Abstract
Deltamethrin (DM) insecticides are currently being promoted worldwide for mosquito control, because of the high efficacy, low mammalian toxicity and less environmental impact. Widespread and improper use of insecticides induced resistance, which has become a major obstacle for the insect-borne disease management. Resistance development is a complex and dynamic process involving many genes. To better understand the possible molecular mechanisms involved in DM resistance, a proteomic approach was employed for screening of differentially expressed proteins in DM-susceptible and -resistant mosquito cells. Twenty-seven differentially expressed proteins were identified by two-dimensional electrophoresis (2-DE) and mass spectrometry (MS). Four members of the ubiquitin-proteasome system were significantly elevated in DM-resistant cells, suggesting that the ubiquitin-proteasome pathway may play an important role in DM resistance. Proteasome subunit beta type 6 (PSMB6) is a member of 20S proteasomal subunit family, which forms the proteolytic core of 26S proteasome. We used pharmaceutical inhibitor and molecular approaches to study the contributions of PSMB6 in DM resistance: the proteasome inhibitor MG-132 and bortezomib were used to suppress the proteasomal activity and siRNA was designed to block the function of PSMB6. The results revealed that both MG-132 and bortezomib increased the susceptibility in DM-resistant cells and resistance larvae. Moreover, PSMB6 knockdown decreased cellular viability under DM treatment. Taken together, our study indicated that PSMB6 is associated with DM resistance in mosquitoes and that proteasome inhibitors such as MG-132 or bortezomib are suitable for use as a DM synergist for vector control.
Introduction
Mosquito-borne diseases, such as malaria, dengue fever, yellow fever, filariasis and encephalitis, cause severe mortality and morbidity around the world, and pose significant threats to public health [1]–[4]. For a long period of time, insecticides have been the primary method for managing mosquito-borne diseases [5], [6]. Pyrethroid, one of the most prevalent insecticides, interacts with ion channels, which disrupt the transmembrane potentials, and damage the insect nervous system [7]. Deltamethrin (DM), a representative synthetic pyrethroid insecticide, is widely used for bed net impregnation and residual spraying for mosquito control [8], [9]. Widespread and improper use of insecticides has induced the development of insecticide resistance [10], [11], which has become the main obstacle for the mosquito-borne disease management [12]–[14].
Insecticide resistance is polygenic inheritance phenomenon, which suggests that multiple genes are associated with resistance [15]. Large-scale transcriptional gene expression profiling based on suppression subtractive hybridization (SSH) and cDNA microarray studies had been carried out to identify DM resistance-associated genes in Culex pipiens pallens [16], [17]. Although novel genes associated with DM resistance have been identified, the mechanisms underlying DM resistance are still not fully understood. Proteomics research, a strategy focusing on protein expression profiling, has great advantages in the study of complicated biological events [18]. Therefore, characterization and comparison of the protein profiles of susceptible and resistant strains will provide valuable information regarding the resistance mechanisms in mosquitoes.
The ubiquitin-proteasome system plays an essential role in cell cycle regulation, stress, carcinogenesis, and DNA repair, through influencing protein stability [19]–[24]. The ubiquitin-conjugating system generated polyubiquitinated proteins serve as substrates for the proteasome [25], [26]. In brief, proteins modified through ubiquitin-activating enzyme E1, ubiquitin-conjugating enzyme E2, and ubiquitin-protein ligase E3 are targeted by the 26S proteasome for degradation [27].
The proteasome 20S core is a multi-subunit protein complex comprised of two α-rings and two β-rings, which have regulatory activity and proteolytic activity, respectively [28], [29]. The 20S proteasome is responsible for the breakdown of shortlived proteins involved in cellular apoptosis, DNA repair, endocytosis, cell cycle regulation and for the rapid removal of misfolded proteins [30]. PSMB6 belongs to the 20S proteasomal subunit family, which participates in catalyzing ubiquitin-protein degradation [31].
This study was designed to isolate differentially expressed proteins identified by protein profiling of DM-susceptible and -resistant mosquito cells, and to study the role of PSMB6 in mosquito deltamethrin resistance.
Materials and Methods
2.1 Cell Culture and Mosquito Strains
Aedes albopictus C6/36 cells were obtained from the China Center for Type Culture Collection (Wuhan, China). The DM-resistant C6/36 strains were selected with increasing dose of DM for hundreds of generations. The resistant strains had a 14.8-fold increase in the 50% lethal concentration (LC50) value compared with the DM-susceptible C6/36 strains. Cells were cultured in DMEM/High Glucose media (Hyclone; UT, USA) including 1% penicillin-streptomycin (Gibco; Carlsbad, USA) and 10% fetal bovine serum (Sijiqing; Zhejiang, China) in a 5% CO2-humidified incubator at 28°C.
A DM-susceptible strain of Cx. pipiens pallen (strain 01, the LC50 was 0.008 mg/L) which had never been exposed to insecticides, was obtained from the Shanghai Insect Institute of the Chinese Academy of Sciences (Shanghai, China). The DM (Sigma; St. Louis, USA) concentration used for selection was determined by LC50, which was calculated by larval bioassays. Two DM-resistant strains (strain 03 and 07) were used in this study. Strain 03 were selected by 0.05 mg/L DM for every generation and strain 07 were selected with increasing dose of DM for generations. For MG-132-treatment (Santa Cruz; Santa Cruz, USA) experiment, mosquitoes were subjected to DM selection for more than 10 generations, the LC50 for strain 03 and strain 07 were 0.08 mg/L and 0.56 mg/L, respectively. For bortezomib-treatment (LC Laboratories; Woburn, USA) experiment, mosquitoes were subjected to DM selection for more than 40 generations, the LC50 for strain 03 and strain 07 were 0.74 mg/L and 3.8 mg/L, respectively. All strains were maintained with approximately 14 h:10 h light/dark cycle at 28°C.
2.2 Two-Dimensional Gel Electrophoresis
DM-susceptible and -resistant cells were lysed in buffer containing 7 M urea, 65 mM dithiothreitol (DTT), 2% (v/v) IPG buffer (pH 3–10, nonlinear), 4% (w/v) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS) (GE Healthcare; Uppsala, Sweden), 2 M thiourea and 1% (v/v) protease inhibitor cocktail (Roche; Rockford, USA). The extracts were centrifuged at 50,000×g for 1 h, and the supernatants were stored at −80°C. The Bradford method was used for protein concentration quantification with BSA as the standard [32]. Sample proteins (120 µg) were loaded by gel rehydration on a 24-cm immobilized (pH 3–10) nonlinear gradient strips for 2-DE. Electrophoretic separation was performed under the following conditions: step-n-hold, 30 V for 6 h and 60 V for 6 h, gradient:500 V for 1 h, 1000 V for 1 h, 3000 V for 3 h and 8000 V for 3 h, step-n-hold: 8000 V for 20 h. Isoelectric focusing (IEF) was performed using IPGphor apparatus (Amersham Bioscience, Piscataway, USA).
