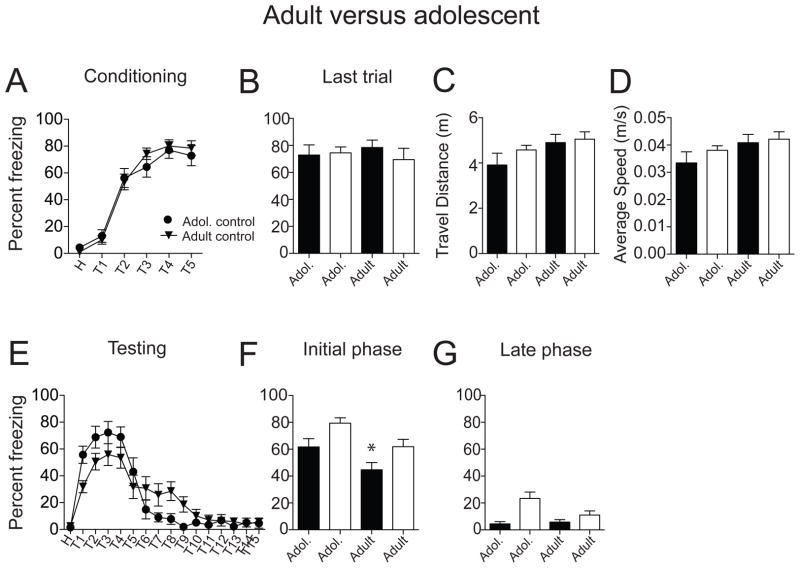
There was no difference between adolescent and adult non-restraint rats in acquisition of fear conditioning (Fig. 7A; F(1,162) = 0.26, p = 0.61, adolescent n = 14 rats, adult n = 15 rats, no significant effect of age, two-way repeated measures ANOVA). At the last conditioning trial, there was no significant difference in the freezing response between adolescent and adult non-restraint rats (Fig. 7B; adolescent: 72.87 ± 7.47 %; adult: 78.44 ± 5.52 %; t = 0.61, df = 27, p = 0.55, unpaired t test). During testing of conditioned freezing after 24 hr, there was no significant effect of age on conditioned freezing overall (Fig. 7E; F(1,432) = 0.01, p = 0.94, adolescent n = 14 rats, adult n = 15 rats, no significant effect of age, two-way repeated measures ANOVA). However, there was noticeably less conditioned freezing in adults during early the early phase of extinction testing, and noticeably slower reduction of freezing over the early course of acquisition of extinction (Fig. 7E; F(15,432) = 2.93, p = 0.0002, significant interaction between age and treatment, two-way repeated measures ANOVA). Adult rats displayed less conditioned freezing than adolescent rats during the initial phase of testing (Fig. 7F; adolescent 61.75 ± 3.81 %, n = 14 rats; adult 44.70 ± 3.37 %, n = 15 rats; t = 3.36, df = 143, p = 0.001, unpaired t test). However, there was no significant difference between these adult and adolescent non-restraint rats in conditioned freezing at the late phase of extinction testing (Fig. 7G; adolescent 4.43 ± 1.35 %; adult 5.81 ± 1.18%; t = 0.77, df = 143, p = 0.44, unpaired t test). Moreover, there was no differences in the total travel distance (Fig. 7C; adolescent 3.91 ± 0.52 m, n = 14 rats; adult 4.90 ± 0.37 m, n = 15 rats; t = 1.57, df = 27, p = 0.12, unpaired t test) and average speed (Fig. 7D; adolescent 0.03 ± 0.004 m/s; adult 0.04 ± 0.003 m/s; t = 1.47, df = 27, p = 0.15, unpaired t test) between adolescent and adult rats during habituation, consistent with similar locomotor activity in the two age groups.
Figure 7. Repeated restraint impaired acquisition of fear extinction in adolescent rats.
(A) Age did not significantly impact the acquisition of fear conditioning under non-restraint conditions. Adolescent and adult rats displayed no significant differences in freezing during conditioning. (B) There was no significant difference in the freezing response between adolescent and adult rats on the last conditioning trial. (C) There was no significant difference between adolescent and adult rats in total travel distance as well as average speed (D), indicating no difference in locomotor activity. (E) During testing after 24 hr, adult rats exhibited less conditioned freezing but slower reduction than adolescent rats. (F) Adult rats exhibited significantly less conditioned freezing during the initial testing phase compared to adolescent rats under non-restraint conditions. (G) There was no significant difference in conditioned freezing responses during the late phase of testing between adolescent and adult non-restraint groups. Black represents non-restraint controls, white represents repeated restraint groups. *p < 0.05 compared between adolescent and adult non-restraint groups.
