Abstract
WRKY transcription factors are reported to be involved in defense regulation, stress response and plant growth and development. However, the precise role of WRKY transcription factors in abiotic stress tolerance is not completely understood, especially in crops. In this study, we identified and cloned 10 WRKY genes from genome of wheat (Triticum aestivum L.). TaWRKY10, a gene induced by multiple stresses, was selected for further investigation. TaWRKY10 was upregulated by treatment with polyethylene glycol, NaCl, cold and H2O2. Result of Southern blot indicates that the wheat genome contains three copies of TaWRKY10. The TaWRKY10 protein is localized in the nucleus and functions as a transcriptional activator. Overexpression of TaWRKY10 in tobacco (Nicotiana tabacum L.) resulted in enhanced drought and salt stress tolerance, mainly demonstrated by the transgenic plants exhibiting of increased germination rate, root length, survival rate, and relative water content under these stress conditions. Further investigation showed that transgenic plants also retained higher proline and soluble sugar contents, and lower reactive oxygen species and malonaldehyde contents. Moreover, overexpression of the TaWRKY10 regulated the expression of a series of stress related genes. Taken together, our results indicate that TaWRKY10 functions as a positive factor under drought and salt stresses by regulating the osmotic balance, ROS scavenging and transcription of stress related genes.
Introduction
Environmental stresses, including drought, salinity and low temperature, are the primary causes of declines in crop yield and quality worldwide. To combat these challenges, plants have evolved sophisticated molecular networks resulting in adaptive responses through physiological and morphological changes [1]. The adaptive responses commonly use transcriptional activation or repression of genes upon signal perception and transduction of the external stimuli [2], [3]. Plant adaptability is mainly operated by the regulation of various transcription factors [4]. Significant progress has been made in understanding the genes response to various stresses [5], [6], [7], and numerous transcription factors and cis-regulatory sequences in plants have been identified [8]. Among them, the plant WRKY transcription factors, comprising a large family of regulatory proteins, are shown to play an important role in response to various stresses.
Since the first WRKY transcription factor was characterized from sweet potato (Ipomoea batatas) [9], many WRKY proteins have been identified from Arabidopsis (Arabidopsis thaliana), rice (Oryza sativa), soybean (Glycine max), barley (Hordeum vulgare), poplar (Populus trichocarpa), pinus (Pinus monticola) and Physcomitrella patens. The characteristic feature of the WRKY family is its highly conserved 60 amino acids, the WRKY domain, which is comprised of a highly conserved WRKYGQK motif at the N-terminus and certain zinc finger motifs at C-terminus [10]. The WRKYGQK motif in various plant species show slight variations in the amino sequences (for example, WRKYGKK and WRKYGEK) [11], [12]. Depending on their domain structures, the WRKY proteins can be divided into three different groups. Proteins with two WRKY domains belong to group I; proteins containing one WRKY domain belong to groups II or III, depending on the type of zinc finger motif [13], [14]. The WRKY factors present high binding affinity to a DNA cis-acting element named as the W box, (C/T)TGAC(T/C), which permits signal transduction to regulate the expressions of stress-related genes, resulting in plant stress tolerance finally [13].
During the process of plant evolution, the WRKY variations facilitate distinct cellular, developmental, and physiological roles. Accumulating evidence has demonstrated that the WRKY factors are central components of many aspects of the innate immune system of plants [15]. In addition, the WRKY factors participate in certain physiological processes, including embryogenesis [16], [17], seed development and germination [18], [19], trichome development [20], biosynthetic pathways regulation [21], [22] and hormone signaling [23], [24], [25]. Recently, attention has focused on biotic stress responsive WRKY transcription factors [21], [26], [27]. However, less progress has been made on understanding the function of WRKY proteins in the abiotic stress, as compared with the biotic stresses research progress [28].
Wheat is the dominant crop for human food and livestock feed. Current and future concerns include improving wheat yield and quality under hostile environments [29]. Considering the diverse roles of WRKY transcription factors under complex environmental conditions, clarifying the functions of certain WRKY members in the abiotic stress response remains a challenge. Overexpression of WRKY genes in Arabidopsis [30], rice [31] and soybean [32] conferred tolerance to abiotic stresses, especially oxidative stress [33], [34]. However, whether WRKYs confer drought and salt stress tolerance through reducing ROS accumulation is not yet to be determined in wheat. In the present study, based on the conserved protein sequence of WRKY transcription factors, expressed sequence tags (ESTs) with high similarity to the WRKYs in wheat genome sequence database were analyzed, collected and assembled into several unigenes. Ten of the TaWRKYs, designated TaWRKY1–TaWRKY10, were successfully identified. Among these genes, TaWRKY10 was observed to confer drought and salt stress tolerance by regulating osmosis and reducing ROS accumulation.
Materials and Methods
Plant Materials and Stress Treatments
Wheat (Triticum aestivum L. cv. Chinese Spring) seeds were treated with 75% (v/v) ethanol for surface-sterilization and washed three times in distilled water. Seeds were germinated on distilled water and cultured in growth chambers (16 h light/8 h dark cycle with a light intensity of 200 µmol·m−2·s−1 at 25°C) for ten days. Polyethylene glycol (PEG) or NaCl treatment were conducted by transferring seedlings into Petri dishes containing 20% PEG6000 or 200 mM NaCl solutions, and incubated under light for 24 h. For cold treatment, seedlings were transferred to cold-chamber with a beaker containing water pre-cooled to 4°C and maintained at 4°C under light for 24 h. For treatment with signaling molecule, seedlings were sprayed with 10 mM H2O2, and incubated under light for 24 h. In all these treatments, wheat seedlings at similar growth states were used, and untreated wheat seedlings were taken as controls [35]. Leaf samples were frozen in liquid nitrogen, then stored at −80°C until RNA extraction. For tissue-specific expression analysis, the roots, stems and leaves of 10-day-old untreated seedlings were also collected.
Cloning and Sequence Analysis of TaWRKY10
Total RNA from wheat leaves tissues was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). After removing the genomic DNA contamination by DNase I (TaKaRa, Dalian, China), 200 ng Poly(A)+ mRNA was converted into cDNA by M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). The cDNA template was used for PCR analysis subsequently [36]. We performed multiple alignment analysis using the wheat unigenes and ESTs are available on the DFCI wheat gene index database (http://compbio.dfci.harvard.edu/cgi-bin/tgi/gireport.pl?gudb=wheat) and wheat genome database (http://www.wheatgenome.org/). The full-length cDNA sequences were identified using DNAMAN software and amplified from wheat Poly (A)+ mRNA by PCR using specific primer pairs (see Table S1). After purification, the PCR products were combined with the pMD-18T plasmid (TaKaRa, Dalian, China) then sequenced. Domain prediction was performed by MEME (http://meme.sdsc.edu/meme/intro.html). This online software was used to create the logo representations of the WRKY domain and the rest of the alignment [37]. Multiple sequence alignment was performed by Clustal W [38] and Mega 4.0 [39]. Subcellular localization was predicted by Euk-mPLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/euk-multi-2/).
Reverse Transcription-polymerase Chain Reaction (RT-PCR)
RT-PCR was used to determine the expression of specific ESTs in the WRKY gene family after treating wheat seedlings with 200 mM NaCl, cold (4°C) or 20% PEG6000. Primers (Table S1) used in RT-PCR had high specificity, as determined by agarose gel electrophoresis, and were also confirmed by sequencing. The RT-PCR reactions were performed using TaKaRa DNA polymerase for 30 cycles (TaKaRa, Dalian, China). Expression levels of target genes were normalized using TaActin as an internal control.
Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
After RNA extraction and reverse transcription as described above, the resulting cDNA was used as the template for amplification with the MJ research Opticon detection system. The Opticon monitor qRT-PCR software was used for data analysis. The appearance of PCR products was monitored by detecting the increase of fluorescence caused by the binding of SYBR green dye (TOYOBO, Osaka, Japan) to dsDNA. The qRT-PCR was performed as described by Zhou et al [35]. The primer pairs’ efficiency and specificity were examined (Primers are provided in Table S1). In all experiments, each reaction had repeated at least three times and negative controls without templates were detected in case of contamination. The expression of TaActin or NtUbiquitin genes were used as the internal controls for normalization. The relative expression of mRNA was calculated using the 2–ΔΔCt formula [40].