An Ettan DALTsix system (GE Healthcare, San Francisco, CA, USA) was applied for the second-dimension gel separation [33]. Silver staining was carried out based on a published protocol [34] except that glutaraldehyde was not included in the sensitizing solution. Gels were scanned and analyzed using ImageMaster 2D Platinum Software (GE Healthcare, San Francisco, CA, USA) as previously described [35]. Statistically significant differences were evaluated with the Student's t-test (ImageMasterTM 2D platinum software, *P<0.05).
2.3 In-Gel Tryptic Digestion and MALDI-TOF/TOF
Silver-stained protein spots were excised, dehydrated in acetonitrile, and dried at room temperature. Proteins were reduced with 10 mM DTT and 25 mM ammonium bicarbonate (NH4HCO3) at 56°C for 1 h and 55 mM iodoacetamide and 25 mM NH4HCO3 at RT for 45 min in the dark. Then gel pieces were completely washed with 25 mM NH4HCO3, 50% acetonitrile and 100% acetonitrile in succession and were thoroughly dried using Speedvac (Concentrator 5301, Eppendorf, Hamburg, Germany). The dried gel pieces were rehydrated with 2 µl trypsin (Promega, Madison, WI, USA) solution (10 mg/L trypsin in 25 mM NH4HCO3) and incubated at 4°C for 40 min. The excess liquid was discarded, and the gel plugs were incubated at 37°C for 12 h before the reaction was stopped by the addition of trifluoroacetic acid (TFA) (Sigma; St. Louis, USA) at a final concentration of 0.1%.
The extracted peptide mixture was then analyzed by MALDI-TOF mass spectrometry and tandem TOF/TOF mass spectrometry which was carried out on a time-of-flight Ultraflex II mass spectrometer (Biflex; Bruker Daltonics, Germany). Peptide mass maps were acquired in positive ion mode using a SmartBeam solid laser (averaging 800 laser shots per MALDI-TOF spectrum and 800 laser shots per TOF/TOF spectrum). Resolution was 15,000 to 20,000. The spectrum was calibrated by bruker calibration mixtures to a mass tolerance within 0.1 Da [33].
2.4 Database Queries and Protein Identifications
The m/z and resolution for mass spectra were ranging from 700 to 4,000 and 10,000 to 20,000, respectively. Results were analyzed using the FlexAnalysis software (version 2.4, Bruker Daltonik GmbH) with the following parameters: peak detection algorithm, Sort Neaten Assign and Place (SNAP); S/N threshold, 3.0; quality factor threshold, 50. The tryptic autodigestion peptides (842.51 and 1,045.56 Da) were used as internal standards. The matrix or auto-proteolytic trypsin fragments and known contaminants (e.g., keratins) were removed. The search conditions used were as described [36]. The detailed principle is available online (http://www.matrixscience.com/pdf/2003WKSHP2.pdf). Search parameters for MS data: 100 ppm for the precursor ion and 0.3 Da for the fragment ions. Covalent modifications and cleavage specificity were recognized to be the same as those described for peptide mass fingerprint (PMF) analysis. Confidence intervals exceeding 95% were considered significant. All significant MS results identified results by Mascot were manually validated for spectral quality, and y and b ion series matches.
2.5 Larvicidal Activity Assay
Three strains of Cx. pipiens pallen (strain 01, 03 and 07) were used for these experiments. In each strain, 180 early fourth instar larvae, randomly divided into three groups, were subjected to different treatments. Larvae were pre-treated with 1 µM MG-132 or 0.125 µM bortezomib for 4 h, followed by DM treatment. The LC50 of each strain was used as the test concentration. The larvae were exposed to these solutions at 28°C for a14 h∶10 h light/dark cycle and the percentage of survival was recorded. MG-132 or bortezomib treatment alone and DM treatment alone were applied as control and each assay was repeated three times.
2.6 RNA extraction and cDNA synthesis
Total RNA of mosquito cells was extracted by TriZol Reagent (Invitrogen; Carlsbad, USA) and the cDNA was reverse transcribed from 1 µg of total RNA by the SuperScript® VILO™ cDNA Synthesis kit (Invitrogen; Carlsbad, USA), according to the manufacturer's instructions.
2.7 Cloning of Cx. pipiens pallen PSMB6 full-length cDNA
Full-length Cx. pipiens pallen PSMB6 cDNA was amplified in separate reactions covering three different regions: the open reading frame (ORF), 5′-cDNA ends (5′-RACE) and 3′-cDNA ends (3′-RACE) of PSMB6. The ORF of PSMB6 was amplified using the following primers: 5′-GGACTAGTGAGATGGAAATGGACATGGCTACTCAAACGACT-3′ (sense) and 5′-CCCTCGAGCTAAGCACGAACGGCGACC-3′ (antisense), designed based on its homologue Cx. quinquefasciatus PSMB6 (GenBank No. XM_001846164.1). PCR reactions were accomplished using KOD-Plus-Neo-401 (TOYOBO; Doushima Hama, Japan) according to the manufacturer's instructions. Amplification of the 5′-RACE and 3′-RACE of PSMB6 was carried out using a SMART™ RACE cDNA Amplification Kit (Clontech; Mountain View, USA). The sequences of 5′-RACE and 3′-RACE adaptor primers supplied by the SMART™ RACE cDNA Amplification Kit were: 5′-CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT-3′ and 5′-CTAATACGACTCACTATAGGGC-3′, respectively. The specific primer sequences for 5′-RACE and 3′-RACE were: 5′-TGTGGTGGGCGTTTCTCCAGTCGT-3′ and 5′-CCATTGGAGGTTCGGGAAGTTCGTACA-3′, respectively. The PCR reactions were carried out using an Advantage®2 PCR Kit (Clontech; Mountain View, USA) following the manufacturer's instructions. PCR products were separated by 1% agarose gel electrophoresis and purified using a QIA quick Gel extraction kit (QIAGEN; GmbH, Germany). The purified products were sequenced by the Shanghai Invitrogen Biotechnology Company (Shanghai, China). Finally, all sequences were assembled to generate the putative full-length cDNA of PSMB6.
2.8 Sequence Alignment and Phylogenetic Analysis
The standard protein/protein BLAST sequence comparison programs (http://beta.uniprot.org/?tab=blast) were used to search sequences with similarities to the translated sequences of PSMB6 in the SWISS-PROT databases. Deduced amino acid sequences were aligned by the ClustalW2 computer program (http://www.ebi.ac.uk/Tools/clustalw2/index.html). The MEGA 5.0 program was used to construct the phylogenic tree.
