Abstract
Purpose
Alveolar soft part sarcoma (ASPS) is a rare, highly vascular tumor, for which no effective standard systemic treatment exists for patients with unresectable disease. Cediranib is a potent, oral small-molecule inhibitor of all three vascular endothelial growth factor receptors (VEGFRs).
Patients and Methods
We conducted a phase II trial of once-daily cediranib (30 mg) given in 28-day cycles for patients with metastatic, unresectable ASPS to determine the objective response rate (ORR). We also compared gene expression profiles in pre- and post-treatment tumor biopsies and evaluated the effect of cediranib on tumor proliferation and angiogenesis using positron emission tomography and dynamic contrast-enhanced magnetic resonance imaging.
Results
Of 46 patients enrolled, 43 were evaluable for response at the time of analysis. The ORR was 35%, with 15 of 43 patients achieving a partial response. Twenty-six patients (60%) had stable disease as the best response, with a disease control rate (partial response + stable disease) at 24 weeks of 84%. Microarray analysis with validation by quantitative real-time polymerase chain reaction on paired tumor biopsies from eight patients demonstrated downregulation of genes related to vasculogenesis.
Conclusion
In this largest prospective trial to date of systemic therapy for metastatic ASPS, we observed that cediranib has substantial single-agent activity, producing an ORR of 35% and a disease control rate of 84% at 24 weeks. On the basis of these results, an open-label, multicenter, randomized phase II registration trial is currently being conducted for patients with metastatic ASPS comparing cediranib with another VEGFR inhibitor, sunitinib.
INTRODUCTION
Alveolar soft part sarcoma (ASPS) is a rare, highly vascular tumor that predominantly affects adolescents and young adults; it accounts for less than 1% of soft tissue sarcomas.1 ASPS is an indolent disease but has a high frequency of metastases, usually to the lungs, brain, and bones. Median survival is reported to be 40 months, with a 5-year survival rate of 20% in patients with unresectable metastatic disease.2,3 Currently, radical surgery is the only known cure; standard cytotoxic chemotherapy regimens used for the treatment of soft tissue sarcomas are ineffective for treating ASPS.4
ASPS is associated with a characteristic unbalanced t(X,17)(p11;q25) translocation, resulting in the formation of the ASPL-TFE3 chimeric transcription factor, which is associated with enhanced MET-related signal transduction.5–7 ASPS is a vascular tumor as visualized by angiography.8 Gene expression profiling studies conducted on surgical samples of ASPS have revealed upregulation of several transcripts associated with angiogenesis, cell proliferation, metastasis, and myogenic differentiation.9,10
Cediranib (AZD2171) is an orally bioavailable, small-molecule inhibitor of all three vascular endothelial growth factor receptor (VEGFR-1, -2, and -3) tyrosine kinases, which mediate angiogenesis and lymphangiogenesis.11,12 Cediranib produced antitumor activity as a single agent in seven patients with metastatic ASPS during phase I and II trials13,14; four patients had a confirmed partial response (PR), and three patients had disease stabilization lasting longer than 200 days.14 On the basis of the vascularity of ASPS and preliminary evidence of therapeutic activity of cediranib, we initiated an open-label, single-arm, phase II trial of cediranib to evaluate the objective response rate (ORR) in patients with metastatic ASPS.
PATIENTS AND METHODS
Patients
Patients with pathologically confirmed metastatic ASPS not curable by surgery were eligible to participate. Patients were required to be ≥ 18 years of age; have an Eastern Cooperative Oncology Group performance status of 0 to 2; and have adequate bone marrow and organ function defined as absolute neutrophil count ≥ 1,500/μL, platelets ≥ 100,000/μL, total bilirubin ≤ 1.5× the upper limit of normal (ULN), ALT and AST less than 2.5× ULN, and creatinine less than 1.5× ULN. There were no restrictions with regard to the number of prior therapies allowed, including other antiangiogenic treatments. All prior therapy must have been completed ≤ 4 weeks before enrollment.
Patients were excluded if they had an uncontrolled intercurrent illness, including uncontrolled hypertension (defined as blood pressure > 150/90 mmHg despite therapy); were pregnant or lactating; had had a myocardial infarction within the past 6 months; or had greater than +1 proteinuria on two consecutive analyses performed no less than 1 week apart.