Southern Blot Analysis
For analysis of wheat genome Southern blot, total wheat genomic DNA was extracted by CTAB method [41]. 10 µg genomic DNA was respectively treated with BamHI, HindIII or SacI restriction enzymes (MBI Fermentas), which cuts outside of the TaWRKY10 coding sequence. For analysis of transgenic (TG) lines, the genomic DNA of TG tobacco lines was digested by HindIII. The TaWRKY10 overexpressing vector was used as a positive control, and the genomic DNA of wild type (WT) tobacco was used as negative control. The digested genomic DNA was separated by electrophoresis and transferred to Hybond-N+ membrane by capillary blotting method. The membrane was hybridized with digoxigenin (DIG) labeled probe (Roche, Basel, Switzerland) (primer pairs are shown in Table S2) then detected by chemiluminescence method, according to Chen, et al [42].
Subcellular Localization Assay
The NcoI and SpeI sites were added to the open reading frame (ORF) of TaWRKY10 (Table S2), respectively. The gene was inserted into the pCAMBIA1304 vector containing the green fluorescent protein (GFP) gene and the Cauliflower Mosaic Virus (CaMV) 35 S promoter. The Subcellular localization of TaWRKY10-GFP fusion protein in onion (Allium cepa L.) epidermal cells was performed using the particle bombardment method (PDS-1000, Bio-Rad, Hercules, CA, USA), according to Hong, et al [43]. The GFP vector was used as a control. The DNA-specific nuclear stain 4',6-diamidino-2-phenylindole (DAPI) was used after bombardment [44]. Transformed cells were cultured for 24 h at room temperature in the dark on MS medium. Fluorescent microscopic images were collected using a fluorescence microscope (Karl Zeiss, Jena, Germany).
Transcriptional Activation Assay
The binding specificity and transactivation activity of the TaWRKY10 protein were investigated in the yeast (Saccharomyces cerevisiae) strain AH109. The full length and deletions of TaWRKY10 (WRKY, WRKY-N1, WRKY-N2, WRKY-C1 and WRKY-C2) were ligated to the yeast expression vector pGBKT7 (pBD) which having His and LacZ reporter genes (primers are provided in Table S2). The plasmid pBD was used as the negative control. These plasmids were transformed into yeast strains and were verified by PCR. The yeast strains were streaked on SD/−Trp and SD/−His plates containing 5 mM 3-amino-1, 2, 4-triazole (3-AT) and X-α-D-Galactoside (X-α-D-gal) [45], [46]. The plates were incubated at 30°C for 3 days.
Anti-TaWRKY10 Polyclonal Antibody Preparation
The ORF of TaWRKY10 was constructed into vector pET32a (Novagen, Billerica, MA, USA) to generate a fusion protein with a hexahistidine tag. The PCR product was digested with BamHI and HindIII (Table S2). The identity of the recombinant pET32a-TaWRKY10 construct was confirmed by DNA sequencing and then transformed into E. coli BL21 (DE3) cells.
The BL21 cells were induced by 1 mM isopropyl-β-D-thiogalactoside (IPTG) for 4 h at 37°C. And then, the cells were harvested by centrifugation, and disrupted by physical fragmentation. Inclusion bodies were dissolved with 6 M urea on ice. The supernatant was filtered through a 0.45-µm membrane and purified by affinity chromatography using a nickel column (Ni-NTA agarose, Roche). The protein was renatured through step dialysis at 4°C for 12 h. The primary polyclonal antibody was produced via the immunization of TaWRKY10 protein in New Zealand rabbit [42].
Western Blot Assay
The total proteins of tobacco overexpressing TaWRKY10 were extracted and separated by SDS-PAGE. After separation, proteins were transferred to a nitrocellulose membrane by electroblotted method. The membrane was blocked overnight at 4°C in TBST buffer with 5% nonfat milk. The membrane was incubated with primary rabbit anti-TaWRKY10 antibody (dilution 1∶10000 in TBST) at room temperature for 2 h. The membrane was washed with TBST buffer for three times and then incubated with 1∶10000 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG antibody for 1 h at 37°C (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The TaActin was used as a control. The signal was scanned with Quantity One software (Bio-Rad).
Generation of Transgenic Tobacco Plants
The tobacco transformation expression vector was constructed through the ligation of the TaWRKY10 to the pSN1301 plasmid controlled by the CaMV 35 S promoter (Table S2). The pSN1301 was used as the vector control (VC). The construct was introduced into Agrobacteria tumefaciens strain LBA4404 competent cells by the freeze-thaw method [41]. Tobacco (Nicotiana tabacum L. cv. SR1) plants were transformed by the Agrobacterium-mediated leaf disc method [47]. The transgenic plants seeds were selected on MS medium [48] containing a final concentration of 40 mg/L of hygromycin. The regenerated seedlings were also confirmed by PCR. Three independent transgenic homozygous T2 line seedlings (Transgenic lines TG 1, TG 5 and TG 7) and the pSN1301 vector control line were used for subsequent experiments.
Germination and Seedling Growth Assays
Approximately 100 seeds from each T2 generation tobacco plants of five independent lines WT, VC, TG 1, TG 5 and TG 7) were surface sterilized with 75% (v/v) ethanol and were sown on MS medium containing 100 mM NaCl, 100 mM mannitol or 5 mM H2O2. Plates were incubated in a 16 h light (25°C) and 8 h dark (20°C) chamber. The germination rate was scored daily for 7 days.
For early growth assessments, 5-day-old seedlings from vertical plates containing MS medium were placed with their roots pointing downwards onto vertically oriented plates with MS medium that were supplemented with NaCl (0, 50, 100, or 200 mM), mannitol (0, 50, 100, or 200 mM) or H2O2 (0, 1, 2 or 5 mM). Each plate containing WT, VC and TG lines, and five replicate plates were used for each treatment. Root lengths were marked at the onset of treatment, and their increases were monitored after 7 days [49].
Drought and Salt Stress Treatments of Transgenic Tobacco Plants
One-month-old transgenic tobacco lines (TG 1, TG 5, TG 7 lines) and WT were subjected to different abiotic stress treatments. Thirty plants for each line were used for drought and salt stress treatment, respectively. For drought stress treatment, plants were withheld from watering for 3 weeks, and were then rewatered. Two weeks later, the rate of leaf yellowing and survival rate were calculated. For the salt stress treatment, plants were irrigated with 400 mM NaCl for 3 weeks, and then returned to the original growth conditions for 2 weeks. The rate of leaf yellowing and survival rate were calculated.
Measurements of Relative Water, Free Proline, Soluble Carbohydrates and Malonaldehyde (MDA) Contents
Leaves of TaWRKY10-overexpression TG and WT tobacco plants were collected 3 weeks after each treatment for measurements. The relative water content (RWC) detection was performed as described by Zhou et al [35]. Proline content analysis was carried out by the ninhydrin reaction method. Fresh leaf material was extracted with sulfosalicylic acid, and the acetic acid and acid ninhydrin reagent were added into the solution and heated at 100°C for 30 min. After cooling to room temperature, the optical density of organic phase was determined at 520 nm. Soluble carbohydrate contents were determined by the phenol reaction method. Leaf tissue was boiled in water for extraction. After cooling to room temperature, the extract solution was mixed with 9% phenol and concentrated sulfuric acid. The tube was then shaken well and left to stand for 30 min. The aqueous extract was determined and recorded at 485 nm. The MDA content analysis was carried out by the thiobarbituric acid method. Fresh leaves were extracted with 10% trichloroacetic acid. After centrifugation at 4,000 g, supernatant was boiled with 0.5% thiobarbituric acid. Optical density readings of organic phase were taken at 532 nm, 600 nm and 450 nm and calculated as described by Draper et al [50].