2.9 Quantitative PCR analysis
Quantitative PCR assays were performed with the ABI PRISM 7300 equipment (Applied Biosystems, Foster City, USA). The primers used are all listed in Table S1. According to the manufacturer's instruction, each reaction was performed in a total volume of 20 µl containing cDNA, specific forward and reverse primers and LightCycler FastStart DNA Master SYBR Green I (Roche; Rockford, USA). A melting curve was generated immediately after the reaction to check the specificity and the data were analyzed with 7300 System SDS Software v1.2.1 (Applied Biosystems). The parameters for PCR were set as: 95°C for 30 s, followed by 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, and the dissociation curve was inspected for quality control purposes. β-actin was used as the internal control. The relative gene expression level was calculated from the threshold cycle (Ct) value of each reaction through Delta-delta Ct method [37].
2.10 Construction of PSMB6-siRNA
Ae. albopictus PSMB6 (GenBank No. GU_371441.1) was amplified with two degenerate primers, which were designed based on the conserved PSMB6 segment of Cx. quinquefasciatus and An. gambiae. Double stranded siRNA molecules corresponding to the partial PSMB6 cDNA sequence were designed and synthesized by the GenePharma Company (Shanghai, China). The sequences of PSMB6 siRNA and scrambled siRNA (used as a negative control) were as follows: PSMB6-siRNA, 5′-UGGCCGGAUUUGAUAACAATT-3′ (sense) and 5′-UUGUUAUCAAAUCCGGCCATT-3′ (antisense); and scrambled siRNA, 5′ –GCGACGAUCUGCCUAAGA-3′ (sense) and 5′-AUCUUAGGCAGAUCGUCG-3′ (antisense).
2.11 Cell Viability Analysis
Resistant and susceptible cells were seeded (2×104/well) in 100 µl per well of complete media in four 96-well plates and incubated for 24 h. Cells were pre-treated with 1 µM MG-132 or 0.1 µM bortezomib. 1% (v/v) Dimethyl sulfoxide (DMSO) (Sigma; St. Louis, USA) alone was used as a negative control. Cells (resistance and susceptible) were then treated with 100 µl MG-132 or bortezomib for 4 h. Other batches of cell were transfected with PSMB6-siRNA for 7 h. siRNA (6 µl) and X-tremeGENE siRNA Transfection Reagent (6 µl) (Roche; Rockford, USA) were mixed in 1.2 ml DMEM in a RNase-free tube for 15 min at room temperature, then 4.8 ml DMEM was added to the transfection mixture, mixed again and added to cells (100 µl per well). Cells transfected with scrambled siRNA were used as negative controls. Cells were treated with 100.5, 101, 101.5, 102, 102.5 mg/L of DM for 68 h. According to the manufacturer's instructions, CCK-8 reagents (Dojindo; Gaithersburg, USA) was added to the medium and incubated for a further 4 h at 28°C. A microplate reader (Biotek Instruments; Winooski, USA) was used to measure the absorbance at 450 nm. Cell viability was calculated as a percentage based upon control cell viability.
2.12 Statistical analysis
All statistical analysis was performed with GraphPad 5.0 (GraphPad Software). Statistically significant differences were evaluated with the Student's t-test (*P<0.05, **P<0.01). All experiments were performed in triplicates on at least three separate occasions.
Results
3.1 Characterization of DM-resistant Mosquito Cells
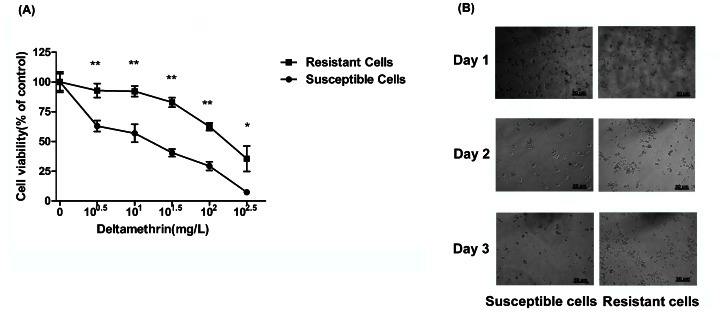
DM-resistance mosquito cells were selected with increasing doses of DM for hundreds of generations to generate the DM-resistance cells and cell viability was analyzed using a modified MTT method (CCK-8). As shown in Figure 1, after DM selection, the LC50 for the resistant cells was 251 mg/L, much higher than 17 mg/L for the susceptible cells (Figure 1A). DM-selection in resistance cells significantly improved cell viability and proliferation compared with susceptible cells (Figure 1B).
Figure 1. Characterization of DM-resistant mosquito cells.
(A) Resistant and susceptible cells were treated with DM at the indicated concentrations, and cell viability was measured after 72 h treatment. The percentage of viable cell is shown relative to the control (DMSO only). The results shown are representative of three independent experiments. After treatment with 50 mg/L DM for 72 h, representative images (B, 200×) of susceptible- and resistant-cells were taken.
3.2 Identification and quantification protein spots on 2-DE gels
A representative 2-DE gel image for protein expression of DM-resistant and -susceptible mosquito cell is presented in Figure S1. Thirty-six proteins were identified to be significantly differently expressed between two groups (P<0.05 with average spot intensity greater than 1.2-fold). All spots were subjected to tryptic digestion and MALDI-TOF/MS analysis. Using PMF analysis, twenty-seven proteins were identified (summary of these proteins, including accession numbers, protein names, scores, sequence coverage, Mr, and pI, are listed in Table 1 and the raw data of each protein are shown in Table S2). The other nine proteins were unidentified because of incomplete polypeptide fragments or low abundance. A further magnified 2-DE gel image of four DM-resistant upregulated proteins: proteasome subunit beta type 6 (PSMB6), 26S proteasome non-ATPase regulatory subunit 14 (PSMD14/POH1), ubiquitin-conjugating enzyme (E2) and ubiquitin-specific protease, putative (USP) was shown in Figure 2. These proteins are components of the ubiquitin-proteasome system. Quantitative PCR confirmed that gene expression of PSMB6, POH1, E2 and USP was 1.59, 1.46, 1.80 and 2.93-fold increased in DM-resistant mosquito strain (strain 07, LC50 was 3.8 mg/L) compared with those of DM-susceptible strain (strain 01) (Figure 2C).