As described above, repeated stress increased conditioned freezing during the initial testing phase in adults (Fig. 5E) and adolescents (Fig. 3E), whereas the late phase of extinction testing was only impaired in adolescents (Fig. 3E). It is possible that this age-dependency of the effects of stress on acquisition of extinction is caused by the smaller conditioned freezing response displayed by adults in general (Fig. 7E). Perhaps if higher levels of conditioned freezing in adults were achieved, repeated restraint stress would also be demonstrated to impair extinction in adults.
3.5 Increased footshock intensity increases the freezing responses in adult rats but does unmask stress-induced impairment of extinction acquisition
To test whether modification of the conditioning procedure to increase conditioned freezing would unmask impaired acquisition of extinction after stress in adult rats, fear conditioning was performed in a separate non-restraint group and repeated restraint group using a footshock intensity that was 0.1 mA more than the threshold footshock intensity for individual animals.
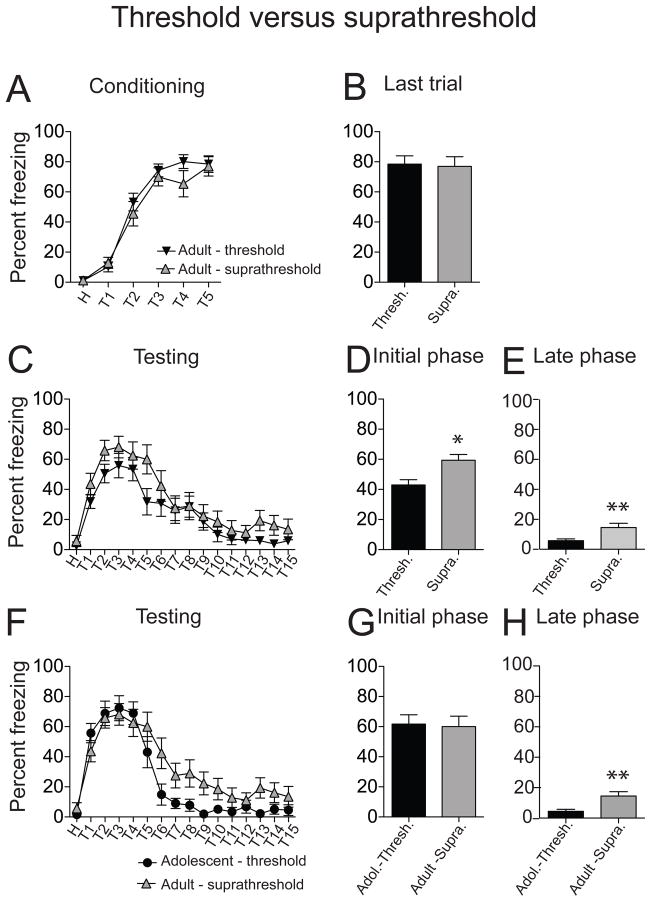
Increased footshock intensity did not significantly increase freezing during fear conditioning in non-restraint rats, compared to the lower footshock intensity (Fig. 8A; F(5,162) = 78.92, p < 0.0001, significant main effect of trials; F(1,162) = 1.90, p = 0.17, no significant effect of footshock intensity; threshold n = 15 rats, suprathreshold n = 14 rats, two-way repeated measures ANOVA), and freezing in the last conditioning trial was similar (Fig. 8B; threshold 78.44 ± 5.52 %; suprathreshold 76.96 ± 6.45 %; t = 0.18, df = 27, p = 0.86, unpaired t test). When conditioned fear during testing was compared, increased footshock intensity (suprathreshold) caused a greater conditioned freezing response compared to the lower footshock intensity (threshold) (Fig. 8C; F(1,432) = 15.64, p < 0.0001, threshold n = 15 rats, suprathreshold n = 14 rats, significant main effect of footshock intensity, two-way repeated measures ANOVA), with significantly greater conditioned freezing during the initial testing phase (Fig. 8D; threshold 44.70 ± 3.37 %; suprathreshold 60.01 ± 3.64 %; t = 3.09, df = 143, p = 0.002, unpaired t test). The level of conditioned freezing achieved during the initial testing phase by increasing the footshock intensity in adults was now equivalent to the adolescent group (Fig. 8F.8G; adolescent threshold 61.75 ± 3.81 %, n = 14 rats; adult suprathreshold 60.01 ± 3.64 %, n = 14 rats; t = 0.33, df = 138, p = 0.74, unpaired t test).