Detection of ROS
The detection of ROS assay was carried out according to the method described by Lee et al. [51]. Briefly, for superoxide (O2 −) staining, tobacco leaves were treated with nitroblue tetrazolium (NBT) in HEPES buffer under vacuum infiltration. In the control treatment, MnCl2 and superoxide dismutase (SOD) were added to the system. For H2O2 staining, tobacco leaves were treated with 3, 3'-diaminobenzidine (DAB) in Tris-acetate buffer under vacuum infiltrated. The ascorbic acid was added to staining system as the control treatment. Samples were incubated over night at room temperature in the dark. After the staining, the tobacco leaves were bleached with 80% ethanol.
Analysis of Downstream Genes Regulated by TaWRKY10
The WT and TG tobacco lines were cultured in soil under unstressed conditions for 30 days. Total RNA of the seedlings was extracted for reverse transcription to generate cDNA. Using quantitative PCR, the expression of stress related genes was detected. The NtUbiquitin gene was used as the internal reference. The sequences of the quantitative PCR primers are listed in Table S2.
Statistical Analysis
Statistical analyses were carried out by Microsoft Excel and SPSS (Chicago, IL, USA). All the experiments were repeated for three times and Student’s t-test was applied for statistical analysis.
Results
The Characteristics of WRKYs in Wheat
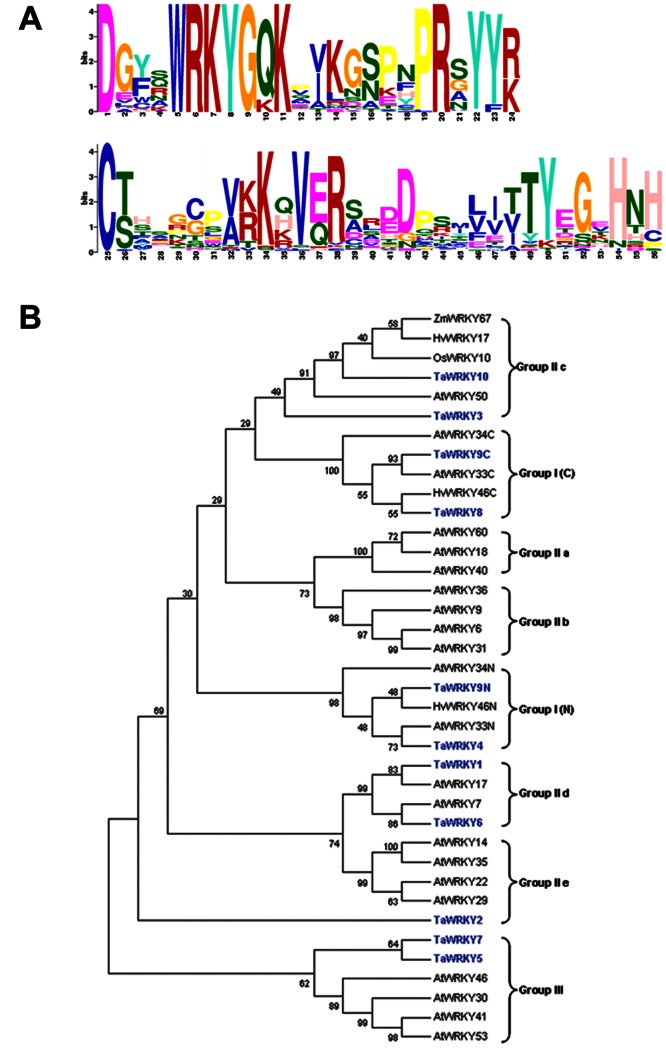
To isolate WRKY genes from wheat, the WRKY conserved sequence was used as a query to search wheat ESTs in the DFCI database and the wheat genome database. After searching for WRKY domains and eliminating repeats, 10 WRKY transcription factors were identified from wheat leaves, of which nine possessed a complete ORF. They were designated TaWRKY1– TaWRKY10, respectively. The characteristics of WRKYs are provided in Table S3. Domain prediction of the full-length deduced proteins of the TaWRKYs clearly showed that these proteins contained the conserved WRKY DNA-binding domain and zinc finger region (Fig. 1A). These WRKYs were further divided into three subgroups: TaWRKY4, 8, and 9 belong to group I; TaWRKY1, 2, 3, 6, and 10 belong to subgroup II; and TaWRKY5 and 7 belong to group III (Fig. 1B). To elucidate the potential function of the 10 WRKY transcription factors in response to various stimuli, the expression patterns were analyzed by RT-PCR under various abiotic stress conditions (Table S4). Among the 10 TaWRKY genes, five WRKYs responded to at least one treatment. TaWRKY10 was apparently induced by multiple treatments, and was chosen for further analysis.
Figure 1. Predicted domains in the TaWRKY1 - TaWRKY10 protein sequences and phylogenetic tree.
(A) Predicted domains in the WRKY protein. The conserved domains were carried out by MEME using the protein sequences of TaWRKYs and other known WRKYs. This online software was used to create the logo representations of the WRKY domain and the zinc finger motif. On the y axis (measured in bits), depicts the overall height of the stack indicating the sequence conservation at that position, while the height of symbols within the stack indicates the relative frequency of each amino or nucleic acid at that position. (B) Phylogenetic tree of the TaWRKYs domains from various plants. The multiple alignments were generated by CLUSTAL W and the phylogenetic tree was constructed with MEGA4.0 using a bootstrap test of phylogeny with minimum evolution test and default parameters. GenBank accession numbers of WRKY proteins used for drawing phylogenetic tree are shown in Table S5.
The TaWRKY10 cDNA is 791 bp in length (GenBank accession no. HQ700327), including a complete ORF of 672 bp encoding a putative protein of 223 amino acids (predicted relative molecular mass of 24.5 kDa). Analyzing the evolutionary relationships among the various species of WRKYs would provide an insight into the evolution of their function. To further characterize the TaWRKY10 protein, 34 WRKY domain proteins in different species were used for alignment. The alignment results revealed that the TaWRKY10 protein contains a conserved DNA-binding domain (WRKY domain) of 60 amino acids and a zinc finger region (C-X4-C-X23-H-X-H), indicating that it belongs to WRKY class II. TaWRKY10 is very similar to other WRKY proteins, such as Phyllostachys edulis (ADF42578.1) (85%), Hordeum vulgare (BAJ98268.1) (86%) and Zea mays (ACN29154.1) (85%). Based on the amino acid sequence alignment, two highly conserved regions were observed in TaWRKY10: four N-myristoylation sites and five Casein kinase II phosphorylation sites predicted by ExPASy Prosite analysis (Fig. S1). Protein myristoylation has been directly detected in Arabidopsis, rice, Lycopersicon esculentum and Cucurbita pepo, and is associated with proteins involved in growth regulation, disease resistance, salt tolerance and endocytosis [52]. These results suggested that TaWRKY10 is a member of the WRKY family in wheat.
Expression of TaWRKY10 is Induced by PEG, NaCl, H2O2 and Cold Treatments
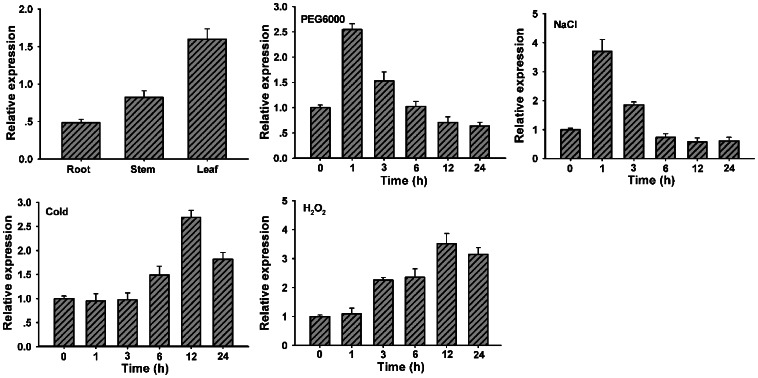
To clarify the tissue expression patterns of TaWRKY10, mRNA isolated from different wheat tissues were using as the templates for qRT-PCR. TaWRKY10 was detected to varying degrees of expression levels in root, stem and leaves of 10-day-old seedlings. To further characterize TaWRKY10, expression patterns of TaWRKY10 under different abiotic stresses as well as signaling molecule were analyzed by qRT-PCR. As shown in Fig. 2, the TaWRKY10 mRNA was induced and reached a maximum at 1 h after treatment with PEG6000 and NaCl. During cold treatment (4°C), TaWRKY10 mRNA accumulated at 6 h after initiation of the treatment and peaked at 12 h. During H2O2 treatment, the expression of TaWRKY10 was increased by 2.8-fold at 3 h and peaked at 12 h. These results suggested that the TaWRKY10 gene is induced by PEG6000, NaCl, cold and H2O2 treatment.
