Abstract
Context
The hypothesis of a serotonin (5-hydroxytryptamine [5-HT]) dysfunction in obsessive-compulsive disorder (OCD) stems largely from the clinical efficacy of 5-HT reuptake inhibitors. Serotonergic abnormalities in the unmedicated symptomatic state, however, remain to be fully characterized.
Objective
To investigate brain regional 5-HT synthesis, as indexed by positron emission tomography and the α-[11C]methyl-L-tryptophan trapping constant (K*), in treatment-free adults meeting criteria for OCD.
Design
Between-group comparison.
Setting
Department of Psychiatry and Montreal Neurological Institute, McGill University, and Department of Psychology, McGill University Health Centre, Quebec, Canada.
Participants
Twenty-one medication-free patients with OCD (15 men with a mean [SD] age of 33.2 [9.3] years and 6 women with a mean [SD] age of 35.8 [7.1] years) and 21 healthy controls matched for age and sex (15 men with a mean [SD] age of 32.9 [10.1] years and 6 women with a mean [SD] age of 36.5.5 [8.6] years).
Main Outcome Measure
The α-[11C]methyl-L-tryptophan brain trapping constant K*, which was analyzed with Statistical Parametric Mapping (SPM8) and with proportional normalization (extent threshold of 100 voxels with a peak threshold of P≤.005).
Results
Compared with healthy controls, the patients with OCD exhibited significantly greater α-[11C]methyl-L-tryptophan trapping in the right hippocampus and left temporal gyrus (Brodmann area 20). In the larger sub-sample of all men, these same differences were also evident, as well as higher K* values in the caudate nucleus. Individual differences in symptom severity correlated positively with K* values sampled from the caudate and temporal lobe of the patients with OCD, respectively. There were no regions where the patients exhibited abnormally low K* values. Volumetric analyses found no morphometric alterations that would account for the group differences.
Conclusion
The results support previous reports of greater striatal and temporal lobe activity in patients with OCD than in healthy controls and suggest that these disturbances include a serotonergic component. Previously reported glucose metabolic disturbances in OCD involving the orbitofrontal and cingulate cortices, in comparison, might reflect postsynaptic changes in the serotonergic system.
Obsessive-compulsive disorder (OCD) is a complex and often disabling disorder characterized by intrusive anxiogenic thoughts (obsessions) and repetitive stereotyped behaviors (compulsions).1 Functional neuroimaging, neurosurgical lesion, and deep brain stimulation studies have implicated a limbic corticostriatal circuit that includes the orbitofrontal cortex, anterior cingulate, and caudate nucleus.2–5
The neurochemistry of OCD is less well understood. Primarily on the basis of the clinical efficacy of serotonin (5-hydroxy-tryptamine [5-HT]) reuptake inhibitors,6–10 disturbance(s) of 5-HT neurotransmission in pathways mediating various components of the behavioral phenotype (repetitive behavior, behavioral control of fear, mental imagery, disinhibition, doubting, metamemory, and cognitive inflexibility) have been proposed, although direct tests of various individual hypothesis have been inconclusive. For example, m-chlorophenylpiperazine (an agonist to 5-HT2C, 5HT1A, and 5-HT1D)11–14 and sumatriptan (an agonist to 5-HT1D)15,16 ex-acerbated symptoms of OCD in some studies, which suggests a 5-HT1D mediated effect.17 However, these compounds failed to do so in other studies.18–20 Moreover, neither obsessions nor compulsions were affected by the 5-HT releaser fenfluramine,21 the 5-HT precursor L-tryptophan,22 or acute tryptophan depletion,23,24 suggesting that short-term changes in presynaptic 5-HT availability and release do not affect symptoms of OCD. Studies using indices of resting 5-HT function have also been equivocal. For example, cerebrospinal fluid levels of the primary 5-HT metabolite, 5-hydroxyindolacetic acid (5-HIAA), are elevated in some OCD studies25,26 but not in others.27,28 Preliminary functional neuroimaging studies have found brain regional densities of the 5-HT transporter that were higher,29 lower,30–33 or not different from controls.34,35 Recently, a meta-analysis of 18 studies, involving 2283 patients with OCD, failed to demonstrate a significant association between 5-HT transporter polymorphism and susceptibility to OCD.36
Failure to identify a consistent 5-HT phenotype in OCD may in part be due to the limited resolution of the available methods of investigation to assess 5-HT function in vivo, the small sample size of most studies, and the inherent complexity and multiple behavioral phenotypes linked to a diagnosis of OCD, as well as the widely distributed and ubiquitous innervation of serotonergic neurons across the brain. Indeed, a parsimonious interpretation of most biological studies to date would argue against the presence of specific regional alterations of 5-HT neurotransmission having pathophysiological relevance for the disorder. Yet, this view is not shared by many and is still a matter of significant controversy.2,24,37 Instead, a model advocating a complex pattern of local facilitatory and inhibitory influences, modulating distinct 5-HT pathways underlying the different components of an OCD phenotype, is preferred. For example, coping or resistance appears a more attractive and plausible alternative; in particular, higher serotonergic input in amygdala is often associated with anxiety-like behaviors,38 whereas lower serotonin function in the orbital frontal cortex is reportedly associated with behavioral disinhibition and impulsivity.39,40
In the past decade, we have developed and validated a method for estimating in vivo brain regional 5-HT synthesis capacity, using positron emission tomography (PET) in combination with a synthetic analog of the 5-HT precursor L-tryptophan, α-[11C]methyl-L-tryptophan (α-[11C]MTrp).41,42 Unlike L-tryptophan, α-[11C]MTrp is not incorporated into protein.43 Like the 5-HT precursor, though, α-[11C]MTrp is carried across the blood-brain barrier by a transport system that is active for large neutral amino acids.44 Once inside the brain, α-[11C]MTrp is taken up into 5-HT neurons where it enters the precursor pool and, eventually, is metabolized into αM-5-HT. With a 2-tissue compartment model, we then use the tracer’s net blood-to-brain clearance (K*, in milliliters per gram per minute) as a proxy to estimate regional rates of 5-HT synthesis.41 This method has been used to study 5-HT synthesis capacity in the brains of healthy adults and children,45–48 as well as in the brains of patients with a history of mood and personality disorders, migraines, autism, alternating hemiplegia during childhood, and serious suicide attempts.40,49–53
In our study, the α-[11C]MTrp/PET method was used to measure in vivo brain regional 5-HT synthesis capacity rates in medication-free patients with OCD compared with age- and sex-matched healthy controls. We specifically focused on the cortical, subcortical, and limbic areas (orbitofrontal cortex, anterior cingulate gyrus, and caudate nucleus), which were reported to be of pathophysiological significance in the various anatomical models derived from functional neuroimaging studies in OCD.54–63 Although specific predictions could be entertained as to the direction and/or location of change, if any, between patients with OCD and healthy controls (eg, greater 5-HT–mediated inhibitory inputs in circuits mediating impulsivity, as an attempt to regain behavioral control, or greater 5-HT–mediated facilitator effects in brain pathways mediating stress-related repetitive behaviors), our study was deemed, in many ways, exploratory rather than hypothesis testing.
METHODS
STUDY POPULATION
The primary entry criteria for the subjects with OCD were as follows: (1) right-handed man or woman, aged 18 to 65 years; (2) current diagnosis of OCD, per the Structured Clinical Interview for DSM-IV Axis I Disorders64; (3) a Yale-Brown Obsessive Compulsive Scale (Y-BOCS)65 score of 19 or higher; (4) a Clinical Global Impressions rating of 3 or higher; (5) a Beck Depression Inventory66 score lower than 16; (6) medication-free for at least 3 weeks or for more than 5 elimination half-lives of the drug, whichever was more; (7) no personal or family history of Tourette syndrome; (8) no history of other Axis I disorders, except for depression secondary to OCD; (9) no current or past substance dependence; and (10) never having used the putative 5-HT neurotoxins 3,4-methylenedioxymeth-amphetamine (MDMA) and 3,4-methylenedioxyamphet-amine (MDA). Twenty-one patients diagnosed with OCD and meeting the entry criteria were referred by psychiatric sites in Montreal and by the OCD clinic (D.S.) at the Department of Psychology, McGill University Health Centre.