Figure 8. Increased footshock intensity increased conditioned freezing in adult rats.
Footshock intensity was increased for conditioning of adult rats to produce greater conditioned freezing levels during testing that were equivalent to adolescent conditioned freezing (0.1 mA above threshold intensity). (A) A small increase (0.1 mA) of footshock intensity did not have a significant effect on acquisition of fear conditioning. (B) There was no significant effect of the small increase of footshock intensity on freezing during the last conditioning trial. (C) Use of suprathreshold footshock intensity caused an increase of conditioned freezing in adult rats measured during fear testing, compared to threshold footshock intensity. (D) There was significantly greater conditioned freezing during the initial testing phase and late testing phase (E) when a suprathreshold footshock was used, compared to a threshold footshock intensity. (F) Suprathreshold footshock intensity led to conditioned freezing in adult rats that was similar to the conditioned freezing of adolescent rats at the threshold intensity. (G) There was no significant difference in the initial conditioned freezing during testing between adult rats conditioned with the suprathreshold intensity and adolescent rats conditioned with the threshold intensity. (H) However, there was greater conditioned freezing during the later phase in adult rats conditioned with suprathreshold footshock compared to adolescent rats conditioned with threshold footshock intensity. Black represents groups using threshold footshock intensity (adolescents or adults), grey represents groups using suprathreshold footshock intensity. *p < 0.05, **p < 0.01 compared between threshold and suprathreshold non-restraint groups.
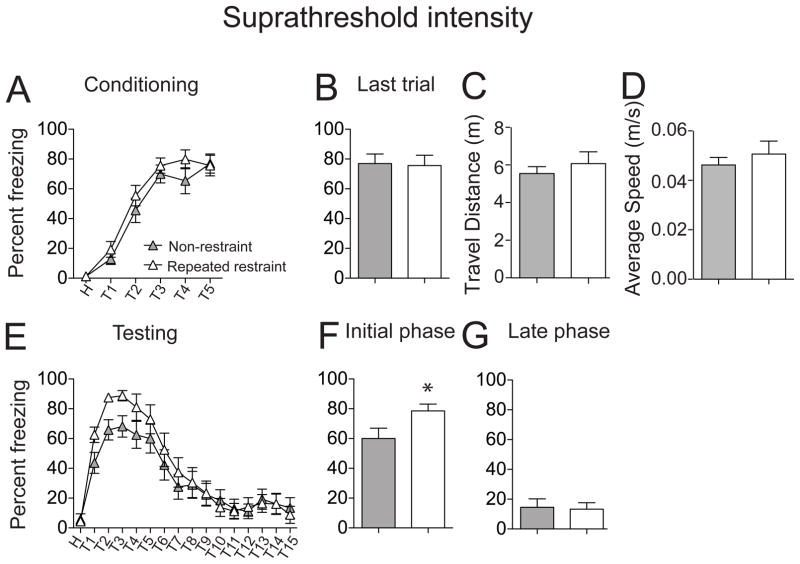
Similar to threshold footshock groups, repeated restraint did not significantly impact acquisition of fear conditioning in suprathreshold groups (Fig. 9A; F(1,156) = 2.91, p = 0.09, non-restraint suprathreshold n = 14 rats, repeated restraint suprathreshold n = 14 rats, no significant effect of treatment, two-way repeated measures ANOVA). There was no significant difference in freezing behavior on the last trial (Fig. 9B; non-restraint 76.96 ± 6.45 %; repeated restraint 75.64 ± 6.90%; t = 0.14, df = 26, p = 0.89, unpaired t test). In addition, there was no significant difference in the total distance travelled (Fig. 9C; non-restraint 5.54 ± 0.37 m, n = 14 rats; repeated restraint 6.07 ± 0.63 m, n = 14 rats; t = 0.73, df = 26, p = 0.47, unpaired t test) as well as average speed (Fig. 9D; non-restraint 0.05 ± 0.003 m/s; repeated restraint 0.05 ± 0.005 m/s, t = 0.73, df = 26, p = 0.47, unpaired t test) between two groups. On the testing day, repeated restraint stress still increased conditioned fear on the testing day at the higher footshock intensity (Fig. 9E; F(1,416) = 6.11, p = 0.01, significant main effect of treatment, two-way repeated measures of ANOVA), and specifically increased conditioned freezing during the initial phases of testing (Fig. 9F; non-restraint 60.01 ± 3.64 %, n = 14 rats; repeated restraint 78.56 ± 3.10 %, n = 14 rats; t = 3.88, df = 138, p = 0.0002, unpaired t test). However, despite the enhancing effects of the increased footshock intensity, repeated restraint stress still did not lead to an impairment of acquisition of extinction (Fig. 9G; non-restraint 14.57 ± 2.78 %; repeated restraint 13.32 ± 2.66 %; t = 0.33, df = 138, p = 0.75, unpaired t test) in adult rats.
