Abstract
Thinopyrum elongatum is an important relative of wheat, it is favored by many researchers for the disease resistant genes that exist in its E genome. Some studies have showed that the 7E chromosome of Th. elongatum contains resistance genes related to Fusarium head blight and wheat rust. Therefore, developing 7E chromosome-specific molecular markers linked to resistance genes will provide an important tool for exploring and using the resistant genes of Th. elongatum. In addition, it would greatly contribute in the effort to cultivate disease-resistant wheat varieties. Featured in high throughput, high-accuracy and low-cost, SLAF-seq technology has been widely used in molecular breeding, system evolution, and germplasm resource detection. Based on SLAF-seq, 518 specific fragments on the 7E chromosome of Th. elongatum were successfully amplified. A total of 135 primers were designed according to 135 randomly selected fragments, and 89 specific molecular markers of Th. elongatum were developed, with efficiencies up to 65.9%. These markers were all detected in a variety of materials, and they are all proved to be specific and stable. These markers can be used not only for detecting the 7E chromosome of Th. elongatum but also for providing an important theoretical and practical basis for wheat breeding by marker-assisted selection (MAS). This paper reports the first application of SLAF-seq technology with a high success rate in developing specific molecular markers for Th. elongatum, providing a strong case for the application of this new technology.
Introduction
Thinopyrum elongatum (syn. Lophopyrum elongatum or Agropyron elongatum) is an important wild relative of wheat, belonging to the tribe Triticeae and genus Elytrigia. It contains three species based on the ploydity: diploid (2n = 2X = 14, EE, syn. EeEe), tetraploid (2n = 4X = 28, EeEeEbEb) and decaploid (2n = 10X = 70, EeEeEbEbExExStStStSt). The E genome of the diploid is the basic genome of Th. elongatum [1], [2]. In addition, hexaploid Th. elongatum (2n = 6X = 42) was alsoreported [3]. Th. elongatum has the same ancestor with common wheat, which exhibits relatively small genetic differentiation between its E and its A, B, and D genomes [1], [2]. Th. elongatum mainly grows in temperate and cold zones, and is a perennial herb with many superior characteristics, such as long spikes, multi-flowers, high grain protein content, strong adaptability and reproductive ability. As it has some useful genes for adverse conditions such as disease, cold, drought and salinity, it is regarded as an important potential gene donor for improving biotic and abiotic stress tolerance in wheat [2], [4]–[12]. Chinese and American scientists have developed several wheat varieties using common wheat and Th. elongatum, such as Xiaoyan 6 [13], [14]. This shows that Th. elongatum can play an important role in the genetic improvement of wheat. After Chinese Spring-Th. elongatum addition and substitution lines were bred successfully, the beneficial characteristics of Th. elongatum, such as stress resistance and good quality, were widely studied at the chromosome level [10]–[12], [15], [16].
Fusarium head blight (FHB) and wheat rust are prevalent wheat diseases and can cause a great reduction in wheat production. Although there are some resistant resources in common wheat germplasm [17], [18], they still cannot control the occurrence of FHB and wheat rust, and they cannot meet the needs of wheat resistance breeding. The study of Th. elongatum has been particularly interesting to researchers world-wide. Studies have shown that the 7E chromosome of Th. elongatum contains some resistance genes [7], [8], [10]–[12], [19], such as the anti-FHB gene FhbLoP [10] and the anti-rust gene Lr19 [10], [20]–[22]. Therefore, fully developing and utilizing the resistance genes in the 7E chromosome of Th. elongatum will greatly enrich wheat resistance resources.
Marker-assisted selection (MAS) is a method to select good linkage genes or breeding multi-gene varieties based on molecular markers [23]. It is necessary and important to develop molecular markers linked to the genes beneficial for plant breeding by MAS. With many excellent genes on the Th. elongatum chromosomes, developing a large number of related, specific molecular markers will improve the chances of obtaining markers tightly linked to anti-disease genes. The markers also can improve the accuracy of anti-disease identification and further accelerate the use of Th. elongatum. In fact, several Th. elongatum chromosome-specific molecular markers have been developed by RAPD [24], [25], SSR [1], [14], [26], RFLP [27], AFLP [20], [28], STS [28], SCAR [22], [25], [29], CAPS [30], RGAP [31], TRAP [14], and SSH [32]. With the high genomic sequence homology between Th. elongatum and common wheat and the weaknesses of current technologies listed above due to high cost, long cycle, and low success rate in molecular marker development, it is difficult to obtain the large amount of markers needed to meet the requirement for breeding anti-disease varieties by MAS.
The SLAF-seq (Specific Length Amplified Fragment Sequencing) was developed based on high-throughput sequencing technology. It allows researchers to design the experimental system through bioinformatics and screen for fragments of a specific length from the constructed SLAF-seq library. The massive sequences were then obtained and analyzed using SLAF_Poly.pl. (Biomarker, Beijing, China). After a sequence comparison using BLAT [33], a large number of specific fragments are selected for specific molecular markers development. SLAF-seq technology has several obvious advantages, such as high throughput, high accuracy, low cost and short cycle, which enable its sequencing results to be directly used for molecular markers development. This technology has been reported for haplotype mapping, genetic mapping, linkage mapping, and polymorphism mapping. It can also provide an important basis for molecular breeding, system evolution and germplasm resource identification. In this paper, SLAF-seq technology was first used to obtain Th. elongatum 7E chromosome-specific fragments and to successfully develop many 7E chromosome-specific molecular markers. The success of developing chromosome- specific molecular markers by SLAF-seq technology provides a strong technical support for its future application.
Materials and Methods
Materials
The genetic stocks employed in the current study are listed in Table 1, including Chinese Spring (CS), diploid Thinopyrum elongatum (Th. elongatum, 2n = 2X), their addition lines (DA lines), ditelo addition lines (DA7ES, DA7EL), substitution lines (DS lines), other wheat varieties, polyploid Th. elongatum, and cross offsprings. The materials were supplied by Dr. Goeger Fedak (Eastern cereal and oilseed research center, Canada), academician Shunhe Cheng (Lixiahe region agricultural scientific research institute, China).
