Abstract
Tauopathies are neurodegenerative disorders characterized by aberrant intracellular aggregation of hyperphosphorylated tau. It has been shown that aggregated tau is phosphorylated at serine, threonine, and tyrosine residues. However, the occurrence of tyrosine phosphorylation on tau proteins at different states of tau aggregation has not been shown. In this report, we utilized the tauopathy mouse model JNPL3 that expresses human 0N4R tau isoform bearing the missense P301L mutation to study the occurrence of tau tyrosine phosphorylation in the course of the development of tau aggregation. These mice develop behavioral and motor deficits and form sarkosyl-insoluble hyperphosphorylated tau in an age-dependent manner. Mass spectrometry analyses of immunopurified brain tau proteins from JNPL3 and Alzheimer’s disease affected individual uncovered novel tau tyrosine-phosphorylated sites. Further studies demonstrated that the abundance of tyrosine-phosphorylated tau increases in an age-dependent manner in JNPL3 mice. Tyrosine-phosphorylated tau was detected in both soluble and sarkosyl-insoluble preparations derived from brain and spinal cord, and localized in neurons containing aggregated tau. The phosphorylation of tyrosine residues in tau appeared to occur along with that of serine and threonine residues and was not detectable in non-transgenic littermates and transgenic mice expressing 0N4R wild-type human tau. The results suggest that tyrosine phosphorylation is as important as phosphorylation of other residues in tauopathy.
Keywords: Tau, Tyrosine phosphorylation, Tauopathy, Transgenic mice
1. Introduction
Tauopathy is a term used to describe a group of neurodegenerative disorders that include Alzheimer’s disease (AD), Pick disease (PiD), progressive supranuclear palsy (PSP), frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), corticobasal degeneration (CBD), and others [11]. These dementia and movement disorders are characterized by formation of fibrillary inclusions containing hyperphosphorylated tau in neurons and glia [1,11]. In addition to phosphorylation, the inclusions have been reported to contain tau modified by ubiquitination, glycation, glycosylation, and nitration [1,7,9,14]. However, the role that these posttranslational modifications may play in the formation and/or stabilization of tau inclusions remains poorly understood.
To overcome the inherent difficulties of human studies, in which the age of disease onset, agonal state, postmortem delay, and other factors can affect the outcome and data interpretation, several animal models have been created to uncover the pathological events leading to pathological aggregation of tau proteins. The tauopathy model developed by Lewis et al., referred to as JNPL3 transgenic mouse, expresses one of the six tau isoforms present in human brain and bears the P301L mutation, a common mutation found in kindred with FTDP-17 [13]. This tau isoform contains zero N-terminal insert and four C-terminal repeats (0N4R). Comparable to human, intracellular tau aggregates were formed in JNPL3 mice in an age-dependent manner. Importantly, JNLP3 mice develop behavioral and motor deficits following the accumulation of tau aggregates.
Aggregated tau in human and animal models of tauopathy is insoluble in the ionic detergent sarkosyl [13,16]. Biochemical analyses of JNPL3 mice have shown an age-dependent increase in the amount of sarkosyl-insoluble tau. The tau proteins present in the sarkosyl-insoluble preparation have an apparent molecular weight of 64 kDa and was demonstrated to possess a filamentous structure [13]. This form of tau is phosphorylated at multiple serine and threonine residues similar to those identified in abnormal tau from AD and related disorders [13,16]. Subsequent studies of JNPL3 mice have indicated that increase in the state of tau phosphorylation is associated with formation and/or stabilization of tau aggregates [16]. At present, more than 20 phosphorylation sites, containing serine and threonine residues, have been identified [1]. Phosphorylation at some of these sites is linked to conformational changes that may promote tau aggregation. Interestingly, in vitro studies have demonstrated that phosphorylation of a tau peptide at tyrosine 394 augments conformational changes in comparison to serine phosphorylation [5]. Limited studies have been focused on determining if aggregated tau is tyrosine phosphorylated and none has determined if such phosphorylation occurs prior to or along with serine and/or threonine phosphorylation on tau. Here we report the identification of two novel tyrosine phosphorylation sites on human tau protein expressed in JNPL3 mice. Such tyrosine-phosphorylated tau proteins are found in the sarkosyl-insoluble fraction and accumulated intraneurally in an age-dependent manner. These results suggest that tyrosine phosphorylation may play a role in the formation of tau inclusions.