This trial was conducted under a National Cancer Institute (NCI) –sponsored investigational new drug application with institutional review board approval, and all participants provided written informed consent. The protocol design and conduct complied with all applicable regulations, guidances, and local policies (ClinicalTrials.gov identifier: NCT00942877).
Study Design
Diagnosis of ASPS was confirmed by pathologists at the NCI in all patients enrolled onto the study. Presence of TFE3 protein was confirmed by immunohistochemistry in 27 of 46 patients. The Division of Cancer Treatment and Diagnosis at the NCI supplied cediranib under a collaborative agreement with AstraZeneca (Wilmington, DE). Cediranib was administered at a dose of 30 mg orally, either 1 hour before or 2 hours after meals, once daily in 28-day cycles. The dose was reduced (20 or 15 mg per day) for grade 3 nonhematologic toxicities (except diarrhea or nausea and vomiting without maximal support or easily correctable electrolyte abnormalities) and/or grade 4 hematologic toxicities (except lymphopenia and anemia). Transient exacerbation of tumor pain did not result in dose reduction.
The study was conducted using an optimal two-stage design to rule out an unacceptably low 5% ORR in favor of a modestly high ORR of 25%. One or more confirmed responses in the first stage of nine patients would result in accrual continuing to a total of 24 patients. After encouraging antitumor activity was observed in the initial cohort of nine and then 24 patients, accrual was expanded to include a replicate cohort of an additional 30 patients, with the option for obtaining paired tumor biopsies for gene profiling.
Assessments
History and physical examination, CBCs, and serum chemistries were performed at baseline, weekly for the first two cycles, and every 2 weeks thereafter. Blood pressure was measured weekly by a health care provider during the first two cycles and then every 2 weeks. Patients were required to maintain a study diary with home blood pressure monitoring twice daily. Adverse events were graded according to NCI Common Terminology Criteria for Adverse Events version 4.0.15
Tumor response was assessed based on RECIST (version 1.0); PR was defined as ≥ 30% reduction in the sum of the longest diameters of target lesions compared with baseline.16 Radiologic evaluations, including [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography scans, were performed at baseline and every two cycles (Data Supplement). Dynamic contrast-enhanced magnetic resonance imaging (MRI) scans of target lesions were performed in patients in the replicate cohort at baseline and during the first week of treatment (between days 3 and 5) to coincide with tumor biopsy, as previously described,17 except with a 3-T MRI system (Achieva; Philips, Andover, MA). The forward contrast transfer rate and the reverse contrast transfer rate were calculated as previously described.17
Patients who agreed to tumor sampling underwent 18-gauge core biopsies under radiologic guidance at baseline and again between days 3 to 5 after initiation of treatment. Biopsy timing was based on observations of increased tumor pain associated with an inflammatory-like response in peripheral tumor lesions, usually during the second week of treatment. Hence, biopsies were obtained in the first week of treatment before the development of clinical signs and symptoms to evaluate gene expression changes. Paired tumor biopsies from eight patients were processed for microarray analysis and quantitative real-time polymerase chain reaction (qRT-PCR; Data Supplement). Microarray analysis was performed using an Affymetrix U133 Plus 2.0 human oligonucleotide microarray (Affymetrix, Santa Clara, CA), and data were evaluated using the Bioconductor (http://bioconductor.org/) and R (http://www.r-project.org/) statistical packages. Primary microarray data are available in the Gene Expression Omnibus database (GSE32569). The top 100 differentially expressed probe sets in response to cediranib treatment, ranked by adjusted P value, were compared and analyzed for enriched pathways and transcriptional networks with Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA).
RESULTS
Patients
Forty-six patients were enrolled between July 17, 2009, and June 30, 2012 (Table 1). Most patients (61%) had received at least one prior systemic therapy. Twelve patients (26%) had received prior antiangiogenic therapy (sunitinib, sorafenib, pazopanib, or bevacizumab). Three patients had received prior MET inhibitor therapy with ARQ 197.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients (N = 46) |
|---|---|
| Sex | |
| Male | 23 |
| Female | 23 |
| Age, years | |
| Median | 27 |
| Range | 19-58 |
| ECOG performance status* | |
| 0 | 4 |
| 1 | 38 |
| 2 | 4 |
| No. of prior systemic therapies | |
| 0 | 18 |
| 1 | 18 |
| 2 | 5 |
| ≥ 3 | 5 |
| Prior resection | 35 |
| Prior radiation therapy | 27 |
| Primary site of disease | |
| Lower extremity | 25 |
| Upper extremity | 7 |
| Pelvis/gluteal area | 5 |
| Chest/chest wall | 3 |
| Axilla | 2 |
| Liver | 1 |
| Base of tongue | 1 |
| Retroperitoneum | 2 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
ECOG performance status is a scale indicating level of patient activity from 0 (fully active) to 5 (dead).
