Abstract
New Delhi metallo-β-lactamase-1 (NDM-1), an acquired class B carbapenemase, is a significant clinical threat due to its extended hydrolysis of β-lactams including carbapenems. In this study, we identified the first confirmed clinical isolate of Escherichia coli BJ01 harboring bla NDM-1 in China. The isolate is highly resistant to all tested antimicrobials except polymyxin. bla NDM-1, bla CTX-M-57, and bla TEM-1 were identified in the isolate. bla NDM-1 was transferable to E. coli EC600 and DH5α in both plasmid conjugation experiments and plasmid transformation tests. BJ01 was identified as a new sequence type, ST224, by multilocus sequence typing. Analysis of genetic environment shows complex transposon-like structures surrounding the bla NDM-1 gene. Genetic analysis revealed that the region flanking bla NDM-1 was very similar to previously identified NDM-positive Acinetobacter spp. isolated in China. The findings of this study raise attention to the emergence and spread of NDM-1-carrying Enterobacteriaceae in China.
Introduction
Antimicrobial resistance is a growing global challenge to human health[1]. The emerging New Delhi metallo-β-lactamase (NDM-1), an acquired class B carbapenemase that was first clinically detected in a patient at a hospital in New Delhi, India, has brought up worldwide public attention again[2]–[4]. It confers resistance to a broad-spectrum of β-lactams, including carbapenems, which are the mainstream treatment for antibiotic-resistant bacterial infections[5]. Although current reports indicate that NDM-1 does not hydrolyze monobactams, most of NDM-1-carrying strains also express enzymes that could hydrolyze monobactams, making NDM-1-producers very difficult to control[6]. The rapid dissemination of NDM-1-producing gram-negative species also contributes to this major concern of public health. It has been reported in >50 countries across five continents, in the last 2 years[2], [4].
NDM-1 is detected mainly in Escherichia coli and Klebsiella pneumonia but occasionally in Klebsiella oxytoca, Citrobacter freundii, Morganella morganii, Providencia spp., Enterobacter cloacae, Proteus spp., Acinetobacter baumannii, Stenotrophomonas maltophilia and Pseudomonas aeruginosa [3], [7], [8]. To our knowledge, NDM-1-producing strains have been seen only in Acinetobacter spp. [9]–[12] and Enterococcus faecium[13] in China. There is no evidence of the emergence of NDM-1-producing Enterobacteriaceae in China at this point, although it was found in the stool samples of a 1-year-old infant and his mother in Hong Kong who once traveled to and were hospitalized in Hunan Province, China[14]. In the present study, we report the first confirmed case of NDM-1-producing E. coli infection in Beijing, China.
Materials and Methods
Patient Information
A 75-year-old Chinese patient with diabetes, who came from the Anhui province in southern China, was admitted for diabetes-related foot complications comprising a swollen peak of the left foot and ulceration of the fourth and fifth toes on September, 2011. The NDM-1-producing E.coli, BJ01, strain was isolated from the ulcer secretion. Since the strain was found to be resistant to almost all tested antibiotics, a combination of intravenous levofloxacin and etimicin was given empirically. Amputation of the fifth metatarsal bones and an incisional drainage operation were performed. The wound started healing nicely. The patient was discharged from the hospital one month after admission. Neither the patient nor the patient's family members had a history of traveling to the Indian subcontinent or other countries. The patient in this manuscript has given written informed consent, as outlined in the PLOS consent form to publication of their case details.
Bacterial Isolate and Phenotypic Screening for MBL
The clinical isolate BJ01 of the metallo-β-lactamase(MBL)-producing E. coli was derived from the ulcer secretion of the patient collected on the admission day. The strain with same phenotype was still positive on day 5 after the operation. The identification and drug susceptibility of the bacteria were tested by using Vitek 2 Compact (bioMerieux, Marcy I''Etoile, France). Minimal inhibitory concentrations (MICs) of 18 antibiotics and combinations of antimicrobials were determined by broth microdilution method in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI)[15]. Most of antimicrobial agents were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP), except for imipenem (Merck Sharp & Dohme, Whitehouse Station, USA), meropenem (Dainippon Sumitomo Pharma, Chuo-ku, Japan) and polymyxin (Sigma, St. Louis, USA). E.coli strain ATCC25922 was used as a quality control for MIC determination. MBL production was tested by comparing diameters of the zones of inhibition for meropenem (10 µg) and imipenem (10 µg) on Mueller-Hinton agar (MHA) versus MHA impregnated with EDTA, an MBL inhibitor. EDTA-impregnated MHA was prepared by spreading 2 mL of 5 mM EDTA on MHA [16].
