Abstract
OBJECTIVE
Describe local changes in the incidence of community-onset and hospital-onset methicillin-resistant Staphylococcus aureus (MRSA) infection and evaluate the impact of MRSA active surveillance on hospital-onset infection.
DESIGN
Observational study using prospectively collected data.
SETTING
Atlanta Veterans Affairs Medical Center (AVAMC).
PATIENTS
All patients seen at the AVAMC over an 8-year period with clinically and microbiologically proven MRSA infection.
METHODS
All clinical cultures positive for MRSA were prospectively identified, and corresponding clinical data were reviewed. MRSA infections were classified into standard clinical and epidemiologic categories. The Veterans Health Administration implemented the MRSA directive in October 2007, which required active surveillance cultures in acute care settings.
RESULTS
The incidence of community-onset MRSA infection peaked in 2007 at 5.45 MRSA infections per 1,000 veterans and decreased to 3.14 infections per 1,000 veterans in 2011 (P ≤ .001 for trend). Clinical and epidemiologic categories of MRSA infections did not change throughout the study period. The prevalence of nasal MRSA colonization among veterans admitted to AVAMC decreased from 15.8% in 2007 to 11.2% in 2011 (P < .001 for trend). The rate of intensive care unit (ICU)–related hospital-onset MRSA infection decreased from October 2005 through March 2007, before the MRSA directive. Rates of ICU-related hospital-onset MRSA infection remained stable after the implementation of active surveillance cultures. No change was observed in rates of non-ICU-related hospital-onset MRSA infection.
CONCLUSIONS
Our study of the AVAMC population over an 8-year period shows a consistent trend of reduction in the incidence of MRSA infection in both the community and healthcare settings. The etiology of this reduction is most likely multifactorial.
The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) has been extensively studied since it was first identified in 1962.1 Initially, MRSA was the cause of sporadic hospital-acquired infection in the 1960s and 1970s. However, in the 1980s, a dramatic increase in the incidence of methicillin resistance among nosocomial S. aureus isolates was observed in the United States,2 and by 2003, 64.4% of hospital-onset S. aureus infections in the intensive care unit (ICU) were methicillin resistant.3 After significant emphasis was placed on the prevention of healthcare-associated MRSA infection by public health agencies and policy makers, a trend of decreasing incidence has been observed among invasive MRSA disease and bloodstream infections.4,5
In the late 1990s, scattered cases of MRSA infection that were not associated with identifiable risk factors began to appear in the general community.6,7 These early outbreaks were the beginning of what is now an epidemic of community-associated MRSA in North America driven by a new MRSA strain, USA300.8–10 Although the majority of community-associated disease is due to skin and soft-tissue infection (SSTI),11 severe and lethal cases of necrotizing pneumonia,6 necrotizing fasciitis,12 and severe sepsis13 have been described. Little is known about the current epidemiology of community-onset (CO) disease, in contrast with that of hospital-acquired infection.14–16 We aim to describe the changing epidemiology of hospital-onset and CO MRSA disease among veterans in Georgia using data that was prospectively collected over an 8-year period.
METHODS
The study population consisted of inpatients and outpatients seen at the Atlanta Veterans Affairs Medical Center (AVAMC) over an 8-year period (October 1, 2003, through September 31, 2011). The AVAMC is a large, integrated healthcare system that encompasses the greater Atlanta metropolitan area with approximately 200 inpatient beds, 8 community-based out-patient clinics, and 1 nursing home care unit.
In an attempt to reduce nosocomial MRSA transmission, the Veterans Health Administration (VHA) issued a directive in 2007 that mandated the use of a MRSA bundle in all acute care settings. The MRSA bundle consists of active surveillance for MRSA nasal colonization in all patients (regardless of earlier colonization or infection status) upon each patient movement within the hospital (admission, transfer, and discharge); contact precautions for patients with MRSA colonization or infection or a history of MRSA colonization or infection; increased emphasis on hand hygiene; and a change in the institutional culture such that infection control and prevention is the responsibility of all employees. At the AVAMC, the MRSA bundle was implemented in April 2007 in the medical ICU, July 2007 in the surgical ICU, and October 2007 for all general wards. No MRSA nasal screening was routinely performed before these dates. Bundles to reduce the incidence of central-line-associated bloodstream infection (CLABSI) and ventilator-associated pneumonia (VAP) were implemented in the ICUs in February 2006 and April 2006, respectively.