Efficacy
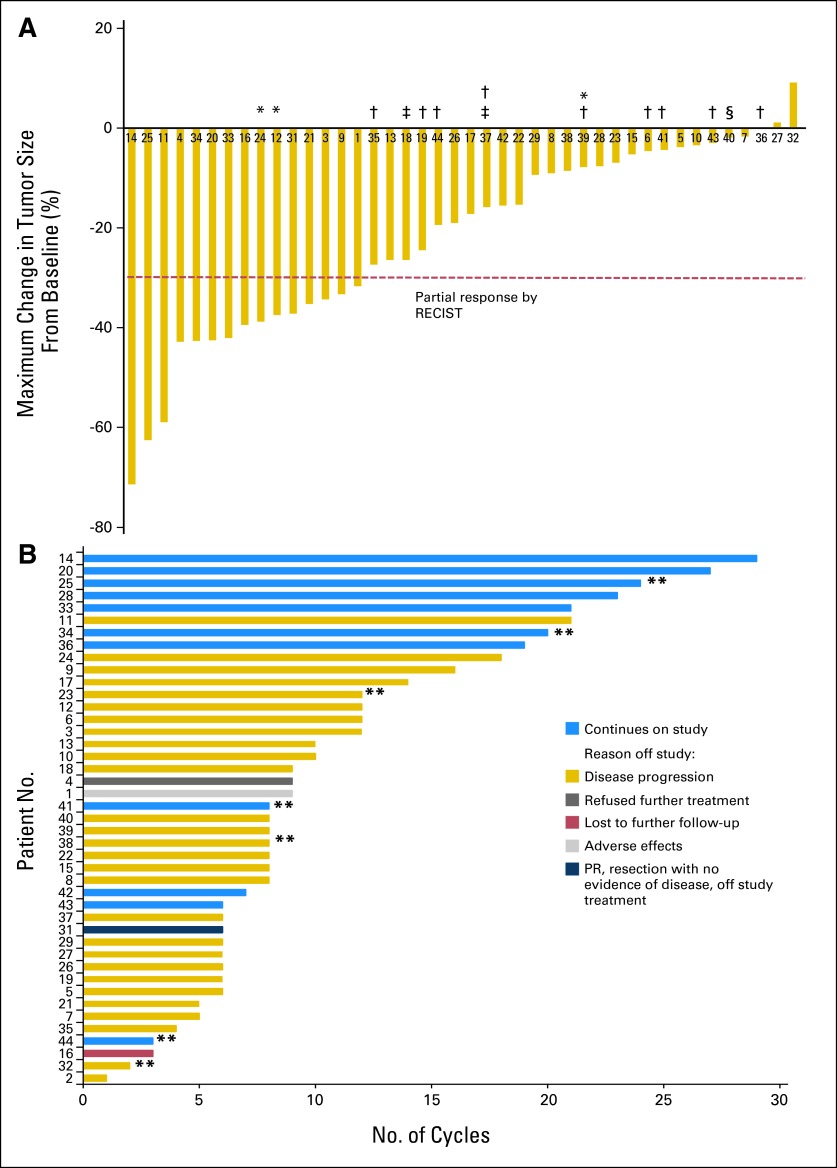
As of June 30, 2012, the date for data analysis, 43 patients met intent-to-treat criteria and were evaluable for assessment of objective response. Two patients have not yet completed two cycles of therapy, and one patient was removed from the trial before receiving any treatment. One patient experienced disease-related complications and died from factors related to tumor invasion of the myocardium. This patient had several treatment interruptions and did not have follow-up radiologic assessment before the fatal event; however, because the patient received treatment on study, data are included for evaluation of response and adverse events. The ORR was 35%, with 15 of 43 patients achieving PR (Figs 1A and 2). One patient with unresectable mediastinal disease achieved a PR, underwent resection, and does not have evidence of disease recurrence 16 months after surgery. Twenty-six patients (60%) had stable disease as the best response. The disease control rate (PR + stable disease) at 24 weeks (six cycles) was 84% (36 of 43 patients). Patients who had not yet completed six cycles and had not experienced progression were not included in the calculation. Nine of 43 patients received 18 or more cycles of treatment on study. Eleven patients were still receiving cediranib at the time of this report (Fig 1B). Of the nine patients who had received prior sunitinib, all had disease stabilization (number of cycles, three to 19); four patients remain on study (Figs 1A and 1B). The three patients who had received prior MET inhibitor treatment derived clinical benefit from cediranib; two patients experienced PRs and one patient had stable disease while receiving eight, 12, or 18 cycles of therapy.