The comparison group consisted of 21 healthy subjects matched for age and sex. These participants were recruited via newspaper advertisements and were interviewed using the Structured Clinical Interview for DSM-IV Axis I Disorders. All were physically healthy, as determined by a physical examination and standard laboratory tests. Exclusion criteria included a personal history of past or current DSM-IV Axis I psychiatric disorder, a DSM-IV Axis I psychiatric disorder in a first-degree relative, and past use of MDMA and/or MDA. On the day of the PET study, all participants tested negative on a urine drug screen (Triage Panel for Drugs of Abuse; Biosite Diagnostics Inc, San Diego, California) that is sensitive to cocaine, opiates, phenyl-cyclohexyl piperidine, tetrahydrocannabinol, barbiturates, benzodiazepines, and amphetamines. All women of fertile age were scanned during their follicular phase. Thus, the findings do not generalize to other phases, such as the luteal phase.
Written informed consent was obtained from all participants. Our study was conducted in accordance with the Declaration of Helsinki (18th World Medical Association General Assembly) and was approved by the Research Ethics Committee of the Montreal Neurological Institute (MNI).
PET AND MAGNETIC RESONANCE IMAGING
The α-[11C]MTrp was prepared as described previously.67 Prior to the PET study, all subjects observed an overnight fast (water allowed ad libitum) preceded by a low-protein diet, the day before the PET study, to reduce interindividual variability in plasma amino acid concentrations.45 The PET studies were conducted in the late morning or early afternoon (between 11 AM and 2 PM) using a whole-body scanner (ECAT HR+; CTI Molecular Imaging, Inc/Siemens, Knoxville, Tennessee). All images were collected and reconstructed in 3-dimensional mode with an intrinsic resolution of 5×5×5-mm full width at half maximum. Before tracer injections, transmission scans for attenuation correction were performed using a 68Ge/Ga source. After the intravenous injection of 10 to 15 mCi (185–740 MBq) of α-[11C]MTrp (dose not scaled to body weight) administered as a 2-minute slow infusion, 60-minute dynamic PET data were acquired. During each PET scan, thirteen 2-mL blood samples were obtained from the antecubital vein of a heated arm to compute the α-[11C]MTrp input function. The input function was derived from intracranial venous sinus radioactivity (0–20 minutes) and “arterialized” venous plasma (20–60 minutes), as described previously.48,68 Three 2-mL blood samples were centrifuged, and ultrafiltrates were stored at −80°C for measurement of free plasma tryptophan concentrations using high-performance liquid chromatography.45,69 Two additional plasma samples were treated with trichloroacetic acid (2:1) for determination by high-performance liquid chromatography of total plasma tryptophan concentrations.
All participants underwent high-resolution magnetic resonance imaging using a 1.5-T superconducting magnet system (Philips Gyroscan; Philips Medical Systems, Eindhoven, the Netherlands). Images were collected using 3-dimensional volume acquisition, T1-weighted (3-dimensional fast-field echo scan: repetition time, 18 milliseconds; echo time, 10 milliseconds; flip angle, 30°) over the whole brain. Magnetic resonance imaging data were stored as a 256×256×160-mm matrix with 1-mm3 isotropic voxels.
CALCULATION OF α-[11C]MTrp TRAPPING (K*)
The Patlak graphic method70 was used to calculate K* (in milliliters per gram per minute) using 40 minutes of dynamic PET data collected between 20 and 60 minutes after tracer injection.48,68 Comparisons of regional normalized K* values between controls and patients with OCD were performed using Statistical Parametric Mapping (SPM8; Wellcome Functional Imaging Laboratory, London, England) and separately via a magnetic resonance image–based region-of-interest method.
SPM8 ANALYSIS
Comparisons of differences in regional normalized K* values between patients with OCD and healthy controls were first performed using a brain-wide voxelwise approach with SPM8. K* images were resampled to the MNI-305 template, 2-mm isotropic stereotaxic space (spatial normalization) using a standard linear automatic algorithm.71 Each functional image in stereotaxic space was smoothed with a gaussian filter (14-mm full width at half maximum) to reduce the effect of individual variability in cortical gyral anatomy. Proportional scaling was applied to remove the effect of global differences on regional values among subjects. Functional images were normalized by setting for each subject the mean global K* value of their gray matter to 100. SPM8 comparisons were restricted to voxels found in the gray matter. The t test was applied voxel-by-voxel to determine regional differences between groups. Statistically significant regional differences were identified using dual criteria. First, the height threshold used to interpret the t test in terms of probability level was set at P=.005 (uncorrected). Second, the extent threshold was set at 100 voxels, suitable for the 14-mm full width at half maximum filter and sufficient to remove small noisy clusters, which may reach significance by chance. SPM8 was also used to identify regions where individual differences in α-[11C]MTrp trapping correlated with Y-BOCS scores using the same criteria for statistical significance.
MAGNETIC RESONANCE IMAGING–BASED REGION-OF-INTEREST ANALYSIS
Comparisons of regional normalized K* values between healthy controls and patients with OCD were also performed using a magnetic resonance image–based region-of-interest approach. Individual magnetic resonance imaging data were corrected for field inhomogeneities72 and resampled in a standard stereotaxic space (MNI-305 template).71 Tissue classification into gray and white matter and cerebrospinal fluid was performed using the series of algorithms known as INSECT (Intensity Normalized Stereotaxic Environment for the Classification of Tissue).73 These data were subsequently submitted to the algorithm known as ANIMAL (Automatic Nonlinear Imaging Matching and Anatomical Labeling)74 for segmentation into 48 anatomical volumes. The bilateral medial and lateral orbitofrontal cortices, the cingulate complex, and the caudate constituted the a priori selected regions of interest. These regions of interest were convolved with a 7-mm full width at half maximum gaussian kernel filter and then resampled into PET acquisition space. Volumes of interest were then applied to dynamic native PET space to extract time-activity curves. Global K* values were compared between subject groups. Subsequently, to minimize the effect of individual global differences on regional values, all regional K* values were normalized by setting the mean global K* values of the gray matter to 100. In addition to comparing functional differences, volumetric comparisons of the aforementioned anatomical volumes in stereotaxic space (MNI-305 template) were performed between healthy controls and patients with OCD.75
RESULTS
DEMOGRAPHICS
Twenty-one patients with OCD (15 men with a mean [SD] age of 33.2 [9.3] years and 6 women with a mean [SD] age of 35.8 [7.1] years) and 21 healthy controls (15 men with a mean [SD] age of 32.9 [10.1] years and 6 women with a mean [SD] age of 36.5.5 [8.6] years) participated in our study (Table). Age did not differ between groups when analyzed as a whole or according to sex. The patients with OCD had significantly higher scores on the Beck Depression Inventory (mean [SD], 13.3 [9.3]) than did the healthy controls (mean[SD], 2.1 [2.8]) (t23=3.07, P≤.005), but their scores were still within the subclinical range, and no patient met diagnostic criteria for major depression. Six patients had a lifetime history of major depression that had occurred secondarily to their OCD symptoms. Of the 21 patients with OCD, 11 had childhood-onset OCD (age, <10 years), 11 had checking behaviors as their predominant compulsion, and 10 had predominantly washing compulsions. None of the subjects included in our study ever had a substance abuse problem, except for 1 patient with OCD who had a lifetime history of cocaine dependence that ended 11 years prior to the study. There were no differences in plasma concentrations of total or free tryptophan between patients with OCD and healthy controls or between men and women.
Table.
Demographic Characteristics of the Study Population
| Characteristic | Patients With OCD (n = 21) | Healthy Controls (n = 21) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 33.95 (8.64) | 33.95 (9.59) |
| Range | 18–53 | 20–56 |
| Y-BOCS score, mean (SD) | 23.57 (5.33) | NA |
| BDI score,a mean (SD) | 13.2 (9.27) | 1.40 (2.19) |
| Disease duration, mean (SD), y | 21.28 (8.73) | NA |
| Childhood-onset OCD (age, <10 y), No. | 11 | NA |
| Predominant compulsion, No. | NA | |
| Washing | 10 | NA |
| Checking | 11 | NA |
| Lifetime history of MDE, No. | 6 | 0 |
| Past SSRI treatment, No. | 2 | 0 |
| Past substance abuse, No. | 1 | 0 |
| Intravenously injected, mean (SD), mCi | 10.16 (3.14) | 9.74 (2.11) |
| Plasma free tryptophan, mean (SD), nmol/L | 8.37 (3.69) | 8.91 (3.76) |
| Global K*, mean (SD), mL/g/min | 5.07 (1.49) | 5.81 (1.96) |
Abbreviations: BDI, Beck Depression Inventory; MDE, major depressive episode; NA, not applicable; OCD, obsessive-compulsive disorder; SSRI, selective serotonin reuptake inhibitor; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale.