Figure 2. Expression patterns of wheat TaWRKY10 in different tissues and under different treatments.
The wheat seedlings were treated with 20% PEG6000, 200 mM NaCl, cold (4°C) or 10 mM H2O2. 200 ng Poly(A)+ mRNA was subjected to reverse transcription, and served as the qRT-PCR template. The y axis indicates the relative expression difference in mRNA level and the data were calculated using the 2–ΔΔCt formula. The transcripts of TaActin in the same samples was using as a reference. Transcript levels of the TaWRKY10 gene in untreated wheat were taken as 1. At least three biological experiments were carried out, which produced similar results.
TaWRKY10 is Present as Three Copies in Wheat Genome
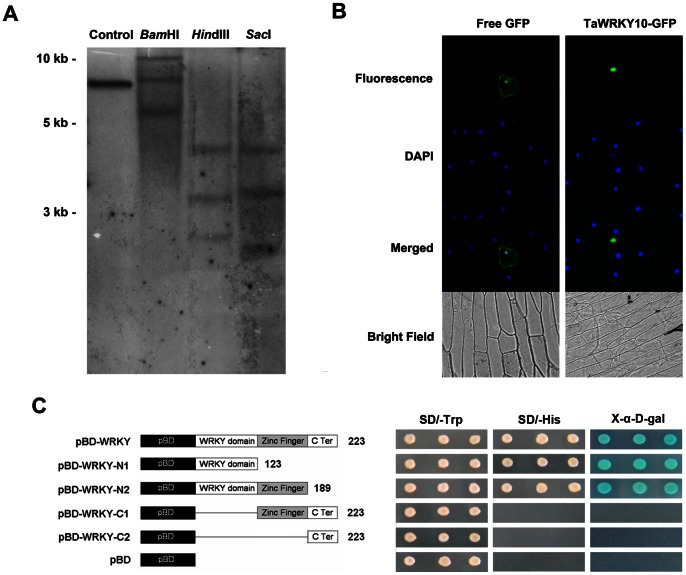
To investigate the genomic architecture of TaWRKY10 in hexaploid wheat, Southern blot assay of genomic DNA was performed. After high stringency washing, three hybridized bands were apparent in each lane. The result revealed that TaWRKY10 was existed as three copies in the genomes of hexaploid wheat (Fig. 3A).
Figure 3. The genomic organization, protein localization and transcriptional activation activity of the TaWRKY10 gene.
(A) Southern blot analysis of the TaWRKY10 gene. 10 µg genomic DNA of hexaploid wheat cv. Chinese Spring was digested completely with the restriction enzymes. The ORF of TaWRKY10 was used as the hybrid probe. The TaWRKY10 overexpressing vector was used as control. The 1 kb DNA Ladder (MBI Fermentas) are indicated on the left. (B) Subcellular localization of TaWRKY10 in onion epidermal cells. Onion epidermal cells were transferred with vector carrying GFP or TaWRKY10-GFP using bombardment method. Free GFP and TaWRKY10-GFP fusion proteins were transiently expressed in onion epidermal cells and observed with an inverted fluorescence microscope. (C) Transactivation activity of the TaWRKY10 protein in Yeast. The schematic diagram demonstrating the TaWRKY10 cDNA fragments encoding different portions of TaWRKY10 that were fused to the yeast vector pGBKT7 (pBD). Transactivation activity analysis of TaWRKY10 was performed using yeast strain AH109. The transformants were streaked on the SD/−Trp or on SD/−His medium. The transformants were examined for growth in the presence of 3-AT and X-α-D-gal. Three biological experiments were carried out, which produced similar results.
The TaWRKY10 is Localized in the Nucleus
To characterize the subcellular localization of the TaWRKY10 protein, the TaWRKY10-GFP fusion protein was bombarded into onion epidermal cells. The subcellular localization of the TaWRKY10-GFP construct was observed via a fluorescence microscope. The nucleus location of TaWRKY10-GFP was confirmed by GFP and DAPI merging images showing a complete match (Fig. 3B). These results suggested that TaWRKY10 is a nuclear protein, possibly serving as a transcription factor.
The TaWRKY10 Functions as a Potential Transcriptional Activator
To determine the TaWRKY10 transcriptional activation activity in eukaryotic cells, a yeast expression system was used. The ORF of TaWRKY10 gene was combined with the DNA-binding domain to identify transcriptional activation activity. The constructs pBD-WRKY, pBD-WRKY-N1, pBD-WRKY-N2, pBD-WRKY-C1 and pBD-WRKY-C2 were transformed into yeast strain AH109, and pBD was used as a control. As shown in Fig. 3C, the yeast cells transformed with pBD-WRKY pBD-WRKY-N1 and pBD-WRKY-N2 grew well in the SD/−His medium and SD/−Trp medium. Meanwhile, yeast cells transformed with pBD-WRKY-C1, pBD-WRKY-C2 and pBD could only survive in the SD/−Trp medium. The staining result showed that the yeast cells turned blue in the presents of X-α-D-gal. The results indicated that the His and LacZ reporter genes were activated and the presence of transcriptional activity in the full-length TaWRKY10 protein and N-terminal domain.
Transgenic Tobacco Plants Obtained
The full-length TaWRKY10 sequence was transformed into WT tobacco line. Seventeen independent TaWRKY10 overexpressing TG lines were obtained and confirmed by genomic PCR. Among the T1 lines, three independent lines (TG 1, TG 5 and TG 7) segregated at a 3∶1 ratio on hygromycin resistance. Moreover, seedlings from all three transgenic T2 lines grew well on MS medium supplemented with hygromycin. Among the T2 lines, the Southern blot assay result showed one copy of the TaWRKY10 was integrated into the genomes of transgenic lines TG 1, TG5 and TG 7 (Fig. 4A). Those transgenic lines showed high expression of the TaWRKY10 protein, as confirmed by Western blotting (Fig. 4B). The results indicated that TaWRKY10 was present as a single copy and was stably inherited in T1–T2 transgenic lines. Three independent T2 lines of the TaWRKY10 transgenic plants were chosen for further analysis.
Figure 4. Identification and the early development assay of the TaWRKY10 transformed tobacco plants.
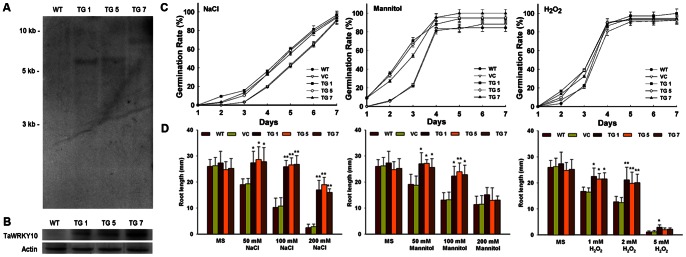
(A) Southern blot confirmation of the TaWRKY10 copy number. The 1 kb DNA Ladder (MBI Fermentas) is indicated on the left. (B) Western blot confirmation of TaWRKY10 protein expression. (C) Germination rate of tobacco overexpressing the TaWRKY10 gene. (D) Root lengths of tobacco plants overexpressing the TaWRKY10 gene. The data present means ± SD of three experiments performed. Significant differences between the TG and control lines are indicated as *p<0.05; **p<0.01.