Figure 9. Suprathreshold footshock did not uncover impaired acquisition of fear extinction in adults.
(A) Repeated restraint did not significantly increase freezing during acquisition of conditioning in adult rats, using a suprathreshold footshock intensity. (B) The amount of freezing during the last conditioning trial was similar in repeated restraint and non-restraint groups. (C) There was no significant difference in total travel distance or average speed (D) between repeated restraint and non-restraint groups. (E) Repeated restraint increased conditioned freezing when tested after 24 hr. (F) Conditioned freezing was significantly greater during the initial testing trials. (G) There was no significant difference in conditioned freezing between groups at the later phase of testing. Grey represents non-restraint group using suprathreshold footshock intensity, white represents repeated restraint group using suprathreshold footshock intensity. *p < 0.05 compared between non-restraint and repeated groups.
4 Discussion
The present study demonstrated an age-dependent effect of repeated restraint on BLA-dependent cued fear conditioning. Repeated restraint facilitated conditioned freezing but disrupted the acquisition of extinction of fear conditioning in adolescent rats. However, in adult rats, repeated restraint facilitated conditioned freezing with no effect on the acquisition of extinction. These findings demonstrate an interaction between age and stress in shaping amygdala-dependent affective behaviors and provide insight into potential differences in the effects of stress on emotion at different ages.
4.1 Amgydala and fear conditioning
Fear conditioning is widely used to study the neurobiological mechanisms and possible treatment of affective disorders. A large body of evidence identifies the amygdala, especially the BLA, as a key structure involved in acquisition, expression and extinction of fear memory [29,36,37,38,39,40,41]. Similarly, in humans, amygdala damage leads to impaired fear conditioning [42] and during fear conditioning, amgydala activity is increased [43,44]. Those findings suggest that the changes in fear conditioning serves as an index of changes in amgydala function.
In the present study, to ensure equivalence of the fear conditioning protocol between groups, each rat experienced a determination of the footshock intensity that leads to forepaw withdrawal in that rat. This was performed immediately prior to fear conditioning (see Methods). Sensitization of fear behavior is a potential confound that needs to be balanced with the benefit of equalizing footshock across individual subjects. However, the footshock used in these experiments is quite mild compared to many other studies. Furthermore, because all rats were subjected to this same procedure, and there was no measured effect of repeated restraint on footshock sensitivity [17], the results of fear conditioning would still exhibit the impact of the repeated restraint on fear conditioning. Importantly, there is no evidence that a single threshold footshock would sensitize rats. It is predicted that low intensity stimuli will not induce sensitization [45], and it has been determined that either a single footshock of moderate intensity (0.6 mA), or multiple footshocks of low intensity (5 footshocks), do not lead to significant sensitization of behavior [46].
