Abstract
Weed populations can have high genetic plasticity and rapid responses to environmental selection pressures. For example, 100-fold amplification of the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene evolved in the weed species Amaranthus palmeri to confer resistance to glyphosate, the world’s most important herbicide. However, the gene amplification mechanism is unknown. We sequenced the EPSPS gene and genomic regions flanking EPSPS loci in A. palmeri, and searched for mobile genetic elements or repetitive sequences. The EPSPS gene was 10,229 bp, containing 8 exons and 7 introns. The gene amplification likely proceeded through a DNA-mediated mechanism, as introns exist in the amplified gene copies and the entire amplified sequence is at least 30 kb in length. Our data support the presence of two EPSPS loci in susceptible (S) A. palmeri, and that only one of these was amplified in glyphosate-resistant (R) A. palmeri. The EPSPS gene amplification event likely occurred recently, as no sequence polymorphisms were found within introns of amplified EPSPS copies from R individuals. Sequences with homology to miniature inverted-repeat transposable elements (MITEs) were identified next to EPSPS gene copies only in R individuals. Additionally, a putative Activator (Ac) transposase and a repetitive sequence region were associated with amplified EPSPS genes. The mechanism controlling this DNA-mediated amplification remains unknown. Further investigation is necessary to determine if the gene amplification may have proceeded via DNA transposon-mediated replication, and/or unequal recombination between different genomic regions resulting in replication of the EPSPS gene.
Introduction
Gene amplification, the reiteration of a coding segment resulting in one or more additional gene copies, is known to be a common process in the evolutionary history of plants and is vital for generating genomic diversity [1]. In addition to being a mechanism of adaptive evolution in mammalian cancer cells [2], bacteria [3], and arthropods [4], gene amplification is an important adaptive mechanism for antibiotic resistance, and the increased expression can offset fitness penalties associated with some resistance mechanisms [5]. Gene amplification and the resulting proportional increase in transcript levels has been implicated in insecticide resistance evolution in 10 different arthropod species, both for genes having a role in increased insecticide metabolism and for genes encoding proteins inhibited by insecticides (reviewed by [4]). Hence, numerous cases have been demonstrated where gene amplification has facilitated adaptive evolution.
Gene amplification is also an adaption in plants conferring resistance to the herbicide glyphosate [6]. Glyphosate is the world’s most important and widely used herbicide and persistent usage is resulting in resistance evolution [7]. An Amaranthus palmeri population highly resistant to glyphosate was found to have from 40- to 100-fold amplification of the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene, and EPSPS gene hybridization signals were observed on each A. palmeri chromosome using fluorescence in-situ hybridisation [6]. The EPSPS gene produces EPSPS, essential in the synthesis of aromatic amino acids, and EPSPS is inhibited by glyphosate [8]. Increased EPSPS expression confers glyphosate resistance [9], and in A. palmeri, the extra EPSPS produced from the amplified gene copies are predicted to enable the plants to survive high glyphosate doses. The inheritance of this gene amplification is complex, as EPSPS copy number in progeny can vary substantially from parental copy number [10], and EPSPS gene amplification and glyphosate resistance can be transferred to related Amaranthus species through cross-pollination [11]. Amplification of the EPSPS gene has also recently been associated with glyphosate resistance in a Lolium population [12] and in A. tuberculatus populations [13].
The mechanistic processes involved in large-scale gene amplification conferring herbicide resistance are currently unknown. A proposed hypothesis for EPSPS gene amplification in A. palmeri is the activity of a mobile genetic element (MGE) [6]. Transposable elements (transposons) are one type of MGE and generate genetic diversity by moving within the genome [14], [15]. Transposons can be grouped into two classes, those that replicate through an RNA intermediate (class 1, retrotransposons) and those that replicate as DNA through a conservative cut-and-paste mechanism (class 2) [16]. Class 2 transposons can increase in copy number and contribute to genome expansion via two mechanisms, 1) transposing from one of two recently replicated chromatids into an un-replicated target site [17], and 2) through gene conversion, a gap repair mechanism that restores a copy of the original sequence to the empty donor site [18], [19]. Class 2 elements can be autonomous, encoding a transposase necessary for replication, or non-autonomous, generally derived from an autonomous element through deletion of internal sequences [16]. Together, transposable elements comprise a large part of the genome of higher organisms and have had major and recent effects on plant genome evolution and organization [16], [20].
Gene amplification may also be due to incorrect recombination or double-strand DNA break repair with subsequent tandem duplications as observed in bacteria, yeast, cancer cells, and plant cell cultures [21], [22], [23], [24]. However, the basis of the amplification mechanism in glyphosate-resistant A. palmeri, how rapidly the initial amplification occurred, and why inheritance of the elevated copy number is difficult to predict [10] are all unanswered questions. If gene amplification occurred via transposon activity, genomic regions flanking amplified EPSPS genes may provide evidence of transposon insertions and the presence of introns may provide evidence for the class of transposon responsible. Therefore, experiments were conducted in A. palmeri to 1) sequence amplified EPSPS genes and genomic regions flanking EPSPS loci using two high-throughput sequencing platforms (454 pyrosequencing and Illumina), 2) identify transposons, other repetitive sequences, and intron sequence diversity, and 3) search for evidence of tandem gene duplications.
Results
EPSPS Intron Analysis
Sequencing Introns
We first examined whether intron sequences were present in EPSPS gene sequences from the previously reported [6] glyphosate-resistant A. palmeri population from Georgia, USA (GA-R) and a glyphosate-susceptible population (GA-S). PCR amplification using primers (Table S1) spanning two predicted introns produced 765 to 767 bp amplicons from GA-R and GA-S (Figure S1), longer than the mature mRNA sequence length of 331 bp. These amplicons were cloned and sequenced and intron sequences were found in both GA-R and GA-S (Figure S1). Intron boundary splice sites matched the intron boundaries in the Arabidopsis EPSPS gene [25]. A phylogenetic tree shows that the GA-R sequences cluster with some GA-S sequences, and other GA-S sequences form a second group (Figure 1). All GA-R sequences contained an Xho I restriction site in intron 5, while GA-S sequences were polymorphic for this restriction site (Figure S1). Intriguingly, the intron sequences from all GA-R clones were identical, while polymorphisms including an A deletion and an AAC insertion were found in GA-S clones (Figure 1, S1). The S population clearly formed two groups of EPSPS sequences (Figure 1), consistent with previously reported evidence for two EPSPS loci in S A. palmeri [26]. Our data suggest that resistance to glyphosate resulted from amplification of only one allele from the two EPSPS loci. It is not known whether expression differs between these two putative EPSPS loci or whether there are any enzymatic differences in the gene products.
Figure 1. Phylogenetic tree of cloned and sequenced PCR products (individual clones labeled a-e) spanning two introns of the EPSPS gene in three glyphosate-resistant (R) and three susceptible (S) A. palmeri individuals supports the existence of two EPSPS loci in A. palmeri, and the amplification of one EPSPS locus in R genomes.