Table 1. The experimental materials used in this study.
| Name of the Materials | Abbreviation of the Materials |
| Diploid Thinopyrum elongatum | Th. elongatum (2n = 2X) |
| Chinese Spring | CS |
| Chinese Spring-Thinopyrum elongatum disomic addition lines | DA1E, DA2E, DA3E, DA4E, DA5E, DA6E, DA7E |
| Chinese Spring-Thinopyrum elongatum telodisomic addition lines | DA7ES,DA7EL |
| Chinese Spring-Thinopyrum elongatum disomic substitution lines | DS1E(1A), DS1E(1B), DS1E(1D), DS2E(2A), DS2E(2B), DS2E(2D), DS3E(3A), DS3E(3B), |
| DS3E(3D), DS4E(4A), DS4E(4B), DS4E(4D), | |
| DS5E(5B), DS5E(5D), DS6E(6A), DS6E(6D), DS7E(7A), DS7E(7B), DS7E(7D) | |
| Langdon | LD |
| Yangmai 10, Yangmai 14, Yangmai 16, Yangmai 18, Yangmai 158 | Y10, Y14, Y16, Y18, Y158 |
| Ningmai 13 | N13 |
| Annong 8455 | An8455 |
| Sumai 3 | Su3 |
| Langdon-Th. elongatum amphidiploid | 8801 |
| Tetraploid Thinopyrum elongatum | Th. elongatum (2n = 4X) |
| Decaploid Thinopyrum elongatum | Th. elongatum (2n = 10X) (PI179162/PI204383) |
| F1 and F2 of Yangmai 16×DS7E(7A) | YD-F1, YD-F2 |
| F1 and F2 of DS7E(7A)×Yangmai 16 | DY-F1, DY-F2 |
SLAF-seq Technology Scheme Design
Based on the GC content, repeat sequences and gene characters, the wheat BAC sequences were analyzed using SLAF_Predict (Biomarker, Beijing, China). The plan for marker development was designed by defining the enzyme digestion scheme, gel cutting ranges and sequencing quantity, which were used to verify the density and homogeneity of the marker being developed and ensure the likelihood of successfully preparing the expected target.
Genomic DNA Extraction
The SDS method [34] was used to extract genomic DNA from young leaves of the genetic stocks. DNA quality and concentration were measured by 0.8% agarose gel electrophoresis, and adjustments were made for a final DNA concentration of 100 ng µL−1.
Genomic DNA Digestion
Genomic DNA (500 ng) from CS, Th. elongatum (2n = 2X) and DA7E were incubated at 37°C with 0.6U MseI (New England Biolabs, Hitchin, Herts, UK), T4 DNA ligase (NEB), ATP (NEB) and MseI adapters. Restriction-ligation reactions were heat-inactivated at 65°C and then digested in an additional reaction with the restriction enzymes HaeIII and BfaI at 37°C.
PCR Reaction and Fragment Amplification
A PCR reaction was performed containing the diluted restriction-ligation samples, dNTP, Taq DNA polymerase (NEB) and MseI-primer containing barcode. The PCR products were purified by E.Z.N.A.® Cycle Pure Kit (Omega) and pooled.
Fragment Selection, Extraction and Amplification
The pooled sample was incubated at 37°C with MseI, T4 DNA ligase, ATP and Solexa adapters. The samples were purified using a Quick Spin column (Qiagen) and then separated on a 2% agarose gel to isolate the fragments between 300 to 500 bp using a Gel Extraction Kit (Qiagen). These fragments were used in a PCR amplification with Phusion Master Mix (NEB) and Solexa amplification primer mix. Phusion PCR settings followed the Illumina sample preparation guide. Samples were gel-purified, and products with appropriate sizes (300 to 500 bp) were excised and diluted for sequencing by Illumina GAIIx (Illumina, San Diego, CA, USA).
Sequencing and Sequence Analysis
The cluster density was optimized to ensure that the SLAFs corresponding with the set requirements, and the PCR amplified products were sequenced using an Illumina GAIIx (Illumina, CA, USA). The SLAFs were identified and filtered to ensure that the original sequencing data were effectively obtained. They were clustered based on similarity using BLAT [33], and their sequences were obtained through focused recognition and correction techniques.
Sequence Comparison and Thinopyrum elongatum 7E Chromosome-specific Fragment Acquisition
The fragments of DA7E and Th. elongatum (2n = 2X) were selected by a specificity comparison. The sequences with good quality from Th. elongatum (2n = 2X) and DA7E were first compared with the CS sequences acquired by SLAF-seq, and they were then compared with the sequences on www.ncbi.nlm.nih.gov and www.cerealsdb.uk.net. Finally, the specific sequences of DA7E and Th. elongatum (2n = 2X) were compared and the 7E chromosome-specific sequences of Th. elongatum were obtained.
7E Chromosome-specific Molecular Markers of Thinopyrum elongatum Development and Stability Detection
Based on these sequences, PCR primers were designed for the amplification of DA7E and CS. The amplified products were electrophoresed in 0.8% agarose gel, and the markers presented in DA7E but absent in CS were identified as the 7E chromosome-specific molecular markers. Then, the stabilities of these markers were detected in DA lines, DA7ES, DA7EL, DS lines, CS, Th. elongatum (2n = 2X), LD, Y10, Y14, Y16, Y18, Y158, N13, An8455, Su3, 8801, Th. elongatum (2n = 4X), Th. elongatum (2n = 10X), YD-F1, YD-F2, DY-F1 and DY-F2, respectively. The PCR system contained 1 µL of genomic DNA (100 ng µL−1), 2.5 µL of loading buffer (10×), 2 µL of dNTP (2.5 mM), 1 µL of primer 1 (10 µM) and primer 2 (10 µM), 0.3 µL of Taq (5 U µL−1) and 18.2 µL of double-distilled water. The PCR procedure was as follows: 94°C for 5 min; followed by 35 cycles of 94°C for 45 s, appropriate anneal temperature (45–60°C) for 1 min, and 72°C for 1.5 min; then 72°C for 10 min.
Results and Analysis
Acquisition of Specific Sequences from the 7E Chromosome of Thinopyrum elongatum
Using the SLAF-seq technology, 70,152, 49,848 and 59,141 effective SLAFs were acquired for CS, Th. elongatum (2n = 2X) and DA7E, respectively. The sequencing depth was more than 9×. The result was optimal and fulfilled the expected requirements. After comparing the CS sequences acquired by SLAF-seq and the sequences in www.ncbi.nlm.nih.gov or www.cerealsdb.uk.net, 20,170 Th. elongatum (2n = 2X) and 4,984 DA7E sequences whose homology with CS and other wheat species was less than 50% were selected as the specific sequences for Th. elongatum (2n = 2X) or DA7E. From those specific ones, 518 DA7E sequences with homologies higher than 80% of Th. elongatum (2n = 2X) were obtained. These DA7E sequences were identified as the 7E chromosome-specific sequences of Th. elongatum.
