Abstract
Blonanserin was developed as an antipsychotic drug in Japan and approved for the treatment of schizophrenia. It belongs to a series of 4-phenyl-2-(1-piperazinyl)pyridines and acts as an antagonist at dopamine D2, D3, and serotonin 5-HT2A receptors. Blonanserin has low affinity for 5-HT2C, adrenergic α1, histamine H1, and muscarinic M1 receptors, but displays relatively high affinity for 5-HT6 receptors. In several short-term double-blind clinical trials, blonanserin had equal efficacy as haloperidol and risperidone for positive symptoms in patients with chronic schizophrenia and was also superior to haloperidol for improving negative symptoms. Blonanserin is generally well tolerated and has a low propensity to cause metabolic side effects and prolactin elevation. We recently reported that blonanserin can improve some types of cognitive function associated with prefrontal cortical function in patients with first-episode and chronic schizophrenia. Taken together, these results suggest that blonanserin may be a promising candidate for a first-line antipsychotic for acute and maintenance therapy for schizophrenia. Further comparative studies are warranted to clarify the benefit/risk profile of blonanserin and its role in the treatment of schizophrenia.
Keywords: blonanserin, schizophrenia, pharmacology, pharmacokinetics, efficacy, safety
Introduction
Schizophrenia is a heterogeneous devastating psychiatric disorder characterized by positive, negative, affective, and cognitive symptoms. It generally presents in late adolescence or early adulthood and is associated with an increased risk of mortality and social or occupational dysfunction.1 Most patients with the illness usually need life-long treatment, and antipsychotic drugs are the mainstay of the pharmacologic treatment for schizophrenia.2
The introduction of second-generation antipsychotics (SGAs) or atypical antipsychotics represented an important advance in the pharmacologic treatment of the disorder.3,4 Accumulating evidence suggests that SGAs have at least equal or superior efficacy as first-generation antipsychotics (FGAs) and may offer some advantages over FGAs such as fewer extrapyramidal symptoms (EPS).5–8 SGAs excluding clozapine are currently recommended in many guidelines as first-line agents for acute and maintenance therapy for schizophrenia.9–11 However, increased concern has arisen regarding the safety profile of SGAs, as problems such as weight gain, metabolic abnormalities, and/or hyperprolactinemia have been noted.3,4 These side effects are associated with potential long-term health risks for patients as well as decreased adherence to treatment regimens. The need for more effective and safer agents has prompted the development of new antipsychotics.12
Blonanserin was developed as a novel antipsychotic drug in Japan and was approved in Japan in 2008 and Korea in 2009 for the treatment of schizophrenia. It is currently under clinical investigation in a Phase III trial in the People’s Republic of China.13 This article reviews the available data about the pharmacological profile, clinical efficacy, safety, and tolerability of blonanserin, and discusses its place in the treatment of schizophrenia as well as future perspectives.
Pharmacological profile
Pharmacodynamics
Blonanserin belongs to a series of 4-phenyl-2-(1-piperazinyl) pyridines and acts as an antagonist at dopamine D2, D3, and serotonin 5-HT2A receptors (Table 1).14 Its affinity for D2 receptors is approximately 6 times greater than that for 5-HT2A receptors. This feature is different from that of most other SGAs. Thus, blonanserin is pharmacologically more similar to FGAs than new generation drugs,4 and in Japan, it is often called a “dopamine-serotonin antagonist”.15 The affinity of blonanserin for D2 receptors is 20- and 94-fold higher than that of haloperidol and risperidone, respectively.
Table 1.
Receptor binding affinities of blonanserin, risperidone, and haloperidol (Ki values, nM)
| Receptor | Preparation | Ligand | Blonanserin | Risperidone | Haloperidol |
|---|---|---|---|---|---|
| Dopamine | |||||
| D1 | Human recombinant | 3H-SCH23390 | 1,070 | 761 | 2,300 |
| D2 | Human recombinant | 3H-spiperone | 0.142 | 13.2 | 2.73 |
| D3 | Human recombinant | 3H-spiperone | 0.494 | ||
| D4 | Human recombinant | 3H-spiperone | 150 | ||
| D5 | Human recombinant | 3H-SCH23390 | 2,600 | ||
| Serotonin | |||||
| 5-HT1A | Human recombinant | 3H-8-OH-DPAT | 804 | ||
| 5-HT2A | Human recombinant | 3H-ketanserin | 0.812 | 1.09 | 45.7 |
| 5-HT2C | Human recombinant | 3H-mesulergine | 26.4 | ||
| 5-HT6 | Human recombinant | 125I-LSD | 41.9 | ||
| 5-HT7 | Human recombinant | 125I-LSD | 183 | ||
| Adrenergic | |||||
| α1 | Rat brain | 3H-prazosin | 26.7 | 0.657 | 8.75 |
| α2 | Rat cortex | 3H-RX821002 | 530 | ||
| Histamine | |||||
| H1 | Human recombinant | 3H-pyrilamine | 765 | ||
| Muscarinic | |||||
| M1 | Human recombinant | 3H-NMS | 100 | ||
Note: Data are mean values. Data reprinted from Jpn J Clin Psychopharmacol, Volume 10, Une T, Kurumiya S, Pharmacological profile of blonanserin, pages 1263–1272. Copyright © 2007, with permission from Seiwa Shoten Publishers Tokyo.