Polymerase Chain Reaction (PCR) Amplification for the Detection of MBL and other β-lactamase genes
The primers used (NDM-F1, 5′-GGCGGAATGGCTCATCACGA-3′; NDM-R1, 5′-CGCAACACAGGCCTGACTTTC-3′) were those recommended by the Chinese Center for Disease Control and Prevention. For the specific detection of bla NDM-1, primers NDM-F2 (5′-ATGGAATTGCCCAATATTATGC-3′) and NDM-R2 (5′-TCAGCGCAGCTTGTCGGCCAT-3′) were used to amplify the entire blaNDM-1 gene. At that time, we performed molecular testing for other MBL, including bla VIM, bla IMP, bla GIM, bla SPM, and bla SIM [17], [18]. PCR was also undertaken for other β-lactamase genes, including bla TEM, bla SHV, bla CTX-M, and bla OXA-1 [19]. bla CTX-M was further identified using the specific primers for the CTX-M group 1 (CTX-M-1grpF, 5′-CCAGAATAAGGAATCCCATG-3′; CTX-M-1grpR, 5′-GCCGTCTAAGGCGATAAAC-3′), CTX-M group 2 (CTX-M-2grpF, 5′-ATGATGACTCAGAGCATTCG-3′; CTX-M-2grpR, 5′-TGGGTTACGATTTTCGCC-3′), and the CTX-M group 9 (CTX-M-9grpF, 5′- ATGGTGACAAAGAGAGTGCA-3′; CTX-M-9grpR, 5′-CCCTTCGGCGATGATTCTC-3′). All positive PCR products were sequenced and the sequencing results were compared with previously reported sequences available in GenBank.
Southern Blot
Southern blot was performed by using DIG High Prime DNA Labeling and Detection Starter Kit II (F. Hoffmann-La Roche Ltd., Basel, Swiss) following manufacturer's protocol. Briefly, genomic DNA was digested with restriction enzyme HindIII and BamHI. The digested DNA fragments were transferred to nylon membrane (Pharmacia & Biotech, Piscataway, USA), and hybridized with digoxigenin-labeled bla NDM-1-specific probes and anti-DIG-AP antibody. The membrane was visualized by applying chemiluminescent substrate CSPD and X-ray exposure.
Conjugation and Transformation
The transfer of carbapenem resistance was tested using a conjugation test (broth mating method). E. coli EC600 (LacZ− Nalr Rifr) was used as the recipient strain. Overnight cultures of the donor strain (300 µL) and recipient strain (100 µL) were mixed with 600 µL of fresh Mueller-Hinton broth and were incubated overnight at 35°C. The mixture was then inoculated on MHA plates containing rifampin (Sigma, St. Louis, USA, 100 µg/mL) plus ceftriaxone (F. Hoffmann-La Roche Ltd., Basel, Swiss, 4 µg/mL) for 24 h at 35°C. The bacterial colonies were then transferred to a plate containing rifampin (Sigma, St. Louis, USA, 100 µg/mL) plus imipenem (Merck Sharp & Dohme, Whitehouse Station, USA, 4 µg/mL) for 24 h at 35°C by replica plating, which maintains the original colony pattern.
Plasmid DNA was extracted using a Qiagen Mini Kit (Qiagen, Hilden, Germany) and was transformed into E. coli DH5α-competent cells (TAKARA, Shanghai, China). Transformants were selected on MHA plates containing IPM (4 µg/mL).
The identification and susceptibility of the transconjugant and transformant were confirmed via using the VITEK 2 system. PCR analysis was used to determine the presence of carbapenemase genes.
Multilocus Sequence Typing (MLST) and Sequencing of Genetic Environment
MLST with seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) was performed according to the protocol described on the E. coli MLST website (http://mlst.ucc.ie/mlst/dbs/Ecoli). The regions flanking the bla NDM-1 gene were sequenced by primer walking strategy, starting from each end of the bla NDM-1 gene in the DH5αNDM-1 transformant. The plasmid containing bla NDM-1 gene was used as a template for primer walking. The first set of primers was designed targeting each end of the bla NDM-1 sequence (5′-GGTCGCCAGTTTCCATTTGC-3′ and 5′-TGCCGACACTGAGCACTAC-3′). The amplification products were sequenced. Then new primers complementary to the known sequence were designed and synthesized for sequencing the unknown DNA sequence next to it. Totally, 13 pairs of primers were synthesized. Sequence files were assembled and aligned by using ContigExpress software.