Fig 1.
Tumor response in 43 evaluable patients. (A) Maximal change in tumor size from baseline assessed according to RECIST (version 1.0), which uses 30% shrinkage in the sum of the longest diameters of target lesions as the threshold for partial response (PR; dashed line). The patient number for each patient entered onto the trial is shown below each bar. (*) Prior ARQ 197 treatment. (†) Prior sorafenib treatment. (‡) Prior sunitinib treatment. (§) Prior bevacizumab treatment. One patient died before follow-up assessment. (B) Duration on study for each evaluable patient through the data analysis date of June 30, 2012. (**) Patients who underwent paired tumor biopsies (see Fig 3).
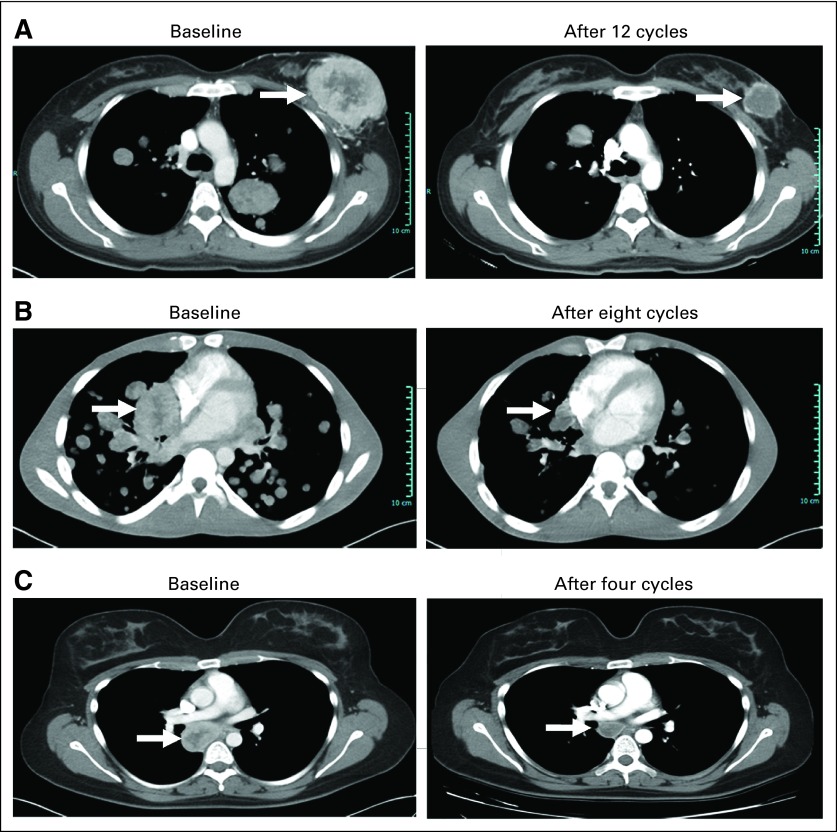
Fig 2.
Computed tomography (CT) scans. (A) CT scans from a 29-year-old woman (patient 24) with metastatic alveolar soft part sarcoma (ASPS) before and after treatment with cediranib; the patient had undergone previous resection and had experienced progression after treatment with ifosfamide plus doxorubicin, gemcitabine with docetaxel, and the MET inhibitor ARQ 197. Significant shrinkage of breast lesions was observed, and the patient received a total of 18 cycles. (B) CT scans from a 25-year-old man (patient 34) with newly diagnosed metastatic ASPS who presented with shortness of breath, was oxygen dependent, and had signs of early hemodynamic compromise from a tumor compressing the heart. Within the first two cycles of cediranib, the patient no longer required oxygen. After eight cycles of therapy, the patient had good exercise tolerance, and significant tumor shrinkage was observed. Patient continues on study, status post 19 cycles. (C) CT scans from a 25-year-old woman (patient 31) who originally presented with a mass in her left calf, underwent resection of primary ASPS followed by radiation, but had disease recurrence in the form of an unresectable subcarinal mass. After six cycles of cediranib, this patient achieved a partial response, underwent resection, and currently has no evidence of disease 16 months later and is off study. Patients did not receive any therapy after resection.