MRSA nasal colonization screening at hospital admission and transfer is performed using the Xpert MRSA assay (Ce-pheid) with nasal cultures performed at hospital discharge. Extranasal sites are not routinely screened for MRSA, and decolonization strategies are not routinely recommended for colonized patients.
Colonization results from the active surveillance program were obtained from the Veterans Health Information Systems and Technology Architecture (VISTA) with the use of TheraDoc (Hospira), a Web-based hospital surveillance system, from October 1, 2007, through September 31, 2011. Annual bed-days-of-care (BDOC) and total unique veterans in care per year were obtained from local administrative data.
As part of routine surveillance at the AVAMC, MRSA infections have been identified prospectively on a monthly basis since 2003 by using the microbiology option for specific organisms in VISTA. This surveillance method captures all clinical cultures performed at AVAMC healthcare facilities but misses positive MRSA cultures performed at non-AVAMC sites. Because most veterans at the AVAMC do not have private insurance coverage and rely solely on the AVAMC for their medical needs, we assume these missed cases are few in number and negligible to the overall trend analysis. Surveillance of MRSA-positive blood cultures began October 1, 2003. Surveillance of MRSA positive cultures from all body sites began October 1, 2005. The study period ended September 31, 2011. All clinical MRSA cultures and corresponding clinical data (anatomic site of culture, radiographic studies, laboratory results, and physician notes) were reviewed by the same experienced infectious disease physician (D.R.) on a monthly basis to identify true infections and exclude cultures representing colonization.
Infections were classified according to the Centers for Disease Control and Prevention criteria.17 A minimum of 30 days between positive culture results was required to define a separate episode of infection, and duplicate cultures from the same clinical infection were excluded, regardless of time. The infections were categorized according to primary site of infection into the following categories: SSTI, bone and joint infection, bloodstream infection, genitourinary infection, lower respiratory tract infection, surgical site infection, and other. Each infection could only be categorized into a single category. A separate database was maintained for MRSA-positive blood cultures regardless of the primary site of infection.
MRSA infections were classified into 3 mutually exclusive epidemiologic categories. An infection was considered to be a hospital-onset (HO) infection if the clinical culture was obtained more than 48 hours after admission to the AVAMC and not present on admission. CO infections were subcategorized into healthcare-associated CO (HACO) or community-associated (CA) infections.18 Infections were considered HACO if MRSA was isolated from an outpatient or within the first 48 hours after admission to the AVAMC and the patient had significant healthcare exposure. Significant healthcare exposure was defined as having at least 1 of the following risk factors: presence of an indwelling device at time of hospital admission or history of surgery, hospitalization, dialysis, or residence in a long-term care facility in the 12 months preceding the culture date. Previous MRSA nasal colonization or infection was not used to define health-care-associated infection, because this discounts the possible community origin of the previous infection.5 Infections were considered to be CA if MRSA was isolated from an outpatient or was isolated within the first 48 hours after admission to the AVAMC without documented healthcare risk factors.
The Emory University institutional review board and the Veterans Affairs (VA) Research and Development Committee approved this study. Because the MRSA directive was a quality-improvement initiative, written informed consent from individual patients was not required. In addition, written informed consent was waived for the review of MRSA infections.
Statistical Analysis
Percentage of patients with at least 1 positive MRSA nasal screening result (either at hospital admission or transfer) per fiscal year was reported in an attempt to control for patients with repeated hospitalizations. Rates of HACO and CA MRSA infections were expressed as the number of infections per 1,000 veterans, and rates of HO infections were expressed as the number of infections per 1,000 BDOCs, stratified on bed type (ICU or non-ICU bed). Surgical-ICU-related HO infections and BDOC from the third quarter of fiscal year 2007 (April 1 through June 30, 2007) were excluded from the analysis to ensure consistency with MRSA directive use in each analyzed time period. CO infections were analyzed on an annual basis, and HO infections were analyzed in 6-month time intervals. Years are expressed as fiscal years (October 1 through September 30), not calendar years.