Abbreviations: LSD, lysergic acid diethylamide; NMS, N-methyl scopolamine.
In contrast, blonanserin has low affinity for 5-HT2C, adrenergic α1, histamine H1, and muscarinic M1 receptors (Table 1).14 These receptor binding profiles may minimize its potential to induce certain adverse effects such as orthostatic hypotension, oversedation, weight gain, metabolic abnormalities, and peripheral anticholinergic side effects.16,17 Blonanserin has a relatively high affinity for 5-HT6 receptors (Ki = 11.7 nM) that is likely involved in the improvement of cognitive function.18 It also shows low inhibitory activity of neuronal re-uptake of dopamine, 5-HT, and norepinephrine.17 A positron emission tomography (PET) study of healthy volunteers showed that blonanserin occupies approximately 80% of striatal D2-like receptors with normal clinical doses.16
A preclinical study demonstrated that systematic administration of blonanserin increases extracellular levels of norepinephrine and dopamine, but not levels of 5-HT, glutamate, or gamma-aminobutyric acid in the prefrontal cortex.19 It also enhances neuronal activity in the locus coeruleus and ventral tegmental area without affecting activity in the dorsal raphe nucleus or the mediodorsal thalamic nucleus. Another study showed that blonanserin attenuates the enhancement of immobility in the forced swimming test induced by repeated treatment with phencyclidine in mice.14 Moreover, it improves apomorphine-induced prepulse inhibition disruption in rats. These effects of blonanserin suggest a potential efficacy for treating negative symptoms and cognitive impairment in schizophrenia.
Pharmacokinetics
Blonanserin is rapidly absorbed from the gastrointestinal tract (with the exception of the stomach) after oral administration and reaches a maximum plasma concentration (Cmax) within 2 hours in healthy volunteers.20 Steady-state levels of blonanserin are attained within 5 days after repeated administration of blonanserin (4 mg/day) in healthy individuals,20 and the plasma concentration is correlated with the administered dose.21 Binding to plasma proteins is almost 100% over the concentration range of 10 ng/mL-2 μg/mL with albumin contributing the most to binding.20 Brain uptake is extensive, and P-glycoprotein, an efflux transporter expressed in the brain, does not actively transport blonanserin as a substrate in humans or mice.22 Results of PET studies, in which D2 receptor occupancy of several antipsychotics in the pituitary and temporal cortex was calculated, demonstrated that blonanserin has the highest brain/plasma concentration ratio (B/P ratio = 3.88) compared with that of olanzapine (B/P ratio = 2.70), haloperidol (B/P ratio = 2.40), risperidone (B/P ratio = 1.61), and sulpiride (B/P ratio = 0.34).23,24 The B/P ratio represents the penetrating capability of antipsychotics across the blood–brain barrier,23 and thus, blonanserin appears to pass into the brain more easily than these other antipsychotics.
Food significantly affects the bioavailability of blonanserin in healthy individuals. Both Cmax and area under the plasma concentration-time curve (AUC) from time 0 to 12 hours after a single oral administration of blonanserin are 2.7-fold higher in the fed state compared with the fasting state, and the time to Cmax (Tmax) is significantly prolonged under fed conditions.20 Thus, blonanserin should be taken after meal intake. However, Saruwatari et al25 demonstrated that the increase in systemic exposure to blonanserin continues until at least 4 hours after food intake, and thus, the drug can be administered immediately before bedtime.
Blonanserin is mainly metabolized by cytochrome P450 (CYP) 3A4 in the liver, and its major metabolites are M-1 (N-deethylated blonanserin) and M-3 (7- and 8-hydroxylated blonanserin).20 M-1 has the highest binding affinity for D2/3 receptors and 5-HT2A receptors, but it shows pharmacological activity several fold lower than that of the parent drug.16
Elimination of blonanserin is predominantly in the urine and feces.20 The terminal elimination half-life (t1/2) of blonanserin was 10.7, 12.0, and 16.2 hours after administration of a single oral dose of 4, 8, and 12 mg, respectively, in healthy volunteers. Thus, the manufacturer’s prescribing information recommends twice-daily administration. However, the elimination t1/2 was 67.9 hours after repeated administration of blonanserin (4 mg/day) for 10 days in healthy individuals.20 Thus, it can be effectively administered once a day in clinical practice.