Safety
The most frequent grade 2 or 3 adverse events were hypertension, diarrhea, transaminitis, proteinuria, hypothyroidism, and tumor pain (Table 2). There were no cediranib-related grade 4 or 5 toxicities. One patient discontinued treatment, despite experiencing a PR, because of chronic grade 1 to 2 GI symptoms. Dose reduction was necessary with continued treatment in 17 (40%) of 43 patients as a result of transaminitis, weight loss, hypertension, and proteinuria. The median number of cycles administered at full doses was two (range, zero to 11 cycles), with 14 (33%) of 43 patients requiring one dose reduction to 20 mg and three (7%) of 43 patients requiring two dose reductions to 15 mg daily. Eight patients developed grade 2 or 3 tumor pain associated with erythema and tenderness at the tumor site. This was observed only in patients with disease in peripheral sites, such as the extremities or chest wall. Exacerbation in tumor pain was typically observed 8 to 14 days after the initiation of study treatment, required increased doses of narcotics, and lasted 48 to 72 hours with relief of pain to baseline or lower levels. Patient 2, who developed grade 3 tumor pain and required narcotics and intravenous steroids for pain control, also developed mental confusion. Evaluation for reversible posterior leukoencephalopathy, including brain MRI, was negative. Mental status changes resolved with reduction in narcotics after improvement in pain symptoms.
Table 2.
Grade ≥ 2 Drug-Related Adverse Events
| Adverse Event | No. of Patients |
|
|---|---|---|
| Grade 2 | Grade 3 | |
| Abdominal pain/distension | 4 | |
| Alkaline phosphatase | 1 | |
| Anorexia | 5 | |
| Confusion | 1 | |
| Diarrhea | 12 | 2 |
| Dyspnea | 1 | |
| Fatigue | 2 | |
| Hand-foot skin reaction | 4 | |
| Headache | 4 | 1 |
| Heartburn/dyspepsia | 2 | |
| Hyperbilirubinemia | 3 | |
| Hypertension | 23 | 8 |
| Hypoalbuminemia | 2 | |
| Hypothyroidism | 15 | |
| Left ventricular systolic dysfunction | 1 | |
| Leukocytes | 1 | |
| Lymphopenia | 2 | 2 |
| Mucositis | 5 | |
| Neutropenia | 3 | 1 |
| Prolonged QTc interval | 1 | |
| Proteinuria | 15 | 1 |
| Rash | 3 | |
| Transaminitis | 6 | 4 |
| Tumor pain | 3 | 3 |
| Vomiting | 1 | |
| Weight loss | 8 | 1 |
NOTE. Worst grade is reported per patient; all adverse events were mapped to Common Terminology Criteria for Adverse Events (version 4.0).
Pharmacodynamics
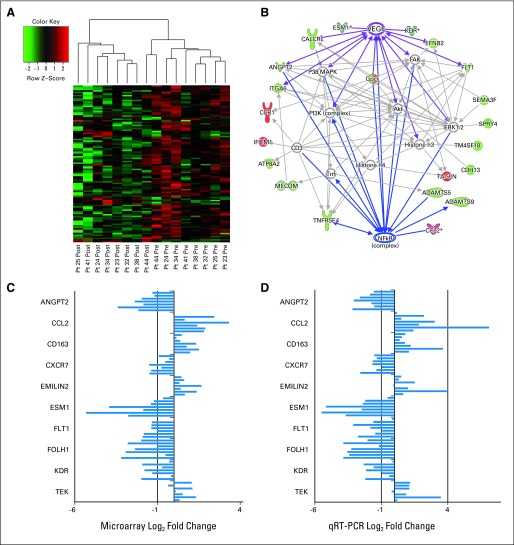
The top 100 differentially expressed genes are shown in Figure 3A and the Data Supplement; 86% of these probe sets were transcriptionally downregulated in response to cediranib. Twenty-nine genes were significantly dysregulated after cediranib treatment (adjusted P value for false discovery rate of P < .05; Data Supplement); all genes but one were downregulated. The top two downregulated probe sets mapped to CDH13 (cadherin) and ANGPT2 (angiopoietin 2). Network analysis identified nodes of response related to vasculogenesis and angiogenesis consistent with VEGFR targeting (Fig 3B). Genes associated with nuclear factor-κB were also identified, which may help to explain the inflammatory changes observed clinically.