Table 1. The Profile of 27 Proteins Identified by 2-DE.
| ID | Acc. no. | Protein description | No. of matched peptides | Score | Sequence coverage(%) | Mr/pI | Fold of resistant/susceptible | P value |
| Upa | ||||||||
| 253 | gi|157125883 | 40S ribosomal protein S12 | 13 | 183 | 68 | 15687/6.20 | 4.23 | 0.0069 |
| 407 | gi|94469190 | proteasome subunit beta type 6 precursor-like protein | 16 | 163 | 56 | 25028/5.45 | 1.83 | 0.0023 |
| 508 | gi|157108608 | pap-inositol-1,4-phosphatase | 13 | 99 | 39 | 33892/5.79 | 4.24 | 0.0034 |
| 536 | gi|157130038 | elongation factor ts | 12 | 69 | 26 | 34245/5.94 | 4.26 | 0.0007 |
| 554 | gi|157112126 | phosphoglycerate mutase | 15 | 149 | 51 | 28595/6.34 | 2.89 | 0.0006 |
| 583 | gi|170043858 | 26S proteasome non-ATPase regulatory subunit 14 | 12 | 114 | 31 | 34696/5.74 | 1.84 | 0.0315 |
| 618 | gi|157114501 | 14-3-3 protein | 11 | 123 | 43 | 28324/4.78 | 5.17 | 0.0002 |
| 672 | gi|157134886 | aldo-keto reductase | 17 | 165 | 43 | 35865/5.20 | 3.58 | 0.0192 |
| 725 | gi|157131170 | l-lactate dehydrogenase | 18 | 176 | 32 | 35812/6.61 | 3.32 | 0.0317 |
| 728 | gi|94469060 | transaldolase | 21 | 169 | 44 | 35909/5.45 | 3.63 | 0.0170 |
| 898 | gi|170036819 | elongation factor ts | 28 | 69 | 10 | 35022/5.88 | 1.81 | 0.0032 |
| 966 | gi|157134639 | eukaryotic translation initiation factor 3 subunit | 10 | 86 | 27 | 36224/5.56 | 1.99 | 0.0335 |
| 991 | gi|157113904 | acetyl-coa acetyltransferase, mitochondrial | 11 | 105 | 30 | 43577/8.60 | 2.27 | 0.0329 |
| 1011 | gi|157130317 | actin | 14 | 134 | 33 | 44980/5.92 | 2.64 | 0.0396 |
| 1095 | gi|157115992 | UDP-glucose 4-epimerase | 25 | 228 | 57 | 39001/6.46 | 2.24 | 0.017 |
| 1153 | gi|157110699 | ethanolamine-phosphate cytidylyltransferase | 14 | 98 | 26 | 42278/6.06 | 3.27 | 0.0229 |
| 1186 | gi|157130058 | hypothetical protein | 8 | 73 | 26 | 44678/5.93 | 4.13 | 0.0089 |
| 1206c | gi|157139358 | ubiquitin-conjugating enzyme E2 | 9 | 68 | 57 | 14187/5.89 | 5.29 | 0.0039 |
| gi|158292977 | AGAP004890-PB | 13 | 81 | 22 | 45597/7.17 | |||
| 1236 | gi|170054846 | mannose-1-phosphate guanylyltransferase | 9 | 67 | 22 | 39381/6.68 | 1.68 | 0.0435 |
| 1348 | gi|157116575 | chaperonin | 9 | 79 | 25 | 53596/5.59 | 2.36 | 0.0135 |
| 1463 | gi|157106871 | phenylalanyl-tRNA synthetase beta chain | 17 | 100 | 28 | 66523/5.85 | 2.92 | 0.0227 |
| 1487 | gi|157110521 | adenylylsulfate kinase | 19 | 93 | 22 | 70212/5.87 | 2.10 | 0.0360 |
| 2235 | gi|212509777 | ubiquitin specific proteinase 54 | 21 | 70 | 12 | 191744/8.95 | 3.62 | 0.0053 |
| Downb | ||||||||
| 83 | gi|170058335 | phosphoglycerate mutase 2 | 15 | 132 | 46 | 28582/6.62 | 0.16 | 0.0012 |
| 245 | gi|170040378 | conserved hypothetical protein | 9 | 67 | 25 | 51872/5.55 | 0.45 | 0.0062 |
| 363 | gi|157115772 | glial maturation factor | 9 | 72 | 59 | 16747/4.92 | 0.51 | 0.0040 |
| 589 | gi|157131660 | methylthioadenosine phosphorylase | 11 | 98 | 44 | 30776/6.07 | 0.71 | 0.0054 |
Profile of differentially expressed proteins in DM-resistant cells. Acc. no.: Swiss-Prot or TrEMBL database accession number; Protein description: name of protein in the Swiss-Prot or TrEMBL database; No of matched peptides: number of peptides matched to the candidate protein (the number of observed peptides); Score: Mowse Score, scores greater than 67 are considered statistically significant (p<0.05); Sequence coverage: identified sequence as a percentage of the complete sequence; Mr: molecular weight; pI: theoretical isoelectric point; Folds of resistant/susceptible: describes the fold changes of the protein expression level in resistant cell compared with that in susceptible cell; P-value: Statistical significance of the fold-change in protein expression;
Up-regulated proteins.
Down-regulated proteins.
This protein spot contains more than one protein.
Figure 2. Four unbiquitin-proteasome proteins were up-regulated in DM-resistant mosquito cells.
(A) Representative magnified images of 2-D gel showing the elevated expression of proteins (PSMB6, PSMD14 (POH1), E2 enzymes and USP) in DM-resistant cells. (B) Relative expression levels of the four ubiquitin-proteasome proteins in DM-resistant cells. Spot density was digitized and the results are shown as the mean±SEM of three independent experiments. *P<0.05, **P<0.01 compared with the DM-susceptible cells. (C) Levels of PSMB6, POH1, E2 and USP mRNA in mosquito strain 07 and strain 01. The relative expression rate was determined by quantitative PCR and normalized with β-actin. Results are expressed as the mean±SEM. *P<0.05, **P<0.01 compared with control. The results shown are representative of three independent experiments.
