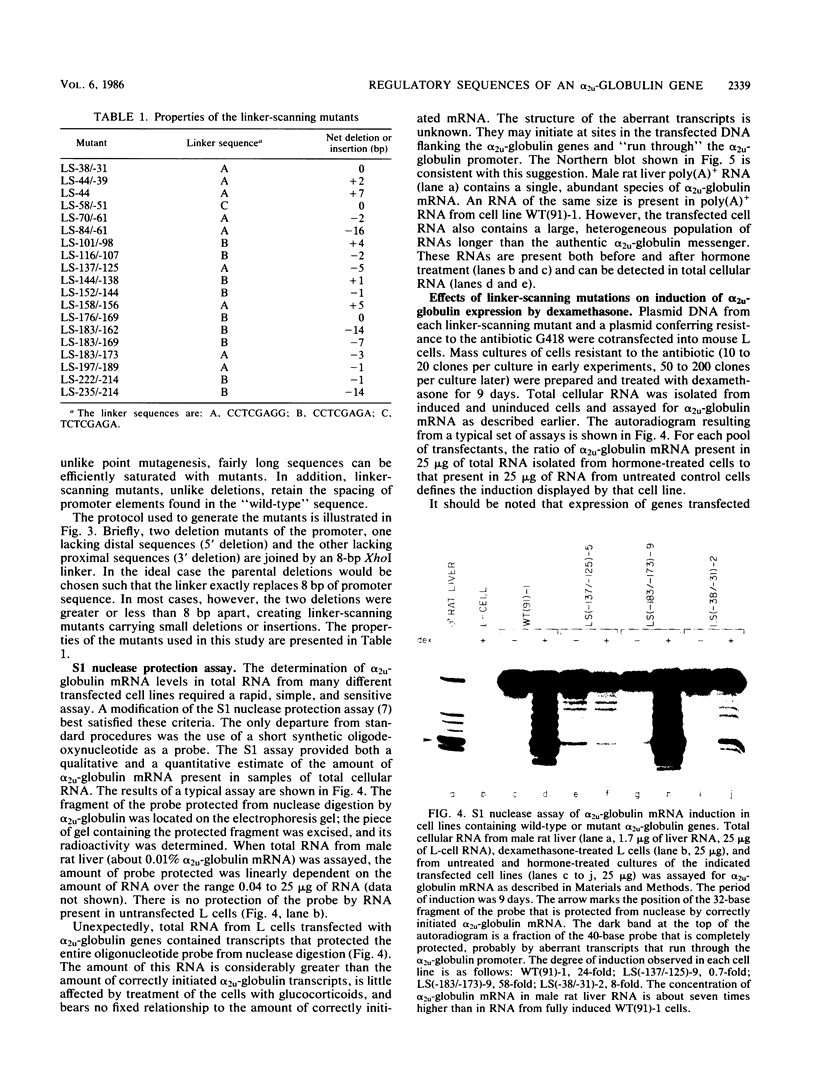
Abstract
alpha 2u-Globulin is a rat protein of as yet unknown function whose synthesis can be induced by glucocorticoids and several other hormones. Induction by glucocorticoids is a secondary response to the hormone: protein synthesis is required before the hormone can exert its stimulatory effect on alpha 2u-globulin transcription. We have used the linker-scanning mutagenesis procedure, followed by transfer of the mutant genes into mouse L-cells for analysis of their phenotype, to determine sequences within a cloned alpha 2u-globulin promoter that are required for its regulation by glucocorticoids. Mutations between positions -115 and -160 abolish or greatly reduce the inducibility of alpha 2u-globulin by the hormone. Mutations just upstream from this region, between positions -177 and -220, have an opposite effect; they increase induction two- to fourfold.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Chihara C., Meltzer P., Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- Ballard P. L., Baxter J. D., Higgins S. J., Rousseau G. G., Tomkins G. M. General presence of glucocorticoid receptors in mammalian tissues. Endocrinology. 1974 Apr;94(4):998–1002. doi: 10.1210/endo-94-4-998. [DOI] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Pfahl M., Baulieu E. E. DNA binding properties of glucocorticosteroid receptors bound to the steroid antagonist RU-486. EMBO J. 1984 Apr;3(4):751–755. doi: 10.1002/j.1460-2075.1984.tb01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram R. J., Kornberg R. D. Specific protein binding to far upstream activating sequences in polymerase II promoters. Proc Natl Acad Sci U S A. 1985 Jan;82(1):43–47. doi: 10.1073/pnas.82.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Geisse S., Wenz M., Westphal H. M., Beato M. The nucleotide sequences recognized by the glucocorticoid receptor in the rabbit uteroglobin gene region are located far upstream from the initiation of transcription. EMBO J. 1984 Dec 1;3(12):2771–2778. doi: 10.1002/j.1460-2075.1984.tb02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Feigelson P. Cycloheximide inhibition of hormonal induction of alpha 2u-globulin mRNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2669–2673. doi: 10.1073/pnas.76.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Ghazal P., Bingham R. W., Barrett D., Bishop J. O. Sequence structures of a mouse major urinary protein gene and pseudogene compared. EMBO J. 1985 Dec 1;4(12):3159–3165. doi: 10.1002/j.1460-2075.1985.tb04059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clever U., Romball C. G. RNA and protein synthesis in the cellular response to a hormone, ecdysone. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1470–1476. doi: 10.1073/pnas.56.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan K. P., Unterman R., McLaughlin M., Nakhasi H. L., Lynch K. R., Feigelson P. The structure and expression of very closely related members of the alpha 2u globulin gene family. J Biol Chem. 1982 Nov 25;257(22):13527–13534. [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Lewis C. D., Felsenfeld G. Interaction of specific nuclear factors with the nuclease-hypersensitive region of the chicken adult beta-globin gene: nature of the binding domain. Cell. 1985 May;41(1):21–30. doi: 10.1016/0092-8674(85)90057-1. [DOI] [PubMed] [Google Scholar]