2. Materials and methods
2.1. Antibodies
Antibodies used in this project are human tau-specific antibodies polyclonal E1 [amino acid residues (aa) 19–22; 1:2000], WKS44 [aa 162–178; 1:500; generated by Dr. Yen’s laboratory [4]], monoclonal Tau12 [aa 9–18; 1:20000; provided by Dr. Lester Binder [8]], and phospho-tau-specific antibodies CP13 (S202 and T205) and PHF-1 (S396 and S404) (both provided by Dr. Peter Davies [6], 1:1000); monoclonal phospho-tyrosine-specific 4G10 antibody (Upstate, NY) (1:200). Secondary antibodies used in Western blot analysis are as follows: peroxidase-conjugated goat anti-rabbit (1:4000) or goat anti-mouse (1:2000) Ig antibodies (Chemicon, Temecula, CA, USA).
2.2. Transgenic mice and AD brain sample preparation
Transgenic and non-transgenic littermates were bred by mating hemizygous JNPL3 or JN25 mice with Swiss Webster mice (Taconic, Germantown, NY, USA [6]). Mice were genotyped for the tau transgene by PCR between exons 9 and 13, using primers directed toward the human tau 0N4R tau isoform. Transgenic and non-transgenic littermates were euthanized at 3, 6, 9, and/or 12 months of age and their brains and spinal cord were collected. Brain samples were homogenized in buffer A (20 mM Tris base, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM PMSF; 5 mM sodium pyrophosphate; 30 mM β-glycerophosphate; 30 mM sodium fluoride) and centrifuge at 21,000 × gavg for 20 min (S1 fraction). The supernatant was collected and the pellet re-homogenized in buffer B (10 mM Tris base, pH 7.4; 0.8 M NaCl; 10% sucrose; 1 mM EGTA; 1 mM PMSF). The re-homogenized pellet was centrifuged at 21,000 × gavg for 20 min and the produced supernatant incubated at 37 °C for 1 h in presence of 1% sarkosyl. After incubation, the supernatant was centrifuged at 60,000 × gavg for 2 h. The supernatant (S2 fraction) was collected and the pellet (P3 fraction), which represents the sarkosyl-insoluble fraction, was resuspended in 1× TBS (20 mM Tris-buffer, pH 7.4, 150 mM NaCl). For human studies, a slice of the temporal lobe of AD brain was incised and homogenized following the same protocol used for mouse brain sample production. The Mayo Foundation’s Institutional Animal Care and Use Committee and Institutional Review Board approved the protocols used for animal and human studies.
2.3. Immunoprecipitation and Western blot analysis
Tau12 and 4G10 antibodies were used to immunoprecipitate tau proteins from S1 (soluble) and P3 (sarkosyl-insoluble) fractions. The S1 fraction (2 mg) and P3 fraction (500 μg) were incubated with protein A-sepharose beads (Pharmacia, USA) for 4 h with constant agitation at 4 °C. The samples were gently centrifuged for 10 s and the supernatant transferred to a clean tube. Tau12 or 4G10 antibodies (3 μg) was added to the pre-cleared supernatant and incubated overnight at 4 °C. The mixtures were incubated with (25 μL) protein A-sepharose beads for 3 h at 4 °C to precipitate the antibody–antigen complex. The precipitates were washed four times with (150 μL) 1× TBS and transferred to a clean tube. The samples were then processed for either Western blot or mass spectrometric analysis.