3.3 Proteasome inhibitor increases the sensitivity of DM-resistant cells to DM treatment
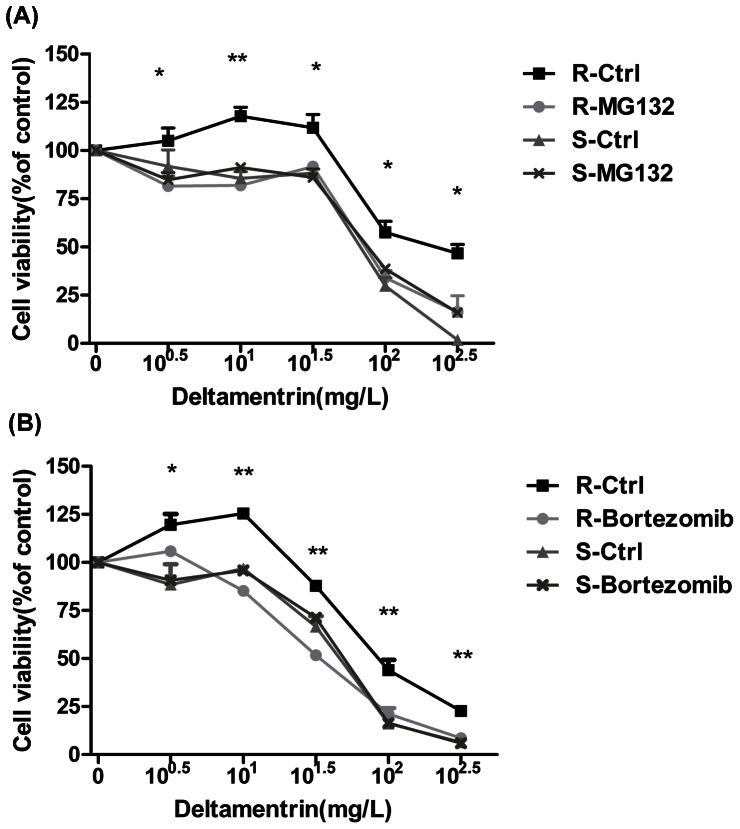
The proteasome inhibitor MG-132 and bortezomib were used to elucidate the involvement of the ubiquitin-proteasome system in DM resistance. A dose-dependent viability experiment was conducted to select the optimum dose of MG-132 or bortezomib. Viabilities and morphology of DM-resistant cells were not notably affected by MG-132 ranging from 0.125 µM to 1 µM (Figure S2A) or bortezomib ranging from 0 µM to 2 µM (Figure S2B). 1 µM and 0.1 µM were chosen as the optimal concentrations for MG-132 and bortezomib, respectively. DM treatment decreased cell viability in a dose-dependent manner, while MG-132(Figure 3A) or bortezomib (Figure 3B) pre-treatment sensitized DM-resistant cells to DM compared with the controls. However, MG-132 or bortezomib treatment has no significant effect on DM-susceptible cell.
Figure 3. Proteasome inhibitor enhanced cell susceptibility to DM.
Resistant and susceptible mosquito cells were exposed to DM at the indicated concentrations after pre-treatment with 1 µM MG-132 (A) or 0.1 µM bortezomib (B) and cell viability was measured. *P<0.05, **P<0.01 compared with the control group. The results shown are representative of three independent experiments.
3.4 Proteasome inhibitor increases mosquito larvae sensitivity to DM
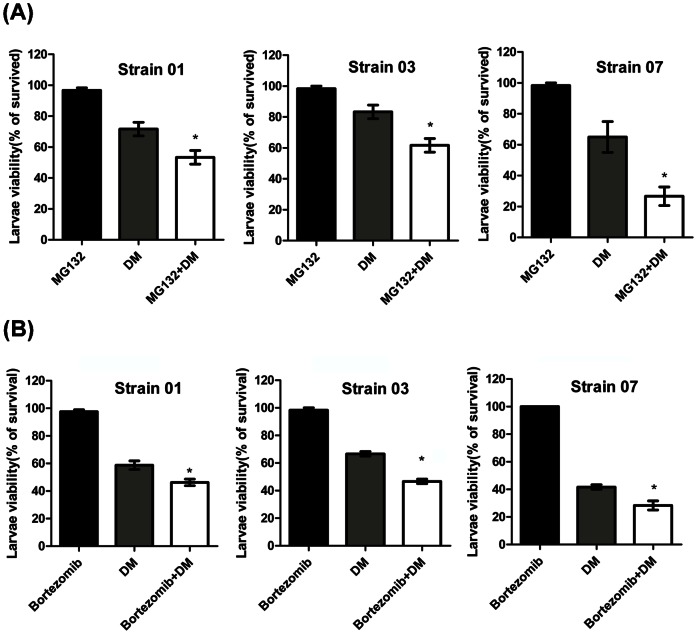
Three Cx. pipiens pallen strains (see Section 2.1) were used in these experiments, 1 µM was used as the optimal concentration for MG-132 (Figure S2C) and 0.125 µM for bortezomib (Figure S2D) (identified in dose-escalation experiments) in larvicidal activity assays. DM treatments reduced mosquito larvae viability in all three strains. MG-132 or bortezomib pre-treatment significantly decreased the mosquito larvae viability compared with the DM treated group in all strains (MG-132: Strain 01, 53% vs. 72%; Strain 03, 62% vs. 83%; Strain 07, 27% vs. 65%; P<0.05 (Figure 4A); Bortezomib: Strain 01, 46% vs. 58%; Strain 03, 47% vs. 67%; Strain 07, 28% vs. 41%; P<0.05 (Figure 4B)), which indicate that MG-132 or bortezomib treatment make mosquitoes more susceptible to DM treatment.
Figure 4. Proteasome inhibitor enhanced susceptibility to DM in mosquito larvae.
Three different DM resistance levels strains (strains 01, 03 and 07) were used in these experiments. Larvae with different levels of DM resistance levels were exposed to the indicated concentrations of DM for 24 h after pre-treatment with 1 µM MG-132 (A) or 0.125 µM bortezomib (B), and the viability was evaluated. *P<0.05, **P<0.01 compared with the DM group. The results shown are representative of three independent experiments.
3.5 Molecular cloning and sequence analysis of PSMB6
PSMB6 is a catalytic subunit of proteasome β-rings [25], [26]. The full-length cDNA of PSMB6 from Cx. pipiens pallen were cloned and submitted to GenBank (GenBank Accession NO: JQ037858) (Figure S3A). The deduced peptide is composed of 227 amino acids. Homology analysis of the Cx. pipiens pallen PSMB6 sequence revealed 99% and 91% and 90% identity with PSMB6 of Cx. Quinquefasciatus, Ae. aegypti and An. Gambiae, respectively (Figure S3B). Phylogenetic analysis showed that Cx. pipiens pallen, Cx. quinquefasciatus, Ae. albopictus, Ae. aegypti and An. gambiae share the most recent common ancestry (Figure S3C).