Primer Design and Marker Development for 7E Chromosome of Thinopyrum elongatum
Based on 135 sequences randomly selected from the specific sequences of the 7E chromosome, 135 pairs of primers were designed for developing specific molecular markers (Table 2). PCR products were amplified from DA lines (DA1E-7E), CS, Th. elongatum (2n = 2X), DA7ES and DA7EL, respectively. A total of 89 of Th. elongatum specific molecular markers were successfully developed (Table 2), with the success rate up to 65.9%. These markers included 61 Th. elongatum 7E chromosome specific markers, 14 genome markers and 14 chromosome markers which also appeared on several other chromosomes including 7E. The 61 specific molecular markers of the 7E chromosome included 35 only appearing on the short arm of the 7E chromosome (Fig. 1A), 24 on the long arm (Fig. 1B), and 2 on both arms (Fig. 1C). The 14 genome markers included 1 marker that only appeared on the short arm, 1 on the long arm and 12 on both arms of the 7E chromosome. The 14 other markers included 8 that only appeared on the short arm, 4 on the long arm and 2 on both arms of the 7E chromosome. The success rate of developing the 7E chromosome-specific molecular markers was as high as 45.2%.
Table 2. The 7E chromosome-specific molecular markers and PCR primers of Thinopyrum elongatum.
| Specific Markers | SpecificPrimers | Sequences of the Special Primers (5′–3′) | AmplifiedChromosomes | Original Fragments | ||
| Forward | Reverse | |||||
| M7E_No.1 | P7E_No.1 | ATCAATCCCTCCACAAAGTC | GCCTTTTTTTTCTAATGTGC | 7E,7ES | SLAF119658 | |
| M7E_No.2 | P7E_No.2 | AGCAAATAAAACGACAGT | GGTTTTCACCAATTACAG | 7E,7EL | SLAF31945 | |
| M7E_No.3 | P7E_No.3 | GGAAAATGGAATCAAGGAGG | GAACTCGTATCCCTTGCCTT | 1E-7E,7ES,7EL | SLAF2140 | |
| M7E_No.4 | P7E_No.4 | TTTTTCTGGTACTTACTAAC | TGTTCTGTGAGATGAATGTT | 7E,7ES | SLAF5720 | |
| M7E_No.5 | P7E_No.5 | AGTCAAGGCAAATTATGGT | ACTGTCTAATGTCTCGTAAT | 7E,7EL | SLAF48385 | |
| M7E_No.6 | P7E_No.6 | GGTGTGTAGAAAAACATAAC | CTTTGAATACACCCTACTTA | 1E,7E,7ES | SLAF45083 | |
| M7E_No.7 | P7E_No.7 | ATTTTGTCCCTATGCT | ATGCCTTCCTGATTTA | 7E,7ES | SLAF49074 | |
| M7E_No.8 | P7E_No.8 | ACCATTCCCCAAAACT | ATTGTCAATTATTTTTTAGC | 1E-4E,7E,7ES,7EL | SLAF20928 | |
| M7E_No.9 | P7E_No.9 | AGTGAACTGAATTCTCTGCT | CTCTTTTGAAGCATTACACA | 7E,7ES,7EL | SLAF50207 | |
| M7E_No.10 | P7E_No.10 | TTTCGCTGCTGAAGAATCTA | GCAAAACCATCATACAATCG | 7E,7ES,7EL | SLAF140297 | |
| M7E_No.11 | P7E_No.11 | TAGAATAGCTTTTAGGAATA | GTGAGCATTCTTAGCATTAC | 7E,7ES | SLAF19795 | |
| M7E_No.12 | P7E_No.12 | AAACCCAAATGAAAGT | CAAACTTATAAGTAGAGACA | 7E,7ES | SLAF25934 | |
| M7E_No.13 | P7E_No.13 | CACTTGTGCATATTCGAGAG | CCATTTTCCAATATATACAA | 7E,7ES | SLAF1998 | |
| M7E_No.14 | P7E_No.14 | GAAATGGCAAGTACCTAA | CAAGTAGAAGTTCAGCAA | 7E,7ES | SLAF47229 | |
| M7E_No.15 | P7E_No.15 | CCAAGAAAAGTCAACCGT | GAGCAACCAAATTCAATAGA | 7E,7EL | SLAF44157 | |
| M7E_No.16 | P7E_No.16 | CCTCGAAATCAATCAATCCT | CCATAAAAAGGCAAAAATCC | 7E,7ES | SLAF21225 | |
| M7E_No.17 | P7E_No.17 | CAGCACCACTGTTTACTTAG | ACACCTGTAAGGCTGTAATA | 1E-7E,7EL | SLAF11089 | |
| M7E_No.18 | P7E_No.18 | ATGTTGTTGTTTTTTGGTGT | TGGAAACTTGTATGAAATGG | 7E,7EL | SLAF237685 | |
| M7E_No.19 | P7E_No.19 | TTTCCATTGGGGTAGT | CGGGGTGATTACTTTC | 7E,7ES | SLAF29032 | |
| M7E_No.20 | P7E_No.20 | CTTTCCATAGTAGGTCCAGT | CGAACTTTGTTTGAAATATG | 7E,7ES | SLAF49101 | |
| M7E_No.21 | P7E_No.21 | GCACAAAAAGAGCAGAATAT | AAGCTTATTACAGGACCATG | 7E,7ES | SLAF22240 | |
| M7E_No.22 | P7E_No.22 | AGTGAATCTGAGATGCATAT | ATTTTGTTTTTACCATTTTT | 7E,7ES | SLAF27230 | |