Statistically significant difference between patients with OCD and healthy controls at P < .001.
GLOBAL AND REGIONAL α-[11C]MTrp TRAPPING
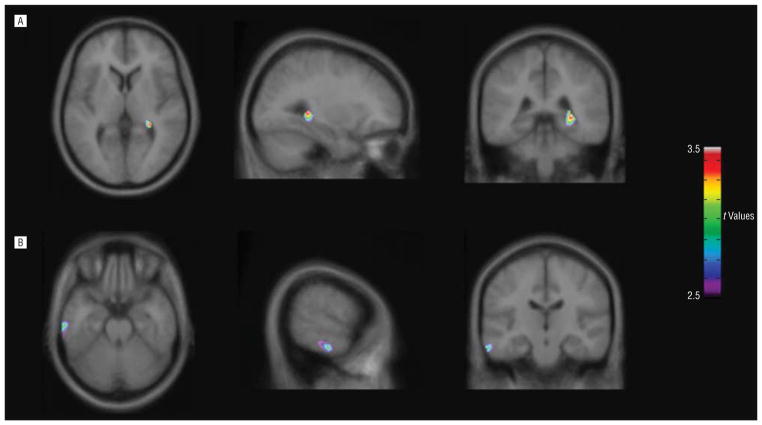
There was no significant group effect in the sample as a whole (t40=1.37, P =.18), or in either sex analyzed separately for global K* values (men: t28=0.095, P =.92; women: t10 = 1.94, P = .08). In comparison, normalized α-[11C]MTrp trapping was significantly higher for patients with OCD than for healthy controls in the right hippocampus (t40=3.37, k =151 voxels, coordinates x, y, z, respectively: 30, −38, 4 mm) and the left temporal gyrus (Brodmann area 20; t40=3.10, k =157 voxels, coordinates x, y, z, respectively: −62, −20, −24 mm). There were no regions with significant lower values in patients with OCD compared with healthy controls (Figure 1).
Figure 1.
Statistical parametric maps (SPM8; Wellcome Functional Imaging Laboratory, London, England) showing brain regions where K* values were significantly higher in patients with obsessive-compulsive disorder (n = 21) than in healthy controls (n = 21). The statistical t-map threshold was 2.70, with P = .005 and an extent threshold of 100 voxels. Significant clusters were found in (A) the right hippocampus (t40 = 3.37, k = 151 voxels, coordinates x, y, z, respectively: 30,−38, 4 mm) and (B) the left inferior temporal gyrus (t40 = 3.10, k = 157, coordinates x, y, z, respectively: −62, −20, −24 mm).
When analyzed separately by sex, in the men (15 patients with OCD vs 15 healthy controls), greater α-[11C]MTrp trapping in patients with OCD compared with controls was present in the same regions as for the whole group (right hippocampus: t28=4.70, k =419 voxels, coordinates x, y, z, respectively: 32, −40, 2 mm; left inferior temporal gyrus [Brodmann area 20]: t28=4.70, k =101 voxels, coordinates x, y, z, respectively: −64, −22, −26 mm), with no regions in the patients with OCD demonstrating values lower than those in healthy controls. In the smaller subgroup of women (6 patients with OCD vs 6 healthy controls), α-[11C]MTrp trapping was significantly higher for the subjects with OCD in the right parahippocampal uncus (Brodmann areas 20 and 36; t10=4.89, k =679 voxels, coordinates x, y, z, respectively: 28, −4, −38 mm) only. Significant decreases in α-[11C]MTrp trapping were also seen in the left cuneus/precuneus (Brodmann area 7; t10=8.89, k=104 voxels, coordinates x, y, z, respectively: −10, −72, 32 mm) and uncus (Brodmann areas 28 and 34; t10=4.40, k=102 voxels, coordinates x, y, z, respectively: −18, 8, −26 mm) for women with OCD.
Region-of-interest analyses confirmed the SPM8-based findings. A group×hemisphere analysis of variance yielded a significant interaction for K* values in the hippocampus (F1,40=14.75, P ≤.001), reflecting greater regional trapping in the patient’s right hemisphere, compared with healthy controls. Region-of-interest analysis also confirmed a significant main effect of group in both the left and right inferior temporal gyrus, with greater tracer trapping in patients with OCD than in healthy controls (F1,40=7.97, P ≤.007). Finally, a group ×hemisphere interaction approached significance in the group as a whole, reflecting higher K* values in the right caudate of patients (F1,40=3.83, P ≤.06). This effect was larger in the men, and a region-of-interest analysis that was restricted to men confirmed the main effect of group (F2,39=9.06, P ≤.001), reflecting higher K* values in the patients with OCD than in healthy controls for both the left (P≤.03) and right caudate nucleus (P≤.001). No volumetric differences were found for any of the regions of interest between patients with OCD and healthy controls.
CORRELATIONS BETWEEN α-[11C]MTrp TRAPPING AND CLINICAL SCORES
In the sample of patients with OCD as a whole (n=21), significant positive correlations between Y-BOCS scores and α-[11C]MTrp trapping were present in the right middle (Brodmann area 21; t19 = 4.24, k = 345 voxels, coordinates x, y, z, respectively: 70, −26, −14 mm) and superior temporal gyrus (Brodmann area 38; t19 =4.40, k = 551 voxels, coordinates x, y, z, respectively: 46, 8, −28 mm) and in the left inferior and middle temporal gyrus (Brodmann areas 20 and 21; t19=3.81, k =183 voxels, coordinates x, y, z, respectively: −60, −6, −26 mm). In the male patients with OCD, a significant positive correlation between Y-BOCS scores and α-[11C]MTrp trapping was seen in the right caudate (t13=4.82, k =145 voxels, coordinates x, y, z, respectively: 16, 8, 20 mm) (Figure 2), whereas in the female patients with OCD, a significant positive correlation was seen in the left pre-cuneus (Brodmann area 19; t4 = 18.32, k = 143 voxels, coordinates x, y, z, respectively: −24, −74, 32 mm), the right inferior and middle temporal gyrus (Brodmann areas 20 and 21; t4=11.04, k =155 voxels, coordinates x, y, z, respectively: 60, −12, −16 mm), and the right inferior and middle frontal gyrus (Brodmann area 47; t4=7.60, k =126 voxels, coordinates x, y, z, respectively: 52, 42, −6 mm).
Figure 2.
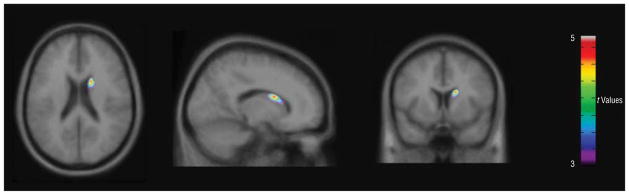
Statistical parametric maps (SPM8; Wellcome Functional Imaging Laboratory, London, England) showing brain regions where K* values correlated positively with Yale-Brown Obsessive-Compulsive Scale scores in male patients with obsessive-compulsive disorder (n = 15). The statistical t-map threshold was 3.01, with P = .005 and an extent threshold of 100 voxels. A significant cluster was found in the right caudate (t13 = 4.82, k = 145 voxels, coordinates x, y, z, respectively: 16, 8, 20 mm).
The Beck Depression Inventory did not correlate with α-[11C]MTrp uptake and trapping. In particular, it did not correlate with any of the brain regions distinguishing patients with OCD from controls, thus ruling out the possibility that current subclinical secondary depression may have confounded the results.
COMMENT
Largely on the basis of the clinical efficacy of 5-HT reuptake inhibitors, it was proposed, more than 20 years ago, that perturbed serotonergic neurotransmission may contribute to the development and expression of OCD.11,25,76 Our study provides some evidence that these putative disturbances may include regionally specific alterations in brain 5-HT synthesis capacity, with medication-free patients with OCD exhibiting, relative to age- and sex-matched controls, significantly elevated normalized α-[11C]MTrp trapping in the right hippocampus and left inferior temporal gyrus (Brodmann area 20). These observations were more robust when the analysis was restricted to male patients with OCD (n = 15), who also demonstrated increased α-[11C]MTrp trapping bilaterally in the caudate nucleus. Individual differences in α-[11C]MTrp trapping in the right caudate and, bilaterally, in the temporal cortex correlated positively with OCD symptom severity in the whole group of patients with OCD. There were no regions of interest where the patients with OCD exhibited significantly lower α-[11C]MTrp trapping values than did controls. The lack of robustness for differences in normalized α-[11C]MTrp trapping in female patients with OCD might be related to the near significance reported (trend) for global K*, between the female patients with OCD and the female healthy controls, as well as to the small number of women studied.