Trends in MRSA infection rates were initially assessed by plotting the observed rates as a function of time. The formal analysis of trends of MRSA infection were based on generalized linear models, assuming a Poisson distribution with a log link function, including fiscal year as a predictor variable and log total number of unique veterans or log BDOC as an offset variable. Model fit was assessed, and regression coefficients for trend were assessed by the partial Wald test, and P values of less than .05 were considered significant. Models were fit for each time period of interest. The null hypothesis for the trend analysis was that the rate of events (ie, infections) did not change over time. An interrupted time series analysis with segmented regression was not used to evaluate the effect of the MRSA directive on HO infection because of limited data from the period before the directive. Distribution of MRSA infection types was analyzed with the χ2 test. Analyses were performed with SAS, version 9.3 (SAS Institute).
RESULTS
From October 2005 through September 2011, 2,028 MRSA infections occurred in 1,620 veterans. A total of 1,858 MRSA infections (91.6%) were CO, and 170 (8.4%) were HO. Of the 1,858 CO infections, 1,108 (59.6%) were HACO, and 750 (40.4%) were CA. Of the 170 HO infections, 54 (31.8%) occurred in the ICUs, and 116 (68.2%) occurred in non-ICU wards.
CO MRSA
Total veterans in care at the AVAMC increased from 58,496 in 2006 to 85,041 in 2011. CO MRSA infections peaked in 2007 at 5.45 MRSA infections per 1,000 veterans and consistently decreased to 3.14 infections per 1,000 veterans in 2011 (Figure 1; P < .001 for trend). HACO infections comprised the majority of CO disease each year, and the distribution of epidemiologic infection categories did not change over the study period (Figure 1; P = .34 by χ2 test). The distribution of clinical infection types per year remained consistent over the study period, with the majority of infections being SSTIs (Figure 2; P = .065 by χ2 test).
FIGURE 1.
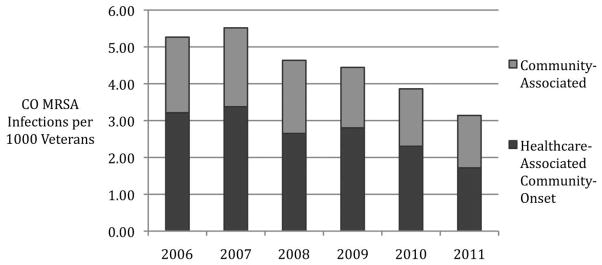
Incidence of community-onset (CO) methicillin-resistant Staphylococcus aureus (MRSA) infection during the period 2006–2011 among Atlanta Veterans (P < .001, for decreasing trend) and distribution of epidemiologic infection categories (P = .34, χ2 for change in distribution of epidemiologic infection categories).
FIGURE 2.
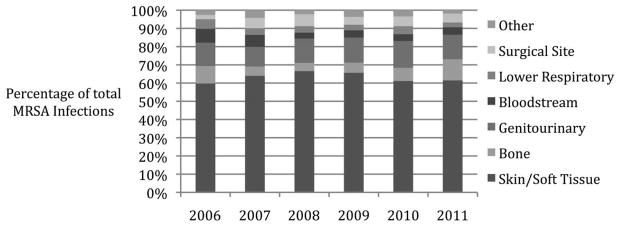
Distribution of community-onset methicillin-resistant Staphylococcus aureus (MRSA) infection types at the Atlanta Veterans Affairs Medical Center, 2006–2011. P = .065, χ2 for a change in distribution of clinical infection categories.