Overexpression of TaWRKY10 Enhances Tolerance to Mannitol, NaCl and H2O2 Treatments during Seed Germination and Root Elongation
To evaluate the stress tolerance of the control and the TG tobacco lines, seed germination was examined. Seeds were sown on MS medium supplemented with appropriate concentrations of mannitol, NaCl or H2O2. Germination rates of WT seeds, VC seeds and TG seeds did not show any significant difference on normal MS medium (data not shown). As shown in Fig. 4C, when seeds were germinated on 100 mM mannitol for 3 days, only 21.9% germination was seen in the WT line and 23.3% in the VC line, whereas the TG lines showed 50%–70% germination. After 7 days of germination, only 84.3% of WT and 88.2% of VC seeds germinated, whereas at least 94.1% germination was observed in the three TG lines. Under mannitol stress, the percentage germination of WT was much lower compared with that of the TG over a 7-day period. When the seeds of WT, VC and TG lines were germinated in the presence of 100 mM NaCl, the TG lines germination rates (TG 1–96.8%, TG 5–94.9% and TG 7–93.8%) were higher than those of WT plants (90.5%) and VC plants (91.5%). When the seeds were germinated on MS medium containing 5 mM H2O2 for 2 days, the germination rates of TG seeds were significantly higher than the WT line and VC line. Only 22.6% of WT seeds and 20.1% of VC seeds germinated at day 2, compared with 38.9%, 39.0% and 32.4% of seeds hosting the TaWRKY10 gene.
Subsequently, root elongation was tested under gradient NaCl, mannitol or H2O2 treatments (Fig. S2). As the concentration of NaCl increased, the root lengths of the control lines were significantly arrested, whereas the TG roots continued to grow. Under mannitol and H2O2 treatments, the TG plants also showed more adaptation to stress compared with the control lines. As shown in Fig. 4D, the growth situation displayed little difference between the WT line and VC line grown on different medium. Thus, these results indicated that overexpression of TaWRKY10 in tobacco increased tolerance to mannitol, NaCl and H2O2 stresses during seed germination and root elongation.
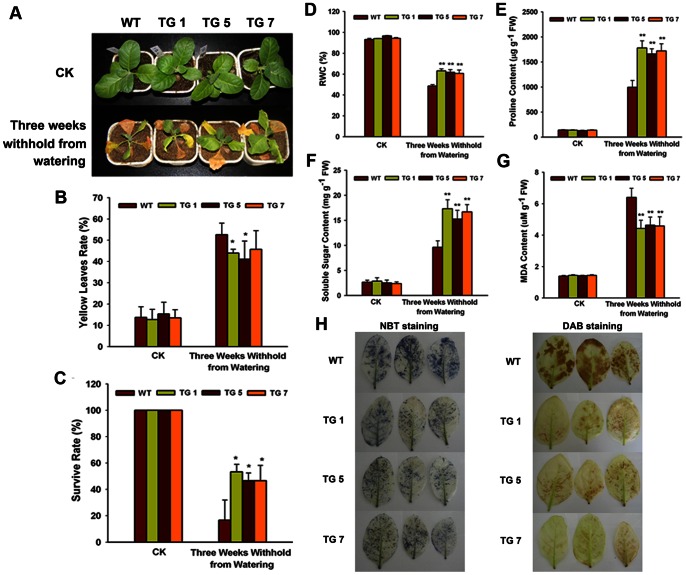
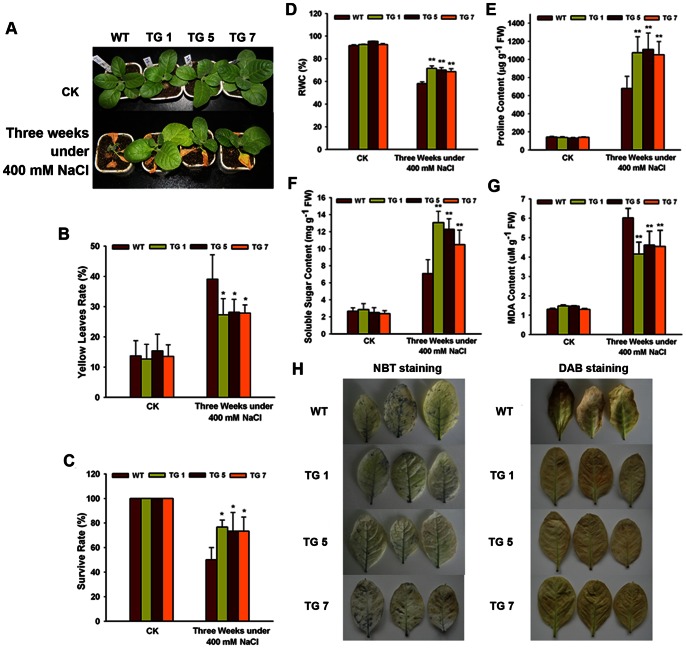
Overexpression of TaWRKY10 Enhances Drought and Salt Tolerance in Transgenic Tobacco Plants
To characterize the performance of TaWRKY10 transgenic lines under drought and salt stress in soil, all three TG lines, and the controls, were tested at the seedling stage. Comparing with the control plants under normal conditions, the TG tobacco plants showed no obvious phenotypic difference in terms of appearance, flowering time or production. However, under stress conditions, the TG plants did show differences in performance. After drought treatment for 3 weeks, compared with TG plants, the WT plants were smaller and more withered (Fig. 5A). After 3 weeks of exposure to 400 mM NaCl, most leaves of the TG tobacco remained green, while leaves of the WT turned yellow (Fig. 6A). The TG lines exhibited lower rates of leaf yellowing (Fig. 5B and 5C) and higher survival rates (Fig. 6B and 6C) than WT lines under drought and salt treatments. The rate of leaf yellowing, plant height and survival rate are the typical phenotypic and physiological parameters used to evaluate resistance in crop plants. Plants that are taller, with higher survival rates and fewer yellow leaves are more tolerant to stresses. The phenotypic characterization suggested that overexpression of TaWRKY10 enhanced drought and salt stress tolerance.
Figure 5. Analysis of the enhanced drought tolerance in transgenic tobacco lines.
(A) Phenotype of WT and TG tobacco lines after 3 weeks of drought treatment. (B) Rate of leaf yellowing. (C) Survival rate. (D) RWC content. (E) Proline content. (F) Soluble sugar content. (G) MDA content. (H) Tissue localization of O2 − generation by NBT staining and tissue localization of H2O2 generation by DAB staining. The data present means ± SD of three experiments performed. Significant differences between the TG and control lines are indicated as *p<0.05; **p<0.01.
Figure 6. Analysis of the enhanced salt tolerance in transgenic tobacco lines.
(A) Phenotype of WT and TG tobacco after 3 weeks of 400 mM NaCl treatment; (B) Rate of leaf yellowing. (C) Survival rate. (D) RWC content. (E) Proline content. (F) Soluble sugar content. (G) MDA content. (H) Tissue localization of O2 − generation by NBT staining and tissue localization of H2O2 generation by DAB staining. The data present means ± SD of three experiments performed. Significant differences between the TG and control lines are indicated as *p<0.05; **p<0.01.
Overexpression of the TaWRKY10 Increases RWC, Proline and Sugar Accumulation, and Decreases MDA and ROS under Drought and Salt Stresses
To investigate the physiological differences between control and TG plants, some important physiological indices were measured. Compared with the WT plants, the TG lines showed remarkably higher levels of RWC (Fig. 5D and 6D), proline (Fig. 5E and 6E) and soluble sugar (Fig. 5F and 6F), but lower levels of MDA (Fig. 5G and 6G) under drought and salt conditions. Subsequently, the presence of ROS in WT lines and TG lines was detected with NBT staining and DAB staining. Under normal conditions, both WT and TG seedlings accumulated less superoxide. Moreover, no staining was detected in WT or TG lines in control treatment. The control treatment result suggested that the staining was caused by superoxide or hydrogen peroxide specifically. After exposure to drought or salt treatments, the ROS level of WT plants accumulated greater than TG seedlings (Figs. 5H and 6H). DAB staining showed that the WT line accumulated more hydrogen peroxide along the main vein under drought and salt stresses. The staining results suggesting that overexpression of TaWRKY10 decreased the accumulation of ROS under drought and salt treatments. In conclusion, the physiological characterization results suggested that overexpression of TaWRKY10 increased RWC, proline and sugar content, and decreased MDA and ROS accumulation under drought and salt conditions.