During the past 2 decades, considerable progress has been made in the dissection of the functional neuroanatomy of OCD, with compelling evidence in support of hyperactivity in parts of a brain circuit loop linking the orbital frontal cortex, the caudate nucleus, and the anterior cingulate gyrus.3 Much less consensus exists, however, as to its significance, whether primary (correlating with symptom severity) or secondary (reflecting resistance and attempts to regain thought and/or behavioral control), or both. A common belief among OCD researchers is that hyperactivity of the frontostriatal loop underscores the motor and cognitive habits progressively evolving toward inflexibility and rigid ritualistic behavior, whereas hyperactivity in the orbitofrontal cortex reflects unsuccessful resistance (ie, failure to inhibit and failed attempts at stress control).3,77 However, primary deficits in orbitofrontal cortex function have also been reported.
The finding of greater α-[11C]MTrp trapping in the caudate nucleus for male patients with OCD than for male healthy controls might reflect serotonergic modulation of a structure known to be hyperactive in patients with current OCD, as noted earlier. Within the striatum, 5-HT exerts primarily, though not exclusively, inhibitory effects.78,79 The greater 5-HT neurotransmission in the striatum of patients with OCD, therefore, could be a contributing factor to the disorder’s pathophysiology and symptom profile and/or locus that might be helped by treatment with selective serotonin reuptake inhibitors.80
The present results do not provide support for the hypothesis of altered 5-HT neurotransmission in the orbitofrontal cortex or other aspects of the frontal lobe via a presynaptic mechanism. Rather, the enhanced glucose metabolic and regional cerebral blood flow changes previously reported in these regions may be the result of post-synaptic 5HT alteration(s). Indeed, the decreased binding potential of the 5-HT2a_ligand [C-11]MDL100.907 in several cortical areas, including the orbitofrontal cortex, represents a specific postsynaptic downregulation of a 5-HT receptor subtype, which mediates an inhibitory response in the rodent brain region equivalent to the human orbitofrontal cortex.81,82 Physiologically, this would translate into a dampened inhibitory tone and thus increased metabolic activity of the orbitofrontal cortex.
There are certainly other possibilities to account for the lack of orbital frontal findings. In particular, increased α-[11C]MTrp trapping was observed in the hippocampus and the rostral parahippocampal uncus, which covers the amygdala’s dorsal surface. These regions have been identified in at least some previous functional neuroimaging studies of OCD,3 and they influence activity in the orbitofrontal cortex via both direct and indirect projections.83,84
The hippocampus and caudate have been proposed to play important roles in 2 independent forms of memory, context-dependent cognitive processes and stimulus-response habit behaviors, respectively.85–87 As part of a serotonergic septohippocampal behavioral inhibition system,88 the hippocampus is also thought to influence anxiety-related behaviors, increasing the salience of negatively valenced affective stimuli and interacting with the amygdala and neocortex to facilitate choices between conflicting alternatives.89,90 Excessive hippocampal output to the striatum might perturb memory-guided coping91,92 and, in the presence of high dopamine levels, promote the perseveration of inflexible, context-dependent habits.87,93,94 This revised model suggests that hippocampal, amygdaloid, and striatal hyperfunction could reflect a tendency to resolve conflicts in terms of a negative bias, potentially contributing to an OCD-susceptible phenotype characterized by danger overestimation, reduced confidence that an act has been completed adequately, and compulsive stereotypies.
To our knowledge, our study describes the only psychiatric population reported to date to exhibit exclusively elevated α-[11C]MTrp trapping values. In comparison, patients with major depression,53 borderline personality disorder,49 and/or a history of serious suicide attempts40 are all reported to have abnormally low α-[11C]MTrp trapping values in limbic regions of the frontal lobe. One potential implication is that patients with OCD who have abnormally low 5-HT activity in these regions might become susceptible to comorbid disorders. The patients in our study were carefully screened to rule out the presence of significant comorbidities.
Subjects with borderline personality disorder are also reported to exhibit lower values in α-[11C]MTrp trapping in the basal ganglia, and individual differences in these K* values correlated negatively with impulsivity scores.49 In comparison, patients with OCD exhibited abnormally high striatal K* values, with individual differences correlating positively with compulsivity. In the presence of other risk factors (eg, hypo- vs hyperfrontality), a continuum of 5-HT activity within the basal ganglia might contribute to a proposed impulsivity-compulsivity dimension.95
The interpretation of our results rests on the following methodological considerations: (1) The significance of the α-[11C]MTrp/PET method has been questioned, and, in particular, Shoaf et al96 have suggested that it might measure blood brain barrier transport of tryptophan rather than synthesis of 5-HT. However, subsequent autoradiography studies on rodents,97 along with analyses of tracer kinetics and the effects of manipulations that selectively increase44,98 vs decrease 5-HT synthesis,99,100 support the general consensus that brain regional α-[11C]MTrp trapping provides an acceptable proxy for 5-HT synthesis.41,42,45,48,101–104 (2) Medication-free patients with OCD did not differ significantly from healthy controls in total and free plasma tryptophan concentrations or in the free plasma tryptophan fraction, making it unlikely that increased normalized K* values reflect group differences in circulating tryptophan concentrations, which otherwise could affect brain 5-HT synthesis.45,105–108 (3) As in other functional neuroimaging studies of psychiatric populations, the sample size is relatively small (21 patients with OCD vs 21 healthy controls), such that general conclusions cannot be drawn until there is an independent replication. A larger sample would also allow one to test whether specific abnormalities are associated with early- vs late-onset OCD or phenotypical subtypes, such as predominantly “checkers” or “washers,” or more recently proposed symptom subtypes such as “symmetry” or “hoarding.”109,110 Other potential confounders, such as body mass index or seasonal variations, could also be more appropriately controlled for in a larger sample. Nevertheless, the sample size is in the upper range of similar neuroimaging studies and is one of the largest in a PET study of patients with OCD reported to date that used a 5-HT system tracer. Healthy controls and patients with OCD were also enrolled and observed in a parallel manner over time. Moreover, precautions included rigorous matching for age and sex, a negative toxicological screen on the day of the scan, and patient selection restricted to medication-free individuals without current comorbid conditions. (4) As frequently seen, 6 of 21 patients had a past history of depression that had developed secondarily to OCD and was in remission at the time of scanning. This, of course, could potentially bias our results because depression is linked to state abnormalities, and perhaps trait 5-HT abnormalities, as well as morphological and structural changes, in particular in the hippocampus. The latter, however, seems to develop with recurrent chronic major depressive disorder,111 which does not correspond to our sample phenotype, in which only some individuals suffered at some point a minor form of depression. Moreover, complementary analyses using Beck Depression Inventory scores did not reveal any significant correlations with K*. In addition, an analysis contrasting OCD patients with and without a history of depression did not reveal any significant differences in regional K*, and removing patients with a history of depression from the analysis did not change the results. (5) In contrast to our results, previous functional neuroimaging studies of the 5-HT transporter have not identified consistent changes in patients with OCD vs controls, either in the midbrain or terminal regions. A recent study,112 though, has measured [18F] altanserin binding values and found evidence of increases in 5-HT2A densities specifically within the caudate nucleus. (6) Under certain pathological conditions, such as inflammatory neurological diseases,113 intractable epilepsy in childhood,114 or brain tumors,115 an increased tryptophan metabolism might reflect the activation of the initial and rate-limiting enzyme of the kynurenine pathway, indolamine 2,3 dioxygenase. Indeed, cases of pediatric auto-immune neuropsychiatric disorders,116 including a subgroup of childhood-onset OCD with or without tics, have been associated with streptococcal infections. Moreover, abnormally increased serotonin synthesis was recently reported in Tourette syndrome.117 Whether neuroinflammatory processes prompted activation of the kynurenine pathway in some OCD cases, thus resulting in the increased rate of uptake and clearance of α-[11C]MTrp reported here, is unknown. (7) Finally, although volumetric differences in patients with OCD have been reported previously, these findings have been inconsistent; for example, the orbitofrontal cortex and striatal tissue volumes have been reported to be abnormally high,118,119 abnormally low,119,120 or to not differ from those in controls.121 Moreover, recent meta-analyses indicate possible structural alterations in parietofrontal areas, the anterior cingulate cortex, the thalamus, lenticular/caudate nuclei, and the putamen, but they do not support the presence of structural alterations in the hippocampus, where we found functional differences.122–124 In our study, careful segmentation (blind to the study group) did not reveal volumetric differences between the OCD and control groups.