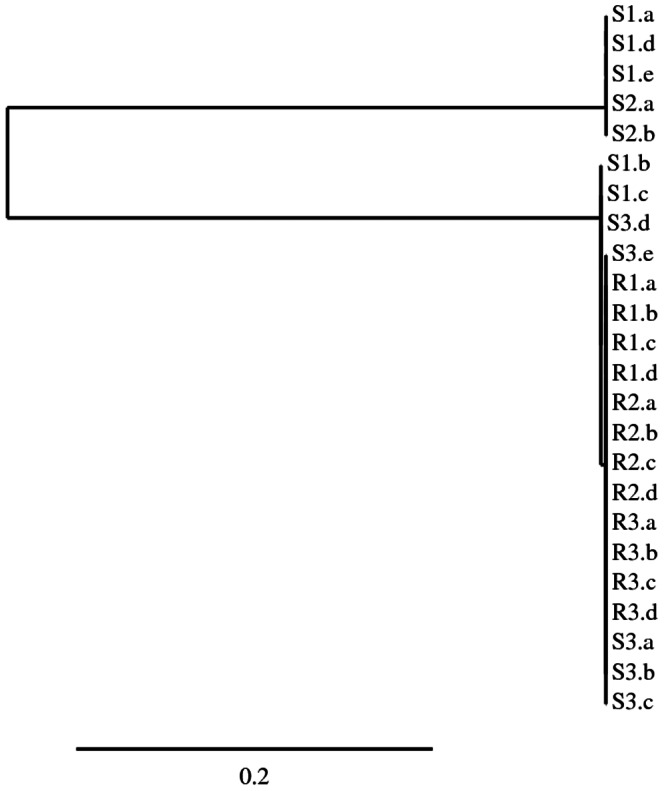
Scale bar represents the number of nucleotide substitutions per site.
Quantitative PCR
Primers for qPCR were designed based on the intron sequences (Table S1). Because individuals from this GA-R A. palmeri population are known to have EPSPS copy numbers of 100-fold or more relative to GA-S, qPCR specific to the intron sequence was used to estimate whether any loci could be detected in GA-R individuals that did not carry introns. Intron-specific primers produced the same estimate of EPSPS genomic copy number as exon-specific primers for all individuals tested (Table 1). These data indicate that all or nearly all of the amplified EPSPS copies in GA-R contain introns.
Table 1. EPSPS genomic copy number measured using qPCR primers within an intron is similar to copy number measured using qPCR primers within an exon for R and S individuals.
| -EPSPS:ALS Relative Genomic Copy Number- | ||||||
| R1 | R2 | R3 | S1 | S2 | S3 | |
| Exon | 108 (4.4) | 84 (8.6) | 60 (0.5) | 1.0 (0.06) | 0.9 (0.02) | 0.9 (0.01) |
| Intron | 106 (1.2) | 73 (3.0) | 57 (5.1) | 0.8 (0.04) | 0.9 (0.07) | 1.0 (0.07) |
Data are means with standard errors in parentheses.
Genomic Sequencing
454 Pyrosequencing
Genomic DNA from a highly glyphosate-resistant GA-R individual with 86-fold EPSPS relative gene amplification (relative copy number determined by qPCR [6]) was de novo sequenced using the Roche GS-FLX 454 platform. More than 800,000 reads with an average length of 560 bp were obtained from shotgun genomic sequencing and assembled into contigs. As expected, numerous hits to the EPSPS gene were obtained. Amplification was specific to the EPSPS gene relative to other herbicide target-site genes (Table 2), and the ratio of EPSPS reads to acetolactate synthase (ALS) reads obtained from 454 sequencing (150-fold more) was similar to the EPSPS:ALS ratio from qPCR (86-fold). A total of 3,278 individual sequence reads were assembled into one large contig of 14,268 bp encompassing the full-length genomic EPSPS gene. Numerous transposable elements were identified in the genomic sequence, the major proportion of which was long terminal repeat (LTR) retrotransposons. Several other general categories of transposable elements were also identified, similar to that reported by Lee et al. [27] for A. tuberculatus. Sequences were identified flanking EPSPS on the 5′ and 3′ ends (Table 3) with high similarity to miniature inverted-repeat transposable elements (MITEs) characterized in the Oryza Repeat Database [28]. A 13 bp imperfect Terminal Inverted Repeat (TIR) and a 3 bp (TAA) duplication were identified in the 454 sequence immediately adjacent to the MITE-homologous regions on the 5′ and 3′ ends of the EPSPS gene (Figure S2A).
Table 2. Hits to EPSPS and other genes encoding herbicide target sites from 454 genomic sequencing in A. palmeri. BLAST searches performed against all raw reads and unigene only (contigs+singletons) databases.
| Herbicide target | Hits to all raw reads | Hits to unigenes |
| Acetolactate synthase (ALS) | 2 | 1 (32%) |
| 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) | 321 | 1 (100%) |
| 4-hydroxyphenylpyruvate dioxygenase (HPPD) | 3 | 4 (80%) |
| Protoporphyrinogen oxidase 2 (PPX2) | 1 | 2 (24%) |
| Protoporphyrinogen oxidase 1 (PPX1) | 2 | 2 (29%) |
Raw read hits were normalized to 1000 bp for each gene query. Unigene searches include full length coding sequences for each gene and percent coverage in parentheses. All BLAST searches were conducted with an E-value cutoff of 10−50 and >90% sequence similarity. Sources and sizes of queries:
ALS: complete cds from A. tuberculatus (Genbank EF157819; 2010 bp).
EPSPS: complete cds from A. palmeri (Genbank FJ861243; 1557 bp).
HPPD: complete cds from A. tuberculatus (Genbank JX259255; 1471 bp).
PPX2: complete cds from A. tuberculatus (Genbank EU024569; 1608 bp).
PPX1: complete cds from A. tuberculatus (Genbank DQ386115; 1659 bp).
Table 3. Sequences in genomic regions flanking EPSPS in A. palmeri have similarity to known transposable elements in rice [28].
| Sequence | Oryza Repeat Database Hit | Strand | Length of Similarity (bp) | Hit Score | % Match |
| 5′ MITE | ORSgTEMT00500849, putative MITE, Gaijin/Gaigin-like | + | 155 | 122 | 60 |
| ORSgTEMT01602459, putative MITE, MITE-adh, type B-like | + | 160 | 128 | 57 | |
| 3′ MITE | ORSgTEMT01600913, putative MITE, MITE-adh, type B-like | – | 209 | 170 | 58 |
| ORSgTEMT02900113, putative MITE, MITE-adh-4-like | – | 298 | 186 | 57 | |
| ORSgTEMT00100233, putative MITE, Tourist-like | – | 140 | 169 | 62 |
Sequences were identified bordering EPSPS in a contig assembled from 454 sequencing of GA-R gDNA.