4.2 Effect of repeated restraint on conditioned freezing
From the current study one cannot determine whether the effects of stress on conditioned freezing are due to modification of consolidation, recall or expression. However, stress did not appear to impact acquisition of conditioning in this study. Previous studies have examined the effects of prepubertal stress on adult fear conditioning [e.g. 47–49]. The current study examined the effects of prepubertal stress on fear conditioning during the same time period, and contrasted this with effects of stress exposure in adult rats. Previous studies demonstrated that chronic stress enhances conditioned freezing [16,17,30]. Consistent with most studies, our results demonstrate that stress does not significantly impact fear acquisition in adolescent or adult rats. However, the effects of stress on fear expression were different across studies. When exposed to stress during adolescence and tested during adolescence, our data demonstrated enhanced conditioned freezing. Rodriguez and Sandi also reported similar results when a 3-day stress was applied [30]. However, others reported reduced conditioned freezing [48]. When exposed to stress during adulthood and tested as adults, studies demonstrated either enhanced [16,17, current study] or no changes [48,50] in conditioned freezing. In addition, when exposed to stress during adolescence and tested as adult, some reported no impact of stress on conditioned freezing [30,50], but others found enhanced conditioned freezing [49]. Variability in stressor type, stress timing, animal strain and fear conditioning procedures may account for these discrepancies. For example, in some studies fear expression was tested on the same day or 48 hours after acquisition and different stressors such as chronic social instability and chronic unpredictable stress were used [43,47].
Many factors could contribute to the effects of stress on fear conditioning. Repeated restraint stress leads to increased number of spines and elongation of dendrites [14,51] and hyperactivity of projection neurons in the BLA [12,13]. The morphological and neurophysiological changes might have significant behavioral consequences. Interestingly, drugs that reduce the excitability of BLA neurons reversed the effects of repeated restraint on fear conditioning in adult rats [17]. Further supporting the electrophysiological changes as a substrate for the behavioral changes, the same repeated stressor leads to age-dependent increases of neuronal activity [27] that parallel the age-dependent increases in conditioned freezing demonstrated here.
Abnormal activation of the corticotrophin releasing factor (CRF) system may contribute to stress-induced enhanced fear conditioning. The amygdala expresses high level of CRF receptors as well as their mRNA [52,53]. CRF receptor activation increases the excitability of BLA projection neurons [54]. In addition, repeated activation of CRF receptors in the BLA leads to the reduction of inhibition with the BLA [55]. Modification of the CRF system during stress may contribute to the electrophysiological and behavioral manifestations. This is further supported by anxiogenic effect of CRF in both human and animal studies, and that many affective disorders, such as depression and posttraumatic stress disorder, are accompanied by increased concentration and responsiveness of CRF [56–58]. Other neurotransmitter systems, such as dopamine, norepinephrine and serotonin are sensitive to the effects of stress, and may contribute to the effects of stress on amygdala function [59–63].
4.3 Effect of repeated restraint on acquisition of extinction of fear conditioning
Impaired extinction of conditioned fear may play a significant role in psychiatric disorders. The current study indicates that repeated restraint leads to resistance to within session acquisition of fear extinction in adolescent rats, but not adult rats. This does not appear to be due to age differences in the amount of conditioned fear displayed, since increased conditioned freezing (via suprathreshold footshock intensity during conditioning) still did not unmask an effect of stress on extinction in adult rats. In addition, it is evident that the age-dependent effects of stress on acquisition of extinction are unlikely due to differences in acquisition of conditioning, as no difference was found in freezing during acquisition between the two age groups. A previous study did not find an effect of chronic stress on extinction in adolescent rats [48]. However, in that study, chronic social instability stress was used, and was designed to measure only initial extinction during retrieval and perhaps a component of between-session extinction. Similar to the current study, several labs did not find an effect of chronic stress on fear extinction in adult rats [50,64] using 7-days repeated restraint or 21-day unpredictable stressors.
Extinction of fear relies upon integrity of the connection between the medial prefrontal cortex (mPFC) and the amygdala [65,66]. Amygdala and PFC are still not mature and undergo developmental changes during adolescence [67–70]. Stressful experience may reshape their structure and function and therefore cause adverse behavioral consequences [71–74]. This is also supported by the findings that amygdala and mPFC pathway is still developing until early adulthood, and therefore likely vulnerable to the effects of stress [75]. The impaired acquisition of extinction in adolescent rats after repeated restraint might be due to disruption of the mPFC-amygdala interaction.