In conclusion, our study revealed elevated normalized α-[11C]MTrp trapping in the right hippocampus, the left inferior temporal gyrus, and the bilateral caudate nucleus in medication-free patients with OCD, relative to age- and sex-matched controls. These findings add to the evidence supporting a serotonergic dysfunction in OCD and, more specifically, point to the critical role played by serotonergic innervation of limbic structures, such as the hippocampus, closely connected to previously identified regions (caudate and orbitofrontal cortex) believed to mediate OCD symptoms. The abnormally high 5-HT synthesis, suggested by the elevated α-[11C]MTrp uptake in the unmedicated symptomatic state, might represent a compensatory mechanism, which could be engaged further in the course of effective anti-OCD treatments.
Acknowledgments
Funding/Support: This study was supported by funding from Fonds de la recherche en Sante de Quebec.
Footnotes
Financial Disclosure: None reported.
Previous Presentation: Presented in part at the 44th Annual Meeting of the American College of Neuropsychopharmacology; December 11–15, 2005; Waikoloa, Hawaii.
Additional Contributions: We thank Rick Fukusawa, Gary Sauchuk, DEC, Dean Jolly, Shadreck Mzengeza, PhD, Mirjana Kovacevic, and Gail Rauw, PhD, for their excellent technical assistance, and Amir Barsoum MD, for clinical expertise and help in patient recruitment.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Aouizerate B, Guehl D, Cuny E, Rougier A, Bioulac B, Tignol J, Burbaud P. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004;72(3):195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, du Montcel ST, Yelnik J, Chéreau I, Arbus C, Raoul S, Aouizerate B, Damier P, Chabardès S, Czernecki V, Ardouin C, Krebs MO, Bardinet E, Chaynes P, Burbaud P, Cornu P, Derost P, Bougerol T, Bataille B, Mattei V, Dormont D, Devaux B, Vérin M, Houeto JL, Pollak P, Benabid AL, Agid Y, Krack P, Millet B, Pelissolo A STOC Study Group. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 5.Rotge JY, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci. 2008;33(5):405–412. [PMC free article] [PubMed] [Google Scholar]
- 6.McDonough M, Kennedy N. Pharmacological management of obsessive-compulsive disorder: a review for clinicians. Harv Rev Psychiatry. 2002;10(3):127–137. doi: 10.1080/10673220216215. [DOI] [PubMed] [Google Scholar]
- 7.Zohar J, Insel TR. Drug treatment of obsessive-compulsive disorder. J Affect Disord. 1987;13(2):193–202. doi: 10.1016/0165-0327(87)90023-1. [DOI] [PubMed] [Google Scholar]
- 8.Chouinard G, Goodman W, Greist J, Jenike M, Rasmussen S, White K, Hackett E, Gaffney M, Bick PA. Results of a double-blind placebo controlled trial of a new serotonin uptake inhibitor, sertraline, in the treatment of obsessive-compulsive disorder. Psychopharmacol Bull. 1990;26(3):279–284. [PubMed] [Google Scholar]
- 9.Benkelfat C, Murphy DL, Zohar J, Hill JL, Grover G, Insel TR. Clomipramine in obsessive-compulsive disorder: further evidence for a serotonergic mechanism of action. Arch Gen Psychiatry. 1989;46(1):23–28. doi: 10.1001/archpsyc.1989.01810010025004. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg BD, Benjamin J, Martin JD, Keuler D, Huang SJ, Altemus M, Murphy DL. Delayed obsessive-compulsive disorder symptom exacerbation after a single dose of a serotonin antagonist in fluoxetine-treated but not untreated patients. Psychopharmacology (Berl) 1998;140(4):434–444. doi: 10.1007/s002130050787. [DOI] [PubMed] [Google Scholar]
- 11.Zohar J, Mueller EA, Insel TR, Zohar-Kadouch RC, Murphy DL. Serotonergic responsivity in obsessive-compulsive disorder: comparison of patients and healthy controls. Arch Gen Psychiatry. 1987;44(11):946–951. doi: 10.1001/archpsyc.1987.01800230026006. [DOI] [PubMed] [Google Scholar]
- 12.Hollander E, DeCaria CM, Nitescu A, Gully R, Suckow RF, Cooper TB, Gorman JM, Klein DF, Liebowitz MR. Serotonergic function in obsessive-compulsive disorder: behavioral and neuroendocrine responses to oral m-chlorophenylpiperazine and fenfluramine in patients and healthy volunteers. Arch Gen Psychiatry. 1992;49(1):21–28. doi: 10.1001/archpsyc.1992.01820010021003. [DOI] [PubMed] [Google Scholar]
- 13.Pigott TA, Zohar J, Hill JL, Bernstein SE, Grover GN, Zohar-Kadouch RC, Murphy DL. Metergoline blocks the behavioral and neuroendocrine effects of orally administered m-chlorophenylpiperazine in patients with obsessive-compulsive disorder. Biol Psychiatry. 1991;29(5):418–426. doi: 10.1016/0006-3223(91)90264-m. [DOI] [PubMed] [Google Scholar]
- 14.Khanna S, John JP, Reddy LP. Neuroendocrine and behavioral responses to mCPP in obsessive-compulsive disorder. Psychoneuroendocrinology. 2001;26(2):209–223. doi: 10.1016/s0306-4530(00)00048-2. [DOI] [PubMed] [Google Scholar]
- 15.Koran LM, Pallanti S, Quercioli L. Sumatriptan, 5-HT(1D) receptors and obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2001;11(2):169–172. doi: 10.1016/s0924-977x(01)00082-7. [DOI] [PubMed] [Google Scholar]
- 16.Gross-Isseroff R, Cohen R, Sasson Y, Voet H, Zohar J. Serotonergic dissection of obsessive compulsive symptoms: a challenge study with m-chlorophenylpiperazine and sumatriptan. Neuropsychobiology. 2004;50 (3):200–205. doi: 10.1159/000079970. [DOI] [PubMed] [Google Scholar]
- 17.Aouizerate B, Guehl D, Cuny E, Rougier A, Burbaud P, Tignol J, Bioulac B. Updated overview of the putative role of the serotoninergic system in obsessive-compulsive disorder. Neuropsychiatr Dis Treat. 2005;1(3):231–243. [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman WK, McDougle CJ, Price LH, Barr LC, Hills OF, Caplik JF, Charney DS, Heninger GR. m-Chlorophenylpiperazine in patients with obsessive-compulsive disorder: absence of symptom exacerbation. Biol Psychiatry. 1995;38(3):138–149. doi: 10.1016/0006-3223(94)00235-U. [DOI] [PubMed] [Google Scholar]
- 19.Ho Pian KL, Westenberg HG, den Boer JA, de Bruin WI, van Rijk PP. Effects of meta-chlorophenylpiperazine on cerebral blood flow in obsessive-compulsive disorder and controls. Biol Psychiatry. 1998;44(5):367–370. doi: 10.1016/s0006-3223(97)00458-7. [DOI] [PubMed] [Google Scholar]
- 20.Boshuisen ML, den Boer JA. Zolmitriptan (a 5-HT1B/1D receptor agonist with central action) does not increase symptoms in obsessive compulsive disorder. Psychopharmacology (Berl) 2000;152(1):74–79. doi: 10.1007/s002130000529. [DOI] [PubMed] [Google Scholar]
- 21.Hollander E, Stein DJ, Saoud JB, DeCaria CM, Cooper TB, Trungold S, Stanley M, Liebowitz MR. Effects of fenfluramine on plasma HVA in OCD. Psychiatry Res. 1992;42(2):185–188. doi: 10.1016/0165-1781(92)90081-d. [DOI] [PubMed] [Google Scholar]
- 22.Charney DS, Goodman WK, Price LH, Woods SW, Rasmussen SA, Heninger GR. Serotonin function in obsessive-compulsive disorder. A comparison of the effects of tryptophan and m-chlorophenylpiperazine in patients and healthy subjects. Arch Gen Psychiatry. 1988;45(2):177–185. doi: 10.1001/archpsyc.1988.01800260095012. [DOI] [PubMed] [Google Scholar]
- 23.Smeraldi E, Diaferia G, Erzegovesi S, Lucca A, Bellodi L, Moja EA. Tryptophan depletion in obsessive-compulsive patients. Biol Psychiatry. 1996;40(5):398–402. doi: 10.1016/0006-3223(95)00393-2. [DOI] [PubMed] [Google Scholar]
- 24.Berney A, Sookman D, Leyton M, Young SN, Benkelfat C. Lack of effects on core obsessive-compulsive symptoms of tryptophan depletion during symptom provocation in remitted obsessive-compulsive disorder patients. Biol Psychiatry. 2006;59(9):853–857. doi: 10.1016/j.biopsych.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Insel TR, Mueller EA, Alterman I, Linnoila M, Murphy DL. Obsessive-compulsive disorder and serotonin: is there a connection? Biol Psychiatry. 1985;20(11):1174–1188. doi: 10.1016/0006-3223(85)90176-3. [DOI] [PubMed] [Google Scholar]