For Western blot analysis, immunoprecipitates were dissolved in 30 μL of sample buffer containing N-ethylmaleimide and no reducing agents. The samples were spin down at 14 krpm for 2 min (Eppendorf centrifuge) and not heated prior to loading into the gel. Equal volume (5 μL) from all samples was loaded on 10% sodium dodecyl sulfate–polyacrylamide gel (SDS–PAGE). Proteins resolved by gel electrophoresis were transferred to nitrocellulose membrane (BioRad, Hercules, CA, USA), which were then incubated in blocking solution (5% dry milk; 0.1% Tween 20 in 1× TBS) for 1 h prior to overnight incubation with the indicated primary antibody. After washing with 1× TBS containing 0.1% Tween 20, the membranes were incubated at room temperature with peroxidase-conjugated goat anti-mouse or anti-rabbit secondary antibodies and washed again. Immunoreacted proteins were visualized with the enhanced chemiluminescence system (ECL plus; Amersham Biosciences, Piscataway, NJ, USA). As control, recombinant tau proteins were used. The 4G10 antibody was found to have a slight cross-reactivity with recombinant tau when the nitrocellulose membranes were overexposed.
2.4. Tryptic digestion and mass spectrometry
For tryptic digestion, the immunoprecipitates were equilibrated in digestion buffer (DB) (50 mM ammonium bicarbonate; 10% acetonitrile), mixed with 1 μg of trypsin (Promega), and incubated overnight at 37 °C [15]. The produced peptides were dried by speed vacuum and resuspended in Loading Solvent (0.1% formic acid, 1.0% acetonitrile (ACN) in HPLC-graded water).
For mass spectrometry analysis, the LCQ-Deca XP Plus (Thermo Electron, San Jose, CA, USA) was used. The peptides were loaded to and eluted from a C18-RP PicoFrit column, using an ACN linear gradient, on line with the mass spectrometer. The mass spectrometer was tuned using a angiotensin I peptide and the following setting were used for analysis: spray voltage 1.70 kV, capillary temperature 170 °C, capillary voltage 27 V, default charge state 2, default isolation width 3.0, normalized collision energy 35%, activation Q = 0.250, activation time 30.0 ms, minimal signal required 8 × 105, minimal MS2 signal 4 × 105. A cycle of one full MS scan followed by three data-dependent MS/MS was repeated throughout the entire elution time (60 min). All MS/MS spectra acquired were analyzed using the Thermo Electron Bioworks Browser, which employs the database search SEQUEST algorithms. The MS/MS spectra were first analyzed using non-redundant protein database, taking into consideration differential mass increase (+79.99) due to phosphorylation at serine, threonine, and tyrosine [2,17]. The MS/MS spectra were re-searched using a subset of the database specific for tau isoforms. Only those identified peptides with XCorr scores higher than 1.5 (+1), 2.0 (+2), or 2.5 (+3) and delta score ≥0.1 where taken into consideration. The MS/MS spectra for the identified peptides were also manually evaluated.
2.5. Immunocytochemistry
The spinal cord and brain tissues were embedded in Tissue-Tek O.C.T. Compound (Ted Pella, Redding, CA), frozen and sectioned into 10-μm-thick slices. Triple fluorescence labeling for WKS44, 4G10, and nuclei was carried out sequentially on the same sections as previously described [2]. Tissue sections were fixed utilizing 4% paraformaldehyde in PBS (pH 7.4) for 15 min at room temperature. After treatment with a blocking solution containing 4% normal goat serum, 0.05% Tween-20 in phosphate-buffered saline (pH 7.5) for 1 h, the sections were incubated overnight at 4 °C with WKS44 (1:500) and 4G10 antibodies (1:200) diluted in 4% normal goat serum in PBS. After washing with 0.05% Tween-20 in PBS-T, the bound immunoglobulins were detected with secondary antibodies, Alexa 488 (goat anti-rabbit IgG) and Alexa 594 (goat anti-mouse IgG), at 1:200 dilution (Molecular Probes, Eugene, OR). Nuclei staining utilizing DAPI (4′,6-diamidio-2-phenylindole; Molecular Probes, Eugene, OR) was employed to confirm the presence of cells. The labeled cells were visualized by confocal microscopy (Olympus BX50). In addition, control experiments utilizing only secondary antibodies were used to determine non-specific labeling and autofluorescence. No non-specific labeling or autofluorescence was detected in experimental conditions. Sections were examined for fluorescent signals using excitation and barrier filters appropriate for selective visualization of fluorescein isothiocyanate and Texas red.