Another potential explanation for the higher freezing response in repeated restraint adolescent during later phases of the extinction testing is that it could be due to greater conditioning or a carryover from greater freezing response in the initial trials. In fact, there is slower acquisition of extinction in adult rats when freezing during early phases of extinction is increased by increasing footshock intensity (suprathreshold group) compared to the threshold footshock intensity group. However, if the apparent deficit in acquisition of extinction is due to greater conditioning, several features would be expected: 1) greater freezing in the 5th pairing of conditioning, 2) greater freezing during the first few trials of the extinction phase, and 3) no differences in extinction when the responses during acquisition and initial testing are equivalent. In adolescent and adult rats, stress caused greater freezing during the first few trials of extinction. However, there were no significant differences between in the freezing observed at the 5th pairing of conditioning, and when acquisition and initial testing were equivalent between control and stress groups (suprathreshold footshock), there was still no significant difference in extinction in adults. Secondly, even when freezing during the initial extinction phase was increased with supratheshold footshock intensity, there was still no effect of repeated stress on the acquisition of extinction in adult rats. This argues that the impaired acquisition of extinction after repeated stress is unique to adolescent rats compared to adult rats, and is not due to increased conditioning or a carryover from increased freezing during early extinction.
4.4 Adolescent and adult rats display differences in conditioned freezing
In this study, adolescent rats displayed significantly more conditioned freezing during the initial testing phase of extinction compared to adult rats. This was observed despite similar acquisition of fear conditioning in adolescent and adult rats, as judged by the freezing response during conditioning. Similar results were also reported in mice [76,77]. Differences in freezing across age have been observed in other studies. For example, Mckinzie et al (1998) found that preweaning (PND 17) rats show less conditioned freezing compared to adult rats, though a much higher footshock intensity (1.0 mA) was used. Interestingly, when a 0.5 mA footshock intensity was used, only adult rats display conditioned freezing if tested 5 min after acquisition [78]. In addition, PND 24 rats display greater eyeblink conditioning compared to PND 17 rats [79]. The age-dependency in conditioned fear is also seen in contextual conditioning paradigms [e.g. 80,81]. In addition, adult rats display contextual conditioning only when footshock and tone were unpaired while adolescent rats displayed contextual freezing whether or not footshock and tone were paired [82]. Age-related differences also exist in the manner of fear expression [83,84], acquisition of conditioning [79], neural circuitries involved in expression of conditioned fear [85] and extinction [86]. These findings all suggest that the expression of learned fear is age-dependent. Developmental changes may contribute to differences in fear conditioning demonstrated in this paper. Differences include thalamic and cortical afferents to BLA [87] and the relative immaturity of inhibitory systems within the BLA in adolescent rats [88,89]. Age-dependent differences in conditioned freezing are unlikely to be due to general differences in locomotion, as adolescent and adult rats displayed similar total travel distance and average speed during the habituation periods in our study. Furthermore, these differences in conditioned freezing in adolescent and adult rats do not explain the age-dependent effects of stress on acquisition of extinction. When the conditioning procedure was modified to produce similar levels of conditioned freezing in adolescent and rats, stress still did not cause impairments of acquisition of extinction in adult rats.
5. Conclusions and implications
Repeated restraint has age-dependent effects on both amgydala physiology and amygdala-dependent affective behavior. Combined with age-dependent effects on the mPFC, these could result in distinct effects on fear extinction across age. Stress has a significant role in anxiety and depressive disorders. These disorders involve disruptions in PFC and amygdala function. Age-dependent effects of stress on these neural circuits, and their associated behaviors, may underlie age-related differences in vulnerability to psychiatric disorders, or the symptomology of psychiatric disorders. Our results provide further support for the hypothesis that psychiatric disorders that have a prominent sensitivity to stress may involve a different pathophysiology in adolescents and adults. It might also be expected that therapeutic targeting of the acquisition of extinction may lead to different results in adolescents and adults.
Highlights.
Repeated restraint enhanced conditioned freezing in both adolescent and adult rats
Repeated restraint impaired extinction of fear conditioning in adolescent rats
Repeated restraint has age-dependent effects on auditory fear conditioning
Acknowledgments
The author would like to thank Drs. Anthony West, Beth Stutzmann, Janice Urban and Michela Marinelli for useful discussion. Grant support provided by the U.S. National Institutes of Health (MH084970) and the Brain Research Foundation.