Fig 3.
Microarray expression analysis of tumor biopsies. (A) Hierarchical clustering of expression changes in biopsies from patients 23, 24, 25, 32, 34, 38, 41, and 44 for the top 100 differentially expressed genes based on adjusted P values before and after treatment with cediranib. Each row represents an Affymetrix probe set (Affymetrix, Santa Clara, CA); red indicates high expression, and green indicates low expression. (B) A representative Ingenuity Pathway Analysis map generated for the differentially expressed probe sets. Red nodes indicate genes that were upregulated by microarray, and green nodes indicate those that were downregulated; the more intense the color, the greater the log2 fold change in expression. (C and D) The log2 fold changes in expression from (C) microarray and (D) quantitative real-time polymerase chain reaction (qRT-PCR) analyses for cediranib-induced changes in individual patient samples. For each gene, results from patients 23, 24, 25, 32, 34, 38, 41, and 44 are listed sequentially from top to bottom; patient 23 did not have sufficient sample volume for qRT-PCR analysis.
Microarray data validation by qRT-PCR was performed for 10 selected genes in samples from seven patients (patient 23 had insufficient tumor for qRT-PCR analysis). Of the 10 genes selected for further examination, ANGPT2, FLT1, FOLH1, and CXCR7 were significantly downregulated by microarray (adjusted P value for false discovery rate of P < .05). Other genes selected for examination have roles in vasculogenesis, angiogenesis, and the inflammatory response (Data Supplement). Analysis by qRT-PCR confirmed that ANGPT2, FLT1, FOLH1, ESM-1, and KDR were downregulated in the tumors of patients treated with cediranib, whereas CCL2, CD163, EMILIN2, and TEK showed modest, but consistent, increases in expression (Figs 3C and 3D).
Changes in [18F]FDG uptake (standardized uptake value) in tumor sites were calculated for the first 30 patients with an evaluable baseline scan and follow-up scan that coincided with the patient's best objective response by computed tomography. Changes in [18F]FDG uptake corresponded with clinical outcome in only 16 (53%) of 30 patients. Significant alterations (> 25%) in both dynamic contrast-enhanced MRI parameters (the forward and reverse contrast transfer rates) were observed in two of nine patients with evaluable pre- and post-treatment scans. No clear pattern of radiologic changes was observed based on clinical outcome.
DISCUSSION
In this largest prospective trial to date of systemic therapy for metastatic ASPS (a challenging disease with no current standard of care), we observed that cediranib has substantial single-agent activity, producing an ORR of 35% and a disease control rate at 6 months of 84%. Cediranib was administered at the dose of 30 mg daily, which is lower than the maximum-tolerated dose of 45 mg established in a phase I trial.18 However, the dose of 45 mg was poorly tolerated in subsequent trials, requiring dose reductions in more than 50% of patients as a result of toxicities.19,20 Overall, treatment with cediranib at 30 mg, a dose used safely in a number of phase II trials, was associated with manageable toxicities that required dose reductions in later cycles in 40% of patients. One patient with unresectable mediastinal disease achieved a PR, underwent resection, and does not have evidence of disease recurrence 16 months after surgery. Although formal quality-of-life assessments were not performed, several patients experienced improvement in symptoms and returned to a more active lifestyle while on study.