Results
Antimicrobial Susceptibility of E. coli BJ01 Strain
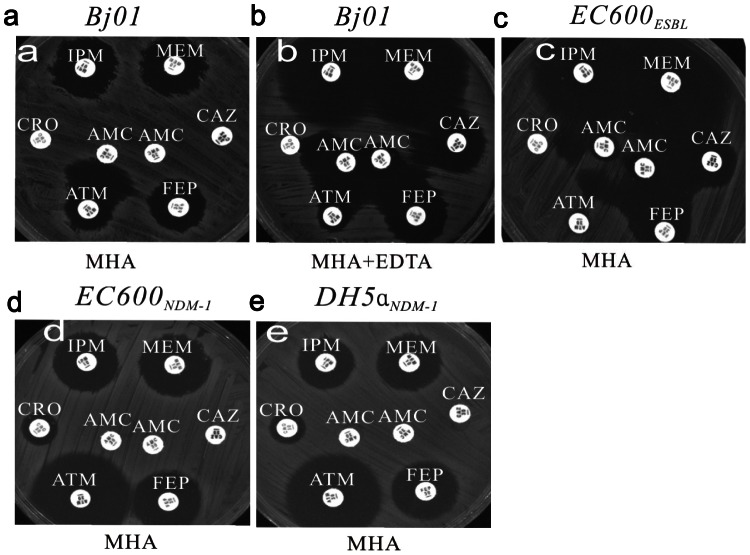
E. coli BJ01 isolated from the patient displayed high resistance to all tested antibiotics, including ampicillin, ampicillin-sulbactam, cefazolin, aztreonam, ceftriaxone, cefepime, imipenem, meropenem, ceftazidime, piperacillin-tazobactam, cefotetan, tobramycin, gentamicin, ciprofloxacin, levofloxacin, amikacin and trimethoprim-sulfamethoxazole, except polymyxin, which showed bacteriostatic activity to BJ01 (MIC 1 µg/ml) ( Table 1 , Fig. 1 ). On MHA, amoxicillin-clavulanic acid showed no synergy with cephalosporins except for aztreonam since metalloenzymes do not hydrolyze aztreonam ( Fig. 1a ). On MHA containing EDTA, metalloenzyme activity was inhibited, the diameters of the inhibition zones for MEM (meropenem) and IPM (imipenem) were both 18 mm greater than on MHA without EDTA, and synergistic function was observed between amoxicillin-clavulanic acid and ceftazidime, ceftriaxone, aztreonam and cefepime ( Fig. 1b ). Above findings are suggestive of ESBL (extended-spectrum β-lactamase) production in E. coli BJ01 [20].
Table 1. Antibiotic susceptibility profiles of different strains (MIC (µg/mL)).
| MIC(µg/mL) | ||||||
| BJ01 | EC600 recipient | EC600ESBLs conjugant | EC600 NDM-1 conjugant | DH5α recipient | DH5αNDM-1 transformant | |
| Ampicilin | >256 | 2 | >256 | >256 | 2 | >256 |
| Ampicillin-sulbactam | >256 | 2 | >256 | >256 | 2 | >256 |
| Cefazolin | >256 | 2 | >256 | >256 | 1 | >256 |
| Aztreonam | 256 | 0.25 | 256 | 0.50 | 0.50 | 0.50 |
| Ceftriaxone | >256 | 0.25 | 256 | 256 | 0.25 | 256 |
| Cefepime | 256 | ≤0.125 | 128 | 128 | ≤0.125 | 128 |
| Imipenem | 32 | ≤0.125 | ≤0.125 | 32 | ≤0.125 | 32 |
| Meropenem | 32 | ≤0.125 | ≤0.125 | 16 | ≤0.125 | 32 |
| Ceftazidime | >256 | 0.25 | 16 | >256 | 0.25 | >256 |
| Piperacillin-tazobactam | 256 | 1 | 1 | 128 | 1 | 128 |
| Cefotetan | 256 | 0.5 | 0.5 | 128 | 1 | 128 |
| Tobramycin | 256 | 0.25 | 128 | 0.5 | 0.25 | 0.5 |
| Gentamicin | 256 | 0.25 | 128 | 0.25 | 0.25 | 0.25 |
| Ciprofloxacin | 32 | 0.25 | 1 | 0.25 | 0.25 | 0.25 |
| Levofloxacin | 16 | 1 | 1 | 0.5 | 0.25 | 0.25 |
| Amikacin | >256 | 0.25 | 256 | 0.50 | 0.50 | 0.50 |
| Trimethoprim-sulfamethoxazole | >32/608 | 0.5/9.5 | 0.5/9.5 | 0.5/9.5 | 0.5/9.5 | 0.5/9.5 |
| Polymyxin | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
MIC, minimum inhibitory concentration; ESBL, extended-spectrum β-lactamase; NDM, New Delhi metallo-β-lactamase-1.