Abbreviations
- ANOVA
Analysis of Variance
- BLA
basolateral amygdala
- CRF
corticotropin-releasing factor
- EPM
elevated plus maze
- HPA axis
hypothalamic-pituitary-adrenal axis
- mPFC
medial prefrontal cortex
- PND
postnatal day
- PTSD
post-traumatic stress disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 2.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–14. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 3.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 4.Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–14. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 5.Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala: a Functional Analysis. Oxford UP; 2000. pp. 213–87. [Google Scholar]
- 6.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 7.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–90. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 9.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 10.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–6. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–36. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–9. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 17.Atchley D, Hankosky ER, Gasparotto K, Rosenkranz JA. Pharmacological enhancement of calcium-activated potassium channel function reduces the effects of repeated stress on fear memory. Behav Brain Res. 2012;232:37–43. doi: 10.1016/j.bbr.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann NY Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 19.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villarreal G, King CY. Brain imaging in posttraumatic stress disorder. Semin Clin Neuropsychiatry. 2001;6:131–45. doi: 10.1053/scnp.2001.21840. [DOI] [PubMed] [Google Scholar]
- 21.Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–65. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- 22.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 23.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 24.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992;56:619–25. doi: 10.1159/000126284. [DOI] [PubMed] [Google Scholar]
- 26.Zanato VF, Martins MP, Anselmo-Franci JA, Petenusci SO, Lamano-Carvalho TL. Sexual development of male Wistar rats. Braz J Med Biol Res. 1994;27:1273–80. [PubMed] [Google Scholar]
- 27.Zhang W, Rosenkranz AJ. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–74. doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–55. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–31. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 30.Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007:71203. doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22:631–4. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- 32.Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–22. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- 33.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:570–7. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–94. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynn DA, Brown GR. The ontogeny of anxiety-like behavior in rats from adolescence to adulthood. Dev Psychobiol. 2010;52:731–9. doi: 10.1002/dev.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 37.Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–74. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- 38.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–91. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 39.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–60. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 40.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Prog Brain Res. 2008;167:79–97. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- 42.Labar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–55. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human Pavlovian fear conditioning: patterns of activation as a function of learning. Neuroreport. 1999;10:3665–70. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- 44.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 45.Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–50. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- 46.Davis M. Sensitization of the acoustic startle reflex by footshock. Behav Neurosci. 1989;103:495–503. [PubMed] [Google Scholar]
- 47.Kendig MD, Bowen MT, Kemp AH, McGregor IS. Predatory threat induces huddling in adolescent rats and residual changes in early adulthood suggestive of increased resilience. Behav Brain Res. 2011;225:405–14. doi: 10.1016/j.bbr.2011.07.058. [DOI] [PubMed] [Google Scholar]
- 48.Morrissey MD, Mathews IZ, McCormick CM. Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiol Learn Mem. 2011;95:46–56. doi: 10.1016/j.nlm.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Yee N, Schwarting RK, Fuchs E, Wöhr M. Juvenile stress potentiates aversive 22-kHz ultrasonic vocalizations and freezing during auditory fear conditioning in adult male rats. Stress. 2012;15:533–44. doi: 10.3109/10253890.2011.646348. [DOI] [PubMed] [Google Scholar]
- 50.Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem. 2008;89:560–6. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–93. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1, receptor mRNA Expression. J Neurosc. 1995;15:6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immuno-cytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–23. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rainnie DG, Fernhout BJ, Shinnick-Gallagher P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. J Pharmacol Exp Ther. 1992;263:846–58. [PubMed] [Google Scholar]