Fosmid library sequencing
A fosmid library was constructed from genomic DNA of an A. palmeri individual with 80-fold increased EPSPS expression (as determined by qPCR). To compare geographically distant populations and different sequencing techniques, this individual was isolated from a second glyphosate-resistant population found in Mississippi, USA (MS-R). Sixteen MS-R fosmid clones containing EPSPS sequence were identified and sequenced with Illumina 50 bp single reads. Sequence coverage was insufficient to permit initial individual assembly, so all sequence reads were first pooled to create a reference sequence (Figure 2A). Next, barcoded sequence reads for each fosmid were assembled individually. Alignment of the contigs from individual assemblies to the reference sequence revealed very few sequence differences among fosmids (Figure 2B). End points of the fosmids were determined by lack of assembly to the consensus beyond a certain point, inclusion of vector sequence with contigs containing insert, and confirmation by PCR. Fosmid insert sequence coverage ranged from 59.8% to 99.8%, aligned reads per fosmid insert ranged from 813 to 46,413, and estimated depth ranged from 1.3-fold to 104-fold coverage.
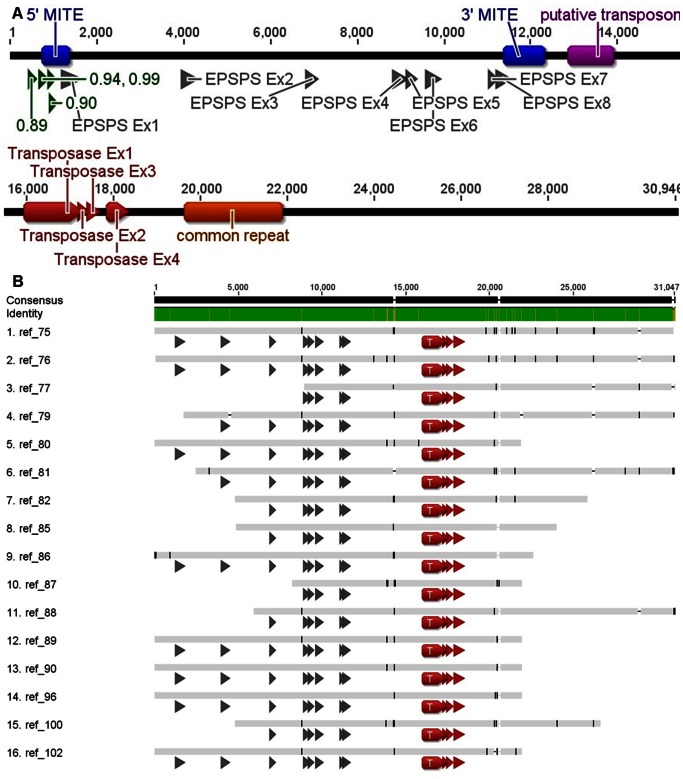
Figure 2. Consensus sequence of A. palmeri EPSPS from 16 MS-R fosmids.
A) exons for EPSPS (gray) and a putative Ac transposase (red), putative promoters (green; numbers indicate promoter prediction score, 1 is maximum possible), MITE-homologous sequence (blue), a putative transposon (purple), and a repetitive sequence motif (orange). B) Alignment of the consensus A. palmeri EPSPS and flanking genomic region sequences for 16 fosmids from MS-R. EPSPS exons (gray arrows) from 1 to 12,000 bp, and exons of a putative Ac transposase (red) from 16,000 to 19,000 bp. Green color in identity bar indicates identical sequences in all fosmids and brown indicates polymorphisms.
Alignment of these 16 clones produced a consensus sequence (GenBank Accession JX564536) of 30,945 base pairs containing the entire EPSPS sequence including 8 exons and 7 introns (Figure 2A). Substantially more sequence was obtained downstream (19,464 bp) than upstream (1,252 bp) of the gene. The EPSPS gene was 10,229 bp long, with the expected coding sequence length of 1,557 bp (A. palmeri EPSPS GenBank Accessions FJ861242.1 and FJ861243.1) containing 8 exons of 333, 245, 154, 215, 118, 211, 62, and 219 bp, and 7 introns of 2416, 2624, 1856, 78, 356, 1242, and 100 bp. All exons were the same sizes as those from both the petunia (Petunia hybrida) and Arabidopsis EPSPS genes, with the exception of the first exon containing the chloroplast transit peptide (327 bp in petunia and 339 bp in Arabidopsis) [25], [29]. The A. palmeri coding region of 10.2 kb is longer than petunia (7.4 kb) and Arabidopsis (2.5 kb) due to longer intron length; for example, the first intron is 1.3 kb in petunia and only 87 bp in Arabidopsis [25], [29].
Predicted promoter motifs and the previously identified MITE-homologous sequences were identified (Figure 2A). A putative transposase, revealed by BLASTn and BLASTp analysis to be similar to several Activator (Ac) transposases, was identified 4.5 kb downstream of EPSPS exon 8 (Figure 2A). It is not known if this putative Ac transposase is expressed or produces a functional gene product. A 256 bp imperfect inverted repeat, referred to as a putative transposon, was identified 1,432 bp downstream (Figure 2A, S3A, S3B) from EPSPS. Assembly of the sequence reads for each individual fosmid did not reveal a sequence divergence point, as all fosmids obtained had nearly identical overlapping sequences (Figure 2B). No tandem duplicated EPSPS genes were observed in the 16 sequenced fosmid inserts. Additional Sanger sequencing for two fosmids, AW88 and AW96, verified the accuracy of the sequence data obtained by the Illumina sequencing procedure (data not shown).
Aligning 454 contigs to the fosmid consensus sequence revealed very high sequence similarity (Figure 3). Within the entire EPSPS gene, the 454 consensus and fosmid consensus differed by only 1 nucleotide, a single T insertion within intron 3 found in the fosmid consensus (Figure 3). Additional contigs from the 454 assembly aligned to the downstream fosmid reference (Figure 3), and these contigs also had a high number of hits (Table S2), confirming that these sequences were amplified in addition to the EPSPS gene sequence in both populations. Some contigs had even more hits than the EPSPS contig (Table S2), suggesting that these sequences occur elsewhere in the genome in addition to flanking amplified EPSPS genes. The fosmid consensus sequence from exon 4 to exon 6, crossing two introns, was identical to the sequence obtained both by 454 sequencing and by PCR from the GA-R population.
Figure 3. Assembly of 454 contigs to the fosmid consensus sequence.
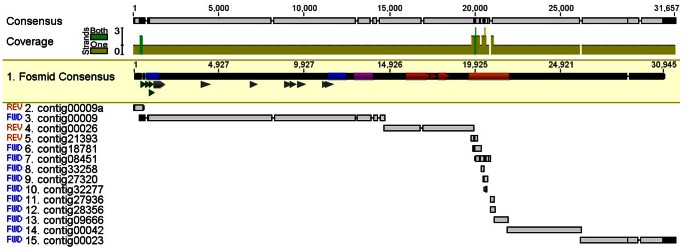
Contigs were identified by running a BLAST of the 454 data against the fosmid consensus sequence. Top sequence highlighted in yellow is the fosmid consensus sequence, remaining sequences are from the 454 data, and sequence at the top is the consensus of the fosmid and 454 data. The EPSPS exons (gray), MITE-homologous sequences (blue), a putative transposon (purple), a putative Ac transposase (red), putative promoters (green triangles), and a repetitive sequence motif (orange) are indicated.