Figure 1. Phenotypic detection of carbapenemases and extended-spectrum β-lactamase (ESBL) in E. coli BJ01.
a. Antibacterial susceptibility testing for BJ01 on the Mueller-Hinton agar (MHA). b. Antimicrobial susceptibility testing for BJ01 on MHA impregnated with 2 mL of ethylenediaminetetraacetic acid (EDTA) 5 mM. c. Antimicrobial susceptibility testing for the EC600 transconjugant (EC600ESBLs). d. Antimicrobial susceptibility testing for the EC600 transconjugant (EC600NDM-1). EC600NDM-1 was resistant to imipenem, meropenem, and cephalosporins except for ATM and showed no synergy between AMC and cephalosporins. e. Antimicrobial susceptibility testing for the DH5α transformant (DH5αNDM-1). The phenotype was the same as that of EC600NDM-1. IMP, imipenem; MEM, meropenem; CRO, ceftriaxone; AMC,amoxicillin-clavulanic acid; CAZ, ceftazidime; ATM, aztreonam; FEP, cefepime.
Transfer of Antibiotic Resistance
In the conjugation experiments, two resistant phenotypes of E. coli EC600 transconjugants (EC600ESBL and EC600NDM-1) were found by using replica plating methods, presenting with different drug resistant patterns ( Table 1 ). The IMP (imipenem) and MEM (meropenem) sensitivity of EC600ESBL and the synergy between AMC (amoxicillin-clavulanic acid) and FEP (cefepime), CAZ (ceftazidime), CRO (ceftriaxone), ATM (aztreonam) on the strain suggested ESBL production ( Fig. 1c ). EC600NDM-1 grew on both MHA containing rifampin plus imipenem and MHA containing rifampin plus ceftriaxone. While, EC600ESBL grew only on MHA containing rifampin plus ceftriaxone. This indicates that the carbapenemase gene and ESBL gene may be on different plasmids and therefore transferred independently. EC600ESBL had the same resistance phenotype as BJ01 on MHA containing EDTA because the carbapenemase in BJ01 was inhibited on MHA impregnated with EDTA. ( Fig. 1c ). The EC600NDM-1 transconjugant was resistant to carbapenems and cephalosporins but sensitive to aminoglycosides, fluoroquinolones, and aztreonam, which indicated that plasmid harboring bla NDM-1 did not carry other ESBL, aminoglycoside-resistance, or quinolone-resistance genes ( Fig. 1d ). The prepared plasmid was transformed into E. coli DH5α. Antimicrobial susceptibility profiles of the DH5αNDM-1 transformant exhibited antibiotic sensitivities similar to the EC600NDM-1 isolate ( Fig. 1e ; Table 1 ).
Detection of Drug Resistance Genes
By PCR and sequencing, we detected the presence of MBL genes in BJ01 strain. The bla NDM-1, bla TEM-1 and bla CTX-M-57 genes detected ( Fig. 2 ), but not any other MBL genes (bla VIM, bla IMP, bla GIM, bla SPM, and bla SIM) or β-lactamase genes (bla SHV and bla OXA-1). The presence of bla NDM-1 gene was further confirmed by Southern blot ( Fig. 3 ). CTX-M-β-lactamases can be divided into five groups based on their amino acid sequence identities. Group I includes CTX-M-1, -3, -10 to -12, -15, -22, -23, -28, -29, etc. CTX-M-57 is a group I CTX-M and shared 99% amino acid identity with CTX-M-15[21], [22], which is one of the most common types of ESBL found in bacterial isolates[23]. The sequences of the bla NDM-1, bla TEM, and bla CTX-M-57 genes were analyzed and deposited in GenBank under accession numbers HQ603057, JX036279, and JX036278. Only bla TEM-1 and bla CTX-M-57 were detected in the EC600ESBL transconjugant and only bla NDM-1 was detected in the EC600NDM-1 transconjugant and DH5αNDM-1 transformant, which indicated that bla TEM-1 and bla CTX-M-57 were located on a different plasmid from bla NDM-1 ( Fig. 2 ).