Drug interactions
As described previously, blonanserin is predominantly metabolized by CYP3A4. Coadministration of potent CYP3A4 inhibitors such as azole antifungal agents and human immunodeficiency virus protease inhibitors with blonanserin may increase the plasma concentration of blonanserin, and coadministration of these drugs is contraindicated.20 In addition, concomitant administration of blonanserin with adrenaline is contraindicated due to the potential risks of serious reductions in blood pressure.
The package inserts also provide cautions about coadministration of blonanserin with other CYP3A4 inhibitors such as erythromycin, clarithromycin, cyclosporine, diltiazem, and grapefruit juice.20 In contrast, CYP3A4 inducers such as phenytoin, carbamazepine, barbiturate, and rifampicin should be carefully administrated with blonanserin, because of the potential for decreased plasma blonanserin concentrations.
The effects of blonanserin may be enhanced after coadministration with central nervous system depressant drugs or alcohol and may be reduced after coadministration with dopamine agonists (eg, levodopa and bromocriptine), and patients should be cautioned to coadminister carefully.20
Therapeutic efficacy
The therapeutic efficacy of blonanserin for the treatment of patients with schizophrenia was evaluated in four comparative short-term trials26–29 and three non-comparative long-term studies.30–32
Short-term efficacy
Four randomized, double-blind, placebo- and active comparator-controlled clinical trials evaluated the short-term efficacy of blonanserin (Table 2).26–29 In a haloperidol-controlled, 8-week Phase III trial,27 a total of 265 patients were randomized. Patients were randomly assigned to receive twice daily doses of blonanserin (8–24 mg/day) or haloperidol (4–12 mg/day). Results for the primary endpoint, the final global improvement rate (percentage of patients graded “markedly improved” or “moderately improved”), were 61.2% improvement in the blonanserin group and 51.3% improvement in the haloperidol group. Blonanserin was non-inferior to haloperidol (P = 0.001). Secondary efficacy measures included scores from the Positive and Negative Syndrome Scale (PANSS) and the Brief Psychiatric Rating Scale (BPRS). No significant differences were found between the treatment groups regarding mean improvements from baseline in PANSS, BPRS total scores, PANSS positive or general psychopathological subscores. However, blonanserin produced significantly greater decreases in the PANSS negative subscale scores (P = 0.025) and the “anergia cluster” score of BPRS (P = 0.022) compared with haloperidol.
Table 2.
Published short-term, randomized, double-blind studies of blonanserin in patients with schizophrenia
| Study (year of publication) | Type of study and duration (weeks) | Diagnoses, n and age of randomized patients | Blonanserin regimen (n of patients; % discontinuation) | Active comparator regimen (n of patients; % discontinuation) | Placebo (n of patients; % discontinuation) | Outcome measures | Main efficacy results (primary outcomes) |
|---|---|---|---|---|---|---|---|
| Murasaki (2007) | Randomized, double-blind, active controlled, 8 weeks | Schizophrenia 265 patients aged 16–64 years | 8–24 mg/day | Haloperidol | NA | Primary: final global improvement rate | Final global improvement ratea (% of patients) |
| (n = 129; 24.0%) | 4–24 mg/day (n= 134; 29.1%) |
Secondary: PANSS, BPRS | B = 61.2 H = 51.3 (P = 0.001)b |
||||
| Miura (2008) | Randomized, double-blind, active controlled 8 weeks | Schizophrenia 302 patients aged ≥ 15 years | 8–24 mg/day | Risperidone | NA | Primary: PANSS-T | PANSS-T score mean change from baseline [baseline] |
| (n = 156; 29.5%) | 2–6 mg/day (n= 145; 25.5%) |
Secondary: PANSS-P, PANSS-N, PANSS-GP, BPRS, final global improvement rate |
B = −11.05 [87.1] R = −11.51 [86.7] (P < 0.001)c |
||||
| Garcia et al (2009) | Randomized, double-blind, placebo- and active controlled 6 weeks | Schizophrenia (with an acute exacerbation) 307 patients aged 18–65 years | 2.5 mg/day | Haloperidol | Placebo | Primary: PANSS-T | PANSS-T score mean change from baselined |
| (n = 61 ; 27.9%) 5 mg/day (n = 58; 20.7%) 10 mg/day (n = 64; 21.9%) |
10 mg/day (n = 60; 18.3%) |
(n = 64; 39.1%) | Secondary: PANSS-P, PANSS-N, BPRS, CGI-S | B 2.5 = −20.60* B 5 = −27.19* B 10 = −30.18* H 10 = −28.16* PI = −12.58 |
|||
| Yang et al (2010) | Randomized, double-blind, active controlled 8 weeks | Schizophrenia 206 patients aged 18–65 years | 8–24 mg/day | Risperidone | NA | Primary: PANSS-T | PANSS-T score mean change from baseline [baseline]e |
| (n = 92; 39.8%) | 2–6 mg/day (n = 91; 28.2%) |
Secondary: BPRS, CGI-I | B =−23.48 [86.91] R = −25.40 [86.49] (P = 0.3014)f |
Notes:
Proportion of patients with an improvement rating of “improved” or “markedly improved” at completion of study treatment;
the noninferiority of blonanserin compared with haloperidol for final global improvement was verified using the handicap method (noninferiority margin of −10%); 95% confidence interval (Cl) −2.7, +22.4;
the predefined criterion for the noninferiority of blonanserin compared with risperidone for the change from baseline in PANSS total score (lower limit of two-sided 95% Cl for the between-group difference of >−7) was met; 95% Cl −4.40, +3.48;
statistical analysis used an analysis of covariance model. Treatment effects were estimated by least squares means;
treatment effects were estimated by least squares means;
Wilcoxon rank sum test;
statistically significant vs placebo (P < 0.001).