- 26.Swedo SE, Leonard HL, Kruesi MJ, Rettew DC, Listwak SJ, Berrettini W, Stipetic M, Hamburger S, Gold PW, Potter WZ, Rapoport JL. Cerebrospinal fluid neurochemistry in children and adolescents with obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49(1):29–36. doi: 10.1001/archpsyc.1992.01820010029004. [DOI] [PubMed] [Google Scholar]
- 27.Altemus M, Pigott T, Kalogeras KT, Demitrack M, Dubbert B, Murphy DL, Gold PW. Abnormalities in the regulation of vasopressin and corticotropin releasing factor secretion in obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49(1):9–20. doi: 10.1001/archpsyc.1992.01820010009002. [DOI] [PubMed] [Google Scholar]
- 28.Leckman JF, Goodman WK, Anderson GM, Riddle MA, Chappell PB, McSwiggan-Hardin MT, McDougle CJ, Scahill LD, Ort SI, Pauls DL, Cohen DJ, Price LH. Cerebrospinal fluid biogenic amines in obsessive compulsive disorder, Tourette’s syndrome, and healthy controls. Neuropsychopharmacology. 1995;12(1):73–86. doi: 10.1038/sj.npp.1380241. [DOI] [PubMed] [Google Scholar]
- 29.Pogarell O, Hamann C, Pöpperl G, Juckel G, Choukèr M, Zaudig M, Riedel M, Möller HJ, Hegerl U, Tatsch K. Elevated brain serotonin transporter availability in patients with obsessive-compulsive disorder. Biol Psychiatry. 2003;54 (12):1406–1413. doi: 10.1016/s0006-3223(03)00183-5. [DOI] [PubMed] [Google Scholar]
- 30.Stengler-Wenzke K, Müller U, Angermeyer MC, Sabri O, Hesse S. Reduced serotonin transporter-availability in obsessive-compulsive disorder (OCD) Eur Arch Psychiatry Clin Neurosci. 2004;254(4):252–255. doi: 10.1007/s00406-004-0489-y. [DOI] [PubMed] [Google Scholar]
- 31.Hesse S, Müller U, Lincke T, Barthel H, Villmann T, Angermeyer MC, Sabri O, Stengler-Wenzke K. Serotonin and dopamine transporter imaging in patients with obsessive-compulsive disorder. Psychiatry Res. 2005;140(1):63–72. doi: 10.1016/j.pscychresns.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Reimold M, Smolka MN, Zimmer A, Batra A, Knobel A, Solbach C, Mundt A, Smoltczyk HU, Goldman D, Mann K, Reischl G, Machulla HJ, Bares R, Heinz A. Reduced availability of serotonin transporters in obsessive-compulsive disorder correlates with symptom severity: a [11C]DASB PET study. J Neural Transm. 2007;114(12):1603–1609. doi: 10.1007/s00702-007-0785-6. [DOI] [PubMed] [Google Scholar]
- 33.Zitterl W, Aigner M, Stompe T, Zitterl-Eglseer K, Gutierrez-Lobos K, Schmidl-Mohl B, Wenzel T, Demal U, Zettinig G, Hornik K, Thau K. [123I]-beta-CIT SPECT imaging shows reduced thalamus-hypothalamus serotonin transporter availability in 24 drug-free obsessive-compulsive checkers. Neuropsychopharmacology. 2007;32(8):1661–1668. doi: 10.1038/sj.npp.1301290. [DOI] [PubMed] [Google Scholar]
- 34.Simpson HB, Lombardo I, Slifstein M, Huang HY, Hwang DR, Abi-Dargham A, Liebowitz MR, Laruelle M. Serotonin transporters in obsessive-compulsive disorder: a positron emission tomography study with [(11)C]McN 5652. Biol Psychiatry. 2003;54(12):1414–1421. doi: 10.1016/s0006-3223(03)00544-4. [DOI] [PubMed] [Google Scholar]
- 35.van der Wee NJ, Stevens H, Hardeman JA, Mandl RC, Denys DA, van Megen HJ, Kahn RS, Westenberg HM. Enhanced dopamine transporter density in psychotropic-naive patients with obsessive-compulsive disorder shown by [123I]beta-CIT SPECT. Am J Psychiatry. 2004;161(12):2201–2206. doi: 10.1176/appi.ajp.161.12.2201. [DOI] [PubMed] [Google Scholar]
- 36.Bloch MH, Landeros-Weisenberger A, Sen S, Dombrowski P, Kelmendi B, Coric V, Pittenger C, Leckman JF. Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: systematic review. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):850–858. doi: 10.1002/ajmg.b.30699. [DOI] [PubMed] [Google Scholar]
- 37.El Mansari M, Blier P. Mechanisms of action of current and potential pharmacotherapies of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(3):362–373. doi: 10.1016/j.pnpbp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63 (9):852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, Trisdorfer R, Haznedar MM, Koenigsberg HW, Flory J, Siever LJ. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32(7):1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- 40.Leyton M, Paquette V, Gravel P, Rosa-Neto P, Weston F, Diksic M, Benkelfat C. alpha-[11C]Methyl-L-tryptophan trapping in the orbital and ventral medial pre-frontal cortex of suicide attempters. Eur Neuropsychopharmacol. 2006;16 (3):220–223. doi: 10.1016/j.euroneuro.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Diksic M, Young SN. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J Neurochem. 2001;78(6):1185–1200. doi: 10.1046/j.1471-4159.2001.00536.x. [DOI] [PubMed] [Google Scholar]
- 42.Chugani DC, Muzik O. Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20(1):2–9. doi: 10.1097/00004647-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL. A new method to measure brain serotonin synthesis in vivo: I, theory and basic data for a biological model. J Cereb Blood Flow Metab. 1990;10(1):1–12. doi: 10.1038/jcbfm.1990.1. [DOI] [PubMed] [Google Scholar]
- 44.Diksic M, Nagahiro S, Chaly T, Sourkes TL, Yamamoto YL, Feindel W. Serotonin synthesis rate measured in living dog brain by positron emission tomography. J Neurochem. 1991;56(1):153–162. doi: 10.1111/j.1471-4159.1991.tb02575.x. [DOI] [PubMed] [Google Scholar]
- 45.Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94(10):5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chugani DC, Muzik O, Rothermel R, Behen M, Chakraborty P, Mangner T, da Silva EA, Chugani HT. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann Neurol. 1997;42(4):666–669. doi: 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- 47.Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with alpha-[C-11]methyl-L-tryptophan. Synapse. 1998;28(1):33–43. doi: 10.1002/(SICI)1098-2396(199801)28:1<33::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 48.Okazawa H, Leyton M, Benkelfat C, Mzengeza S, Diksic M. Statistical mapping analysis of serotonin synthesis images generated in healthy volunteers using positron-emission tomography and alpha-[11C]methyl-L-tryptophan. J Psychiatry Neurosci. 2000;25(4):359–370. [PMC free article] [PubMed] [Google Scholar]
- 49.Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, Young SN, Blier P, Benkelfat C. Brain Regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. Am J Psychiatry. 2001;158(5):775–782. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- 50.Chugani DC, Niimura K, Chaturvedi S, Muzik O, Fakhouri M, Lee ML, Chugani HT. Increased brain serotonin synthesis in migraine. Neurology. 1999;53 (7):1473–1479. doi: 10.1212/wnl.53.7.1473. [DOI] [PubMed] [Google Scholar]
- 51.Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. Developmental changes in brain serotonin synthesis capacity in autistic and non-autistic children. Ann Neurol. 1999;45(3):287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 52.Pfund Z, Chugani DC, Muzik O, Juhász C, Behen ME, Lee J, Chakraborty P, Mangner T, Chugani HT. Alpha[11C] methyl-L-typtophan positron emission tomography in patients with alternating hemiplegia of childhood. J Child Neurol. 2002;17(4):253–260. doi: 10.1177/088307380201700403. [DOI] [PubMed] [Google Scholar]
- 53.Rosa-Neto P, Diksic M, Okazawa H, Leyton M, Ghadirian N, Mzengeza S, Nakai A, Debonnel G, Blier P, Benkelfat C. Measurement of brain regional alpha-[11C]methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-free patients with major depression. Arch Gen Psychiatry. 2004;61 (6):556–563. doi: 10.1001/archpsyc.61.6.556. [DOI] [PubMed] [Google Scholar]