Figure 2.
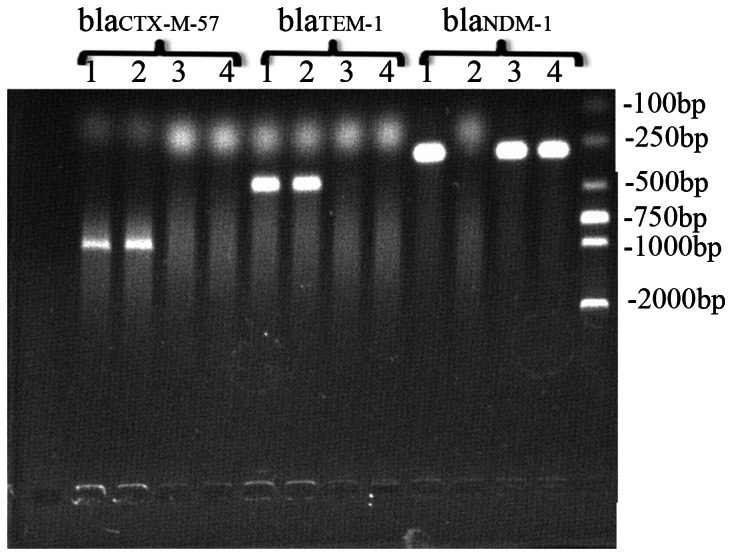
Polymerase chain reaction-amplified CTX-M-57, TEM-1, and NDM-1 genes in BJ01 (1), EC600ESBL (2), EC600NDM-1 (3), and DH5αNDM-1 (4). CTX-M-57 and TEM-1 were detected in BJ01 and EC600ESBL; blaNDM-1 was detected in BJ01, E600NDM-1, and DH5αNDM-1.
Figure 3. Southern blot hybridization on bla NDM-1 gene of BJ01.

The band marked with the white arrow indicated positive signals by Southern blot hybridization with the specific NDM-1 probe.
MLST genotype analysis
MLST revealed that E. coli BJ01 belonged to a new sequence type 224 (ST224).
Genetic Environment of the bla NDM-1 gene
A 17,924-bp fragment was obtained from plasmid DNA of strain E. coli BJ01, and sequenced by primer walking. The sequence has been deposited in GenBank with accession No. JX296013. Sequencing of the bla NDM-1 upstream region identified the presence of an ISAba125 insertion sequence. Further sequence analysis revealed that the region flanking the ISAba125 was the IS3000 gene. The trpF gene flanked the 3′ end of bla NDM-1, followed by dsbC, truncated cut1 gene disrupted by IS26, umuD and a transposase gene. ( Fig. 4 )
Figure 4. Analysis of the bla NDM-1 gene environment.
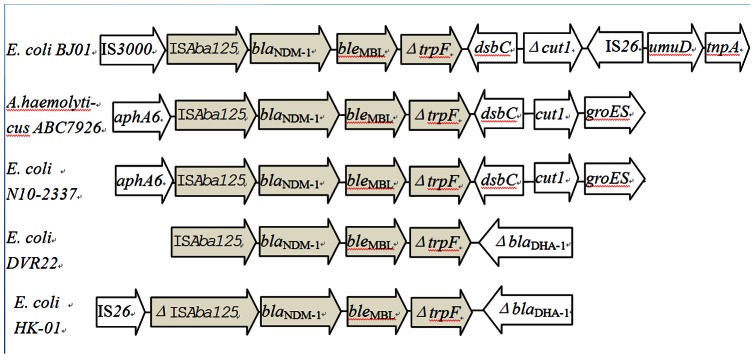
Schematic drawing comparing the genetic elements surrounding the bla NDM-1 gene in Escherichia coli BJ01, Acinetobacter haemolyticus ABC7926(GenBank JQ080305), E. coli N10-2337(GenBank JF714412), E. coli HK-01(GenBank NC_019063), and E. coli DVR22(GenBank JF922606). Δ, truncated gene. The arrows indicate the orientation of each open reading frame. Similarity regions of the flanking region of NDM-1 gene between BJ01 and other NDM-1-carrying isolates were labeled in grey color.