Abbreviations: n, number; B, blonanserin; H, haloperidol; R, risperidone; PI, placebo; PANSS-T (P, N, GP), Positive and Negative Syndrome scale-Total (Positive, Negative, and General Psychopathology subscales); BPRS, Brief Psychiatry Rating Scale; CGI-S (I), Clinical Global Impression of Severity Scale (Improvement); P, P-value; NA, not available.
Garcia et al26 conducted a 6-week, placebo- and haloperidol-controlled, international, multicenter study in 307 patients with an acute exacerbation of schizophrenia. Patients were randomized into one of five treatment groups (blonanserin 2.5, 5, or 10 mg, haloperidol 10 mg, or placebo once daily). All active treatments produced significantly greater reductions from baseline in the PANSS total and positive scores at week 6 compared with placebo (P < 0.001). Blonanserin (5 and 10 mg/day) was superior to haloperidol for treating the negative symptoms of schizophrenia.
In an 8-week, risperidone-controlled, Phase III trial conducted in Japan,28 302 patients with chronic schizophrenia were randomly assigned to receive twice daily doses of blonanserin (8–24 mg/day) or risperidone (2–6 mg/day). Blonanserin was as effective as risperidone regarding mean improvements from baseline in the PANSS total score and each of the subscale scores as well as the BPRS total and cluster scores.
Yang et al29 conducted an 8-week, risperidone-controlled trial in 206 Korean patients with chronic schizophrenia. Patients were randomly assigned to take twice daily doses of blonanserin (8–24 mg/day) or risperidone (2–6 mg/day). Blonanserin showed equal efficacy as risperidone regarding mean improvements from baseline in the PANSS total score and the subscale scores as well as the BPRS total and cluster scores.
Kishi et al13 recently performed a systematic review and meta-analysis of these four studies and found no significant differences in discontinuation due to any cause (P = 0.29) or due to ineffectiveness (P = 0.32) between blonanserin and other pooled antipsychotics. Moreover, they did not find significant heterogeneity in the response rate between blonanserin and other antipsychotics.
In summary, blonanserin had equal short-term efficacy as haloperidol and risperidone regarding positive symptoms in patients with chronic schizophrenia. It was also superior to haloperidol for improving negative symptoms.
Long-term efficacy
Three open-label, non-comparative studies were conducted in Japan to evaluate the long-term efficacy of blonanserin.30–32
Data are available from two studies (n = 6130 and 32131) that were both conducted for 28 and 52–56 weeks of treatment. Of the 61 patients eligible for analysis, 48 patients (78.7%) received blonanserin for 28 weeks, and 38 patients (62.3%) were treated for up to 56 weeks.30 Of the 321 patients eligible for analysis, 264 patients (82.2%) received blonanserin for 28 weeks, and 155 patients (48.3%) were treated for more than 52 weeks.31 The final global improvement rate was 52%–87% after 28 or 52–56 weeks of treatment.30,31 Blonanserin produced significant improvements from baseline in the PANSS total score and the subscale scores as well as the BPRS total score (P < 0.0001).
In an extended long-term trial, nine (42.9%) of 21 patients who were enrolled in the study completed over 6 years of treatment with blonanserin.32 The final mean dose was 14.2 mg/day, and the final global improvement rate was 86%. Blonanserin produced significant reductions from baseline in the BPRS total score (P < 0.001).
In summary, these data suggest that blonanserin may have long-term efficacy for the treatment of schizophrenia. However, data on direct head-to-head comparison with other antipsychotics are lacking for the maintenance treatment of the illness.