One notable feature was a repetitive sequence motif (identified as ‘common repeat’ in Figure 2A). The motif was identified by stacking of a large number of Illumina sequence reads over a 2.1 kb region after assembly of Illumina reads to the reference sequence (Figure S4). Several 454 contigs aligned to the motif (Figure 3) and had a higher number of hits than the EPSPS contig (Table S2). Observed restriction fragment lengths of fosmid inserts did not match predicted lengths based on the sequence within the assembled 2.1 kb ‘common repeat’ region. A repeated motif of 551 bp occurred twice within the ‘common repeat’ region, and it contained an internal 26 bp direct repeat and an internal 15 bp inverted repeat. The actual length of this section is likely longer than assembled in the reference, and additional sequencing will be necessary to resolve this region.
The 454 EPSPS sequence (contig 00009) had nearly 100% sequence homology upstream of EPSPS with the fosmid library sequence until a divergence point just upstream of the 5′ MITE-homologous sequence (Figure 3, S2A). The 5′ TIR and TAA duplication identified in the 454 sequence (Figure S2A) were not present in the fosmid sequence. The fosmid sequence contains a TATA box (Figure S2A) that is absent from the 454 sequence. Additionally, the assembled sequence in the 454 contig 00009 upstream of the TIR aligned in reverse orientation to the assembled fosmid sequence in this position (contig 00009a, Figure 3). The 454 sequence assembly was confirmed by PCR on GA-R individuals, as primers specific to the 454 contig 00009 sequence (Table S1) amplified products of the predicted size with a reverse primer in EPSPS exon 1, while primers specific to the fosmid (MS-R) sequence produced no PCR products with a reverse primer in EPSPS exon 1 (Figure S2B). The 454 sequence and fosmid library sequence had 100% sequence homology around the 3′ MITE-homologous sequence, with no divergence points identified (Figure 3, S2A).
Gene Amplification Structure Analysis
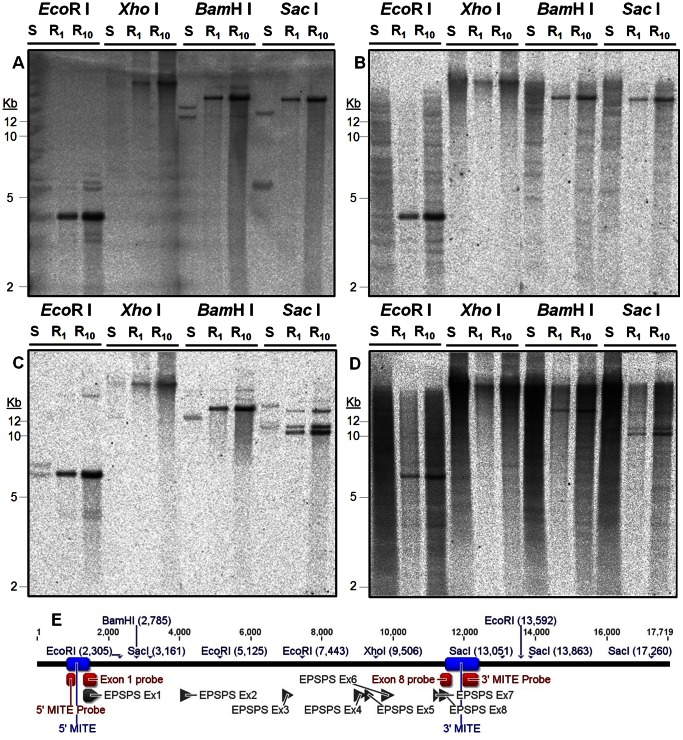
DNA blot hybridizations were conducted using probes in Exon 1 and Exon 8 of EPSPS (the first and last exons, respectively) on GA-R and GA-S restriction-digested DNA. The expected pattern of much higher hybridization signal intensity in GA-R than in GA-S was observed (Figure 4A, C). Patterns in GA-S support the existence of 2 distinct EPSPS loci, consistent with the intron sequence phylogeny results (Figure 1). If EPSPS loci were arranged as tandem duplications in GA-R with inter-genic regions ≤20 kb, then we had expected to observe both the Exon 1 and the Exon 8 probes hybridizing to the same fragment, with the assumption that the expected flanking restriction sites (Figure 4E) would have been lost in a tandem duplication event. This was not observed, however, as no bands were common between Exon 1 and Exon 8 for all four restriction enzymes in GA-R. Combining the sizes of the observed BamH I bands suggests the size of the amplified EPSPS locus is at least 30 kb (Figure 4A, C).
Figure 4. Southern blot analysis of EPSPS gene amplification in GA-S (S, 20 µg gDNA) and GA-R (R1, 1 µg gDNA and R10, 10 µg gDNA) A. palmeri.
Hybridizations with 33P-dCTP labeled probes for A) EPSPS first exon, B) 5′ MITE, C) EPSPS last exon, and D) 3′ MITE; E) probe and exon locations and expected restriction sites.
Additional hybridizations (Figure 4B, D) were conducted with probes for the MITE-homologous sequences identified next to the EPSPS gene in the 454 and fosmid library sequencing (probe sequence shown in Figure S2A). Hybridization with these probes occurred to the same size fragments as the respective Exon 1 and Exon 8 probes in GA-R, but the MITE-homologous probes did not hybridize to the same fragments as Exon 1 and Exon 8 in GA-S (Figure 4B, D). Additionally, the MITE-homologous probes hybridized to multiple fragments of the same size in both GA-S and GA-R, with additional fragments observed in GA-S but not in GA-R (Figure 4B, D). PCR experiments indicated both 5′ and 3′ MITE probes could be amplified from GA-S and GA-R (Figure S5A, B). Amplification from both 5′ and 3′ MITE-homologous sequences to EPSPS occurred only in GA-R (Figure S5C, D) and not in 16 GA-S individuals (Figure S5E, F, G) or in 5 S individuals from a North Carolina A. palmeri population (Figure S5H). Therefore, it appears that the genomic sequence flanking EPSPS within approximately 500 bp in GA-R is different from the two loci predicted in GA-S due to the insertion of sequence that also occurs at other locations in the genome.