3. Results
3.1. Immunoprecipitated tau from S1 fraction is tyrosine phosphorylated
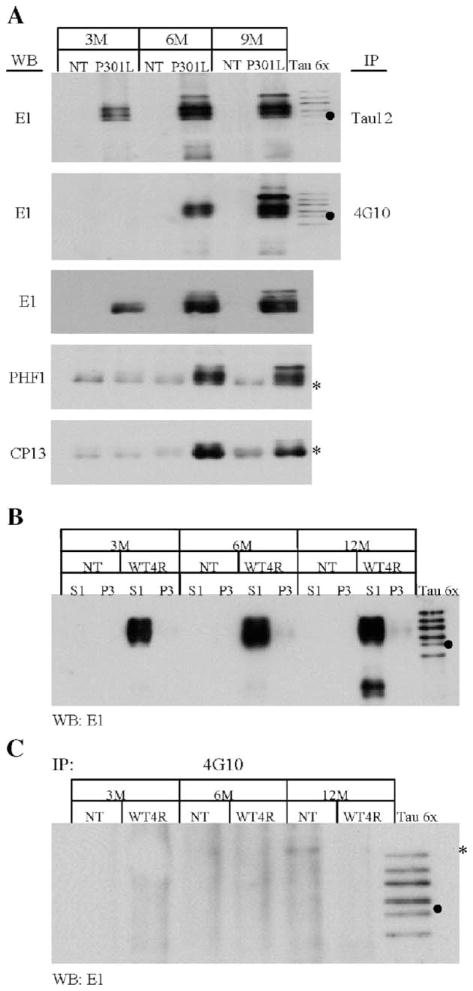
Tau proteins were immunoprecipitated from S1 (soluble) and P3 (sarkosyl-insoluble) fractions from brain extract of 10-month-old JNPL3 mice utilizing the human tau-specific monoclonal Tau12 antibody (aa 9–18). As revealed by Western blotting analysis using human tau-specific polyclonal E1 antibody (aa 19 – 33), tau proteins were efficiently immunoprecipitated from both S1 and P3 fractions (Fig. 1A, IP). The immunoprecipitated proteins correspond to the tau proteins observed in S1 and P3 fractions obtained from 9-month-old JNPL3 mouse brain extract (Fig. 1A, compare In and IP). As control, protein A-sepharose beads incubated with S1 and P3 fractions (Fig. 1A, beads control) and Tau12 immunoprecipitates derived from NT littermate mice (Fig. 1C, NT) were used. The Tau12 immunoprecipitates from 9-month-old JNPL3 mice, but not protein A-sepharose beads and NT littermates, contain tau proteins readily detectable with antibody E1 (Figs. 1A and B). Furthermore, analysis of the supernatant after immunoprecipitation (Fig. 1B, Sup) indicates that most tau proteins were efficiently precipitated. These results indicate that Tau12 specifically immunoprecipitates human tau proteins expressed in JNPL3 transgenic mice.
Fig. 1.