Effect of blonanserin on cognitive function
Cognitive impairment is a core feature of schizophrenia33,34 and is present early in the course of the illness.35,36 A number of studies have reported a 1–2 standard deviation decline in performance on tests of multiple cognitive domains, including attention, executive function, memory, and processing speed, compared to healthy individuals.35,37–39 These cognitive deficits largely determine social and occupational functioning, as well as quality of life (QOL) in patients with schizophrenia.40
We previously conducted an 8-week, randomized, double-blind comparison of blonanserin (n = 10) and risperidone (n = 12) to evaluate the effects of these antipsychotics on cognitive functions in patients with chronic schizophrenia.41 Both antipsychotics significantly improved some types of verbal memory, whereas greater improvement was seen with blonanserin for attention and processing speed.
We recently conducted an 8-week, open-label study to evaluate the effects of blonanserin on clinical efficacy including cognitive function and subjective QOL in patients with antipsychotic-naïve first-episode schizophrenia.42 Cognitive function was assessed with the Brief Assessment of Cognition in Schizophrenia Japanese-language version (BACS-J),43 and subjective QOL was assessed with the Schizophrenia Quality of Life Scale Japanese-language version (SQLS-J).44
Twenty-four patients were recruited and tested at baseline. Of these, 20 completed the study. The mean daily doses of blonanserin at the start after baseline assessment and at 8 weeks were 2.9 mg/day and 7.2 mg/day, respectively. Results of paired t-tests demonstrated that z-scores of the letter fluency score (verbal fluency) and the Tower of London score (executive function) assessed with BACS-J were significantly increased after treatment with blonanserin (P < 0.05 each) (Figure 1). The effect sizes for these changes were in the moderate range (0.58 and 0.62, respectively). All items on the PANSS, SQLS-J, and Clinical Global Impression-Severity of Illness Scale significantly improved after 8 weeks of treatment (all P < 0.01).42 These results suggest that blonanserin improves some types of cognitive function (letter fluency and executive function) that are associated with prefrontal cortical function in patients with first-episode schizophrenia. Moreover, blonanserin may have beneficial effects on psychiatric symptoms and subjective QOL in this population. Further studies with a large sample size and longer duration of treatment are warranted to confirm our findings.
Figure 1.
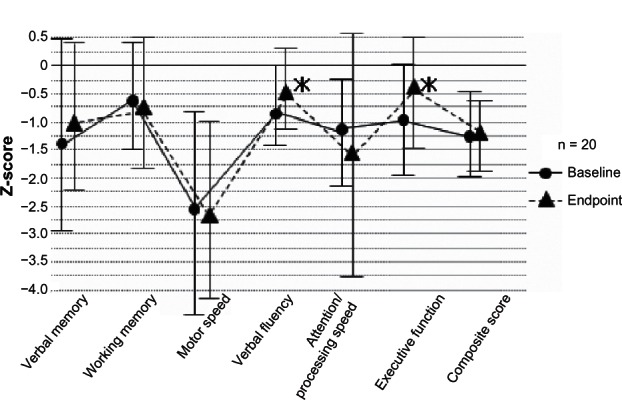
Changes in z-score of BACS-J subscales at baseline and endpoint.
Notes: The data are the mean ± standard deviation. Z-score was calculated by using the mean raw scores and standard deviation of each assessment in pooled healthy controls (n = 340). A composite score was calculated by averaging all z-scores of the six subscale measures. Paired t-tests were used to compare changes in z-score.
Abbreviation: BACS-J, Brief Assessment of Cognition in Schizophrenia in a Japanese-language version.
Safety and tolerability
Short-term treatment
In a meta-analysis of four short-term double-blind trials in 1080 patients with schizophrenia, blonanserin had a 0.31 lower risk of hyperprolactinemia than risperidone and haloperidol (P < 0.00001, numbers-needed-to-harm (NNH) = not significant).13 Blonanserin had lower rates of dizziness (risk ratio (RR) = 0.47, P = 0.03, NNH = not significant) and akathisia (RR = 0.54, P = 0.02, NNH = 7) than haloperidol. However, it demonstrated a 1.62 higher risk of akathisia than risperidone (P = 0.003, NNH = 3). No significant differences were found in the rate of other adverse events between blonanserin and other pooled antipsychotics. In addition, no differences were found in discontinuation due to adverse events (P = 0.56) or death (P = 0.33).
Long-term treatment
The long-term safety and tolerability of blonanserin were evaluated in three open-label, non-comparative studies in Japan.30–32 However, the number of patients who completed the longest-term study32 was very small (n = 9), and thus, these data are not discussed in this section.