Additional bands with lower intensity were observed using Exon 1 and 8 probes in EcoR I and BamH I digested gDNA, both shorter and longer than expected (Figure 4A, C, E). This could indicate the existence of a few amplified loci with length polymorphisms due to other insertions or deletions. All other observed hybridizations were consistent with expected results based on the predicted restriction sites obtained from fosmid library sequencing (Figure 4E), except for the results obtained with the Sac I digest and the Exon 8 probe in GA-R. Three Sac I restriction sites were predicted downstream from Exon 8 prior to the first expected BamH I site (Figure 4C, E). However, three major bands (10, 11, and 14 kb) were observed for Sac I and a single major band was observed for BamH I (15 kb) (Figure 4C). Both Sac I sites are located within a large inverted repeat (Figure S3A) referred to as a putative transposon (Figure 2A) and if none, one, or two Sac I sites were disrupted, the expected fragment sizes using the Exon 8 probe would be 9.9, 10.7, and 14 kb, matching the observed sizes (Figure 4C). As Sac I is sensitive to cytosine methylation at GAGmCTC, the observed restriction fragment length polymorphism could be due to differences in DNA methylation status, or it could suggest that some sequence differentiation has occurred among amplified loci within the putative transposon.
Discussion
DNA-mediated amplification of the EPSPS gene has occurred recently in glyphosate-resistant A. palmeri. No intron sequence variation was detected within GA-R and MS-R individuals, two EPSPS loci were detected in the S A. palmeri genome, and it appears that only one EPSPS locus was amplified in R individuals. Amplification of the entire EPSPS gene is supported by fosmid library data, as all fosmids positive for EPSPS contained introns. Both Southern blot and fosmid sequencing results suggest that no small tandem EPSPS duplications are present, although tandem duplications greater than 30 kb could be possible. Very few polymorphisms were detected among fosmids sequenced using Illumina short read technology. The fosmid, 454, and Southern blot data support DNA-mediated amplification in that at least 30 kb of sequence containing the EPSPS gene has been amplified. An RNA-mediated gene amplification process would likely insert a mature mRNA, i.e., with introns spliced out, into the genomic DNA. A process inserting an immature EPSPS mRNA, with introns still intact, would presumably leave short 5′ and 3′ untranslated regions, and neither RNA-mediated scenario is consistent with the fosmid library sequencing results. The haploid genome size of A. palmeri is estimated to be approximately 450 Mbp [30]. Therefore a 100-fold amplification of a 30 kb fragment would represent 3 million additional bp, a 0.67% increase in total genome size.
Sequence differences were observed in the first 500 bp flanking both sides of EPSPS between GA-S and both GA-R and MS-R individuals, where sequences with homology to known MITEs were associated with the EPSPS gene only in glyphosate-resistant individuals. The MITE-homologous sequences were detected elsewhere in the S and R genomes, but not next to the EPSPS gene in S. MITEs are one type of non-autonomous class 2 (DNA-mediated) transposons, characterized by TIR of between 10 and 20 bp and Target Site Duplications (TSD) generally of 3 bp (including TAA), and often inserting in AT rich regions [31], [32]. Although MITEs have not previously been shown to acquire and duplicate functional gene sequences, Mutator-like elements (MULEs) in Arabidopsis [33] and rice [34] commonly acquire and duplicate short host gene fragments. Both TIR and a TSD-like sequence motif were identified adjacent to the MITE-homologous sequences flanking amplified EPSPS genes in the GA-R population, and different sequences were found upstream from the 5′ MITE-homologous sequence between the GA-R and MS-R populations. The absence in the MS-R fosmid sequence of the TIR and TSD identified in the GA-R 454 sequence, and the reversed alignment of GA-R 454 sequence to the MS-R fosmid sequence upstream of the TIR, may be due to population differences between GA-R and MS-R, may indicate separate origins of the gene amplification mechanism, or may indicate the border of the amplified unit. Given that the length of the amplification extends at least 18 kb past the 3′ MITE-homologous sequence, we cannot conclude that the identified MITE-homologous sequences are mechanistically responsible for the gene amplification and the amplification mechanism remains unknown. Further investigation is necessary to determine if the MITE-homologous sequences are simply passengers in the amplified DNA sequence, or if they have a role in the amplification mechanism.
The active MITE family known as mPing was first identified in rice [35] and can rapidly increase in copy number each generation without negative effects. The element preferentially inserts close to genes (within 5 kb) but less frequently in exons or introns [36]. Novel mPing insertions in 5′ and 3′ flanking regions, often within 1 kb, can influence gene expression regulation, particularly resulting in increased gene expression under stress conditions [37]. Increased mPing activity was associated with adaptation to an extremely different temperate environment during rice domestication [35], suggesting that MITE amplification can generate adaptive genetic diversity. A MITE in Brassica was found to preferentially accumulate in gene regulatory regions but not in coding regions [38], which is also the case in the present study. MITEs were found to flank 58% of genes in the rice genome [39], and 15% of MITEs have presence/absence polymorphisms among selected rice cultivars.
Both transposons and repetitive sequences have been found flanking amplified genes conferring insecticide resistance. An insecticide resistant Culex mosquito population had duplicated copies of a cytochrome P450 (CytP450) gene, over 260-fold increased expression, and the insertion of a MITE-like element was found upstream of both copies [40]. Daborn et al. [41] showed that increased expression of a CytP450 gene in Drosophila was sufficient for resistance, and the increased expression was due to insertion of an Accord transposable element upstream of the gene. Both an increase in copy number and insertions of transposable elements in regulatory regions have contributed to insecticide resistance, occurring in multiple steps and permitting adaptation of D. melanogaster to insecticides [42]. Currently it is unknown whether the MITE insertion in the Culex population is conferring cis-mediated increased expression as in Drosophilia, but it could be possible, both for Culex and for A. palmeri. An esterase B1 gene was found to be amplified around 250-fold in Culex mosquitos [43]. The amplified gene had neighboring repetitive sequences that were also found in other parts of the R genome, and also found in the S genome, but not near the esterase gene. The esterase B1 genes were present as single copies in a 25 kb sequence that was highly conserved in amplified copies, and flanked by larger, more heterogenous regions, with the entire amplicon up to 100 kb in total. Myzus aphids had amplified esterase genes occurring on multiple chromosomes [44], and the authors postulated that this was due to reciprocal interchange between chromosomes, or possibly due to the activity of transposable elements.
In summary, amplification of the EPSPS gene in glyphosate-resistant A. palmeri has occurred through a DNA-mediated mechanism. Our data support the presence of two EPSPS loci in S A. palmeri, and only one EPSPS locus has been amplified in glyphosate-resistant A. palmeri. We have shown that sequences with homology to MITEs (non-autonomous class 2 transposons) are present in the genomes of both S and R individuals, but are associated with EPSPS gene copies only in R individuals from two different populations. Additionally, a predicted Ac transposase and a large repetitive sequence were found downstream of amplified EPSPS copies. The mechanism directing the DNA-mediated gene amplification remains unknown. The large size of the amplified sequence (>30 kb), association with various types of genetic elements, and the previously reported unpredictable copy number inheritance patterns [10] are all intriguing, and raise questions such as whether the EPSPS gene amplification is an inducible adaptive mutation via a transposon-mediated process. Additional bordering DNA sequence of amplified regions from A. palmeri and other plant species with EPSPS gene amplification [12], [13] should provide insight into candidate mechanisms such as DNA-mediated transposon activity and/or unequal recombination between different genomic regions resulting in replication of the EPSPS gene.