- 55.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 57.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 58.Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 59.Jedema HP, Sved AF, Zigmond MJ, Finlay JM. Sensitization of norepinephrine release in medial prefrontal cortex: effect of different chronic stress protocols. Brain Res. 1999;830:211–7. doi: 10.1016/s0006-8993(99)01369-4. [DOI] [PubMed] [Google Scholar]
- 60.Bland ST, Twining C, Watkins LR, Maier SF. Stressor controllability modulates stress-induced serotonin but not dopamine efflux in the nucleus accumbens shell. Synapse. 2003;49:206–8. doi: 10.1002/syn.10229. [DOI] [PubMed] [Google Scholar]
- 61.Buffalari DM, Grace AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol. 2009;12:95–107. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasheed N, Ahmad A, Pandey CP, Chaturvedi RK, Lohani M, Palit G. Differential response of central dopaminergic system in acute and chronic unpredictable stress models in rats. Neurochem Res. 2010;35:22–32. doi: 10.1007/s11064-009-0026-5. [DOI] [PubMed] [Google Scholar]
- 63.Yohe LR, Suzuki H, Lucas LR. Aggression is suppressed by acute stress but induced by chronic stress: immobilization effects on aggression, hormones, and cortical 5-HT(1B)/striatal dopamine D(2) receptor density. Cogn Affect Behav Neurosci. 2012;12:446–59. doi: 10.3758/s13415-012-0095-9. [DOI] [PubMed] [Google Scholar]
- 64.Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–8. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 68.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–8. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 70.Chareyron LJ, Lavenex PB, Lavenex P. Postnatal development of the amygdala: A stereological study in rats. J Comp Neurol. 2012;520:3745–63. doi: 10.1002/cne.23132. [DOI] [PubMed] [Google Scholar]
- 71.Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, Braun K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proc Natl Acad Sci USA. 2003;100:16137–42. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silva-Gómez AB, Rojas D, Juárez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–36. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- 73.Teicher HM. Am Acad Child Adolesc Psychiary. Vol. 25. Toronto, Ontario: 2005. Childhood abuse and regional brain development: evidence for sensitive periods; p. 78. [Google Scholar]
- 74.Jacobson-Pick S, Elkobi A, Vander S, Rosenblum K, Richter-Levin G. Juvenile stress-induced alteration of maturation of the GABAA receptor alpha subunit in the rat. Int J Neuropsychopharmacol. 2008;11:891–903. doi: 10.1017/S1461145708008559. [DOI] [PubMed] [Google Scholar]
- 75.Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518:2693–709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–5. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear learning across development in both mouse and human. Proc Natl Acd Sci USD. 2012;109:16318–23. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McKinzie DL, Chen WJ, Spear NE. Ontogenetic differences in the expression of conditioned stimulus conditioning: effects of retention interval. Behav Neurosci. 1998;112:920–8. doi: 10.1037//0735-7044.112.4.920. [DOI] [PubMed] [Google Scholar]
- 79.Stanton ME, Freeman JH, Skelton RW. Eyeblink conditioning in the developing rat. Behav Neurosci. 1992;106:657–65. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- 80.Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107:887–91. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- 81.Mckinzie DL, Spear NE. Ontogenetic differences in conditioning to context and CS as a function of context saliency and CS-US interval. Learning & Behavior. 1995;23:304–313. [Google Scholar]
- 82.Esmorís-arranz FJ, Spear NE. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behav Presses. 2008;78:340–50. doi: 10.1016/j.beproc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barnet RC, Hunt PS. The expression of fear-potentiated startle during development: integration of learning and response systems. Behav Neurosci. 2006;120:861–72. doi: 10.1037/0735-7044.120.4.861. [DOI] [PubMed] [Google Scholar]
- 84.Yap CS, Richardson R. The ontogeny of fear-potentiated startle: effects of earlier-acquired fear memories. Behav Neurosci. 2007;121:1053–62. doi: 10.1037/0735-7044.121.5.1053. [DOI] [PubMed] [Google Scholar]
- 85.Li S, Kim JH, Richardson R. Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. Behav Neurosci. 2012;126:217–25. doi: 10.1037/a0027151. [DOI] [PubMed] [Google Scholar]
- 86.Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–8. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan BX, Ito W, Morozov A. Divergence between thalamic and cortical inputs to lateral amygdala during juvenile-adult transition in mice. Biol Psychiatry. 2009;66:964–71. doi: 10.1016/j.biopsych.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brummelte S, Witte V, Teuchert-Noodt G. Postnatal development of GABA and calbindin cells and fibers in the prefrontal cortex and basolateral amygdala of gerbils (Meriones unguiculatus) Int J Dev Neurosci. 2007;25:191–200. doi: 10.1016/j.ijdevneu.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Dávila JC, Olmos L, Legaz I, Medina L, Guirado S, Real MA. Dynamic patterns of colocalization of calbindin, parvalbumin and GABA in subpopulations of mouse basolateral amygdalar cells during development. J Chem Neuroanat. 2008;35:67–76. doi: 10.1016/j.jchemneu.2007.06.003. [DOI] [PubMed] [Google Scholar]