| M7E_No.23 | P7E_No.23 | GCTCTTGAGCGTCTACAGTG | TTCGTATGGTTTTTTCTGGC | 7E,7ES | SLAF65372 | |
| M7E_No.24 | P7E_No.24 | CAAAATGAAAAGATAAAACT | AGATTCAAAATTTTAGTTAT | 7E,7ES | SLAF91582 | |
| M7E_No.25 | P7E_No.25 | CAGCTCCATCGAAACTCT | GGACGACCTGCTAATACA | 5E,7E,7EL | SLAF46357 | |
| M7E_No.26 | P7E_No.26 | TGTGTCGTAGAGATGTGTTG | GGGTGATAGACATATGCAAT | 7E,7EL | SLAF46632 | |
| M7E_No.27 | P7E_No.27 | GTCGTGGATATGTCATTGTA | TTGTATGGATGCTTTGGT | 1E,2E,4E,5E,7E,7ES | SLAF258417 | |
| M7E_No.28 | P7E_No.28 | ATTCTGATGTGTATTGAGCC | AACGTGTCCACTAACAACTT | 7E,7ES | SLAF31380 | |
| M7E_No.29 | P7E_No.29 | CTACTGCTTTAGGGTGTTGA | CCAAGAATAGCACAAACAAC | 7E,7ES | SLAF22799 | |
| M7E_No.30 | P7E_No.30 | AAGTTCAAGTTTGCAGGTAC | AGCATTAGTATTTGAGAAGC | 4E,5E,7E,7ES | SLAF238 | |
| M7E_No.31 | P7E_No.31 | CTACCCTTACCACCTCG | CCACTGGATGCTGTTTAT | 7E,7ES | SLAF14034 | |
| M7E_No.32 | P7E_No.32 | CTGAGCTGCGTCGGTA | CCAGAAATTGCTAAAATCTT | 1E-7E,7ES,7EL | SLAF35615 | |
| M7E_No.33 | P7E_No.33 | TGTTTAGTAGAGGGTTCATT | GTGTGGGTAATATTTTTGTA | 1E-7E,7ES | SLAF44977 | |
| M7E_No.34 | P7E_No.34 | AAAATCAGCGGTGCCT | ACCTGTAGATTGAAATGCCT | 7E,7EL | SLAF49963 | |
| M7E_No.35 | P7E_No.35 | GACCAATGGAAAGAAAATGT | CAACACTCTTGTCTTCCTTT | 7E,7EL | SLAF42598 | |
| M7E_No.36 | P7E_No.36 | TGTTTCTTAGTTGTTTTGTT | GCCTTGACCACCATAC | 1E-7E,7ES,7EL | SLAF12623 | |
| M7E_No.37 | P7E_No.37 | GGTAAGCTTGAAATACATGA | TCCAAGTGATATTGTAGTCG | 7E,7ES | SLAF32494 | |
| M7E_No.38 | P7E_No.38 | GTGGAATTGGACTTTTTTTG | AGATTTCCTGTTATCCCAAG | 1E-7E,7ES,7EL | SLAF231806 | |
| M7E_No.39 | P7E_No.39 | TTTATAAGTTGATGAGGGGG | AAGGCTTTACCGAAAATCAT | 7E,7EL | SLAF216573 | |
| M7E_No.40 | P7E_No.40 | CTCGTCCTCGTCCTCCTTGT | AGCATAACTTGCCAATCCCC | 7E,7ES | SLAF5918 | |
| M7E_No.41 | P7E_No.41 | AAAGTGCTTCATCCCAAAT | AGGATGATATGAATGCTTTT | 7E,7EL | SLAF14218 | |
| M7E_No.42 | P7E_No.42 | TTAGCATATGCTTTTTAGGC | GCAAATCAGTTCAGTGAACC | 1E,3E-5E,7E,7ES,7EL | SLAF12080 | |
| M7E_No.43 | P7E_No.43 | GCCCAGTGTAGTTCGCTCGT | TTCTCAGGCGAGGAAGTGGA | 7E,7ES | SLAF39853 | |
| M7E_No.44 | P7E_No.44 | ACAGATGCCTAAAAGC | CACAAAATCTTGGGTC | 7E,7EL | SLAF7994 | |
| M7E_No.45 | P7E_No.45 | TTGTTTGTTGGACTTGAATG | GCACAAAATAGTGAGAAGGC | 7E,7ES | SLAF8447 | |
| M7E_No.46 | P7E_No.46 | GTCTAACTTGTTGTGTGTGC | CACTCAGGAACTAAATTTGC | 7E,7EL | SLAF12583 | |
| M7E_No.47 | P7E_No.47 | ATGTTGTACTCCATTCAGAT | GAGATACAAAAATTTGAGTG | 1E,5E,7E,7ES | SLAF24261 | |
| M7E_No.48 | P7E_No.48 | CATGGGTGATGAAAAGAAGA | GCCAACTATGTGGTTTCAAG | 7E,7ES | SLAF236334 | |
| M7E_No.49 | P7E_No.49 | ATACTTGAGGTGATTTCGGT | GGTGCAAAGTTTTTACAATG | 7E,7ES | SLAF236809 | |
| M7E_No.50 | P7E_No.50 | TAAAGTGGAGGTAAAATGAC | AAAGATTCGAAAAATTAGTT | 7E,7EL | SLAF200585 | |
| M7E_No.51 | P7E_No.51 | TACACAGAAGGAAAGCATTA | CATCAGAAATTTTCTTTTGA | 7E,7EL | SLAF1699 | |
| M7E_No.52 | P7E_No.52 | ACAAGTCCATTCATTACAAC | TACTACTTTTGTGACAGCAG | 7E,7ES | SLAF43910 | |
| M7E_No.53 | P7E_No.53 | GTCAAGAGTTGGCTTTATTC | ATTTGCTAATTCTCGTCATA | 7E,7ES | SLAF6445 | |
| M7E_No.54 | P7E_No.54 | CATGCGACCTACAATAAATT | GTAATTTTTTGTCATGTGCC | 7E,7EL | SLAF251157 | |
| M7E_No.55 | P7E_No.55 | ATTATTTACGTTTCTTGAGC | CTTCCCCACTCTTTGACT | 1E,2E,5E-7E,7EL | SLAF140771 | |
| M7E_No.56 | P7E_No.56 | TTACACTAACCCATGGTGTT | GCAGAGAATGAAGCAAAATC | 7E,7ES | SLAF12105 | |
| M7E_No.57 | P7E_No.57 | CTTTTATGTATTTGAGAGCA | CGCAACTCCAATATGA | 7E,7ES | SLAF22820 | |
| M7E_No.58 | P7E_No.58 | CAAATCTGTTGAACTGTCTT | TGCGATACAAGTATAAAATG | 5E,7E,7ES,7EL | SLAF15563 | |
| M7E_No.59 | P7E_No.59 | TTGCTACAAATATTGAGTCA | GTACTTGTGCATCCCTTC | 7E,7ES | SLAF130591 | |