Discussion
In contrast to most other countries where NDM has been mainly identified in Enterobacteriaceae, in China to date NDM has only been reported in Acinetobacter [9], [11], [12], [24] and Enterococcus faecium [13]. However, other types of MBLs, such as IMP, and other classes of acquired carbapenemases, such a KPC, have been reported in Enterobacteriaceae in China[25]–[29], but isolates of NDM-1-producing E.coli have not previously been identified in China.
NDM-1-harboring strains could be highly multidrug resistant[3]. Previous reports on NDM-1-producing Acinetobacter spp. have demonstrated that strains resistant to all available antimicrobials except colistin were common in China[10], [30]. Our results showed resistant to almost all tested antimicrobials of the identified NDM-1-producing E.coli isolate. The patient had a good general condition and the infection was localized. Ultimately, the patient survived the infection. But this case should have raised public concerns again over increasing incidence of highly multidrug resistant NDM-1-harboring strains in China.
Travelers contribute significantly to the global movement of microbes and resistance genes [31]. Although nosocomial transmission of NDM-1 has occurred in many countries[26], [32], [33], medical tourism plays a significant role in the spread of NDM-1[34], and traveling to the Indian subcontinent is a significant risk factor of infection with an NDM-1-producing bacterium[35]. Patients in China who were found to have bla NDM-1-carrying bacteria had no history of traveling to the Indian subcontinent or another country[9], [11], [13], [24]. In this study, the patient carrying the bla NDM-1-positive E. coli strain had no foreign travel history.
The identified BJ01 strain demonstrated phenotypes of both ESBL and NDM-1 ( Table 1 ). This is consistent to previous reports on NDM-1-producers. For example, a study from China indicates that all 4 identified NDM-1-carrying A. baumannii isolates expressed both genes[30]. Data from the SMART study shows that bla NDM-1 positive enterobacteriaceae isolates often carry additional β-lactamase genes. Among 33 bla NDM-1-carrying strains, bla CTX-M-15 is detected in 30[36]. However, our data also suggested that ESBL and NDM-1 genes may be carried by distinct plasmids and could be transferred separately ( Fig. 1 ). This has to be further confirmed by sequence analysis.
In our study, MLST analysis revealed that BJ01 belonged to sequence type 224 (ST224), which was different from the NDM-1-producing ST101 E. coli isolated from Australia [37], Germany [38], Canada[39], the UK [40], and New Zealand [41]. This is the first reported ST224 E.coli strain producing NDM-1.
Analysis of the genetic environment of bla NDM-1 in BJ01 reveals that the region flanking bla NDM-1 is very similar to some Acinetobacter spp. isolated in China [10], [11], [24] and E. coli plasmid pNDM102337 (GenBank: JF714412) isolated in Canada, in which DsbC and cutI are located downstream of the bla NDM-1. cutI is truncated by a transposon-like structure. In contrast with E. coli HK-01 (GenBank: HQ451074) and E. coli DVR22 (GenBank: JF922606.1), there was no bla DHA downstream of bla NDM-1 in BJ01( Fig. 4 ). This finding indicates a potential link between Acinetobacter spp. and Enterobacteriaceae in term of the bla NDM-1 gene in China. More complex transposon-like structures are detected around bla NDM-1. IS3000 and ISAba125 are located upstream of the bla NDM-1, while IS26 and a transposase gene are located downstream of the bla NDM-1. Transposons should play an important role in horizontal transfer of the bla NDM-1 between different species of bacteria, such as from Acinetobacter spp. to E. coli.
In conclusion, in mainland China, this is the first reported case of infection due to a NDM-1-producing E. coli, which is highly drug-resistant and comes with a new MLST genotype in E. coli. The patient carrying this strain does not have a travel history The detection of the NDM-1-positive E. coli isolate in our study indicates immediate importance of strengthening surveillance to prevent the nosocomial infection and dissemination of NDM-1 in China.