Materials and Methods
EPSPS Intron Analysis
Sequencing Introns
Genomic DNA (gDNA) was extracted from 3 glyphosate-resistant (GA-R) and 3 -susceptible (GA-S) A. palmeri individuals from Georgia, USA, as previously described [6]. Primers Ex4F and Ex6R1 (Table S1) were designed based on the A. palmeri EPSPS sequence (GenBank Accessions FJ861242.1 and FJ861243.1) to amplify from exon 4 to exon 6 of EPSPS, crossing 2 introns. The expected coding sequence from the cDNA was 331 bp long, and it was expected that longer amplicons would contain introns. PCR was conducted using Phusion High-Fidelity DNA Polymerase (New England Biolabs) in 25 µL reactions with 1X HF buffer, 200 µM each dNTP, 0.5 µM each primer, 0.5 Units polymerase, and 10 ng template DNA, with initial denaturation at 98°C for 30 sec and then 25 cycles with 98°C for 15 sec, 55°C for 15 sec, and 72°C for 15 sec. Amplicons were separated on 1% agarose gel and gel extracted for cloning. Amplicons were cloned using the StrataClone Blunt PCR cloning kit (Agilent) according to the manufacturer’s instructions. Plasmids were isolated (Qiagen Plasmid Mini Kit) from white clones, the presence of an insert was confirmed by EcoR I digestion, and positive plasmids were submitted for sequencing at the Australian Genome Research Facility (AGRF). Sequences were aligned and a phylogenetic tree was constructed using Phylogeny.fr [45].
Quantitative PCR
Primers In5F and Ex6R2 (Table S1) were designed to produce a 155 bp amplicon within the identified intron sequences for quantitative PCR (qPCR). Previously described primers and qPCR protocols [6] were used to measure EPSPS genomic copy number relative to the ALS gene. Primers within an EPSPS exon were used, which have previously shown in glyphosate-susceptible individuals an EPSPS copy number relative to ALS of one [6]. After verifying for amplification of the expected intron PCR product, the intron primers were used in addition to the exon primers to measure EPSPS genomic copy relative to ALS genomic copy number.
Genomic Sequencing
454 Pryosequencing
Genomic DNA was extracted from one individual from the GA-R population using the Plant DNEasy kit (Qiagen) for use in 454 pyrosequencing (schematic in Figure S6). The individual plant was selected because of its high EPSPS copy number (86-fold, relative to ALS, based on qPCR). One-half of a pico-titer plate was sequenced on the Roche GS-FLX 454 at the W.M. Keck Center for Comparative and Functional Genomics at the University of Illinois. Initial quality control was performed before base calling, and assembly was performed as previously described [27]. Hits to the EPSPS gene and to other herbicide target genes including ALS, 4-hydroxyphenylpyruvate dioxygenase (HPPD), and protoporphyrinogen oxidase (PPX1 and PPX2) were compared to assess whether gene amplification was specific to EPSPS. Additionally, assembled contigs were searched for transposable elements using RepeatMasker (http://www.repeatmasker.org). All contig sequences were searched against the Oryza and Arabidopsis repeat libraries using default settings. Outputs were compared to results from similar searches performed with A. tuberculatus 454-derived sequence data [27].
Fosmid Library and Sequencing
A fosmid library was prepared from gDNA from a different glyphosate-resistant A. palmeri population from Mississippi, USA (MS-R). A glyphosate-resistant individual was identified with 80-fold increased EPSPS expression as determined by qPCR [6]. Genomic DNA was extracted using the Masterpure DNA purification kit (Epicentre) and the fosmid library was constructed as described in Methods S1. Sanger sequencing of fosmid inserts was performed by the USDA-ARS GBRU. Fosmid insert DNA for Illumina library preparation was fragmented to 100–300 bp following the protocol for the dsDNA fragmentase (New England Biolabs), and fragmented DNA was prepared for sequencing using the NEXTflex DNA Sequencing kit (Bioo Scientific). Each fosmid was labeled with a barcode prior to pooling of the libraries for sequencing. Libraries were sequenced on an Illumina HiSeq 2000 with 50 bp single reads and the raw data were analyzed by the USDA-ARS GBRU. Assemblies were performed using Geneious Pro [46] and CLC Bio [47]. Promoters were identified using a neural network promoter predictor from the Berkley Drosophila Genome Project [48], using default parameters.
Gene Amplification Structure Analysis
Genomic DNA from one GA-R and one GA-S individual was extracted and digested with restriction enzymes, transferred to a membrane, and hybridized with probes as described in Methods S1. Probes were hybridized in the order of EPSPS Exon 1, Exon 8, 5′ MITE, and 3′ MITE, and the blot was stripped after each hybridization (Methods S1).
Supporting Information
Sequence alignment of EPSPS genomic sequence (exon 4 to exon 6) from representative glyphosate-resistant (R) and –susceptible (S) A. palmeri cloned PCR products. Primers Ex4F and Ex6R1 are underlined, and XhoI polymorphism is highlighted with a square. Intron sequences are in lower case.
(DOCX)
Alignment of fosmid consensus sequence (MS-R), 454 consensus sequence (GA-R), and the probes used for Southern blots (5′ MITE and 3′ MITE from GA-R). A 13 bp imperfect Terminal Inverted Repeat (TIR) is underlined with one non-matching base in lower case, and a 3 bp duplicated sequence similar to known Target Site Duplications (TSD) is shown in bold and italics.
(DOCX)
A 256 bp imperfect inverted repeat identified in fosmid sequence from MS-R gDNA; A, alignment showing identity between inverted repeat on ends of the putative transposon (Figure 4) and B, sequence of the inverted repeat.
(DOCX)
Assembly of Illumina reads to the fosmid reference sequence, and the presence of read stacking in a region from approximately 20,000 bp until 22,000 bp. The sequence in this region contains a repeated 551 bp sequence.
(TIF)
PCR evidence for existence of putative MITE sequences in both GA-R and GA-S, but present flanking EPSPS only in GA-R and not in GA-S or NC-S A. palmeri individuals. PCR on gDNA of 3 GA-R and 3 GA-S A. palmeri with primers A) 5′ MITE.F by 5′ MITE.R, B) 3′ MITE.F by 3′ MITE.R, C) 5′ MITE.F by Ex1R and D) Ex8F by 3′ MITE.R; PCR on gDNA of 16 GA-S and 1 GA-R A. palmeri with primers E) 5′ MITE.F by Ex1R, F) Ex8F by 3′ MITE.R, and G) Ex1F by Ex1R as a positive PCR control; H) PCR on gDNA of 5 NC-S (North Carolina) and 1 GA-R A. palmeri with primers (left to right) Ex1F by Ex1R as a positive PCR control, 5′ MITE.F by Ex1R, and Ex8F by 3′ MITE.R. Negative controls (templates without primers and primers without template) were evaluated separately and no PCR products were observed.
(TIF)
Schematic diagram of steps used in EPSPS intron analysis and genomic sequencing of A. palmeri populations.