- 54.Baxter LR, Jr, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, Fair-banks L. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am J Psychiatry. 1988;145(12):1560–1563. doi: 10.1176/ajp.145.12.1560. [DOI] [PubMed] [Google Scholar]
- 55.Nordahl TE, Benkelfat C, Semple WE, Gross M, King AC, Cohen RM. Cerebral glucose metabolic rates in obsessive compulsive disorder. Neuropsychopharmacology. 1989;2(1):23–28. doi: 10.1016/0893-133x(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 56.Sawle GV, Hymas NF, Lees AJ, Frackowiak RS. Obsessional slowness: functional studies with positron emission tomography. Brain. 1991;114(pt 5):2191–2202. doi: 10.1093/brain/114.5.2191. [DOI] [PubMed] [Google Scholar]
- 57.Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, Bellodi L, Smeraldi E, Fazio F. [18F]FDG PET study in obsessive-compulsive disorder. A clinical/metabolic correlation study after treatment. Br J Psychiatry. 1995;166(2):244–250. doi: 10.1192/bjp.166.2.244. [DOI] [PubMed] [Google Scholar]
- 58.Saxena S, Brody AL, Ho ML, Alborzian S, Ho MK, Maidment KM, Huang SC, Wu HM, Au SC, Baxter LR., Jr Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biol Psychiatry. 2001;50(3):159–170. doi: 10.1016/s0006-3223(01)01123-4. [DOI] [PubMed] [Google Scholar]
- 59.Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM. Local cerebral glucose metabolic rates in obsessive-compulsive disorder: patients treated with clomipramine. Arch Gen Psychiatry. 1990;47(9):840–848. doi: 10.1001/archpsyc.1990.01810210048007. [DOI] [PubMed] [Google Scholar]
- 60.Baxter LR., Jr Neuroimaging studies of obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15(4):871–884. [PubMed] [Google Scholar]
- 61.Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL, Rapoport SI, Rapoport JL, Grady CL. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder: revisualization during pharmacotherapy. Arch Gen Psychiatry. 1992;49(9):690–694. doi: 10.1001/archpsyc.1992.01820090018003. [DOI] [PubMed] [Google Scholar]
- 62.Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N, Ho MK, Huang SC, Wu HM, Baxter LR., Jr Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry. 2002;59(3):250–261. doi: 10.1001/archpsyc.59.3.250. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz JM, Stoessel PW, Baxter LR, Jr, Martin KM, Phelps ME. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53(2):109–113. doi: 10.1001/archpsyc.1996.01830020023004. [DOI] [PubMed] [Google Scholar]
- 64.First M, Spitzer R, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID I/P, Version 2.0) New York, NY: Biometrics Research Dept, New York State Psychiatric Institute; 1995. [Google Scholar]
- 65.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale: I, development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 66.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 67.Mzengeza S, Venkatachalam TK, Diksic M. Asymmetric radiosynthesis of alpha-[11C]methyl-L-tryptophan for PET studies. Nucl Med Biol. 1995;22(3):303–307. doi: 10.1016/0969-8051(94)00116-2. [DOI] [PubMed] [Google Scholar]
- 68.Nishizawa S, Leyton M, Okazawa H, Benkelfat C, Mzengeza S, Diksic M. Validation of a less-invasive method for measurement of serotonin synthesis rate with alpha-[11C]methyl-tryptophan. J Cereb Blood Flow Metab. 1998;18 (10):1121–1129. doi: 10.1097/00004647-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Gharib A, Balende C, Sarda N, Weissmann D, Plenevaux A, Luxen A, Bobillier P, Pujol JF. Biochemical and autoradiographic measurements of brain serotonin synthesis rate in the freely moving rat: a reexamination of the alpha-methyl-L-tryptophan method. J Neurochem. 1999;72(6):2593–2600. doi: 10.1046/j.1471-4159.1999.0722593.x. [DOI] [PubMed] [Google Scholar]
- 70.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 71.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 72.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 73.Zijdenbos A, Forghani R, Evans A. Automatic quantification of MS lesions in 3D MRI brain data sets: validation of INSECT. In: Wells WM, Colchester A, Delp S, editors. Proceedings (Lecture Notes in Computer Science); Medical Image Computing and Computer-Assisted Intervention: MIC-CAI’98: First International Conference; Cambridge, MA, USA. October 11–13, 1998; New York, NY: Springer-Verlag; 1998. pp. 439–448. [Google Scholar]
- 74.Collins DL, Zijdenbos AP, Baare WFC, Evans AC. ANIMAL+INSECT: improved cortical structure segmentation. In: Kuba A, Samal M, Todd-Pokropek A, editors. Proceedings (Lecture Notes in Computer Science); Information Processing in Medical Imaging: 16th International Conference, IPMI’99; Visegrad, Hungary. June 28-July 2, 1999; New York, NY: Springer-Verlag; 1999. pp. 210–223. [Google Scholar]
- 75.MacDonnald D. Program for Display and Segmentation of Surfaces and Volumes. Montreal, Quebec: McConell Brain Imaging Centre, Montreal Neurological Institute; 1996. [Google Scholar]
- 76.Lieberman J. Evidence for a biological hypothesis of obsessive-compulsive disorder. Neuropsychobiology. 1984;11(1):14–21. doi: 10.1159/000118043. [DOI] [PubMed] [Google Scholar]
- 77.Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia. 2007;45(4):654–662. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 78.Di Cara B, Samuel D, Salin P, Kerkerian-Le Goff L, Daszuta A. Serotonergic regulation of the GABAergic transmission in the rat basal ganglia. Synapse. 2003;50(2):144–150. doi: 10.1002/syn.10252. [DOI] [PubMed] [Google Scholar]
- 79.Anguiano-Rodríguez PB, Gaytán-Tocavén L, Olvera-Cortés ME. Striatal serotonin depletion facilitates rat egocentric learning via dopamine modulation. Eur J Pharmacol. 2007;556(1–3):91–98. doi: 10.1016/j.ejphar.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 80.Insel TR. Toward a neuroanatomy of obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49(9):739–744. doi: 10.1001/archpsyc.1992.01820090067011. [DOI] [PubMed] [Google Scholar]
- 81.Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, Matarrese M, Carpinelli A, Bellodi L, Fazio F. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42(1):306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- 82.Rueter LE, Tecott LH, Blier P. In vivo electrophysiological examination of 5-HT2 responses in 5-HT2C receptor mutant mice. Naunyn Schmiedebergs Arch Pharmacol. 2000;361(5):484–491. doi: 10.1007/s002109900181. [DOI] [PubMed] [Google Scholar]
- 83.Turner DA, Buhl EH, Hailer NP, Nitsch R. Morphological features of the entorhinal-hippocampal connection. Prog Neurobiol. 1998;55(6):537–562. doi: 10.1016/s0301-0082(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 84.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 85.White NM, Salinas JA. Mnemonic functions of dorsal striatum and hippocampus in aversive conditioning. Behav Brain Res. 2003;142(1–2):99–107. doi: 10.1016/s0166-4328(02)00402-3. [DOI] [PubMed] [Google Scholar]
- 86.Packard MG, Teather LA. Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiol Learn Mem. 1998;69(2):163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 87.Packard MG, Cahill L. Affective modulation of multiple memory systems. Curr Opin Neurobiol. 2001;11(6):752–756. doi: 10.1016/s0959-4388(01)00280-x. [DOI] [PubMed] [Google Scholar]
- 88.Depue RA, Spoont MR. Conceptualizing a serotonin trait: a behavioral dimension of constraint. Ann N Y Acad Sci. 1986;487:47–62. doi: 10.1111/j.1749-6632.1986.tb27885.x. [DOI] [PubMed] [Google Scholar]
- 89.Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Inquiry Into the Functions of the Septo-Hippocampal System (Oxford Psychology Series) New York, NY: Oxford University Press; 2000. [Google Scholar]