The incidence of CO MRSA-positive blood cultures, regardless of the primary site of infection, decreased from 0.68 positive blood cultures per 1,000 veterans in care in 2006 to 0.21 positive blood cultures per 1,000 veterans in care in 2011 (P < .001 for trend). Primary bloodstream infections comprised 47.6%–63.2% of all positive blood culture results per year, whereas skin and soft tissue and bone were the primary sites of infection for 15.8%–27.8% of MRSA-positive blood culture results per year.
HO MRSA
Total BDOC (31,000–37,000 BDOC per year) and total number of admissions to acute care (5,306–5,967 admissions per year) at the AVAMC have remained stable from 2006 through 2011. The admitted veteran population in Atlanta is mostly male (95%) and either white (54%) or black (44%). The mean age of veterans admitted to AVAMC in 2007 was 63.4 years.
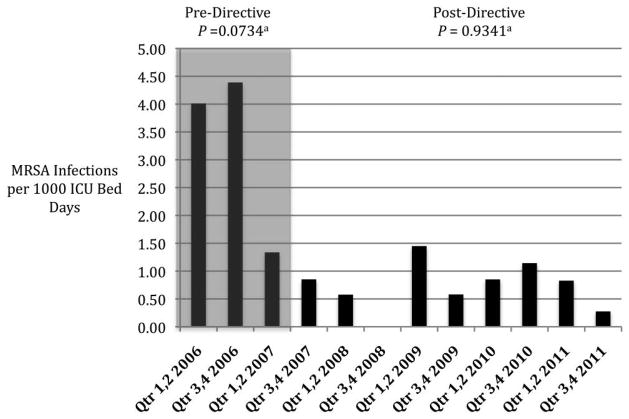
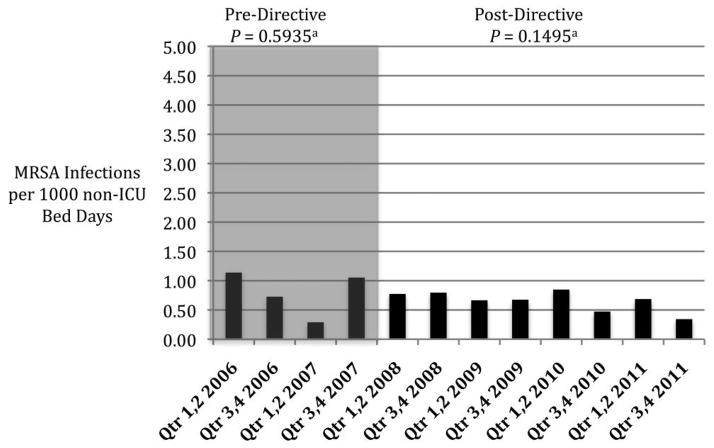
The rate of ICU-related HO MRSA infection was decreasing before the implementation of the MRSA directive (Figure 3; P = .0734 for trend) and remained stable after the directive was implemented (Figure 3; P = .9341 for trend). No change in non-ICU-related HO infection rates was observed before or after the implementation of the directive (Figure 4). Because of the very low incidence of CLABSIs and VAPs at the AVAMC, a separate analysis to determine the effectiveness of bundles and their impact on HO MRSA infection rates was not possible.
FIGURE 3.
Rates of hospital-onset, intensive care unit (ICU)–related methicillin-resistant Staphylococcus aureus (MRSA) infection at the Atlanta Veterans Affairs Medical Center (AVAMC) during the period 2006–2011, before and after implementation of the MRSA directive. Qtr, quarter. aP values for trend (null hypothesis is rate is unchanged over time).
FIGURE 4.
Rates of hospital-onset methicillin-resistant Staphylococcus aureus (MRSA) infection not related to the intensive care unit (ICU) at the Atlanta Veterans Affairs Medical Center (AVAMC) during the period 2006–2011, before and after implementation of the MRSA directive. aP values for trend (null hypothesis is rate is unchanged over time).
MRSA Colonization
In 2008, 15.8% of veterans admitted to AVAMC had at least 1 admission or transfer nasal screening result that was positive for MRSA. Over the subsequent 3 years, rates of MRSA nasal colonization decreased to 11.2% in 2011 (P < .001 for trend). Compliance with admission nasal MRSA screening has exceeded 90% since the MRSA initiative was fully implemented in October 2007.