| M7E_No.60 | P7E_No.60 | TTTTCCAGCTTCCTAATT | TTGACTGCTTCATTCTTC | 5E,7E,7ES,7EL | SLAF160814 | |
| M7E_No.61 | P7E_No.61 | TAAGTTGATAGATGTGCTG | TTGAATTGTAGCTAAAGTAA | 1E-7E,7ES,7EL | SLAF15482 | |
| M7E_No.62 | P7E_No.62 | CCAAGATGGTATGACACTAT | AGTACTCGGATGATTTTCTC | 7E,7ES | SLAF29906 | |
| M7E_No.63 | P7E_No.63 | ACAAGCAGAATCGGAACG | GCACATCCAATTGTCACACT | 1E-7E,7ES,7EL | SLAF105525 | |
| M7E_No.64 | P7E_No.64 | ATTTTATGACCAAGGACT | ACACACACTTCTACTTTC | 4E,5E,6E,7E,7ES | SLAF137880 | |
| M7E_No.65 | P7E_No.65 | CACACACTTCTACTTTCG | GGGTTGGTTCCATCACAT | 7E,7ES | SLAF137880 | |
| M7E_No.66 | P7E_No.66 | GGGTTTACCTCCGCATCG | GCAAATTATTATCAGCCACCAA | 2E-7E,7ES | SLAF3970 | |
| M7E_No.67 | P7E_No.67 | ATTTTGTCAGTGGAATGGAT | AATAAATCAAATCCTGCTCA | 4E,5E,7E,7ES | SLAF251334 | |
| M7E_No.68 | P7E_No.68 | CAATGGTACATATCACACT | ATGCACGATTCTACAGT | 7E,7ES | SLAF774 | |
| M7E_No.69 | P7E_No.69 | TTTCTGTAAGCCGATGC | AAGAACTACCTGGTGAAATAC | 1E-7E,7ES,7EL | SLAF45682 | |
| M7E_No.70 | P7E_No.70 | AATGGAGCCCAAGGAG | CCATCCAACGGAAGTG | 1E-7E,7ES,7EL | SLAF16926 | |
| M7E_No.71 | P7E_No.71 | GTCTTGCCTGTCCTCG | ATTTTCAAAGTTCTCACAAG | 7E,7EL | SLAF252555 | |
| M7E_No.72 | P7E_No.72 | GGACTTGGACTCTATCTTC | GACCCAACAATTTCGA | 7E,7EL | SLAF45552 | |
| M7E_No.73 | P7E_No.73 | ACTCATACCAATCCCGTCTA | TTGTTATTTTCGCACTATGG | 1E-7E,7ES,7EL | SLAF40006 | |
| M7E_No.74 | P7E_No.74 | CGTGCCTGTGGTTATGT | TTGCCTTCAGTCATTTCA | 7E,7EL | SLAF9221 | |
| M7E_No.75 | P7E_No.75 | TTCAAAGGAACATTTACAAG | CTACCCGGTCCTTCTC | 1E-7E,7ES,7EL | SLAF362764 | |
| M7E_No.76 | P7E_No.76 | AGCATAGGGACCACTTC | TTACTGATGGATTGGCA | 1E-7E,7ES,7EL | SLAF23848- | |
| M7E_No.77 | P7E_No.77 | TGTTGTAGTTTCGTCCCT | TGGTGGATGAGGAAGAC | 7E,7ES | SLAF4571 | |
| M7E_No.78 | P7E_No.78 | AATTACTATGTGCATCGG | TGTAATCAAAATATCAGTCG | 7E,7EL | SLAF129639 | |
| M7E_No.79 | P7E_No.79 | GTAGTATCTCGCCGATGTCGT | TCTGGCGTGATTATTGTGGC | 1E-7E,7ES,7EL | SLAF9285 | |
| M7E_No.80 | P7E_No.80 | GCTTGGAGGAGTTGAT | TTCTTCTATGTGTTTTATTG | 7E,7EL | SLAF32358 | |
| M7E_No.81 | P7E_No.81 | ACACAAAGGTGAGTGAAAAC | GAGTAGCAAAAATCTCAACA | 7E,7EL | SLAF72555 | |
| M7E_No.82 | P7E_No.82 | AGTATTGTGCCAGTATTC | ATCAAGAGGGTATAACTG | 7E,7ES | SLAF34164 | |
| M7E_No.83 | P7E_No.83 | AGACTATCTTATCAACCATT | CAACTACACGCTAAACC | 7E,7ES | SLAF38680 | |
| M7E_No.84 | P7E_No.84 | CGAAGGGTCTTTGATT | GCAAACATCTGACAAGG | 7E,7E | SLAF2228 | |
| M7E_No.85 | P7E_No.85 | CATGTTTACGTCCTAATTCT | TCAAACTGCTTGCTCTG | 7E,7EL | SLAF3153 | |
| M7E_No.86 | P7E_No.86 | CACCATTGCAAGTTTGA | AAGCCCACCTCTATTGA | 7E,7EL | SLAF69129 | |
| M7E_No.87 | P7E_No.87 | ACAAACCAATGGAAAGG | CGGAGCAACTACAGACG | 2E,6E,7E,7ES | SLAF35412 | |
| M7E_No.88 | P7E_No.88 | ATGTTCTTTCTTTCGGTT | GCTTACTCAACAGAAAAAAC | 7E,7ES | SLAF1128 | |
| M7E_No.89 | P7E_No.89 | TGCAATGTCCTTGATAGA | GCTCTGTAAAGGTAAAATCT | 7E,7EL | SLAF240072 | |
A list of the names of the specific markers is shown, where M7E_No.1 stands for the first (No.1) molecular marker (M) of the Thinopyrum elongatum 7E-chromosome (7E). A list of the name of the specific primers is shown, where P7E_No.1 stands for the first pair (No.1) of primers (P) of the Thinopyrum elongatum 7E-chromosome (7E). Additionally, the name of the original fragments is listed, where SLAF119658 stands for specific (S) length (L) amplified (A) fragment (F), and its number is 119658.
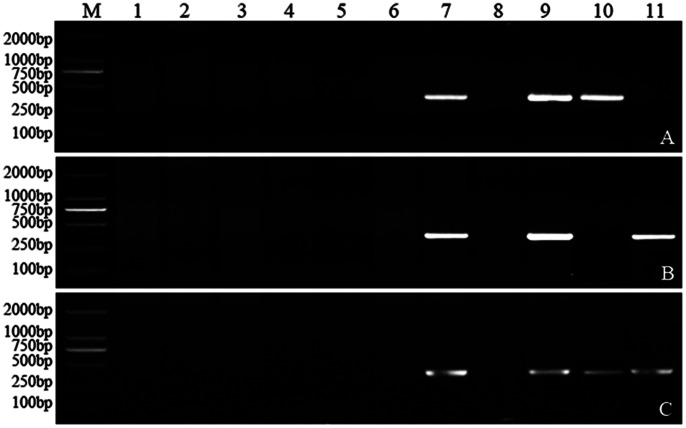
Figure 1. The PCR amplification of M7E_No.1 (A), M7E_No.2 (B) and M7E_No.9 (C) in CS- Thelongatum disomic addition and 7E telodisomic addition lines.
M: Marker (DL2000); 1–7: DA1E-DA7E; 8: CS; 9: Th. elongatum (2n = 2X); 10: DA7ES; 11: DA7EL.