Long-term treatment with blonanserin is generally well tolerated. The overall incidence of adverse drug reactions did not increase during blonanserin therapy for 56 weeks (72.1%30 and 68.5%31). Serious adverse reactions were observed in 9.8%30 and 5.9%31 of patients. However, only two patients reported serious adverse events, including stupor, anxiety, irritability, and akathisia, which were considered to be related to treatment with blonanserin.31
The most frequent adverse reactions with blonanserin were EPS, which were observed in 52.5%30 and 35.8%31 of patients. The most commonly (ie, incidence > 10%) reported EPS were akathisia (32.8%30 and 17.1%31), tremor (21.3%30 and 15.9%31), and hypokinesia (13.1%30 and 10.9%31). However, significant mean decreases (P < 0.01) from baseline were noted in the Drug-Induced Extrapyramidal Symptoms Scale total score and overall severity score after 28 and 52–56 weeks of treatment with blonanserin.30,31 Newly occurring tardive dyskinesia was not found during the studies.30,31
Other frequently reported adverse drug reactions were hyperprolactinemia (34.4%30 and 20.9%31), insomnia (18.0%30 and 17.4%31), somnolence (16.4%30 and 12.8%31), thirst (14.8%30), constipation (12.8%31), and dizziness (11.5%30). Of note, at week 52 or 56, mean changes from baseline in serum prolactin levels were −8.4 ng/mL30 and −5.0 ng/mL.31 Moreover, the incidence of abnormal menstruation was low (6.6%30 and 1.9%31).
Weight gain was reported in 8.2%30 and 5.0%31 of patients. However, no significant changes from baseline in body weight were noted at week 52 or 56 (from 60.5 to 61.5 kg30 and 62.3 to 62.5 kg31). Results also showed no significant changes in fasting glucose, cholesterol, or triglycerides.30,31 The incidence of QTc interval prolongation was very low; only one of 321 (0.3%) blonanserin recipients experienced this adverse event.31
The place of blonanserin in therapy and future perspectives
Although a number of antipsychotic drugs are currently available on the market, certain needs in the treatment of schizophrenia have clearly not yet been met.3 Available antipsychotics are considerably effective for positive symptoms of schizophrenia, but they do little to improve negative symptoms and cognitive impairments, which are recognized as core features of schizophrenia and play a greater role in poor functional outcome.1
Furthermore, many of the available FGAs can cause acute and chronic EPS, and SGAs can cause considerable weight gain and metabolic abnormalities.3,4 Accordingly, an antipsychotic drug that can alleviate negative and cognitive symptoms as well as positive symptoms of schizophrenia and that is metabolically neutral and has a low propensity for EPS is absolutely needed. Several SGAs already meet some of these conditions, but an alternative would be helpful.12,45
As described previously, oral blonanserin showed short-and long-term efficacy for treating both positive and negative symptoms of schizophrenia in several randomized and non-comparative studies. Blonanserin was as effective as haloperidol and risperidone for primary endpoints in three 8-week, randomized, double-blind trials,27–29 although it appeared to be better than haloperidol in ameliorating negative symptoms.27 Moreover, blonanserin improved some types of cognitive function associated with prefrontal cortical function in patients with first-episode and chronic schizophrenia.41,42
Blonanserin appears to be generally well tolerated and has minimal effects on weight, metabolic parameters, and QTc interval. It may have tolerability benefits regarding prolactin elevation compared with risperidone. Taken together, these data suggest that blonanserin may be a promising candidate for a first-line antipsychotic for patients with first-episode and chronic schizophrenia.
However, one limitation is that individuals who participated in the Phase III trials may be dissimilar to patients routinely encountered in clinical practice in terms of comorbid psychiatric and somatic conditions. No evidence currently exists to demonstrate that blonanserin may be useful as an alternative to clozapine in treatment-resistant schizophrenia. Additional clinical studies with a broader population of patients with schizophrenia will be useful.
Conclusion
Blonanserin appears to be effective for a wide range of symptoms in adult patients with first-episode and multiple episodes of schizophrenia. The data provide initial support for a potential cognitive benefit of blonanserin in schizophrenia. The drug has a low propensity to cause weight gain, metabolic abnormalities, and prolactin elevation. Further large-scale long-term trials comparing blonanserin with other SGAs, especially regarding relapse prevention, QOL, and functional ability of patients, are warranted to establish its efficacy, safety, and effectiveness for the treatment of schizophrenia.