(DOCX)
PCR primers used in experiments to sequence introns, conduct qPCR on introns, synthesize Southern blot probes, and amplify MITE-homologous sequences.
(DOCX)
Contigs from 454 sequencing that align with fosmid reference sequence (see Figure 3) have a high number of hits. Raw read hits for each contig were normalized for size to 1000 bp to facilitate comparisons across contigs.
(DOCX)
(DOCX)
Acknowledgments
The authors thank Dr. Hiromichi Ishihara for assistance with PCR product cloning, Dr. Brian Scheffler for advice and consultations, Dr. O.P. Perera for assistance in generating the Illumina libraries, Darci Giacomini for genomic DNA preparation, Mary Duke for assistance in Illumina sequencing, Fanny Liu for assistance with fosmid DNA extraction and Sanger sequencing, Linda Ballard for assistance with bioinformatics analyses, and Dr. Jeffrey Bennetzen for helpful comments on the manuscript.
Funding Statement
This work was supported in part by United States Department of Agriculture-Agricultural Research Service (USDA-ARS) project number 6402-21000-050-00D. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1. Flagel LE, Wendel JF (2009) Gene duplication and evolutionary novelty in plants. New Phytol 183: 557–564. [DOI] [PubMed] [Google Scholar]
- 2. Schimke RT (1986) Methotrexate resistance and gene amplification: Mechanisms and implications. Cancer 57: 1912–1917. [DOI] [PubMed] [Google Scholar]
- 3. Hastings PJ (2007) Adaptive amplification. Crit Rev Biochem Mol Biol 42: 271–283. [DOI] [PubMed] [Google Scholar]
- 4. Bass C, Field LM (2011) Gene amplification and insecticide resistance. Pest Manag Sci 67: 886–890. [DOI] [PubMed] [Google Scholar]
- 5. Sandegren L, Andersson DI (2009) Bacterial gene amplification: implications for the evolution of antibiotic resistance. Nat Rev Microbiol 7: 578–588. [DOI] [PubMed] [Google Scholar]
- 6. Gaines TA, Zhang W, Wang D, Bukun B, Chisholm ST, et al. (2010) Gene amplification confers glyphosate resistance in Amaranthus palmeri . Proc Natl Acad Sci USA 107: 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64: 319–325. [DOI] [PubMed] [Google Scholar]
- 8. Amrhein N, Schab J, Steinrücken HC (1980) The mode of action of the herbicide glyphosate. Naturwissenschaften 67: 356–357. [Google Scholar]
- 9. Shah DM, Horsch RB, Klee HJ, Kishore GM, Winter JA, et al. (1986) Engineering herbicide tolerance in transgenic plants. Science 233: 478–481. [DOI] [PubMed] [Google Scholar]
- 10. Gaines TA, Shaner DL, Ward SM, Leach JE, Preston C, et al. (2011) Mechanism of resistance of evolved glyphosate-resistant Palmer amaranth (Amaranthus palmeri). J Agric Food Chem 59: 5886–5889. [DOI] [PubMed] [Google Scholar]
- 11. Gaines TA, Ward SM, Bukun B, Preston C, Leach JE, et al. (2012) Interspecific hybridization transfers a previously unknown glyphosate resistance mechanism in Amaranthus species. Evol Appl 5: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salas RA, Dayan FE, Pan Z, Watson SB, Dickson JW, et al. (2012) EPSPS gene amplification in glyphosate-resistant Italian ryegrass (Lolium perenne ssp. multiflorum) from Arkansas. Pest Manag Sci 68: 1223–1230. [DOI] [PubMed] [Google Scholar]
- 13. Tranel PJ, Riggins CW, Bell MS, Hager AG (2011) Herbicide resistances in Amaranthus tuberculatus: a call for new options. J Agric Food Chem 59: 5808–5812. [DOI] [PubMed] [Google Scholar]
- 14. McClintock B (1951) Chromosome organization and genic expression. Cold Spring Harbor Symp Quant Biol 16: 13–47. [DOI] [PubMed] [Google Scholar]
- 15. McClintock B (1984) The significance of responses of the genome to challenge. Science 226: 792–801. [DOI] [PubMed] [Google Scholar]
- 16. Feschotte C, Jiang N, Wessler SR (2002) Plant transposable elements: Where genetics meets genomics. Nat Rev Genet 3: 329–341. [DOI] [PubMed] [Google Scholar]
- 17.Fedoroff N (1989) Maize transposable elements. In: Berg DE, Howe MM, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology. 377–411.
- 18. Engels WR, Johnson-Schlitz DM, Eggleston WB, Sved J (1990) High-frequency P element loss in Drosophila is homolog dependent. Cell 62: 515–525. [DOI] [PubMed] [Google Scholar]
- 19. Lisch D, Chomet P, Freeling M (1995) Genetic characterization of the Mutator system in maize: Behavior and regulation of Mu transposons in a minimal line. Genetics 139: 1777–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. SanMiguel P, Gaut BS, Tikhonov A, Nakajima Y, Bennetzen JL (1998) The paleontology of intergene retrotransposons of maize. Nat Genet 20: 43–45. [DOI] [PubMed] [Google Scholar]
- 21. Hyppa RW, Smith GR (2010) Crossover Invariance Determined by Partner Choice for Meiotic DNA Break Repair. Cell 142: 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slack A, Thornton PC, Magner DB, Rosenberg SM, Hastings PJ (2006) On the mechanism of gene amplification induced under stress in Escherichia coli . PLoS Genet 2: 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suh H, Hepburn AG, Kriz AL, Widholm JM (1993) Structure of the amplified 5-enolpyruvylshikimate-3-phosphate synthase gene in glyphosate-resistant carrot cells. Plant Mol Biol 22: 195–205. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe T, Tanabe H, Horiuchi T (2011) Gene amplification system based on double rolling-circle replication as a model for oncogene-type amplification. Nucleic Acids Res 39: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klee HJ, Muskopf YM, Gasser CS (1987) Cloning of an Arabidopsis thaliana gene encoding 5-enolpyruvylshikimate-3-phosphate synthase: Sequence analysis and manipulation to obtain glyphosate-tolerant plants. Mol Gen Genet 210: 437–442. [DOI] [PubMed] [Google Scholar]
- 26. Burgos NR, Alcober EAL, Sales MA, Lawton-Rauh A, Rauh B, et al. (2008) The spread and population genetics of glyphosate-resistant Palmer amaranth in Arkansas. University of Arkansas Agricultural Experiment Station Research Series 573, Summaries of Arkansas Cotton Research 2008: 103–109. [Google Scholar]
- 27. Lee RM, Thimmapuram J, Thinglum KA, Gong G, Hernandez AG, et al. (2009) Sampling the waterhemp (Amaranthus tuberculatus) genome using pyrosequencing technology. Weed Sci 57: 463–469. [Google Scholar]