Immunoprecipitated tau from JNPL3 mice is tyrosine phosphorylated. (A) Brain samples from JNPL3 (P301L) and non-transgenic (NT) littermate were homogenized and subjected to fractionation as outlined in Materials and methods. The S1 (soluble) and P3 (sarkosyl-insoluble) fractions were subjected to immunoprecipitation experiments, using monoclonal tau-specific antibody Tau12. As control, protein A-sepharose beads (A, beads control) were used to determine the specificity of Tau12 antibodies (T12). The protein lysate used (In) and Tau12-immunoprecipitated proteins [A and B; (IP)] were visualized using polyclonal tau-specific antibody E1 (A –B). The supernatant after Tau12 immunoprecipitation was used to determine the immunopurification efficiency of tau proteins (B, Sup). (C) The immunoprecipitated proteins from both NT (lanes 1 and 2) and JNPL3 (lanes 3 and 4) mice were resolved by SDS –PAGE and subjected to Western blotting analysis, using phospho-tyrosine-specific antibody 4G10. The two asterisks to the left denote two weakly labeled bands in NT samples.
In order to determine whether tyrosine phosphorylation on tau proteins takes place in vivo, immunoprecipitated tau proteins from the S1 fraction were subjected to Western blot analysis utilizing the phosphotyrosine-specific 4G10 antibody. The 4G10 immunoreactive proteins are comparable to the apparent molecular weight of tau protein, ranging from 50 to 64 kDa (Fig. 1C). In contrast, immunoprecipitates derived from non-transgenic littermates showed two low molecular weight bands weakly labeled with 4G10 antibodies (Fig. 1C, lanes 1–2, asterisks). These bands do not correspond in size to the human tau proteins observed in Tau12 immunoprecipitates from JNPL3 mice (Figs. 1A and B). It is possible that such labeling of NT controls samples is due to non-specific binding of proteins to the beads or weak cross-reactivity of Tau12 with mouse (endogenous) tau. Nevertheless, this result indicates that immunopurified human tau proteins from the S1 (soluble) fraction of aged JNPL3 mice brain extract are tyrosine phosphorylated.
3.2. Tyrosine-phosphorylated tau is enriched in an age-dependent manner
Serine and threonine phosphorylation of tau in JNPL3 mice has been demonstrated to increase in an age-dependent manner [13,16]. To further confirm tau tyrosine phosphorylation and determine whether this type of phosphorylation increases in an age-dependent manner, soluble brain tau proteins (S1 fraction) from 3-, 6-, and 9-month-old NT and JNPL3 mice were subjected to immunoprecipitation using either Tau12 or 4G10 antibody. The immunoprecipitates were analyzed by Western blot utilizing human tau-specific E1 antibody (Fig. 2A). Tau12 immunoprecipitates prepared from 3 months and older JNPL3 mice were demonstrated to contain E1 immunoreactive tau (Fig. 2A, IP-Tau12). In contrast, 4G10 immunoprecipitates from 3-month-old mice did not display E1 immunoreactivity. Interestingly, however, there is an increase in 4G10-immunoprecipitated tau from 6- to 9-month-old mice in comparison to comparable levels of Tau12-immunoprecipitated tau (compare Fig. 2A, IP-Tau12 and IP-4G10). By comparing the relative abundance of immunoprecipitated tau with 4G10 and Tau12, or the increase of tyrosine-phosphorylated tau versus that of total tau in different animals (Fig. 2A, WB-E1 and IP-Tau12), it is evident that tyrosine-phosphorylated tau is more abundant in 9-month-old than 6-month-old JNPL3 mice (Fig. 2A, WB-E1 and IP-4G10). These results suggest that there is an age-dependent increase of tyrosine-phosphorylated tau in the JNPL3 mice.
Fig. 2.