- 90.McNaughton N, Wickens J. Hebb, pandemonium and catastrophic hypermnesia: the hippocampus as a suppressor of inappropriate associations. Cortex. 2003;39(4–5):1139–1163. doi: 10.1016/s0010-9452(08)70882-7. [DOI] [PubMed] [Google Scholar]
- 91.Radomsky AS, Rachman S, Hammond D. Memory bias, confidence and responsibility in compulsive checking. Behav Res Ther. 2001;39(7):813–822. doi: 10.1016/s0005-7967(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 92.van den Hout M, Kindt M. Obsessive-compulsive disorder and the paradoxical effects of perseverative behaviour on experienced uncertainty. J Behav Ther Exp Psychiatry. 2004;35(2):165–181. doi: 10.1016/j.jbtep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Goto Y, Grace AA. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex. 2008;18(6):1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hollander E, editor. Obsessive-Compulsive Related Disorders. Washington, DC: American Psychiatric Publishing, Inc; 1993. [Google Scholar]
- 96.Shoaf SE, Carson RE, Hommer D, Williams WA, Higley JD, Schmall B, Herscovitch P, Eckelman WC, Linnoila M. The suitability of [11C]-alpha-methyl-L-tryptophan as a tracer for serotonin synthesis: studies with dual administration of [11C] and [14C] labeled tracer. J Cereb Blood Flow Metab. 2000;20(2):244–252. doi: 10.1097/00004647-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 97.Diksic M, Tohyama Y, Takada A. Brain net unidirectional uptake of alpha-[14c]methyl-L-tryptophan (alpha-MTrp) and its correlation with regional serotonin synthesis, tryptophan incorporation into proteins, and permeability surface area products of tryptophan and alpha-MTrp. Neurochem Res. 2000;25(12):1537–1546. doi: 10.1023/a:1026654116999. [DOI] [PubMed] [Google Scholar]
- 98.Nishikawa M, Kumakura Y, Young SN, Fiset P, Vogelzangs N, Leyton M, Benkelfat C, Diksic M. Increasing blood oxygen increases an index of 5-HT synthesis in human brain as measured using alpha-[(11)C]methyl-L-tryptophan and positron emission tomography. Neurochem Int. 2005;47(8):556–564. doi: 10.1016/j.neuint.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Tohyama Y, Takahashi S, Merid MF, Watanabe A, Diksic M. The inhibition of tryptophan hydroxylase, not protein synthesis, reduces the brain trapping of alpha-methyl-L-tryptophan: an autoradiographic study. Neurochem Int. 2002;40(7):603–610. doi: 10.1016/s0197-0186(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 100.Hasegawa S, Kanemaru K, Gittos M, Diksic M. The tryptophan hydroxylase activation inhibitor, AGN-2979, decreases regional 5-HT synthesis in the rat brain measured with alpha-[14C]methyl-L-tryptophan: an autoradiographic study. Brain Res Bull. 2005;67(3):248–255. doi: 10.1016/j.brainresbull.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 101.Muzik O, Chugani DC, Chakraborty P, Mangner T, Chugani HT. Analysis of [C-11]alpha-methyl-tryptophan kinetics for the estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab. 1997;17(6):659–669. doi: 10.1097/00004647-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Chugani DC, Chugani HT. PET: mapping of serotonin synthesis. Adv Neurol. 2000;83:165–171. [PubMed] [Google Scholar]
- 103.Leyton M, Diksic M, Benkelfat C. Brain regional alpha-[11C]methyl-L-tryptophan trapping correlates with post-mortem tissue serotonin content and [11C]5-hydroxytryptophan accumulation. Int J Neuropsychopharmacol. 2005;8(4):633–634. doi: 10.1017/S1461145705005420. [DOI] [PubMed] [Google Scholar]
- 104.Lundquist P, Hartvig P, Blomquist G, Hammarlund-Udenaes M, Långström B. 5-Hydroxy-L-[beta-11C]tryptophan versus alpha-[11C]methyl-L-tryptophan for positron emission tomography imaging of serotonin synthesis capacity in the rhesus monkey brain. J Cereb Blood Flow Metab. 2007;27(4):821–830. doi: 10.1038/sj.jcbfm.9600381. [DOI] [PubMed] [Google Scholar]
- 105.Booij L, Van der Does W, Benkelfat C, Bremner JD, Cowen PJ, Fava M, Gillin C, Leyton M, Moore P, Smith KA, Van der Kloot WA. Predictors of mood response to acute tryptophan depletion: a reanalysis. Neuropsychopharmacology. 2002;27(5):852–861. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- 106.Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action: reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47(5):411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 107.Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46(3):243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 108.Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. J Psychiatr Res. 1997;31(4):393–432. doi: 10.1016/s0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- 109.Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(2):228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- 110.Bloch MH, Landeros-Weisenberger A, Rosario MC, Pittenger C, Leckman JF. Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am J Psychiatry. 2008;165(12):1532–1542. doi: 10.1176/appi.ajp.2008.08020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 112.Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, Bolwig TG, Knudsen GM. Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol. 2005;8(3):391–401. doi: 10.1017/S1461145705005055. [DOI] [PubMed] [Google Scholar]
- 113.Heyes MP, Saito K, Major EO, Milstien S, Markey SP, Vickers JH. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease: attenuation of synthesis from L-tryptophan by 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain. 1993;116(pt 6):1425–1450. doi: 10.1093/brain/116.6.1425. [DOI] [PubMed] [Google Scholar]
- 114.Juhász C, Chugani DC, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT. Alpha-methyl-L-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology. 2003;60(6):960–968. doi: 10.1212/01.wnl.0000049468.05050.f2. [DOI] [PubMed] [Google Scholar]
- 115.Juhász C, Chugani DC, Muzik O, Wu D, Sloan AE, Barger G, Watson C, Shah AK, Sood S, Ergun EL, Mangner TJ, Chakraborty PK, Kupsky WJ, Chugani HT. In vivo uptake and metabolism of alpha-[11C]methyl-L-tryptophan in human brain tumors. J Cereb Blood Flow Metab. 2006;26(3):345–357. doi: 10.1038/sj.jcbfm.9600199. [DOI] [PubMed] [Google Scholar]
- 116.Snider LA, Swedo SE. PANDAS: current status and directions for research. Mol Psychiatry. 2004;9(10):900–907. doi: 10.1038/sj.mp.4001542. [DOI] [PubMed] [Google Scholar]
- 117.Saporta AS, Chugani HT, Juhász C, Makki MI, Muzik O, Wilson BJ, Behen ME. Multimodality neuroimaging in Tourette syndrome: alpha-[11C] methyl-L-tryptophan positron emission tomography and diffusion tensor imaging studies. J Child Neurol. 2010;25(3):336–342. doi: 10.1177/0883073809339394. [DOI] [PubMed] [Google Scholar]
- 118.Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, Chang KH, Kwon JS. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- 119.Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61(7):720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 120.Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, Koreen A, Cole K, Bogerts B. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52(5):393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- 121.Aylward EH, Harris GJ, Hoehn-Saric R, Barta PE, Machlin SR, Pearlson GD. Normal caudate nucleus in obsessive-compulsive disorder assessed by quantitative neuroimaging. Arch Gen Psychiatry. 1996;53(7):577–584. doi: 10.1001/archpsyc.1996.01830070021006. [DOI] [PubMed] [Google Scholar]
- 122.Rotge JY, Langbour N, Guehl D, Bioulac B, Jaafari N, Allard M, Aouizerate B, Burbaud P. Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology. 2010;35(3):686–691. doi: 10.1038/npp.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65(1):75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 124.Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67(7):701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]