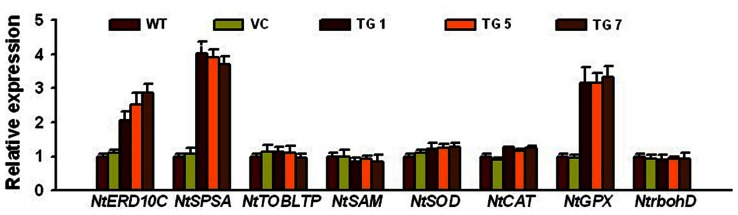
Overexpression of the TaWRKY10 Significantly Activates the Expression of Stress Related Genes in Tobacco Plants
To gain a deeper understanding of the function of TaWRKY10 during drought and salt stress, we detected the expression of eight osmotic stress related genes in the control and TG lines by qRT-PCR. The expression of three genes in overexpressed seedlings was obviously higher in TG lines than in control plants (Fig. 7). Genes selected for this analysis include NtERD10C and NtSPSA related to osmotic stress, and NtGPX (encoding glutathione peroxidase) involved in scavenging ROS, suggesting that TaWRKY10 constitutively induced the expression of osmotic stress and oxidative stress genes in tobacco plants.
Figure 7. Overexpression of the TaWRKY10 gene in tobacco enhances the expression of stress related genes.
The y axis indicates the relative expression difference in mRNA levels of these genes in TG tobacco, and the data were calculated using the 2–ΔΔCt formula. The transcripts of NtUbiquitin in the same samples was using as a reference. Three biological experiments were performed, which produced similar results.
Discussion
Recently, more attention has been paid to WRKY transcription factors in regulating plant growth and development and biotic stress; however, their precise functions remain uncertain. Moreover, compared with the progress on biotic stresses, less progress has been made on understanding the function of WRKY proteins in the abiotic stress [25]. Wheat is the foremost staple food crop in the world which provides both calories and proteins to over 35% of the human population [53]. The production of wheat is affected by multiple environmental stresses, including drought, salinity and extreme temperatures. However, only two wheat WRKYs have been characterized; TaWRKY2 and TaWRKY19 are involved in abiotic stresses responses [28]. In the present study, 10 non-redundant wheat WRKYs were identified and TaWRKY10 was characterized to function as a positive factor in abiotic stresses.
TaWRKY10 is a Member of the WRKY Family in Wheat
The conserved primary structural features of the TaWRKYs were identified using MEME (Fig. 1a). All 10 deduced WRKY proteins have WRKY domains and zinc finger motifs. Notably, TaWRKY4 and TaWRKY8, which carry only one WRKY domain, belong to group I WRKY proteins (Fig. 1b). This might be explained by the secondary loss of an N-terminal WRKY domain [13]. The result of subcellular localization of TaWRKY10-GFP indicated that the construct was located in the nucleus (Fig. 3B), which is consistent with previous studies on WRKY transcription factors from other species [54]. The localization data were consistent with the results of bioinformatics analysis, which showed that TaWRKY10 contained a nuclear localization signal. This implies that it acts as part of a transcription-regulating complex, as predicted by Euk-mPLoc 2.0 (data not shown). Transcriptional activation analysis showed that the transcriptional activity in the presence of full-length TaWRKY10 protein and N-terminal domain. Activating regions are typically acidic and probably form part of an α-helix. The N-terminal region of TaWRKY10 is acidic (amino acid 31–70, pI = 3.62), which has transcriptional activity. These data conclude that the TaWRKY10 is a member of the WRKY family in wheat and may serve as a transcription activator.
TaWRKY10 Plays a Positive Role during Abiotic Stresses
In our study, quantitative PCR demonstrated that TaWRKY10 was induced by multiple stresses, including PEG6000, NaCl, cold (4°C) and H2O2 (Fig. 2). Responses to abiotic stimuli of WRKY transcription factors are often extremely rapid and transient. The WRKY transcription factors mediate signals transduction via activating adaptive responses and regulation of downstream genes. During responses to multiple stresses, a single WRKY gene often participates various signaling pathways, indicating its diverse regulatory mechanism. The expressions of certain stress-induced genes have been demonstrated to be associated with stress tolerance [25]. The response of TaWRKY10 to a broad range of environmental stresses implied that TaWRKY10 might be involved in different stress signaling pathways as a connection point.
The in vivo role of TaWRKY10 in plant resistance was clearly demonstrated in transgenic tobacco lines transformed with an overexpression construct for TaWRKY10. Under drought and salt stress conditions, the WT plants were smaller, more withered and more yellow, while the TG tobaccos were more able to adapt to those stresses (Figs. 5 and 6). The rate of leaf yellowing and the survival rate are typical phenotypic and physiological parameters used for evaluating plants resistance. Plants with higher survival rate and fewer yellow leave have higher tolerance and are more resistant to stresses. Our findings were consistent with previous results reporting that BcWRKY46, TaWRKY2, TaWRKY19, and HvWRKY38 conferred drought and salt stresses, and enhanced stress tolerance, in transgenic plants [28], [55], [56]. Data showed that the overexpression of TaWRKY10 in tobacco led to adaptation to drought and salt stresses.
Overexpression of the TaWRKY10 in Tobacco Increases Drought and Salt Resistance
After drought or salt treatment for 3 weeks, TG lines accumulated higher levels of RWC, proline and soluble sugar (Figs. 5 and 6). RWC is a relevant index for measuring plant water status under drought tolerance. Plants accumulate several metabolites, such as proline, and a variety of sugars and sugar alcohols to prevent these detrimental changes [57]. The accumulations of free proline and soluble sugar in plants play highly protective roles under stresses conditions. Studies have shown that plants with higher proline and soluble sugar had better stress resistances [58]. Thus, our results indicated that the TG lines increase drought and salt resistance by osmoregulation.
It has been reported that abiotic stress causes lipid peroxidation, leading to MDA accumulation [59], [60], [61]. MDA content could be used as a measure of the damage caused by abiotic stresses [62]. Our results demonstrated that TaWRKY10 might protect the plant by decreasing the accumulation of MDA. In the second experiment, TaWRKY10-overexpressing plants exhibited lower O2− and H2O2 accumulation than WT plants under drought and salt stress conditions (Figs. 5H and 6H). ROS, which comprise O2−, H2O2, singlet oxygen (1O2) and hydroxyl radicals (HO·), are highly reactive and toxic to cells, and finally result in oxidative damages [63]. Plants have to maintain their ROS balance to minimize cellular damage caused by stress. Among the variety of ROS compounds, the present study investigated the effect of superoxide (O2−) and hydrogen peroxide (H2O2) in abiotic stress resistant. The results showed that overexpression of TaWRKY10 reduced cellular injuries caused by ROS in TG seedlings. Consequently, we infer that overexpression of TaWRKY10 in tobacco confers drought and salt stress tolerance by reducing ROS accumulation.
TaWRKY10 Confers Drought and Salt Stress through Stress Related Genes
To gain a further insight into the function of TaWRKY10 in stress tolerance at the molecular level, the expressions of 8 stress related genes were investigated (Fig. 7). NtERD10C belongs to LEA-like proteins, which are highly hydrophilic and glycine-rich [64]. The LEA-like proteins pervade throughout all organisms and can be induced by osmotic stress suggests that LEA-like proteins may acclimatize to osmotic stress [65], [66], [67]. SPSA is critical in the synthesis of sucrose in photosynthetic and non-photosynthetic tissues. Diverse choices for posttranslational modification of the SPSA protein allow enzyme activity adaptate to severe environment rapidly [68]. This is especially important in actively photosynthesizing leaves during adaptation to salt stress [69]. The results in this study suggested that TaWRKY10 constitutively regulated the expression of genes that involved in osmoregulation in TG seedlings. Subsequently, we examined the expression of genes response to oxidative stress. GPXs are enzymes that catalyze decreases in hydrogen peroxide, organic hydroperoxide and lipid hydroperoxide, and protect cells against oxidative radicals [70]. Plant GPX proteins or transcripts increased in response to several stresses, including biotic and abiotic stresses [71]. Our results indicated that TaWRKY10 alters the expression of oxidative related genes in response to stress. Thus, it might be that abiotic stresses regulate the expression of TaWRKY10; the accumulation of the TaWRKY10 then most likely upregulates the transcription of downstream stress-inducible genes, and the obtained proteins resulted in the increased the resistance to stress conditions.