3.6 PSMB6 is essential for DM resistance in mosquito cells
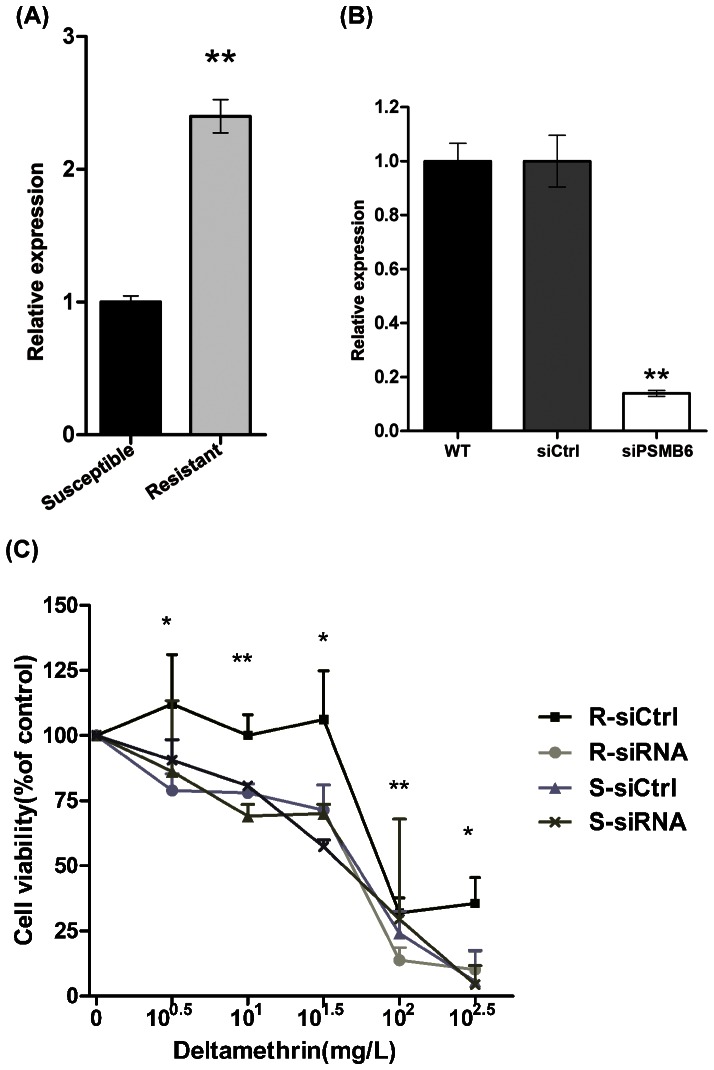
The expression of PSMB6 in DM-resistant cell was shown to be 2.5-fold higher than that in DM-susceptible cell assessed by quantitative PCR (Figure 5A). Combined with the 2-DE results, these results indicated that PSMB6 expression is upregulated at both the transcriptional and translational levels in DM-resistant cells. Subsequent investigation of the role of PSMB6 in DM resistance was conducted using PSMB6 siRNA. The knockdown efficiency of PSMB6 was confirmed by quantitative PCR (Figure 5B). Cell viability over a wide range of DM concentrations (100.5, 101, 101.5, 102, 102.5 mg/L) of DM was measured using the CCK-8 method. As shown in Figure 5C, transient knock down of PSMB6 expression in DM-resistant cells resulted in decreased cell viability, indicating that cell lack of PSMB6 became more sensitized to DM treatment. No significant difference in cell viability was observed between control and PSMB6 siRNA-treated groups of DM-susceptible cells.
Figure 5. PSMB6 played an important role in DM resistance.
(A) Levels of PSMB6 mRNA in DM-susceptible and -resistant mosquito cells. The relative expression rate was determined by quantitative PCR and normalized with β-actin. Results are expressed as the mean±SEM. **P<0.01 compared with the control. The results shown are representative of three independent experiments. (B) The knockdown efficiency of siPSMB6. DM-resistant mosquito cells were transfected with siPSMB6 or a scrambled siRNA as a control. Results are expressed as the mean±SEM. **P<0.01 compared with the WT and siCtrl groups. (C) Transient knockdown of PSMB6 decreased DM resistance in resistant mosquito cells. DM-resistant and -susceptible cells were transfected with siPSMB6 and siCtrl and exposed to DM at the indicated concentrations. Cell viability was measured after 72 h. The percentage of viable cells is shown relative to the control (DMSO only). *P<0.05, **P<0.01 compared with the DM-susceptible group.
Discussion
In the present study, a global comparative proteomic analysis was performed between DM-susceptible and -resistant mosquito cells. Twenty-seven DM resistance-associated candidate proteins, involved in metabolism, energy generation, translation and signalling transduction, were identified. Four proteins (PSMB6, POH1, E2 enzymes and USP) involved in different steps of ubiquitin-proteasome degradation process were found to be upregulated. Together with the upregulated transcription of these genes, our results suggested that ubiquitin-proteasome system may play critical roles in DM-resistance.
E2 is involved in the formation of ubiquitin chains during protein ubiquitination. Ubiquitin specific proteinase (USP) is an important regulator that mediates the removal, and recycling of ubiquitin to maintain adequate free ubiquitin levels [38]–[40]. POH1 is a component of the proteasomal complex [41]. PSMB6 is a member of the proteasome β-type family, which participate in the formation of proteolytic centers of proteasome machinery [42]. The upregulated expression of these ubiquitin-proteasome proteins involved in each stage of the degradation process suggests that this ubiquitin-proteasome system is functionally enhanced and may contribute to DM resistance in mosquitoes.
Enhanced proteasomal activity has been demonstrated as a mediator of resistance to chemotherapy [20]. Overexpression of members of the ubiquitin-proteasome pathway has been implicated in cancer chemotherapy resistance. Inhibiting E2 enzyme activity with CDC34 could enhance the anti-cancer activity of bortezomib, dexamethasone and 2-methoxyestradiol [43]. Smith et al (2007) reported that the over-expression of the PSMB1 proteasomal subunit is associated with resistance to cisplatin in cancer cell lines [44]. Furthermore, PSMB7 has been proved to be associated with anthracycline-resistance in breast cancer [45]. Overexpression of the POH1 subunit confers resistance to vinblastine, cisplatin, doxorubicin and paclitaxel in mammalian cells [43]. In this study, we identified PSMB6 as a DM-resistance mediator in mosquitoes for the first time.
MG-132 and Bortezomib are both reversible and cell-permeable proteasome inhibitor [46]–[48]. Bortezomib is reported to be more specific with no significant inhibitory activity towards other enzymes or receptors [48]. As we anticipated, pre-treatment with MG-132 or bortezomib was associated with significantly decreased viability of DM-resistant cells or mosquito larvae indicating that proteasome do involved in DM-resistant. PSMB6 was highly at transcriptional level in DM-resistant cells, which was consistent with the protein profile, indicating that transcriptional upregulation of PSMB6 leads to translational upregulation of the protein. PSMB6 silencing resulted in significantly decreased cell viability under DM stress. Inhibition of proteasome activity using pharmaceutical inhibitor or knocking down the expression of PSMB6 through molecular methods resulted in sensitization of mosquito cells to DM-treatment, which strongly suggests that ubiquitin-proteasome system maybe involved in the DM resistance. Taken together, it is possible to manage DM resistance by regulating the ubiquitin-proteasome activity.