Several observations have emerged from our trial that could influence future treatment strategies for ASPS. This study is the first, to our knowledge, to report gene expression changes in ASPS after cediranib treatment; the significant downregulation of a gene related to angiogenesis (ANGPT2) and of the FLT1 gene, which encodes VEGFR-1, is consistent with drug-induced modulation of vascular physiology,11 which may be critical to disease control. It is interesting to note, furthermore, that although the tumor-specific ASPL-TFE3 fusion transcription factor is known to directly upregulate MET,7 patients with ASPS treated with the MET inhibitor ARQ 197 have not demonstrated evidence of tumor shrinkage.21 This could be a result of upregulation of compensatory pathways for growth and survival, because crosstalk between MET and a number of signaling pathways, such as the epidermal growth factor receptor, ERBB2, insulin-like growth factor 1 receptor, and WNT–β-catenin pathway, has been demonstrated.22 Thus, inhibition of MET alone may not be sufficient to cause tumor shrinkage. MET signaling also plays a role in angiogenesis, and hypoxia promotes expression of MET in tumors. Inhibition of MET has been shown to suppress the development of resistance to VEGFR inhibitors.23 Two patients in our trial who had received ARQ 197 achieved objective responses to cediranib, which supports testing the therapeutic potential of combined inhibition of VEGFR and MET in patients with ASPS.
Changes in the expression of inflammatory pathway genes, such as those controlled by nuclear factor-κB,24 are consistent with the inflammatory response (erythema and tumor pain) we observed during the second week of study treatment. The clinical observation of an inflammatory response only in patients with peripheral lesions may be a result of our ability to observe this effect only in peripheral sites or of differences in the enervation of peripheral versus visceral organ sites of metastases. However, because of the modest number of affected patients, no definitive correlations can be drawn between induction of an inflammatory response, changes in gene expression, and tumor shrinkage.
Other clinical results support the utility of targeting VEGFR in this highly vascular tumor. The multitargeted kinase inhibitor sunitinib, which also inhibits VEGFR, has demonstrated preliminary evidence of activity in patients with ASPS; five of nine patients have been reported to have experienced a PR after sunitinib therapy.25 Of note, several patients on our trial whose disease had progressed while receiving sunitinib exhibited prolonged disease stabilization on study. We have recently performed additional molecular studies suggesting that cediranib and sunitinib have somewhat different effects on gene expression in human umbilical vein endothelial cells and in an ASPS-1 cell line (Data Supplement). Specifically, cediranib inhibits ESM-1 expression in these cells, whereas sunitinib enhances ESM-1 at the mRNA level.
Given the substantive clinical benefit demonstrated in this study, the therapeutic value of cediranib for metastatic ASPS is currently being evaluated in an NCI-sponsored, multicenter phase II trial in which patients are randomly assigned to receive either open-label cediranib or sunitinib, with cross over at disease progression. The objectives of this study include determining the ORR and 6-month progression-free survival for each drug; data from this trial will provide comparative information on the relative roles of these agents in the treatment of advanced ASPS and will address the optimal sequencing of treatment with cediranib. With the opening of the randomized trial, accrual of adult patients to the single-arm cediranib trial was put on hold. The single-arm trial has been amended with a revised pediatric dosing schema, and the remaining slots are reserved for pediatric patients (age ≤ 16 years). Data from this cohort will be analyzed separately.
Supplementary Material
Acknowledgment
We thank Gina Uhlenbrauck, Heather Gorby, and Yvonne A. Evrard, SAIC-Frederick, for editorial assistance in the preparation of this article, and Giovanna Speranza, Marcie Weil, Yvonne Horneffer, Lamin Juwara, and Janelle Bingham of the Developmental Therapeutics Clinical Trial Group, National Cancer Institute.