DISCUSSION
We describe the decreasing incidence of MRSA infection and colonization among Atlanta veterans over an 8-year period using patient-level data and chart review to identify and classify infections. The decreasing incidence of CO MRSA infection (CA and HACO), HO MRSA infection, and MRSA nasal colonization in Atlanta veterans is a significant finding that may or may not reflect more than a localized trend in the current national MRSA epidemic.
Current population-based prospective studies evaluating the epidemiology of both invasive and noninvasive CO MRSA infection are limited. Our study is the first to evaluate recent trends in CO disease by assessing patient-level data. Landrum et al19 recently published a report on the incidence of CO and HO MRSA infections among Department of Defense beneficiaries from 2005 through 2010 using a medical record database of positive microbiologic cultures without supporting clinical data. In this report, the incidence of CO MRSA bacteremia showed a similar pattern of decrease when compared with our Atlanta population. A statistically significant decrease in the incidence of SSTI was not detected, but a peak incidence of SSTIs occurred in 2008, and 2010 had the lowest incidence of SSTI since the start of the study, which was also similar to our Atlanta data.
Two recent studies evaluated CO MRSA incidence using International Classification of Diseases, Ninth Revision (ICD-9), diagnosis codes to identify cases of MRSA infection.16,20 Contrary to our findings, both of these studies found stable or increasing rates of MRSA infection through the year 2009. The ICD-9 codes, although useful in gathering large quantities of data, have been shown to be poorly sensitive in identifying MRSA infections.21,22
In parallel to the reduction of CO MRSA infection cases, the rates of MRSA nasal colonization in our study population have also decreased in the past 4 years, from 15% to 11%. Although the association of nasal colonization and CO infection has been called into question,23–25 the reduction in the incidence of nasal colonization at admission is additional evidence of the decreasing community burden of MRSA among Atlanta veterans.
CO MRSA infection rates have continued to decrease throughout the first half of fiscal year 2012 in Atlanta. The etiology of the reduction in CO MRSA rates remains unclear and speculative in nature. Because the incidence of both CA and HACO infections are decreasing, interventions within the healthcare system are unlikely to be the primary cause of this reduction, but it is conceivable that reduced MRSA transmission within healthcare facilities could lead to reduced HACO and colonization rates. Other factors, such as increased community awareness of MRSA and improved hand hygiene, improved antibiotic prescribing practices, and the natural course of an epidemic are likely to be involved. With our data, we are unable to determine how the reduction of CO MRSA has affected the incidence of CO methicillin-susceptible S. aureus infections or the molecular epidemiology of MRSA.
In addition to a decreasing incidence of CO MRSA infection, a reduction in ICU-related MRSA infection was also observed from 2006 to 2011. This reduction in HO MRSA infection is consistent with other reports of decreasing invasive healthcare-associated infection5,19 and CLABSI in US ICUs.4 Our data add to the current literature by demonstrating a similar decrease in HO MRSA disease up to 2008 with subsequent stabilization of rates from 2008 to 2011.
The greatest observed reduction in ICU-related infection occurred before implementation of the MRSA directive and was followed by stable, low rates of infection. No significant change in the incidence of HO MRSA infection was seen among non-ICU patients. In this single-center, observational study, the national VHA MRSA directive did not appear to have a significant impact on the incidence of HO MRSA infection.