Footnotes
Disclosure
Dr Tenjin has received speaker’s honoraria from Dainippon Sumitomo. Dr Miyamoto is a consultant for Dainippon Sumitomo and has received advisory board honoraria from Chugai Pharmaceutical and speaker’s honoraria from Dainippon Sumitomo and Eli Lilly. Dr Miyake has received speaker’s honoraria from Dainippon Sumitomo, Eli Lilly, Mitsubishi Tanabe, Otsuka, and Yoshitomi. Drs Tenjin, Miyamoto, and Miyake declare that they have no direct conflict of interest or grant support that is directly related to the content of this article. Dr Yamaguchi has received advisory board and/or speaker’s honoraria from Daiichi Sankyo, Eizai, Eli Lilly, Janssen, Otsuka, and Takeda. The other authors have no conflicts of interest to declare.
References
- 1.Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto S, Duncan GE, Goff DC, Lieberman JA. Therapeutics of Schizophrenia. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 775–807. [Google Scholar]
- 3.Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17(12):1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto S, Fleischhacker WW, Lieberman JA. Pharmacologic treatment of schizophrenia. In: Lieberman JA, Murray RM, editors. Comprehensive Care of Schizophrenia (Second Edition): A Textbook of Clinical Management. New York: Oxford University Press; 2012. pp. 77–138. [Google Scholar]
- 5.Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63(10):1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 6.Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 7.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318–378. doi: 10.3109/15622975.2012.696143. [DOI] [PubMed] [Google Scholar]
- 11.Lehman AF, Lieberman JA, Dixon LB, et al. Practice Guideline for the Treatment of Patients with Schizophrenia. Am J Psychiatry. (2nd ed) 2004;161(Suppl 2):1–56. [PubMed] [Google Scholar]
- 12.Miyake N, Miyamoto S, Jarskog LF. New serotonin/dopamine antagonists for the treatment of schizophrenia: are we making real progress? Clin Schizophr Relat Psychoses. 2012;6(3):122–133. [PubMed] [Google Scholar]
- 13.Kishi T, Matsuda Y, Nakamura H, Iwata N. Blonanserin for schizophrenia: Systematic review and meta-analysis of double-blind, randomized, controlled trials. J Psychiatr Res. 2013;47(2):149–154. doi: 10.1016/j.jpsychires.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Une T, Kurumiya S. Pharmacological profile of blonanserin. Jpn J Clin Psychopharmacol. 2007;10(7):1263–1272. [Google Scholar]
- 15.Murasaki M. Preclinical and clinical features of blonanserin. Jpn J Clin Psychopharmacol. 2008;11(5):855–868. [Google Scholar]
- 16.Deeks ED, Keating GM. Blonanserin: a review of its use in the management of schizophrenia. CNS Drugs. 2010;24(1):65–84. doi: 10.2165/11202620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Oka M, Noda Y, Ochi Y, et al. Pharmacological profile of AD-5423, a novel antipsychotic with both potent dopamine-D2 and serotonin-S2 antagonist properties. J Pharmacol Exp Ther. 1993;264(1):158–165. [PubMed] [Google Scholar]
- 18.Murasaki M, Nishikawa H, Ishibashi T. Dopamine-serotonin antagonist: Receptor binding profile of a novel antipsychotic blonanserin. Jpn J Clin Psychopharmacol. 2008;11(5):845–854. [Google Scholar]
- 19.Ohoyama K, Yamamura S, Hamaguchi T, et al. Effect of novel atypical antipsychotic, blonanserin, on extracellular neurotransmitter level in rat prefrontal cortex. Eur J Pharmacol. 2011;653(1–3):47–57. doi: 10.1016/j.ejphar.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Lonasen® (blonanserin) [interview form] Osaka, Japan: Dainippon Sumitomo Pharma Co, Ltd; 2012. [Google Scholar]
- 21.Suzuki H, Gen K. The relationship between the daily dose, the plasma concentration of blonanserin, and its plasma anti-dopamine D2 and anti-serotonin 5-HT2A activity. Hum Psychopharmacol. 2010;25(4):342–346. doi: 10.1002/hup.1124. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Osada K, Tagawa M, et al. Blonanserin, a novel atypical antipsychotic agent not actively transported as substrate by P-glycoprotein. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):156–162. doi: 10.1016/j.pnpbp.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Arakawa R, Okumura M, Ito H, et al. Positron emission tomography measurement of dopamine D(2) receptor occupancy in the pituitary and cerebral cortex: relation to antipsychotic-induced hyperprolactinemia. J Clin Psychiatry. 2010;71(9):1131–1137. doi: 10.4088/JCP.08m04307yel. [DOI] [PubMed] [Google Scholar]
- 24.Tateno A. PET evaluation for dopamine D2 receptor occupancy of blonanserin in schizophrenia patients. Jpn J Clin Psychopharmacol. 2011;14(2):334–341. [Google Scholar]