Footnotes
See accompanying editorial on page 2246
Supported in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology, June 3-7, 2011, Chicago, IL.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00942877
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Shivaani Kummar, Anne Monks, Eric C. Polley, Curtis D. Hose, S. Percy Ivy, Robert J. Kinders, Richard Simon, James H. Doroshow, Lee Helman
Financial support: James H. Doroshow
Administrative support: James H. Doroshow
Provision of study materials or patients: James H. Doroshow
Collection and assembly of data: Shivaani Kummar, Deborah Allen, Anne Monks, Eric C. Polley, Curtis D. Hose, S. Percy Ivy, Ismail B. Turkbey, Robert J. Kinders, Peter Choyke, James H. Doroshow, Lee Helman
Data analysis and interpretation: Shivaani Kummar, Deborah Allen, Anne Monks, Eric C. Polley, Curtis D. Hose, S. Percy Ivy, Ismail B. Turkbey, Scott Lawrence, Robert J. Kinders, Peter Choyke, Seth M. Steinberg, James H. Doroshow, Lee Helman
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Zarrin-Khameh N, Kaye KS. Alveolar soft part sarcoma. Arch Pathol Lab Med. 2007;131:488–491. doi: 10.5858/2007-131-488-ASPS. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman PH, Brennan MF, Kimmel M, et al. Alveolar soft-part sarcoma: A clinico-pathologic study of half a century. Cancer. 1989;63:1–13. doi: 10.1002/1097-0142(19890101)63:1<1::aid-cncr2820630102>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Portera CA, Jr,, Ho V, Patel SR, et al. Alveolar soft part sarcoma: Clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer. 2001;91:585–591. doi: 10.1002/1097-0142(20010201)91:3<585::aid-cncr1038>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Reichardt P, Lindner T, Pink D, et al. Chemotherapy in alveolar soft part sarcomas: What do we know? Eur J Cancer. 2003;39:1511–1516. doi: 10.1016/s0959-8049(03)00264-8. [DOI] [PubMed] [Google Scholar]
- 5.Ladanyi M, Lui MY, Antonescu CR, et al. The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene. 2001;20:48–57. doi: 10.1038/sj.onc.1204074. [DOI] [PubMed] [Google Scholar]
- 6.Argani P, Antonescu CR, Illei PB, et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: A distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol. 2001;159:179–192. doi: 10.1016/S0002-9440(10)61684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuda M, Davis IJ, Argani P, et al. TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res. 2007;67:919–929. doi: 10.1158/0008-5472.CAN-06-2855. [DOI] [PubMed] [Google Scholar]
- 8.Lorigan JG, O'Keeffe FN, Evans HL, et al. The radiologic manifestations of alveolar soft-part sarcoma. AJR Am J Roentgenol. 1989;153:335–339. doi: 10.2214/ajr.153.2.335. [DOI] [PubMed] [Google Scholar]
- 9.Stockwin LH, Vistica DT, Kenney S, et al. Gene expression profiling of alveolar soft-part sarcoma (ASPS) BMC Cancer. 2009;9:22. doi: 10.1186/1471-2407-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazar AJ, Das P, Tuvin D, et al. Angiogenesis-promoting gene patterns in alveolar soft part sarcoma. Clin Cancer Res. 2007;13:7314–7321. doi: 10.1158/1078-0432.CCR-07-0174. [DOI] [PubMed] [Google Scholar]
- 11.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 12.Smith NR, James NH, Oakley I, et al. Acute pharmacodynamic and antivascular effects of the vascular endothelial growth factor signaling inhibitor AZD2171 in Calu-6 human lung tumor xenografts. Mol Cancer Ther. 2007;6:2198–2208. doi: 10.1158/1535-7163.MCT-07-0142. [DOI] [PubMed] [Google Scholar]
- 13.Fox E, Aplenc R, Bagatell R, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28:5174–5181. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner K, Judson I, Leahy M, et al. Activity of cediranib, a highly potent and selective VEGF signaling inhibitor, in alveolar soft part sarcoma. J Clin Oncol. 2009;27(suppl 15S):541s. abstr 10523. [Google Scholar]
- 15.National Cancer Institute: Common Terminology Criteria for Adverse Events version 4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Kummar S, Gutierrez ME, Chen A, et al. Phase I trial of vandetanib and bevacizumab evaluating the VEGF and EGF signal transduction pathways in adults with solid tumours and lymphomas. Eur J Cancer. 2011;47:997–1005. doi: 10.1016/j.ejca.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 19.Matulonis UA, Berlin S, Ivy P, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27:5601–5606. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulders P, Hawkins R, Nathan P, et al. Cediranib monotherapy in patients with advanced renal cell carcinoma: Results of a randomised phase II study. Eur J Cancer. 2012;48:527–537. doi: 10.1016/j.ejca.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Wagner AJ, Goldberg JM, Dubois SG, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: Results of a multicenter phase 2 trial. Cancer. 2012;118:5894–5902. doi: 10.1002/cncr.27582. [DOI] [PubMed] [Google Scholar]
- 22.Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: Rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 23.Sennino B, Ishiguro-Oonuma T, Wei Y, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Li Z, Bai L, et al. NF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front Biosci. 2011;16:1172–1185. doi: 10.2741/3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stacchiotti S, Negri T, Zaffaroni N, et al. Sunitinib in advanced alveolar soft part sarcoma: Evidence of a direct antitumor effect. Ann Oncol. 2011;22:1682–1690. doi: 10.1093/annonc/mdq644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.