- 28. Ouyang S, Buell CR (2004) The TIGR Plant Repeat Databases: a collective resource for the identification of repetitive sequences in plants. Nucleic Acids Res 32: D360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gasser CS, Winter JA, Hironaka CM, Shah DM (1988) Structure, expression, and evolution of the 5-enolpyruvylshikimate-3-phosphate synthase genes of petunia and tomato. J Biol Chem 263: 4280–4289. [PubMed] [Google Scholar]
- 30. Rayburn AL, McCloskey R, Tatum TC, Bollero GA, Jeschke MR, et al. (2005) Genome size analysis of weedy Amaranthus species. Crop Sci 45: 2557–2562. [Google Scholar]
- 31. Casacuberta JM, Santiago N (2003) Plant LTR-retrotransposons and MITEs: control of transposition and impact on the evolution of plant genes and genomes. Gene 311: 1–11. [DOI] [PubMed] [Google Scholar]
- 32. Wessler SR, Bureau TE, White SE (1995) LTR-retrotransposons and MITEs: important players in the evolution of plant genomes. Curr Opin Genet Dev 5: 814–821. [DOI] [PubMed] [Google Scholar]
- 33. Yu Z, Wright SI, Bureau TE (2000) Mutator-like elements in Arabidopsis thaliana: Structure, diversity, and evolution. Genetics 156: 2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang N, Bao ZR, Zhang XY, Eddy SR, Wessler SR (2004) Pack-MULE transposable elements mediate gene evolution in plants. Nature 431: 569–573. [DOI] [PubMed] [Google Scholar]
- 35. Jiang N, Bao Z, Zhang X, Hirochika H, Eddy SR, et al. (2003) An active DNA transposon family in rice. Nature 421: 163–167. [DOI] [PubMed] [Google Scholar]
- 36. Naito K, Cho E, Yang GJ, Campbell MA, Yano K, et al. (2006) Dramatic amplification of a rice transposable element during recent domestication. Proc Natl Acad Sci USA 103: 17620–17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naito K, Zhang F, Tsukiyama T, Saito H, Hancock CN, et al. (2009) Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature 461: 1130–1134. [DOI] [PubMed] [Google Scholar]
- 38. Sarilar V, Marmagne A, Brabant P, Joets J, Alix K (2011) BraSto, a Stowaway MITE from Brassica: recently active copies preferentially accumulate in the gene space. Plant Mol Biol 77: 59–75. [DOI] [PubMed] [Google Scholar]
- 39. Lu C, Chen J, Zhang Y, Hu Q, Su W, et al. (2012) Miniature inverted-repeat transposable elements (MITEs) have been accumulated through amplification bursts and play important roles in gene expression and species diversity in Oryza sativa . Mol Biol Evol 29: 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Itokawa K, Komagata O, Kasai S, Okamura Y, Masada M, et al. (2010) Genomic structures of Cyp9m10 in pyrethroid resistant and susceptible strains of Culex quinquefasciatus . Insect Biochem Mol Biol 40: 631–640. [DOI] [PubMed] [Google Scholar]
- 41. Daborn PJ, Yen JL, Bogwitz MR, Le Goff G, Feil E, et al. (2002) A single P450 allele associated with insecticide resistance in Drosophila . Science 297: 2253–2256. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt JM, Good RT, Appleton B, Sherrard J, Raymant GC, et al. (2010) Copy number variation and transposable elements feature in recent, ongoing adaptation at the Cyp6g1 locus. PLoS Genet 6: 998–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mouches C, Pauplin Y, Agarwal M, Lemieux L, Herzog M, et al. (1990) Characterization of amplification core and esterase B1 gene responsible for insecticide resistance in Culex . Proc Natl Acad Sci USA 87: 2574–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blackman RL, Spence JM, Field LM, Devonshire AL (1999) Variation in the chromosomal distribution of amplified esterase (FE4) genes in Greek field populations of Myzus persicae (Sulzer). Heredity 82: 180–186. [Google Scholar]
- 45. Dereeper A, Guigon V, Blanc G, Audic S, Buffet S, et al. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36: W465–W469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geneious version 5.4 created by Biomatters. Available: http://www.geneious.com. Accessed 2012 January 20.
- 47.CLC Bio website. Available: http://www.clcbio.com. Accessed 2011 December 14.
- 48.Berkley Drosophila Genome Project website. Available: http://www.fruitfly.org/seq_tools/promoter.html. Accessed 2012 June 10.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of EPSPS genomic sequence (exon 4 to exon 6) from representative glyphosate-resistant (R) and –susceptible (S) A. palmeri cloned PCR products. Primers Ex4F and Ex6R1 are underlined, and XhoI polymorphism is highlighted with a square. Intron sequences are in lower case.
(DOCX)
Alignment of fosmid consensus sequence (MS-R), 454 consensus sequence (GA-R), and the probes used for Southern blots (5′ MITE and 3′ MITE from GA-R). A 13 bp imperfect Terminal Inverted Repeat (TIR) is underlined with one non-matching base in lower case, and a 3 bp duplicated sequence similar to known Target Site Duplications (TSD) is shown in bold and italics.
(DOCX)
A 256 bp imperfect inverted repeat identified in fosmid sequence from MS-R gDNA; A, alignment showing identity between inverted repeat on ends of the putative transposon (Figure 4) and B, sequence of the inverted repeat.
(DOCX)
Assembly of Illumina reads to the fosmid reference sequence, and the presence of read stacking in a region from approximately 20,000 bp until 22,000 bp. The sequence in this region contains a repeated 551 bp sequence.
(TIF)
PCR evidence for existence of putative MITE sequences in both GA-R and GA-S, but present flanking EPSPS only in GA-R and not in GA-S or NC-S A. palmeri individuals. PCR on gDNA of 3 GA-R and 3 GA-S A. palmeri with primers A) 5′ MITE.F by 5′ MITE.R, B) 3′ MITE.F by 3′ MITE.R, C) 5′ MITE.F by Ex1R and D) Ex8F by 3′ MITE.R; PCR on gDNA of 16 GA-S and 1 GA-R A. palmeri with primers E) 5′ MITE.F by Ex1R, F) Ex8F by 3′ MITE.R, and G) Ex1F by Ex1R as a positive PCR control; H) PCR on gDNA of 5 NC-S (North Carolina) and 1 GA-R A. palmeri with primers (left to right) Ex1F by Ex1R as a positive PCR control, 5′ MITE.F by Ex1R, and Ex8F by 3′ MITE.R. Negative controls (templates without primers and primers without template) were evaluated separately and no PCR products were observed.
(TIF)
Schematic diagram of steps used in EPSPS intron analysis and genomic sequencing of A. palmeri populations.
(DOCX)
PCR primers used in experiments to sequence introns, conduct qPCR on introns, synthesize Southern blot probes, and amplify MITE-homologous sequences.
(DOCX)
Contigs from 454 sequencing that align with fosmid reference sequence (see Figure 3) have a high number of hits. Raw read hits for each contig were normalized for size to 1000 bp to facilitate comparisons across contigs.
(DOCX)
(DOCX)