Under continuous selective pressure from insecticides, mosquitoes have attained stable inheritance of DM-resistance through over-expression of ubiquitin-proteasome proteins. It can be speculated that hyperactivation of the proteasome degradation pathway is associated with pyrethroid resistance, although further studies are required to elucidate the underlying mechanism.
This study provides compelling evidences that PSMB6 is associated with DM resistance, which indicated the potential of proteasome inhibitors as synergistic agent for insecticides.
Supporting Information
Representative 2-DE images of susceptible and resistant mosquito cell lysates. The 27 differential protein spots identified by MS are marked with arrows.
(TIF)
Effect of proteasome inhibitors on cell or larvae viability. DM-resistant mosquito cells were treated with MG-132 (A) or bortezomib (B) at the indicated concentrations for 72 h and the cell viability was measured by CCK-8 assay. Results are expressed as the mean±SEM. *P<0.05, **P<0.01 compared with the DMSO control. Larvae of the early fourth instar were exposed to MG-132 (C) or bortezomib (D) at the indicated concentrations for 24 h before the survival was calculated. The results shown are representative of three independent experiments.
(TIF)
Molecular cloning and sequence analysis of PSMB6 . (A).Three fragments of the cDNA sequence of PSMB6. The PCR product was cloned from Cx. pipiens pallen and separated by electrophoresis. (B) Homology analysis of PSMB6 cloned from Cx. pipiens pallen. (C) Phylogenetic relationship of PSMB6 with other species.
(TIF)
Sequences of primers used for quantitative PCR.
(DOC)
Acknowledgments
We thank Dr. Jiahao Sha and Ling Wang for their expert technical assistance in 2-DE and MS.
Funding Statement
This work was supported by grants from the National Institutes of Health of US (2R01AI075746-05), National Science and Technology Major Project of China (No. 2008ZX10004-010), National Natural Science Foundation of China (No. 30972564, No. 30901244, No. 801101279 and No. 81171900), Natural Science Foundation of Jiangsu province (No. BK2011768), Research Fund for the Doctoral Program of Higher Education of China (No. 20113234120007), and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Butler D (2007) Poor follow-up hampers malaria projects. Nature 450: 144–145. [DOI] [PubMed] [Google Scholar]
- 2. Lounibos LP (2002) Invasions by insect vectors of human disease. Annu Rev Entomol 47: 233–266. [DOI] [PubMed] [Google Scholar]
- 3. Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, et al. (2007) Late-acting dominant lethal genetic systems and mosquito control. BMC Biol 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rivero A, Vezilier J, Weill M, Read AF, Gandon S (2010) Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathog 6: e1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL (2006) The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol 22: 308–312. [DOI] [PubMed] [Google Scholar]
- 6. Mahande AM, Dusfour I, Matias JR, Kweka EJ (2012) Knockdown Resistance, rdl Alleles, and the Annual Entomological Inoculation Rate of Wild Mosquito Populations from Lower Moshi, Northern Tanzania. J Glob Infect Dis 4: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vais H, Williamson MS, Devonshire AL, Usherwood PN (2001) The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels. Pest Manag Sci 57: 877–888. [DOI] [PubMed] [Google Scholar]
- 8. Roberts DR (1998) DDT versus deltamethrin impregnated bed nets. Trans R Soc Trop Med Hyg 92: 687. [DOI] [PubMed] [Google Scholar]
- 9. Boonyuan W, Kongmee M, Bangs MJ, Prabaripai A, Chareonviriyaphap T (2011) Host feeding responses of Aedes aegypti (L.) exposed to deltamethrin. J Vector Ecol 36: 361–372. [DOI] [PubMed] [Google Scholar]
- 10. WHO (1992) Vector resistance to pesticides. Fifteenth Report of the WHO Expert Committee on Vector Biology and Control. World Health Organ Tech Rep Ser 818: 1–62. [PubMed] [Google Scholar]
- 11. Casimiro S, Coleman M, Hemingway J, Sharp B (2006) Insecticide resistance in Anopheles arabiensis and Anopheles gambiae from Mozambique. J Med Entomol 43: 276–282. [DOI] [PubMed] [Google Scholar]
- 12. Denholm I, Devine GJ, Williamson MS (2002) Evolutionary genetics. Insecticide resistance on the move. Science 297: 2222–2223. [DOI] [PubMed] [Google Scholar]
- 13. Hemingway J, Field L, Vontas J (2002) An overview of insecticide resistance. Science 298: 96–97. [DOI] [PubMed] [Google Scholar]
- 14. Wilding CS, Smith I, Lynd A, Yawson AE, Weetman D, et al. (2012) A cis-regulatory sequence driving metabolic insecticide resistance in mosquitoes: functional characterisation and signatures of selection. Insect Biochem Mol Biol 42: 699–707. [DOI] [PubMed] [Google Scholar]
- 15. David JP, Strode C, Vontas J, Nikou D, Vaughan A, et al. (2005) The Anopheles gambiae detoxification chip: a highly specific microarray to study metabolic-based insecticide resistance in malaria vectors. Proc Natl Acad Sci U S A 102: 4080–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu H, Tian H, Wu G, Gretchen L, Jonathan K, et al. (2004) Culex pipiens pallens: identification of genes differentially expressed in deltamethrin-resistant and -susceptible strains. Pesticide Biochemistry and Physiology 79: 75–83. [Google Scholar]
- 17. Liu N, Liu H, Zhu F, Zhang L (2007) Differential expression of genes in pyrethroid resistant and susceptible mosquitoes, Culex quinquefasciatus (S.). Gene 394: 61–68. [DOI] [PubMed] [Google Scholar]
- 18. Pandey A, Mann M (2000) Proteomics to study genes and genomes. Nature 405: 837–846. [DOI] [PubMed] [Google Scholar]
- 19. Geng F, Tansey WP (2012) Similar temporal and spatial recruitment of native 19S and 20S proteasome subunits to transcriptionally active chromatin. Proc Natl Acad Sci U S A 109: 6060–6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith L, Lind MJ, Drew PJ, Cawkwell L (2007) The putative roles of the ubiquitin/proteasome pathway in resistance to anticancer therapy. Eur J Cancer 43: 2330–2338. [DOI] [PubMed] [Google Scholar]