During the height of the hospital-based MRSA epidemic in the 1990s, the use of active surveillance cultures (ASCs) to identify and control the reservoir of MRSA was strongly supported by the Society of Healthcare Epidemiology of America, which cited multiple studies on the benefits of ASC.26 More recently, as states have legislated for mandatory ASC and the VHA has instituted a directive calling for mandatory ASC, very little high-quality evidence has been presented to support the use of ASC during periods of disease endemicity.27,28 The two largest studies to evaluate ASC to date were recently published and reported discrepant results. Huskins et al29 reported on the first randomized controlled trial evaluating ASC and barrier precautions in 19 adult ICUs. In this well-designed study, ASC for all ICU patients and subsequent barrier precautions for those colonized with MRSA was not effective in reducing the incidence of MRSA colonization or infection. However, Jain et al30, using aggregated data from 153 VA hospitals across the United States, reported that the VHA MRSA directive (as described above) significantly reduced the incidence of HO MRSA infections. A recent mathematical modeling study using the parameters from the Jain et al30 study calls into question whether the VHA MRSA directive is causally linked to the reduction of HO infections.31 The use of universal ASC in nonepidemic settings will continue to be hotly debated given the continued use of expensive, legislatively mandated programs that lack consistent, high-quality evidence to support them.
The reduction in HO MRSA infections at the AVAMC cannot be attributed to a single specific practice but rather should be attributed to an increased focus on infection control practices throughout the institution. Since 2003, ICU-related bloodstream infection rates (all organisms) have decreased and have reached levels well below the National Healthcare Safety Network’s median rates. Central-line insertion techniques, VAP prevention bundles, and installation of hand sanitizer dispensers in patient care areas have all likely contributed to this reduction.
Our study has several unique strengths. All infections during the study period were analyzed prospectively using a consistent clinical and microbiologic definition and reviewed by the same experienced infectious disease physician. Also, our longitudinal trend analysis used a consistent cohort followed over the entire analysis period in a healthcare population with high retention.
However, our study does have several limitations. First, the changes appreciated in Atlanta may not be applicable to other regions of the United States where population characteristics are different.32–34 Second, although the majority of veterans receive care primarily at the AVAMC, infections treated and cultured at non-VA medical centers would have been missed and caused a reduction in estimates. Third, our definition of MRSA infection required a positive clinical culture in addition to clinical findings consistent with infection. We assume that the rate of culturing of clinical specimens did not change throughout the study. Also, nonpurulent SSTIs due to MRSA were likely missed because of the lack of a culturable specimen. Fourth, data on compliance with hand hygiene and barrier precautions is lacking. Although not suspected, poor compliance with directive measures could potentially account for no change in infection rates. Finally, low numbers of HO infections that required extended time intervals for rate calculations combined with limited predirective data prevented a more robust statistical analysis with segmented regression.
Our study of the AVAMC population shows a consistent trend of MRSA reduction in both the community and health-care settings. These reductions in infection rates cannot be attributed to a single intervention and are the likely result of a multitude of factors. With MRSA infection rates at very low levels in the hospital and the community burden decreasing, the use of expensive, labor-intensive control programs targeting a single organism must be reevaluated.
Acknowledgments
Financial support. Public Health Service grant UL RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
Presented in part: 49th Annual Meeting of the Infectious Diseases Society of America; Boston, Massachusetts; October 20–23, 2011.