- 25.Saruwatari J, Yasui-Furukori N, Inoue Y, Kaneko S. Effect of dose timing in relation to food intake on systemic exposure to blonanserin. Eur J Clin Pharmacol. 2010;66(9):899–902. doi: 10.1007/s00228-010-0834-1. [DOI] [PubMed] [Google Scholar]
- 26.Garcia E, Robert M, Peris F, Nakamura H, Sato N, Terazawa Y. The efficacy and safety of blonanserin compared with haloperidol in acute-phase schizophrenia: a randomized, double-blind, placebo-controlled, multicentre study. CNS Drugs. 2009;23(7):615–625. doi: 10.2165/00023210-200923070-00006. [DOI] [PubMed] [Google Scholar]
- 27.Murasaki M. Clinical evaluation of blonanserin for schizophrenia: a randomized controlled study comparing blonanserin with haloperidol. Jpn J Clin Psychopharmacol. 2007;10(11):2059–2079. [Google Scholar]
- 28.Miura S. Clinical evaluation of blonanserin for schizophrenia: a randomized controlled study comparing blonanserin with risperidone. Jpn J Clin Psychopharmacol. 2008;11(2):297–314. [Google Scholar]
- 29.Yang J, Bahk WM, Cho HS, et al. Efficacy and tolerability of Blonanserin in the patients with schizophrenia: a randomized, double-blind, risperidone-compared trial. Clin Neuropharmacol. 2010;33(4):169–175. doi: 10.1097/WNF.0b013e3181dcda50. [DOI] [PubMed] [Google Scholar]
- 30.Murasaki M. Long-term clinical study of blonanserin for schizophrenia: a multicenter open study to determine safety and effectiveness in schizophrenic patients (Kanagawa Region Clinical Psychopharmacology Study Group) Jpn J Clin Psychopharmacol. 2007;10(12):2241–2257. [Google Scholar]
- 31.Kinoshita T. Long-term clinical study of blonanserin for schizophrenia: a multicenter open study to determine safety and effectiveness in schizophrenic patients (Japan-wide study) Jpn J Clin Psychopharmacol. 2008;11(1):135–153. [Google Scholar]
- 32.Osada K, Miyamoto S, Maruta S, Miyake N, Nakano M, Yamaguchi N. Long-term clinical study of blonanserin for schizophrenia: a multicenter open study to assess the safety and efficacy in patients with schizophrenia (Continuation of two long-term studies by request from patients) Jpn J Clin Psychopharmacol. 2009;12(11):2337–2351. [Google Scholar]
- 33.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 34.Gold JM, Harvey PD. Cognitive deficits in schizophrenia. Psychiatr Clin North Am. 1993;16(2):295–312. [PubMed] [Google Scholar]
- 35.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 36.Bozikas VP, Andreou C. Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust N Z J Psychiatry. 2011;45(2):93–108. doi: 10.3109/00048674.2010.541418. [DOI] [PubMed] [Google Scholar]
- 37.Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157(4):549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 38.Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 39.Wolwer W, Brinkmeyer J, Riesbeck M, et al. Neuropsychological impairments predict the clinical course in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258( Suppl 5):28–34. doi: 10.1007/s00406-008-5006-2. [DOI] [PubMed] [Google Scholar]
- 40.Matsui M, Sumiyoshi T, Arai H, Higuchi Y, Kurachi M. Cognitive functioning related to quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):280–287. doi: 10.1016/j.pnpbp.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Miyake N, Miyamoto S, Takeuchi A, et al. Effect of new-generation antipsychotic blonanserin on cognitive impairment in schizophrenia: A randomized double-blind comparison with risperidone. Jpn J Clin Psychopharmacol. 2008;11(2):315–326. [Google Scholar]
- 42.Tenjin T, Miyamoto S, Miyake N, et al. Effect of blonanserin on cognitive function in antipsychotic-naive first-episode schizophrenia. Hum Psychopharmacol. 2012;27(1):90–100. doi: 10.1002/hup.1276. [DOI] [PubMed] [Google Scholar]
- 43.Kaneda Y, Sumiyoshi T, Keefe R, Ishimoto Y, Numata S, Ohmori T. Brief assessment of cognition in schizophrenia: validation of the Japanese version. Psychiatry Clin Neurosci. 2007;61(6):602–609. doi: 10.1111/j.1440-1819.2007.01725.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaneda Y, Imakura A, Fujii A, Ohmori T. Schizophrenia Quality of Life Scale: validation of the Japanese version. Psychiatry Res. 2002;113(1–2):107–113. doi: 10.1016/s0165-1781(02)00240-8. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto S, Merrill DB, Lieberman JA, Fleischhacker WW, Marder SR. Antipsychotic Drugs. In: Tasman A, Kay J, Lieberman JA, First MB, Maj M, editors. Psychiatry. 3rd ed. Chichester: John Wiley & Sons; 2008. pp. 2161–2201. [Google Scholar]