- 21. Stitzel ML, Durso R, Reese JC (2001) The proteasome regulates the UV-induced activation of the AP-1-like transcription factor Gcn4. Genes Dev 15: 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeuchi J, Toh-e A (1997) [Regulation of cell cycle by proteasome in yeast]. Tanpakushitsu Kakusan Koso 42: 2247–2254.9366204 [Google Scholar]
- 23. Walters KJ, Lech PJ, Goh AM, Wang Q, Howley PM (2003) DNA-repair protein hHR23a alters its protein structure upon binding proteasomal subunit S5a. Proc Natl Acad Sci U S A 100: 12694–12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zimmermann J, Lamerant N, Grossenbacher R, Furst P (2001) Proteasome- and p38-dependent regulation of ERK3 expression. J Biol Chem 276: 10759–10766. [DOI] [PubMed] [Google Scholar]
- 25. Bochtler M, Ditzel L, Groll M, Hartmann C, Huber R (1999) The proteasome. Annu Rev Biophys Biomol Struct 28: 295–317. [DOI] [PubMed] [Google Scholar]
- 26. Smalle J, Vierstra RD (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 55: 555–590. [DOI] [PubMed] [Google Scholar]
- 27. Kim HM, Yu Y, Cheng Y (2011) Structure characterization of the 26S proteasome. Biochim Biophys Acta 1809: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. da Fonseca PC, He J, Morris EP (2012) Molecular model of the human 26S proteasome. Mol Cell 46: 54–66. [DOI] [PubMed] [Google Scholar]
- 29. Murata S, Yashiroda H, Tanaka K (2009) Molecular mechanisms of proteasome assembly. Nat Rev Mol Cell Biol 10: 104–115. [DOI] [PubMed] [Google Scholar]
- 30. Cardozo C, Michaud C (2002) Proteasome-mediated degradation of tau occur independently of the chymotrypsin-like activity by a nonprocessive pathway. Arch Biochem Biophys 408: 103–1. [DOI] [PubMed] [Google Scholar]
- 31. Wu X, Wang Y, Sun Y (2011) Molecular characterization, expression analysis and association study with immune traits of porcine PSMB6 gene. Mol Biol Rep 38: 5465–5470. [DOI] [PubMed] [Google Scholar]
- 32. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 33. Wu J, Wang F, Gong Y, Li D, Sha J, et al. (2009) Proteomic analysis of changes induced by nonylphenol in Sprague-Dawley rat Sertoli cells. Chem Res Toxicol 22: 668–675. [DOI] [PubMed] [Google Scholar]
- 34. Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858. [DOI] [PubMed] [Google Scholar]
- 35. Zhu YF, Cui YG, Guo XJ, Wang L, Bi Y, et al. (2006) Proteomic analysis of effect of hyperthermia on spermatogenesis in adult male mice. J Proteome Res 5: 2217–2225. [DOI] [PubMed] [Google Scholar]
- 36. Ma M, Guo X, Wang F, Zhao C, Liu Z, et al. (2008) Protein expression profile of the mouse metaphase-II oocyte. J Proteome Res 7: 4821–4830. [DOI] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 38. Tobias JW, Varshavsky A (1991) Cloning and functional analysis of the ubiquitin-specific protease gene UBP1 of Saccharomyces cerevisiae. J Biol Chem 266: 12021–12028. [PubMed] [Google Scholar]
- 39. Gilchrist CA, Gray DA, Baker RT (1997) A ubiquitin-specific protease that efficiently cleaves the ubiquitin-proline bond. J Biol Chem 272: 32280–32285. [DOI] [PubMed] [Google Scholar]
- 40. Quesada V, Diaz-Perales A, Gutierrez-Fernandez A, Garabaya C, Cal S, et al. (2004) Cloning and enzymatic analysis of 22 novel human ubiquitin-specific proteases. Biochem Biophys Res Commun 314: 54–62. [DOI] [PubMed] [Google Scholar]
- 41. Spataro V, Toda T, Craig R, Seeger M, Dubiel W, et al. (1997) Resistance to diverse drugs and ultraviolet light conferred by overexpression of a novel human 26 S proteasome subunit. J Biol Chem 272: 30470–30475. [DOI] [PubMed] [Google Scholar]
- 42. Jager S, Groll M, Huber R, Wolf DH, Heinemeyer W (1999) Proreasome beta-type subunits: unequal roles of propeptides in core particle maturation and a hierarchy of active site function. J Mol Biol 291: 997–1013. [DOI] [PubMed] [Google Scholar]
- 43. Chauhan D, Li G, Hideshima T, Podar K, Shringarpure R, et al. (2004) Blockade of ubiquitin-conjugating enzyme CDC34 enhances anti-myeloma activity of Bortezomib/Proteasome inhibitor PS-341. Oncogene 23: 3597–3602. [DOI] [PubMed] [Google Scholar]
- 44. Smith L, Welham KJ, Watson MB, Drew PJ, Lind MJ, et al. (2007) The proteomic analysis of cisplatin resistance in breast cancer cells. Oncol Res 16: 497–506. [DOI] [PubMed] [Google Scholar]
- 45. Munkacsy G, Abdul-Ghani R, Mihaly Z, Tegze B, Tchernitsa O, et al. (2010) PSMB7 is associated with anthracycline resistance and is a prognostic biomarker in breast cancer. Br J Cancer 102: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee DH, Goldberg AL (1996) Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae. J Biol Chem 271: 27280–27284. [DOI] [PubMed] [Google Scholar]
- 47. Kisselev AF, Goldberg AL (2001) Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8: 739–758. [DOI] [PubMed] [Google Scholar]
- 48. Verbrugge SE, Assaraf YG, Dijkmans BA, Scheffer GL, Al M, et al. (2012) Inactivating PSMB5 mutations and P-glycoprotein (multidrug resistance-associated protein/ATP-binding cassette B1) mediate resistance to proteasome inhibitors: ex vivo efficacy of (immuno) proteasome inhibitors in mononuclear blood cells from patients with rheumatoid arthritis. J Pharmacol Exp Ther 341: 174–182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative 2-DE images of susceptible and resistant mosquito cell lysates. The 27 differential protein spots identified by MS are marked with arrows.
(TIF)
Effect of proteasome inhibitors on cell or larvae viability. DM-resistant mosquito cells were treated with MG-132 (A) or bortezomib (B) at the indicated concentrations for 72 h and the cell viability was measured by CCK-8 assay. Results are expressed as the mean±SEM. *P<0.05, **P<0.01 compared with the DMSO control. Larvae of the early fourth instar were exposed to MG-132 (C) or bortezomib (D) at the indicated concentrations for 24 h before the survival was calculated. The results shown are representative of three independent experiments.
(TIF)
Molecular cloning and sequence analysis of PSMB6 . (A).Three fragments of the cDNA sequence of PSMB6. The PCR product was cloned from Cx. pipiens pallen and separated by electrophoresis. (B) Homology analysis of PSMB6 cloned from Cx. pipiens pallen. (C) Phylogenetic relationship of PSMB6 with other species.
(TIF)
Sequences of primers used for quantitative PCR.
(DOC)