References
- 1.Jevons MP, Coe AW, Parker MT. Methicillin resistance in staphylococci. Lancet. 1963;1(7287):904–907. doi: 10.1016/s0140-6736(63)91687-8. [DOI] [PubMed] [Google Scholar]
- 2.Panlilio AL, Culver DH, Gaynes RP, et al. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975–1991. Infect Control Hosp Epidemiol. 1992;13(10):582–586. doi: 10.1086/646432. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis. 2006;42(3):389–391. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 4.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301(7):727–736. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 5.Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA. 2010;304(6):641–648. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]
- 6.Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus: Minnesota and North Dakota, 1997–1999. MMWR Morb Mortal Wkly Rep. 1999;48(32):707–710. [PubMed] [Google Scholar]
- 7.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279(8):593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JK, Khoie T, Shurland S, Kreisel K, Stine OC, Roghmann MC. Skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus USA300 clone. Emerg Infect Dis. 2007;13(8):1195–1200. doi: 10.3201/eid1308.061575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12(11):1715–1723. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talan DA, Krishnadasan A, Gorwitz RJ, et al. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis. 2011;53(2):144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- 12.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 13.Moellering RC, Jr, Abbott GF, Ferraro MJ. Case records of the Massachusetts General Hospital. Case 2–2011. A 30-year-old woman with shock after treatment of a furuncles. N Engl J Med. 2011;364(3):266–275. doi: 10.1056/NEJMcpc1003886. [DOI] [PubMed] [Google Scholar]
- 14.Hota B, Ellenbogen C, Hayden MK, Aroutcheva A, Rice TW, Weinstein RA. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167(10):1026–1033. doi: 10.1001/archinte.167.10.1026. [DOI] [PubMed] [Google Scholar]
- 15.Hota B, Lyles R, Rim J, et al. Predictors of clinical virulence in community-onset methicillin-resistant Staphylococcus aureus infections: the importance of USA300 and pneumonia. Clin Infect Dis. 2011;53(8):757–765. doi: 10.1093/cid/cir472. [DOI] [PubMed] [Google Scholar]
- 16.Tracy LA, Furuno JP, Harris AD, Singer M, Langenberg P, Roghmann MC. Staphylococcus aureus infections in US veterans, Maryland, USA, 1999–2008. Emerg Infect Dis. 2011;17(3):441–448. doi: 10.3201/eid1703.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 19.Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012;308(1):50–59. doi: 10.1001/jama.2012.7139. [DOI] [PubMed] [Google Scholar]
- 20.Caffrey AR, Laplante KL. Changing epidemiology of methicillin-resistant Staphylococcus aureus in the Veterans Affairs Healthcare System, 2002–2009. Infection. 2012;40(3):291–297. doi: 10.1007/s15010-011-0232-3. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer MK, Ellingson K, Conover C, et al. Evaluation of international classification of diseases, ninth revision, clinical modification codes for reporting methicillin-resistant Staphylococcus aureus infections at a hospital in Illinois. Infect Control Hosp Epidemiol. 2010;31(5):463–468. doi: 10.1086/651665. [DOI] [PubMed] [Google Scholar]
- 22.Tracy LA, Furuno JP, Harris AD, Singer M, Langenberg P, Roghmann MC. Predictive ability of positive clinical culture results and international classification of diseases, ninth revision, to identify and classify noninvasive Staphylococcus aureus infections: a validation study. Infect Control Hosp Epidemiol. 2010;31(7):694–700. doi: 10.1086/653206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(5):752–760. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 24.Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39(10):1446–1453. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 25.Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 26.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003;24(5):362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 27.Weber SG, Huang SS, Oriola S, et al. Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enter-ococci: position statement from the Joint SHEA and APIC Task Force. Infect Control Hosp Epidemiol. 2007;28(3):249–260. doi: 10.1086/512261. [DOI] [PubMed] [Google Scholar]
- 28.McGinigle KL, Gourlay ML, Buchanan IB. The use of active surveillance cultures in adult intensive care units to reduce methicillin-resistant Staphylococcus aureus-related morbidity, mortality, and costs: a systematic review. Clin Infect Dis. 2008;46(11):1717–1725. doi: 10.1086/587901. [DOI] [PubMed] [Google Scholar]
- 29.Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407–1418. doi: 10.1056/NEJMoa1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 31.Gurieva T, Bootsma MC, Bonten MJ. Successful Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections revisited. Clin Infect Dis. 2012;54(11):1618–1620. doi: 10.1093/cid/cis272. [DOI] [PubMed] [Google Scholar]
- 32.Hudson LO, Murphy CR, Spratt BG, et al. Differences in meth-icillin-resistant Staphylococcus aureus (MRSA) strains isolated from pediatric and adult patients from hospitals in a large Cal-ifornia county. J Clin Microbiol. 2012;50(3):573–579. doi: 10.1128/JCM.05336-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin Infect Dis. 2008;46(11):1637–1646. doi: 10.1086/587893. [DOI] [PubMed] [Google Scholar]
- 34.Laupland KB, Church DL, Mucenski M, Sutherland LR, Davies HD. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J Infect Dis. 2003;187(9):1452–1459. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]