Repeatablity, Stability and Specificity of the 7E Chromosome- specific Molecular Markers of Thinopyrum elongatum
To test for the repeatability, stability and specificity, the molecular markers were amplified using all materials listed in Table 1, including DA lines, DA7ES, DS7EL, DS lines, CS, Th. elongatum, other wheat varieties, polyploid Th. elongatum, and cross offsprings. The results showed that the markers developed by the SLAF-seq technology were repeatable, stable and specific. For example, M7E_No.2 appeared consistently in DA7E, DA7EL, Th. elongatum (2n = 2X) (Fig. 1B), DS7E(7A), DS7E(7B), DS7E(7D) (Fig. 2), 8801, Th. elongatum (2n = 4X), Th. elongatum (2n = 10X) (Fig. 3), YD-F1, DY-F1, parts of YD-F2 and DY-F2 (Fig. 4), while it did not appear in the materials lacking the 7E chromosome. This indicated that M7E_No.2 is a repeatable, stable and specific molecular marker of Th. elongatum 7E chromosome.
Figure 2. The stability of M7E_No.2 in CS- Th. elongatum disomic substitution lines.

M: Marker (DL2000); 1: DS1E (1A); 2: DS1E (1B); 3: DS1E (1D); 4: DS2E (2A); 5: DS2E (2B); 6: DS2E(2D); 7: DS3E(3A); 8: DS3E(3B); 9: DS3E(3D); 10: DS4E(4A); 11: DS4E(4B); 12: DS4E(4D); 13: DS5E(5B); 14: DS5E(5D); 15: DS6E(6A); 16: DS6E(6D); 17: DS7E(7A); 18: DS7E(7B); 19: DS7E(7D); 20: CS; 21: Th. elongatum (2n = 2X).
Figure 3. The stability of M7E_No.2 in other wheat, amphidiploid and polyploid Th. Elongatum.

M: Marker (DL2000); 1: LD; 2: Y10; 3: Y14; 4: Y16; 5: Y18; 6: Y158; 7: N13; 8: An 8455; 9: Su 3; 10∶8801; 11: Th. elongatum (2n = 4X); 12: Th. elongatum (2n = 10X, PI179162); 13: Th. elongatum (2n = 10X, PI204383); 14: CS; 15: Th. elongatum (2n = 2X).
Figure 4. The stability of M7E_No.2 in F1 and F2 of orthogonal (A) and reciprocal (B) cross offspring of Y16 and DS7E (7A).
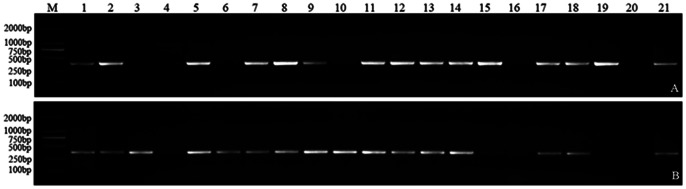
M:Marker (DL2000); 1: YD-F1 (A) or DY-F1 (B); 2–19: YD-F2 (A) or DY-F2 (B); 20: Y16; 21: DS7E(7A).
Analysis of the 7E Chromosome-specific Molecular Markers of Thinopyrum elongatum
PCR products of ten markers randomly selected from the 89 specific molecular markers of Th. elongatum were re-sequenced and compared with common wheat sequences. As expected, the lengths of the specific molecular markers of Th. elongatum developed by SLAF-seq were between 300 bp to 500 bp, and they hadlittle sequence homology with common wheat. To confirm these findings, M7E_No.2. was re-sequenced and compared with wheat common sequences in www.ncbi.nlm.nih.gov or www.cerealsdb.uk.net. It showed that the 339 bp M7E_No.2 marker (Table 3) had low sequence homology with CS or other common wheat varieties.
Table 3. The DNA sequences and length of the Thinopyrum elongatum 7E chromosome-specific molecular marker M7E_No.2.
| Name | Sequences(5′-3′) | Length |
| M7E_No.2 | AGCAAATAAAACGACAGTGCAGCTCGTGGTTAGTATGAAAATTTACTTTAGTATACTACTATCCGCATCTAATGCATGTATGGATGCACCAAAATTTGTACTAATAAAGGAGCATTATCATATTTGTTTAGCAAGCGAACCGTGGTACTTATTGCAGCAGAACACTTCTGAATAAATTCAATGCGGGAGAGAGGTGTTACCTTCTTAGCATTCAGGTAGCTGTCCTTGGGTAGCTCGGTAAAGGTATTTTTCAAAGGAGTTCTCGACCCGGTGCTCCATGGTGCAGTATCCAGTGACGATGCAATTAGCAGACAGCCCCGGCTGTAATT GGTGAAAACC | 339 bp |
Discussion
The Feasibility and Advantages of SLAF-seq Technology in Chromosome-specific Molecular Marker Development
SLAF-seq technology is highly automated because it was developed using bioinformatics for high-throughput sequencing technology applications. It can generate large amounts of sequence information and handle any whole genome density distributions. In this study, 518 specific fragments of the 7E chromosome of Th. elongatum were obtained by the SLAF-seq technology. Based on 135 randomly selected fragments, 89 specific molecular markers including 61 7E-chromosome specific molecular markers were developed. SLAF-seq technology was capable of developing Th. elongatum specific markers with high success rate and low cost. On the other hand, the success rate of developing Th. elongatum genome- or chromosome-specific molecular markers by conventional methods were quite low [24], [26], [28], [32]. For example, 94 Th. elongatum specific fragments were obtained using 26 pair of RAPD primers [24] with only 3 1E or 3E chromosome-specific molecular markers obtained. 108 Th. elongatum specific fragments were obtained using 40 SSR primers [26] with only 1 genome-specific molecular markers obtained. 28 Th. elongatum specific fragments were obtained using 5 pair of AFLP primers [28] with only 4 chromosome-specific molecular markers obtained. In addition, 65 Th. elongatum specific fragments were obtained using SSH, but only 1 chromosome-specific molecular marker was developed [32]. The SLAF-seq technique cost 1/8 of that of AFLP while the efficiency was 27 times (www.biomarker.com.cn). Therefore, compared to RAPD [24], AFLP [28] or SSH [32], the SLAF-seq technology is much better in developing plant chromosome-specific molecular markers with higher success rate, specificity, stability, and lower cost.