Tyrosine-phosphorylated tau is enriched in an age-dependent manner. (A) The S1 (soluble) fractions from non-transgenic (NT) and JNPL3 (P301L) mouse brains at 3, 6, and 9 months (M) of age were subjected to immunoprecipitation and Western blot analysis. Tau proteins in these fractions were immunoprecipitated (IP) with either Tau12 or 4G10 antibodies. The immunoprecipitated proteins were analyzed by Western blotting using E1 antibodies. The S1 fractions from both NT and P301L mice were also analyzed by Western blotting using E1 and phospho-tau-specific antibodies PHF1 and CP13. The asterisks denote a non-specific cross-reacting band observed when either PHF1 or CP13 monoclonal antibodies were used. (B) The S1 and P3 fractions, from both NT and JN25 (WT4R) mouse brains, were analyzed by Western blotting, using antibody E1 to determine the abundance of tau proteins at 3, 6, and 12 months of age. (C) The S1 fraction was then subjected to IP using antibody 4G10. The immunoprecipitated proteins were analyzed by Western blotting using antibody E1. The asterisk indicates a non-specific cross-reacting band. The lanes designated Tau6x contain six isoforms of recombinant tau. The filled circles indicate the 0N4R tau isoform.
The development of intracellular tau aggregates has been linked to hyperphosphorylation of tau at serine and threonine residues. Based on this observation, it would be important to determine whether phosphorylation of these residues and tyrosine follows a similar temporal sequence. In the present study, we focused on the phosphorylation of S205/T205 and S396/S404, which are recognized by monoclonal antibodies CP13 and PHF1, respectively. Antibodies CP13 and PHF-1 have been recognized as useful immunoprobes for detecting tau inclusions [1,6]. Our Western blot analysis of S1 fraction from mice demonstrated an increase of serine and threonine-phosphorylated tau in JNPL3 mice of 6 months and older, but not in control NT mice (Fig. 2A, WB-PHF1 and CP13). A weak cross-reacting band in the NT animals migrates at slight lower molecular weight than tau proteins (Fig. 2A, asterisk). Since CP13 and PHF1 are mouse-monoclonal antibodies, it is possible that the band observed in NT mice is a product of the cross-reaction between the goat anti-mouse-HRP secondary antibody and endogenous mouse antibodies. Nevertheless, the increase in CP13 and PHF1 immunoreactivity in 6-month-old JNPL3 mice coincides with that of tyrosine phosphorylation. These results suggest that tyrosine phosphorylation takes place concurrently with serine and threonine phosphorylation.
To confirm that tyrosine phosphorylation of tau observed in JNPL3 mice is not simply due to overexpression of human tau, we analyzed 3-, 6-, and 12-month-old JN25 mice, which express wild-type human tau at level comparable to, if not more than, that in age-matched JNPL3 mice (Fig. 2B, S1). JN25 and NT littermates have no or very little accumulation of sarkosyl-insoluble tau (Fig. 2B, P3). The S1 fraction from JN25 mice was used for immunoprecipitation with 4G10 and analyzed by Western blot using E1 antibodies. The immunoprecipitates derived from these mice (Fig. 2C) were shown to contain no detectable levels of E1-immunoreactive tau, indicating that the observed tyrosine phosphorylation of tau in JNPL3 mice is not simply due to tau overexpression (Fig. 2A). Furthermore, this result also demonstrates that tau proteins containing tyrosine phosphorylation are specifically immunoprecipitated by 4G10 antibodies (compare Figs. 2A and C).