In conclusion, our results clearly demonstrated that TaWRKY10 is a stress-inducible wheat transcription factor, and that the TaWRKY10 was up-regulated by PEG, NaCl, cold and H2O2. TaWRKY10 could enhance drought and salt stress tolerances in transgenic tobacco plants. These functions are achieved by accumulating water, proline and soluble sugar contents, and by reducing plant ROS and MDA contents. TaWRKY10 confers drought and salt stress tolerance by regulating the expression of stress related genes, thus protecting plants from damage. However, further evidences are needed to verify whether these genes are direct targets of TaWRKY10s. Thus, exploring the specific and direct genes regulated by TaWRKY10, together with its interacting partners will reveal the exact mechanisms of plant responses to abiotic stresses.
Supporting Information
Sequence analysis of the TaWRKY10 protein. Boxes represent casein kinase II phosphorylation sites. Sequences with single underlined indicate N-myristoylation sites, sequence marked with double underlines refer to WRKY domain. Zinc-finger motif is marked with asterisk.
(DOC)
Root lengths of tobacco plants overexpressing the TaWRKY10 gene under different stress conditions. The WT, VC and TG lines were cultured in MS medium under a 16 h light/8 h dark cycle at 25°C for 1 week, and then the seedlings were transplanted to fresh MS medium or MS medium supplied with 100 mM NaCl or 100 mM Mannitol or 2 mM H2O2 for 1 week. Then the photographs were taken. Three biological experiments were carried out, which produced similar results.
(DOC)
Gene specific primers used for isolating wheat WRKY genes and RT-PCR analysis.
(DOC)
Primers for TaWRKY10 used in this article.
(DOC)
Characteristics of WRKYs from wheat.
(DOC)
TaWRKY1 - TaWRKY10 expression patterns in wheat ( Triticicum aestivum cv Chinese Spring) under abiotic stresses.
(DOC)
The GenBank accession numbers of WRKY proteins used for drawing phylogenetic tree.
(DOC)
Acknowledgments
We thank Central Shared Research Platform for Biological Sciences of Huazhong University of Science and Technology (HUST) for supplying the fluorescence microscope. We would like to thank Dr. Mark Wilkinson, Rothamsted Research, UK for his helpful advice on the manuscript.
Funding Statement
This work was supported by the International Science and Technology Cooperation Key Projects of the Ministry of Science and Technology of China (Grant No. 2009DFB30340), the National Genetically Modified New Varieties of Major Projects of China (2011ZX08002-004, 2011ZX08010-004), the Key Projects of Science and Technology Research of the Ministry of Education of China (Grant No. 109105) and the Wuhan Municipal Science and Technology research project (Grant No. 201120922286). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7: 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang YO, Shah J, Klessig DF (1997) Signal perception and transduction in defense responses. Genes Dev 11: 1621–1639. [DOI] [PubMed] [Google Scholar]
- 3. Wang HH, Hao JJ, Chen XJ, Hao ZN, Wang X, et al. (2007) Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol Biol 65: 799–815. [DOI] [PubMed] [Google Scholar]
- 4. Pandey SP, Somssich IE (2009) The Role of WRKY Transcription Factors in Plant Immunity. Plant Physiol 150: 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Molec Biol 51: 463–499. [DOI] [PubMed] [Google Scholar]
- 6. Reddy ASN, Ali GS, Celesnik H, Day IS (2011) Coping with Stresses: Roles of Calcium- and Calcium/Calmodulin-Regulated Gene Expression. Plant Cell 23: 2010–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiong YQ, Liu TY, Tian CG, Sun SH, Li JY, et al. (2005) Transcription factors in rice: A genome-wide comparative analysis between monocots and eudicots. Plant Mol Biol 59: 191–203. [DOI] [PubMed] [Google Scholar]
- 9. Ishiguro S, Nakamura K (1994) Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5' upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol Gen Genet 244: 563–571. [DOI] [PubMed] [Google Scholar]
- 10. Latchman DS (1997) Transcription factors: An overview. Int J Biochem Cell Biol 29 (12): 1305–1312. [DOI] [PubMed] [Google Scholar]
- 11. Zhang YJ, Wang LJ (2005) The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends in Plant Sci 15: 247–258. [DOI] [PubMed] [Google Scholar]
- 13. Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends in Plant Sci 5: 199–206. [DOI] [PubMed] [Google Scholar]
- 14. Proietti S, Bertini L, Van der Ent S, Leon-Reyes A, Pieterse CMJ, et al. (2011) Cross activity of orthologous WRKY transcription factors in wheat and Arabidopsis . J Exp Bot 62: 1975–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin in Plant Biol 10: 366–371. [DOI] [PubMed] [Google Scholar]
- 16. Ueda M, Zhang Z, Laux T (2011) Transcriptional activation of Arabidopsis axis patterning genes WOX8/9 links zygote polarity to embryo development. Dev Cell 20: 264–270. [DOI] [PubMed] [Google Scholar]
- 17. Lagace M, Matton DP (2004) Characterization of a WRKY transcription factor expressed in late torpedo-stage embryos of Solanum chacoense. Planta 219: 185–189. [DOI] [PubMed] [Google Scholar]
- 18. Jiang W, Yu D (2009) Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol 9: 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie Z, Zhang Z-L, Hanzlik S, Cook E, Shen QJ (2007) Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol Biol 64: 293–303. [DOI] [PubMed] [Google Scholar]
- 20. Johnson CS, Kolevski B, Smyth DR (2002) TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 14: 1359–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guillaumie S, Mzid R, Mechin V, Leon C, Hichri I, et al. (2010) The grapevine transcription factor WRKY2 influences the lignin pathway and xylem development in tobacco. Plant Mol Biol 72: 215–234. [DOI] [PubMed] [Google Scholar]
- 22. Wan JR, Zhang SQ, Stacey G (2004) Activation of a mitogen-activated protein kinase pathway in Arabidopsis by chitin. Mol Plant Pathol 5: 125–135. [DOI] [PubMed] [Google Scholar]
- 23. Antoni R, Rodriguez L, Gonzalez-Guzman M, Pizzio GA, Rodriguez PL (2011) News on ABA transport, protein degradation, and ABFs/WRKYs in ABA signaling. Curr Opin Plant Biol 14: 547–553. [DOI] [PubMed] [Google Scholar]
- 24. Makandar R, Nalam V, Chaturvedi R, Jeannotte R, Sparks AA, et al. (2010) Involvement of salicylate and jasmonate signaling pathways in Arabidopsis interaction with Fusarium graminearum . Mol Plant Microbe In 23: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen L, Song Y, Li S, Zhang L, Zou C, et al. (2012) The role of WRKY transcription factors in plant abiotic stresses. BBA-Gene Regul 1819: 120–128. [DOI] [PubMed] [Google Scholar]
- 26. Ishihama N, Yamada R, Yoshioka M, Katou S, Yoshioka H (2011) Phosphorylation of the Nicotiana benthamiana WRKY8 Transcription Factor by MAPK Functions in the Defense Response. Plant Cell 23: 1153–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mao G, Meng X, Liu Y, Zheng Z, Chen Z, et al. (2011) Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niu C, Wei W, Zhou Q, Tian A, Hao Y, et al. (2012) Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ 35: 1156–1170. [DOI] [PubMed] [Google Scholar]
- 29. Shewry PR (2009) Wheat. J Exp Bot 60: 1537–1553. [DOI] [PubMed] [Google Scholar]
- 30. Chen H, Lai ZB, Shi JW, Xiao Y, Chen ZX, et al. (2010a) Roles of arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol 10: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song Y, Jing S, Yu D (2009) Overexpression of the stress-induced OsWRKY08 improves osmotic stress tolerance in Arabidopsis. Chinese Sci Bull 54: 4671–4678. [Google Scholar]
- 32. Zhou Q, Tian A, Zou H, Xie Z, Lei G, et al. (2008) Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J 6: 486–503. [DOI] [PubMed] [Google Scholar]