- Feinstein S. C., Ross S. R., Yamamoto K. R. Chromosomal position effects determine transcriptional potential of integrated mammary tumor virus DNA. J Mol Biol. 1982 Apr 15;156(3):549–565. doi: 10.1016/0022-2836(82)90266-2. [DOI] [PubMed] [Google Scholar]
- Giniger E., Varnum S. M., Ptashne M. Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell. 1985 Apr;40(4):767–774. doi: 10.1016/0092-8674(85)90336-8. [DOI] [PubMed] [Google Scholar]
- Gubits R. M., Lynch K. R., Kulkarni A. B., Dolan K. P., Gresik E. W., Hollander P., Feigelson P. Differential regulation of alpha 2u globulin gene expression in liver, lachrymal gland, and salivary gland. J Biol Chem. 1984 Oct 25;259(20):12803–12809. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hynes N., van Ooyen A. J., Kennedy N., Herrlich P., Ponta H., Groner B. Subfragments of the large terminal repeat cause glucocorticoid-responsive expression of mouse mammary tumor virus and of an adjacent gene. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3637–3641. doi: 10.1073/pnas.80.12.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Kreuzer K. N., Alberts B. M. Site-specific recognition of bacteriophage T4 DNA by T4 type II DNA topoisomerase and Escherichia coli DNA gyrase. J Biol Chem. 1984 Apr 25;259(8):5339–5346. [PubMed] [Google Scholar]
- Kulkarni A. B., Gubits R. M., Feigelson P. Developmental and hormonal regulation of alpha 2u-globulin gene transcription. Proc Natl Acad Sci U S A. 1985 May;82(9):2579–2582. doi: 10.1073/pnas.82.9.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Chan K. M., Feigelson P. Glucocorticoid induction of hepatic alpha2u-globulin synthesis and messenger RNA level in castrated male rats in vivo. J Biol Chem. 1978 Nov 10;253(21):7886–7890. [PubMed] [Google Scholar]
- Kurtz D. T. Hormonal inducibility of rat alpha 2u globulin genes in transfected mouse cells. Nature. 1981 Jun 25;291(5817):629–631. doi: 10.1038/291629a0. [DOI] [PubMed] [Google Scholar]
- Kurtz D. T., McCullough L., Bishop D. K., Manos M. M. DNA sequences required for hormonal induction of rat alpha 2u-globulin genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):985–988. doi: 10.1101/sqb.1983.047.01.112. [DOI] [PubMed] [Google Scholar]
- Kurtz D. T. Rat alpha 2u globulin is encoded by a multigene family. J Mol Appl Genet. 1981;1(1):29–38. [PubMed] [Google Scholar]
- Laperche Y., Lynch K. R., Dolan K. P., Feigelson P. Tissue-specific control of alpha 2u globulin gene expression: constitutive synthesis in the submaxillary gland. Cell. 1983 Feb;32(2):453–460. doi: 10.1016/0092-8674(83)90465-8. [DOI] [PubMed] [Google Scholar]