Funding Statement
This work was supported by Internal Funding of Guang'anmen Hospital (Grant# 2011S242) and Beijing You'an Project for Liver diseases and AIDS (Grant# JYAN-2011-064). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10: S122–129. [DOI] [PubMed] [Google Scholar]
- 2. Bonomo RA (2011) New Delhi metallo-beta-lactamase and multidrug resistance: a global SOS? Clin Infect Dis 52: 485–487. [DOI] [PubMed] [Google Scholar]
- 3. Moellering RC Jr (2010) NDM-1–a cause for worldwide concern. N Engl J Med 363: 2377–2379. [DOI] [PubMed] [Google Scholar]
- 4. Rolain JM, Parola P, Cornaglia G (2010) New Delhi metallo-beta-lactamase (NDM-1): towards a new pandemia? Clin Microbiol Infect 16: 1699–1701. [DOI] [PubMed] [Google Scholar]
- 5. Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, et al. (2009) Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53: 5046–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shakil S, Azhar EI, Tabrez S, Kamal MA, Jabir NR, et al. (2011) New Delhi metallo-beta-lactamase (NDM-1): an update. J Chemother 23: 263–265. [DOI] [PubMed] [Google Scholar]
- 7. Wilson ME, Chen LH (2012) NDM-1 and the Role of Travel in Its Dissemination. Curr Infect Dis Rep 14: 213–226. [DOI] [PubMed] [Google Scholar]
- 8. Jovcic B, Lepsanovic Z, Suljagic V, Rackov G, Begovic J, et al. (2011) Emergence of NDM-1 metallo-beta-lactamase in Pseudomonas aeruginosa clinical isolates from Serbia. Antimicrob Agents Chemother 55: 3929–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Cui Y, Pu F, Jiang G, Zhao X, et al. (2012) Draft genome sequence of an Acinetobacter genomic species 3 strain harboring a bla(NDM-1) gene. J Bacteriol 194: 204–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu H, Hu Y, Pan Y, Liang H, Wang H, et al. (2012) Novel plasmid and its variant harboring both a bla(NDM-1) gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob Agents Chemother 56: 1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou Z, Guan R, Yang Y, Chen L, Fu J, et al. (2012) Identification of New Delhi metallo-beta-lactamase gene (NDM-1) from a clinical isolate of Acinetobacter junii in China. Can J Microbiol 58: 112–115. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Wu C, Zhang Q, Qi J, Liu H, et al. (2012) Identification of New Delhi metallo-beta-lactamase 1 in Acinetobacter lwoffii of food animal origin. PLoS One 7: e37152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X, Liu W, Zou D, Li X, Wei X, et al. (2013) High rate of New Delhi metallo-beta-lactamase 1-producing bacterial infection in China. Clin Infect Dis 56: 161–162. [DOI] [PubMed] [Google Scholar]
- 14.Ho PL, Li Z, Lai EL, Chiu SS, Cheng VC (2012) Emergence of NDM-1-producing Enterobacteriaceae in China. J Antimicrob Chemother 2012 Mar 21. doi:10.1093/jac/dks095.Epub ahead of print. [DOI] [PubMed]
- 15.CaLSI (CLSI) (2012) Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. CLSI document M100–S22.
- 16. Birgy A, Bidet P, Genel N, Doit C, Decre D, et al. (2012) Phenotypic screening of carbapenemases and associated beta-lactamases in carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 50: 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shibata N, Doi Y, Yamane K, Yagi T, Kurokawa H, et al. (2003) PCR typing of genetic determinants for metallo-beta-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J Clin Microbiol 41: 5407–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Queenan AM, Bush K (2007) Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 20: : 440–458, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fang H, Ataker F, Hedin G, Dornbusch K (2008) Molecular epidemiology of extended-spectrum beta-lactamases among Escherichia coli isolates collected in a Swedish hospital and its associated health care facilities from 2001 to 2006. J Clin Microbiol 46: 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NCfCLS (NCCLS) (1999) Performance standards for antimicrobial susceptibility testing. NCCLS approved standard M100–S9.