- 33. Davletova S, Rizhsky L, Liang HJ, Zhong SQ, Oliver DJ, et al. (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, et al. (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141: 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou S, Hu W, Deng X, Ma Z, Chen L, et al. (2012) Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. Plos One 7: e52439 doi:10.1371/journal.pone.0052439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu W, Yuan Q, Wang Y, Cai R, Deng X, et al. (2012) Overexpression of a Wheat Aquaporin Gene, TaAQP8, Enhances Salt Stress Tolerance in Transgenic Tobacco. Plant and Cell Physiology 53: 2127–2141. [DOI] [PubMed] [Google Scholar]
- 37. Wilkinson MD, Castells-Brooke N, Shewry PR (2013) Diversity of sequences encoded by the Gsp-1 genes in wheat and other grass species. J Cereal Sci 57: 1–9. [Google Scholar]
- 38. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 40. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 41. Chen L, Tu Z, Hussain J, Cong L, Yan Y, et al. (2010b) Isolation and heterologous transformation analysis of a pollen-specific promoter from wheat (Triticum aestivum L.). Mol Biol Rep 37: 737–744. [DOI] [PubMed] [Google Scholar]
- 42. Chen P, Wang C, Li K, Chang J, Wang Y, et al. (2008) Cloning, expression and characterization of novel avenin-like genes in wheat and related species. J Cereal Sci 48: 734–740. [Google Scholar]
- 43. Hong JP, Kim WT (2005) Isolation and functional characterization of the Ca-DREBLP1 gene encoding a dehydration-responsive element binding-factor-like protein 1 in hot pepper (Capsicum annuum L. cv. Pukang). Planta 220: 875–888. [DOI] [PubMed] [Google Scholar]
- 44. Han Q, Zhang J, Li H, Luo Z, Ziaf K, et al. (2012) Identification and expression pattern of one stress-responsive NAC gene from Solanum lycopersicum . Mol Biol Rep 39: 1713–1720. [DOI] [PubMed] [Google Scholar]
- 45. Shieh MW, Wessler SR, Raikhel NV (1993) Nuclear targeting of the maize R protein requires two nuclear localization sequences. Plant Physiol 101: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang G, Chen M, Li L, Xu Z, Chen X, et al. (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J Exp Bot 60: 3781–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SC, et al. (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231. [DOI] [PubMed] [Google Scholar]
- 48. Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- 49. Tseng MJ, Liu CW, Yiu JC (2007) Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol Bioch 45: 822–833. [DOI] [PubMed] [Google Scholar]
- 50. Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, et al. (1993) A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radical Bio Med 15: 353–363. [DOI] [PubMed] [Google Scholar]
- 51. Lee BH, Lee HJ, Xiong LM, Zhu JK (2002) A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14: 1235–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Podell S, Gribskov M (2004) Predicting N-terminal myristoylation sites in plant proteins. BMC Genomics 5: 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nevo E, Korol AB, Beiles A, Fahima T (2002) Evolution of wild emmer and wheat improvement: Population genetics, genetic resources, and genome organization of wheat's progenitor, Triticum dicoccoides. i-xxii, 1–364 p.
- 54. Zou CS, Jiang WB, Yu DQ (2010) Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J Exp Bot 61: 3901–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang F, Hou XL, Tang J, Wang Z, Wang SM, et al. (2012) A novel cold-inducible gene from Pak-choi (Brassica campestris ssp chinensis), BcWRKY46, enhances the cold, salt and dehydration stress tolerance in transgenic tobacco. Mol Biol Rep 39: 4553–4564. [DOI] [PubMed] [Google Scholar]
- 56. Xiong X, James VA, Zhang H, Altpeter F (2010) Constitutive expression of the barley HvWRKY38 transcription factor enhances drought tolerance in turf and forage grass (Paspalum notatum Flugge). Mol Breeding 25: 419–432. [Google Scholar]
- 57. Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16: 123–132. [DOI] [PubMed] [Google Scholar]
- 58. Shao HB, Chen XY, Chu LY, Zhao XN, Wu G, et al. (2006) Investigation on the relationship of proline with wheat anti-drought under soil water deficits. Colloid Surface B 53: 113–119. [DOI] [PubMed] [Google Scholar]
- 59. Liu JP, Zhu JK (1997) Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol 114: 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kong X, Pan J, Zhang M, Xing X, Zhou Y, et al. (2011) ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ 34: 1291–1303. [DOI] [PubMed] [Google Scholar]
- 61. Wu L, Zhang Z, Zhang H, Wang X-C, Huang R (2008) Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol 148: 1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sathiyaraj G, Lee OR, Parvin S, Khorolragchaa A, Kim YJ, et al. (2011) Transcript profiling of antioxidant genes during biotic and abiotic stresses in Panax ginseng C. A. Meyer. Mol Biol Rep 38: 2761–2769. [DOI] [PubMed] [Google Scholar]
- 63. Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends in Plant Sci 9: 490–498. [DOI] [PubMed] [Google Scholar]
- 64. Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25: 131–139. [DOI] [PubMed] [Google Scholar]
- 65. Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bray EA (1997) Plant responses to water deficit. Trends in Plant Sci 2: 48–54. [Google Scholar]
- 67. Garay-Arroyo A, Covarrubias AA (1999) Three genes whose expression is induced by stress in Saccharomyces cerevisiae . Yeast 15: 879–892. [DOI] [PubMed] [Google Scholar]
- 68. Baxter CJ, Foyer CH, Rolfe SA, Quick WP (2001) A comparison of the carbohydrate composition and kinetic properties of sucrose phosphate synthase (SPS) in transgenic tobacco (Nicotiana tabacum) leaves expressing maize SPS protein with untransformed controls. Ann Appl Biol 138: 47–55. [Google Scholar]
- 69. Chen S, Hajirezaei M, Bornke F (2005) Differential expression of sucrose-phosphate synthase isoenzymes in tobacco reflects their functional specialization during dark-governed starch mobilization in source leaves. Plant Physiol 139: 1163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Eshdat Y, Holland D, Faltin Z, BenHayyim G (1997) Plant glutathione peroxidases. Physiol Plantarum 100: 234–240. [Google Scholar]
- 71. Takemoto D, Yoshioka H, Doke N, Kawakita K (2003) Disease stress-inducible genes of tobacco: expression profile of elicitor-responsive genes isolated by subtractive hybridization. Physiol Plantarum 118: 545–553. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence analysis of the TaWRKY10 protein. Boxes represent casein kinase II phosphorylation sites. Sequences with single underlined indicate N-myristoylation sites, sequence marked with double underlines refer to WRKY domain. Zinc-finger motif is marked with asterisk.
(DOC)
Root lengths of tobacco plants overexpressing the TaWRKY10 gene under different stress conditions. The WT, VC and TG lines were cultured in MS medium under a 16 h light/8 h dark cycle at 25°C for 1 week, and then the seedlings were transplanted to fresh MS medium or MS medium supplied with 100 mM NaCl or 100 mM Mannitol or 2 mM H2O2 for 1 week. Then the photographs were taken. Three biological experiments were carried out, which produced similar results.
(DOC)
Gene specific primers used for isolating wheat WRKY genes and RT-PCR analysis.
(DOC)
Primers for TaWRKY10 used in this article.
(DOC)
Characteristics of WRKYs from wheat.
(DOC)
TaWRKY1 - TaWRKY10 expression patterns in wheat ( Triticicum aestivum cv Chinese Spring) under abiotic stresses.
(DOC)
The GenBank accession numbers of WRKY proteins used for drawing phylogenetic tree.
(DOC)