Repeatability, Stability and Specificity of the Thinopyrum elongatum 7E Chromosome-specific Molecular Markers Developed by SLAF-seq
M7E_No.2, one 7E chromosome-specific molecular marker, uniquely appeared in all the materials containing the 7E chromosome but not in others (Fig. 1B, 2, 3 and 4). This suggested that M7E_No.2 was reliable and the fact M7E_No.2 stably appeared not only in the diploid Th. elongatum but also in the polyploid Th. elongatum proved that the E genome of the diploid Th. elongatum was the basic genome of the polyploid Th. elongatum (Fig. 3). M7E_No.2 was detected in some progenies of YD-F2 and DY-F2, and its segregation of positive and negative was nearly 3∶1, strictly consistent with Mendel’s law (Fig. 4).
All the specific molecular markers of Th. elongatum were also detected, and the results, especially those of the 60 7E-chromosome specific molecular markers, were the same as that of M7E_No.2. This finding showed that the specific molecular markers of Th. elongatum developed by the SLAF-seq technology were all repeatable, stable and specific. The result of the 14 genome markers and the other 14 chromosome markers also appearing in the materials having some E chromosomes confirmed that all the E chromosomes of Th. elongatum had high DNA sequence homology with each other which might be caused by chromosomal rearrangement [27].
The Application Value of the 7E Chromosome-specific Molecular Markers of Thinopyrum elongatum
After DA lines and DS lines were crossed successfully, the positive characteristics of Th. elongatum were widely studied at the chromosome level [10]–[12], [15], [16]. Dvorák et al. found that different chromosomes of Th. elongatum had different effects, whereas the 7E chromosome affected the number of days to heading, maturity and seed yield, decreased the plant height, and increased the seed weight [15], [16]. Many studies also showed that there were anti-FHB genes [7], [8], [10]–[12], [19] and anti-rust genes [10], [20], [21], such as FhbLoP or Lr19, located on the 7E chromosome of Th. elongatum. If the resistance genes are fully explored and used, they would greatly enrich the resistance germplasm resources for wheat.
The 7E chromosome-specific molecular markers of Th. elongatum developed in this study are dominant markers, which provides a good basis for their subsequent applications. Based on molecular markers, FhbLoP has been mapped to the very distal region of the long arm of 7E chromosome within a 3.71 cM interval flanked by Xcfa2240 and Xswes19, which accounts for 30.46% of the phenotypic variance. Lr19 has been bracketed by Xwmc273 and XBE404744, with a map distance of 1.54 and 1.43 cM from either side, respectively [10]. The closely linked markers to anti-disease genes will be helpful for marker-assisted introgression of the genes of interest, such as anti-FHB genes, into elite cultivars of the common wheat. The development of a genetic map will accelerate the map-based cloning of these genes. Hybridizing or back-crossing between DS lines and cultivated wheat, or using Ph gene mutation, small fragments containing resistance genes of Th. elongatum E genome will translate into wheat which can be performed rapidly and accurately to obtain the resistance offspring by MAS [14], [35]. It was reported that radiating the hybrid offspring between DS lines and cultivated resulted in the chromosome fragments to break and reclose, allowing the generation of Th. elongatum translocation lines. Using the MAS, these translocation lines can be used to breed anti-desease wheat varieties [14], [36].
Developing a large number of Th. elongatum 7E chromosome-specific molecular markers is very valuable, not only for the identification of Th. elongatum 7E chromosomes but also for the acceleration of the exploration and usage of the useful genes of Th. elongatum with high agronomical or anti-disease value, such as FhbLoP and Lr19. This finding further enriches the resistance resources for wheat and provides a basis for anti-disease or anti-stress wheat breeding.
Acknowledgments
We thank Dr. Goerge Fedak (Eastern cereal and oilseed research center, Canada), academician Shunhe Cheng (Lixiahe region agricultural scientific research institute, China) for supplying us with the materials used in this experiment.
Funding Statement
This study was supported by the National Natural Science Foundation of China (31071406), Specialized Research Fund for the Doctoral Program of Higher Education (20123250110010) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Li N, Wang XP, Cao SH, Zhang XQ (2005) Genome constitution of Agropyron elongatum 4x by biocheical and SSR markers.Acta. Genetica Sinica 32(6): 571–578. [PubMed] [Google Scholar]
- 2. Yan XD, Li JL, Zhang YM (2010) Application Study of Genetic Resources in Elytrigia. Biotechnol. Bull. 6: 18–21. [Google Scholar]
- 3. Mao PS, Huang Y, Wang XG, Meng L, Mao PC, et al. (2010) Cytological evaluation and karyotype analysis in plant germplasms of Elytrigia Desv. Agr Sci China 9(11): 1553–1560. [Google Scholar]
- 4. Garg M, Tanaka H, Ishikawa N, Takata K, Yanaka M, et al. (2009) Agropyron elongatum HMW-glutenins have a potential to improve wheat end-product quality through targeted chromosome introgression. J. Cereal Sci 50: 358–363. [Google Scholar]
- 5. McDonald MP, Galwey NW, Ellneskog-Staam P, Colmer TD (2001) Effects on growth and development of individual chromosomes from slow-growing Lophopyrum elongatum Löve when incorporated into bread wheat (Triticum aestivum L.).Annals of Botany. 2: 215–223. [Google Scholar]
- 6. Lammer D, Cai XW, Arterburn M, Chatelain J, Murray T, et al. (2004) A single chromosome addition from Thinopyrum elongatum confers a polycarpic, perennial habit to annual wheat. J. Exp Bot 55(403): 1175–1720. [DOI] [PubMed] [Google Scholar]
- 7. Shen XR, Ohm H (2006) Fusarium head blight resistance derived from Lophopyrum elongatum chromosome 7E and its augmentation with Fhb1 in wheat. Plant Breeding 125: 424–429. [Google Scholar]