- 21. Hopkins TA, Boyd DA, Xia Y, Shifflett GM, Hess FM, et al. (2008) Infrared spectroscopic study of C(2)F(6) monolayers and bilayers on graphite. J Chem Phys 128: 154714. [DOI] [PubMed] [Google Scholar]
- 22. Bonnet R (2004) Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother 48: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sowmiya M, Malathi J, Madhavan HN (2012) Screening of ocular enterobacteriaceae isolates for presence of chromosomal blaNDM-1 and ESBL genes: a 2-year study at a tertiary eye care center. Invest Ophthalmol Vis Sci 53: 5251–5257. [DOI] [PubMed] [Google Scholar]
- 24.Fu Y, Du X, Ji J, Chen Y, Jiang Y, et al.. (2012) Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J Antimicrob Chemother. [DOI] [PubMed]
- 25. Chen S, Hu F, Liu Y, Zhu D, Wang H, et al. (2011) Detection and spread of carbapenem-resistant Citrobacter freundii in a teaching hospital in China. Am J Infect Control 39: e55–60. [DOI] [PubMed] [Google Scholar]
- 26. Espinal P, Fugazza G, Lopez Y, Kasma M, Lerman Y, et al. (2011) Dissemination of an NDM-2-producing Acinetobacter baumannii clone in an Israeli rehabilitation center. Antimicrob Agents Chemother 55: 5396–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi DS, Wang WP, Kuai SG, Shao HF, Huang M (2012) Identification of bla (KPC-2) on different plasmids of three Morganella morganii isolates. Eur J Clin Microbiol Infect Dis 31: 797–803. [DOI] [PubMed] [Google Scholar]
- 28. Zhang R, Wang XD, Cai JC, Zhou HW, Lv HX, et al. (2011) Outbreak of Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae with high qnr prevalence in a Chinese hospital. J Med Microbiol 60: 977–982. [DOI] [PubMed] [Google Scholar]
- 29. Hu F, Chen S, Xu X, Guo Y, Liu Y, et al. (2012) Emergence of carbapenem-resistant clinical Enterobacteriaceae isolates from a teaching hospital in Shanghai, China. J Med Microbiol 61: 132–136. [DOI] [PubMed] [Google Scholar]
- 30. Chen Y, Zhou Z, Jiang Y, Yu Y (2011) Emergence of NDM-1-producing Acinetobacter baumannii in China. J Antimicrob Chemother 66: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 31. O′Brien TF, Pla MP, Mayer KH, Kishi H, Gilleece E, et al. (1985) Intercontinental spread of a new antibiotic resistance gene on an epidemic plasmid. Science 230: 87–88. [DOI] [PubMed] [Google Scholar]
- 32. Gaibani P, Ambretti S, Berlingeri A, Cordovana M, Farruggia P, et al. (2011) Outbreak of NDM-1-producing Enterobacteriaceae in northern Italy, July to August 2011. Euro Surveill 16: 20027. [PubMed] [Google Scholar]
- 33. D′Andrea MM, Venturelli C, Giani T, Arena F, Conte V, et al. (2011) Persistent carriage and infection by multidrug-resistant Escherichia coli ST405 producing NDM-1 carbapenemase: report on the first Italian cases. J Clin Microbiol 49: 2755–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Struelens MJ, Monnet DL, Magiorakos AP, Santos O′Connor F, Giesecke J (2010) New Delhi metallo-beta-lactamase 1-producing Enterobacteriaceae: emergence and response in Europe. Euro Surveill 15.. [DOI] [PubMed] [Google Scholar]
- 35. Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, et al. (2010) Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10: 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lascols C, Hackel M, Marshall SH, Hujer AM, Bouchillon S, et al. (2011) Increasing prevalence and dissemination of NDM-1 metallo-beta-lactamase in India: data from the SMART study (2009). J Antimicrob Chemother 66: 1992–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poirel L, Lagrutta E, Taylor P, Pham J, Nordmann P (2010) Emergence of metallo-beta-lactamase NDM-1-producing multidrug-resistant Escherichia coli in Australia. Antimicrob Agents Chemother 54: 4914–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfeifer Y, Witte W, Holfelder M, Busch J, Nordmann P, et al. (2011) NDM-1-producing Escherichia coli in Germany. Antimicrob Agents Chemother 55: 1318–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA (2011) New Delhi metallo-beta-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg Infect Dis 17: 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen JB, Hansen F, Littauer P, Schonning K, Hammerum AM (2012) An NDM-1-producing Escherichia coli obtained in Denmark has a genetic profile similar to an NDM-1-producing E. coli isolate from the UK. J Antimicrob Chemother. [DOI] [PubMed]
- 41. Williamson DA, Sidjabat HE, Freeman JT, Roberts SA, Silvey A, et al. (2012) Identification and molecular characterisation of New Delhi metallo-beta-lactamase-1 (NDM-1)- and NDM-6-producing Enterobacteriaceae from New Zealand hospitals. Int J Antimicrob Agents 39: 529–533. [DOI] [PubMed] [Google Scholar]