- Liao Y. C., Taylor J. M., Vannice J. L., Clawson G. A., Smuckler E. A. Structure of the rat alpha 1-acid glycoprotein gene. Mol Cell Biol. 1985 Dec;5(12):3634–3639. doi: 10.1128/mcb.5.12.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Marks A. R., Buckley D. I., Kapler G., Payvar F., Goodman H. M. The first intron of the human growth hormone gene contains a binding site for glucocorticoid receptor. Proc Natl Acad Sci U S A. 1985 Feb;82(3):699–702. doi: 10.1073/pnas.82.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Pöche H., Junghahn I., Geissler E., Bielka H. Cycloheximide resistance in Chinese hamster cells. III. Characterization of cell-free protein synthesis by polysomes. Mol Gen Genet. 1975;138(2):173–177. [PubMed] [Google Scholar]
- Reinke R., Feigelson P. Rat alpha 1-acid glycoprotein. Gene sequence and regulation by glucocorticoids in transfected L-cells. J Biol Chem. 1985 Apr 10;260(7):4397–4403. [PubMed] [Google Scholar]
- Renkawitz R., Schütz G., von der Ahe D., Beato M. Sequences in the promoter region of the chicken lysozyme gene required for steroid regulation and receptor binding. Cell. 1984 Jun;37(2):503–510. doi: 10.1016/0092-8674(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Regulation of mouse mammary tumor virus gene expression by glucocorticoid hormones. Curr Top Microbiol Immunol. 1983;106:79–103. doi: 10.1007/978-3-642-69357-1_4. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G. Control of gene expression by glucocorticoid hormones. Biochem J. 1984 Nov 15;224(1):1–12. doi: 10.1042/bj2240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. K., Chatterjee B., Prasad M. S., Unakar N. J. Role of insulin in the regulation of the hepatic messenger RNA for alpha 2u-globulin in diabetic rats. J Biol Chem. 1980 Dec 10;255(23):11614–11618. [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. An analysis of the kinetics of rat liver tryptophan pyrrolase induction: the significance of both enzyme synthesis and degradation. Biochem Biophys Res Commun. 1964 Mar 26;15(3):214–219. doi: 10.1016/0006-291x(64)90148-2. [DOI] [PubMed] [Google Scholar]
- Schmid W., Scherer G., Danesch U., Zentgraf H., Matthias P., Strange C. M., Röwekamp W., Schütz G. Isolation and characterization of the rat tryptophan oxygenase gene. EMBO J. 1982;1(10):1287–1293. doi: 10.1002/j.1460-2075.1982.tb00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Scherer G., Schmid W., Zentgraf H., Schütz G. Isolation and characterization of the rat tyrosine aminotransferase gene. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1346–1350. doi: 10.1073/pnas.81.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Ringold G. M., McLean J. W., Taylor J. M. Induction of the acute-phase reactant, alpha 1-acid glycoprotein, by glucocorticoids in rat hepatoma cells. DNA. 1983;2(3):205–212. doi: 10.1089/dna.1983.2.205. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Widman L. E., Chasin L. A. Multihormonal induction of alpha 2u-globulin in an established rat hepatoma cell line. J Cell Physiol. 1982 Sep;112(3):316–326. doi: 10.1002/jcp.1041120303. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yaniv M. Regulation of eukaryotic gene expression by transactivating proteins and cis acting DNA elements. Biol Cell. 1984;50(3):203–216. doi: 10.1111/j.1768-322x.1984.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]