3.3. Sarkosyl-insoluble tau is tyrosine phosphorylated
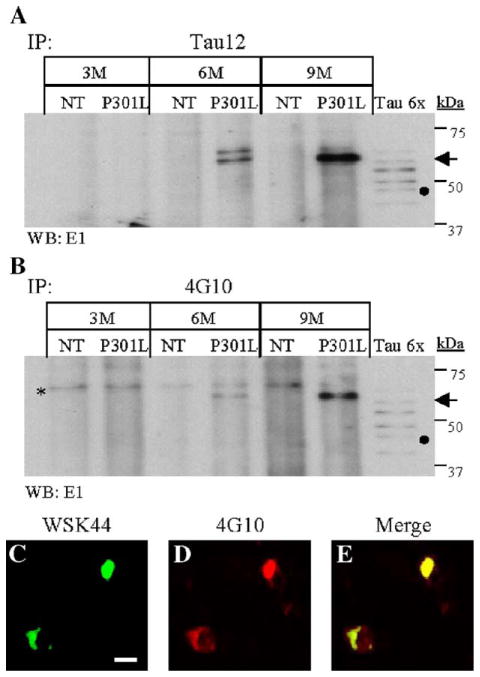
The sarkosyl-insoluble fraction (P3) has been shown to contain filamentous structures consisting of hyperphosphorylated tau [16]. In JNPL3 mice, sarkosyl-insoluble tau has an apparent molecular weight of 64 kDa due to its hyperphosphorylation. In order to determine whether this fraction also contains tyrosine-phosphorylated tau, immunoprecipitation experiments were conducted using 4G10 and Tau12 antibodies. Western blot utilizing tau-specific E1 antibodies confirmed the presence of tau proteins in the immunoprecipitates (Fig. 3). Molecular weight markers and the six recombinant tau isoforms (Tau 6×) were used as references to estimate the apparent molecular weight of immunoprecipitated sarkosyl-insoluble tau (Figs. 3A and B). The major tau species present in these samples has an apparent molecular weight of approximately 64 kDa and is present in JNPL3 mice (Fig. 3A, P301L) but not in NT littermates (Fig. 3A, NT). Consistently, sarkosyl-insoluble tau was enriched in preparations derived from older JNPL3 mice (Fig. 3A, arrow). Western blotting of 4G10 immuno-precipitated proteins with antibody E1 also demonstrated the presence of the 64-kDa specie of human tau (Fig. 3B, arrow). As observed in Tau12 immunoprecipitates, the 4G10 antibody immunoprecipitated more sarkosyl-insoluble tau in older JNPL3 mice (Figs. 3A and B). In contrast, no tau proteins were detected in JNPL3 sarkosyl-insoluble fractions incubated with protein A-sepharose beads alone (Fig. 1A, beads control; data not shown). These results suggest that tyrosine-phosphorylated tau is a component of tau inclusions.
Fig. 3.
Sarkosyl-insoluble tau and tau inclusions display phosphotyrosine immunoreactivity. Tau proteins present in the sarkosyl-insoluble fraction of NT and JNPL3 (P301L) mouse brains, from 3, 6, and 9 months of age, were immunoprecipitated (IP) using either Tau12 (A) or 4G10 antibody (B). (A –B) IP tau proteins were detected by Western blot analysis using E1 antibody. The lanes designated Tau6x contain six isoforms of recombinant tau. The filled circle on panels A and B marked the potion of the 0N4R tau isoform. The arrows indicate the 64-kDa tau species in panels A and B. The asterisk on panel B marks cross-reacting labeled band in both NT and P301L samples. (C –E) Spinal cord transverse sections from a 10-month-old JNPL3 (C– E) mice were used for immunofluorescence analysis using tau-specific antibody WSK44 (C-green) and phospho-tyrosine-specific antibody 4G10 (B-red). Neurons immunoreactive to both antibodies display yellow signal (E-merge). The cells were visualized by confocal microscopy at 0.1 μm. The scale bar in panel C = 100 μm.
To verify that tyrosine-phosphorylated tau is a component of intracellular tau inclusions, we labeled spinal cord sections (Figs. 3C and E) from 10-month-old JNPL3 with 4G10, polyclonal anti-tau WKS44 antibodies, and nuclear stain DAPI (not shown). The stained sections were visualized by confocal microscopy at 0.1 μm. The WKS44 antibody (Fig. 3C) labeled neuronal cell bodies in JNPL3 mice, particularly those located at the dorsal and ventral horns of spinal cords. Consistent with the immunoprecipitation experiments, both 4G10 (Fig. 3D) and WKS44 antibodies co-labeled the same structure (Fig. 3E). Similar results were also observed in JNPL3 brain sections, but not in spinal cord and brain sections from NT and JN25 aged-matched controls (data not shown). Together, these results indicate that tyrosine-phosphorylated tau accumulates in intracellular aggregates, suggesting that tyrosine phosphorylation may affect tau filament formation.