- 8. Shen XR, Ohm H (2007) Molecular mapping of Thinopyrum-derived Fusarium head blight resistance in common wheat. Mol. Breed 20: 131–140. [Google Scholar]
- 9. Oliver RE, Xu SS, Stack RW, Friesen TL, Jin Y, et al. (2006) Molecular cytogenetic characterization of four partial wheat-Thinopyrum ponticum amphiploids and their reactions to Fusarium head blight, tan spot, and Stagonospora nodorum blotch. Theor. Appl. Genet 112(8): 1473–1479. [DOI] [PubMed] [Google Scholar]
- 10. Zhang XL, Shen XR, Hao YF, Cai JJ, Ohm HW, et al. (2011) A genetic map of Lophopyrum ponticum chromosome 7E,harboring resistance genes to Fusarium head blight and leaf rust.Theor. Appl. Genet 122 (2): 263–270. [DOI] [PubMed] [Google Scholar]
- 11. Fu SL, Lv ZL, Qi B, Guo X, Li J, et al. (2012) Molecular cytogenetic characterization of Wheat-Thinopyrum elongatum addition,substitution and translocation lines with a novel source of resistance to Wheat Fusarium head blight. J.Genet Genomics 39(2): 103–110. [DOI] [PubMed] [Google Scholar]
- 12. Chen SQ, Huang ZF, Zhang Y, Ge JY, Zhu X, et al. (2012) Chromosomal location of the genes associated with FHB resistance of Lophopyrum elongatum in Chinese Spring background. J. Triticeae crops 32(5): 839–845. [Google Scholar]
- 13. Li ZS (1977) Common wheat and Agropyron elongatum (2n = 70) hybrid breeding and genetic analysis. Acta. genetic Sinica 4 (4): 279–283. [Google Scholar]
- 14. Jauhar PP, Peterson TS, Xu SS (2009) Cytogenetic and molecular characterization of a durum alien disomic addition line with enhanced tolerance to Fusarium head blight.Genome. 52(5): 467–483. [DOI] [PubMed] [Google Scholar]
- 15. Dvorák J, Knott DR (1974a) Disomic and ditelosomic additions of diploid Agropyron elongatum chromosomes to Triticum aestivum. Can.J.Genet.Cytol. 16(2): 399–417. [Google Scholar]
- 16. Dvorák J, Sosulski FW (1974b) Efects of additions and sustitutions of Agropyron elongatum chromosomes on characters in wheat. Can.J.Genet.Cytol. 16: 627–637. [Google Scholar]
- 17. Cuthbert PA, Somers DJ, Brule-Babel A (2007) Mapping of Fhb2 on chromosome 6BS:a gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theor.Appl.Genet 114: 429–437. [DOI] [PubMed] [Google Scholar]
- 18. Qi LL, Pumphrey MO, Friebe B, Chen PD, Gill BS (2008) Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat.Theor.Appl.Genet. 117: 1155–1166. [DOI] [PubMed] [Google Scholar]
- 19. Wang JR, Wang L, Gulden S, Rocheleau H, Balcerzak M, et al. (2010) RNA profiling of fusarium head blight-resistant wheat addition lines containing the Thinopyrum elongatum chromosome 7E. Can. J. Plant Pathol 32(2): 188–214. [Google Scholar]
- 20. Prins R, Groenewa JZ, Marais GF, Snape JW, Koebner R MD (2001) AFLP and STS tagging of Lr19,a gene conferring resistance to leaf rust in wheat. Theor.Appl.Genet. 103: 618–624. [Google Scholar]
- 21. Gennaro A, Koebner RMD, Ceoloni C (2009) A candidate for Lr19,an exotic gene conditioning leaf rust resistance in wheat. Funct Integr Genomics 9: 325–334. [DOI] [PubMed] [Google Scholar]
- 22. Yan HF, Yang WX, Chen YF, Meng QF, Liu DQ (2009) Specificity and stability of E-chromosome specific SCAR marker from Thinopyrum spp. . for Lr19.Acta Phytopathologica Sinica 39(1): 76–81. [Google Scholar]
- 23. Du JK, Yu GH, Wang XE, Ma HX (2010) Development and validation of a SSCP marker for Fusarium head blight resistance QTL region in wheat. J. Triticeae Crops 30(5): 829–834. [Google Scholar]
- 24. Liu SB, Jia JZ, Wang HG, Kong LR, Zhou RH (1998) Special chromosome markers for E genome and DNA polymorphism between Agropyron elongatum (2n = 14) and common wheat detected by RAPD markers. Acta Agron Sin 24(6): 687–690. [Google Scholar]
- 25. You MS, Li BY, Tang ZH, Liu SB, Song JM, et al. (2002) Establishme t of E-genome specific RAPD and SCAR markers for Thinopyrum spp. J. China Agric. Univ 7(5): 1–6. [Google Scholar]
- 26. You MS, Li BY, Tian ZH, Tang ZH, Liu SB, et al. (2003) Development of specific SSR marker for Ee-genome of Thinopymm sp. by using wheat microsatellites. J.Agric Biotechnol 11(6): 577–581. [Google Scholar]
- 27. Liu SB, Jia JZ, Wang HG, Kong LR, Zhou RH (1999) Identification of homoeology between the Elytrigia elongatum (2n = 14, EE) and wheat chromosomes using biochemical and molecular markers. Acta Agron Sin 26(1): 37–4227. [Google Scholar]
- 28. Zhang L, Yan ZH, Zheng YL, Liu DC, Dai SF, et al. (2008) Development of Ee-chromosome specific AFLP and STS molecular marker for Lophopyrum elongatum in Chinese Spring wheat background. J.Agric Biotechnol 16(3): 465–473. [Google Scholar]
- 29. Prabhu KV, Gupta SK, Charpe A, Koul S (2004) SCAR marker tagged to the alien leaf rust resistance gene Lr19 uniquely marking the Agropyron elongatum-derived gene Lr24 in wheat:a revision. Plant Breeding 123(5): 417–420. [Google Scholar]
- 30. Li XM, Lee BS, Mammadov AC, Koo B C, Mott IW, et al. (2007) CAPS markers specific to Eb, Ee,and R genomes in the tribe Triticeae . Genome 50(4): 400–411. [DOI] [PubMed] [Google Scholar]
- 31. Chen GY, Dong P, Wei YM, He K, Li W, et al. (2007) Development of Ee-chromosome -specific RGAP markers for Lophopyrum elongatum (Host) in wheat background by using resistance gene analog polymorphism. Acta Agron Sin 33(11): 1782–1787. [Google Scholar]
- 32. Ge JY, Chen SQ, Gao YY, Gao Y, Huang ZF, et al. (2012) Development of Genome- specific molecular markers for Lophopyrum elongatum based on Suppression Subtractive Hybridization. Acta Agronomica Sinica 38(10): 1818–1826. [Google Scholar]
- 33. Kent W J (2002) BLAT–The BLAST-Like Alignment Tool. Genome Res 12: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang JX, Sun Y, Gao WJ (2000) A simple of practical method for extracting plant total DNA. J.Shanxi Univ 23(3): 271–272. [Google Scholar]
- 35. Ma JX, Zhou RH, Dong YC, Jia JZ (1999) Chromosomal location of thegenes resistant to wheat stripe rust from Lophopyrum elongatum. Chinese Sci Bull. 44(1): 65–69. [Google Scholar]
- 36. Sears ER (1993) Use of radiation to transfer alien segments to wheat. Crop Sci. 33: 897–901. [Google Scholar]