3.4. Identification of novel tyrosine phosphorylation sites on tau proteins
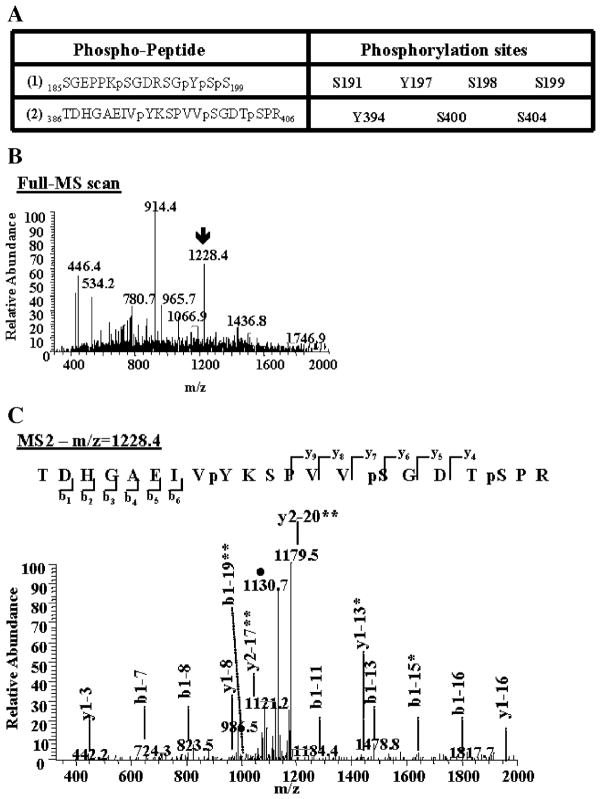
To identify tyrosine phosphorylation sites on tau proteins, mass spectrometry analyses of samples prepared from 10-month-old JNPL3, NT, and JN25 mice were performed. Tau proteins from S1 brain fractions were immunoprecipitated with antibody Tau12, enzymatically cleaved with trypsin, and analyzed by mass spectrometry as described in Materials and methods. The identity of the peptides and the sites of phosphorylation were determined by correlating MS-MS (MS2) spectra with sequences from the NCBI non-redundant protein database using the SEQUEST algorithms, and searching parameters included differential mass modification due to phosphorylation [15,17]. In the immunoprecipitates from JNPL3 mice, we identified two tau peptides containing tyrosine (Y) and serine (S) phosphorylation (Fig. 4A). We also detected several other non-phosphorylated and phosphorylated tau-specific peptides (data not shown). Immunoprecipitates derived from NT mice controls did not contain detectable amounts of tau peptides (data not shown). The two identified phospho-peptides (Fig. 4A) correspond to amino acid residues 185–199 and 386–402, based on the sequence of the longest 2N4R tau isoform peptide sequence. The corresponding full MS and MS2 spectra for these phospho-peptides were also manually verified. Figs. 4B and C correspond to the MS and MS2 of the second peptide listed on Fig. 4A, respectively. The MS2 spectrum (Fig. 4C) shows the identification of b and y ions produced from the fragmentation of its precursor ion (Fig. 4B, m/z = 1228.4). As shown in Fig. 4A, the first phospho-peptide (Fig. 4A, 1) was phosphorylated at S191, Y197, S198, and S199 and the second peptide (Fig. 4A, 2) was phosphorylated at Y394, S400, and S404. Phosphorylation of S191, Y197, and Y394 has not been reported previously, hence are novel phosphorylation sites, whereas S198, S199, S400, and S404 are known phosphorylated sites. These novel tyrosine-phosphorylated sites were found in tau proteins purified from, at least, three different JNPL3 mice. Importantly, no tyrosine-phosphorylated tau peptides were identified from both NT and JN25 mice controls (data not shown). These results confirm the presence of tyrosine-phosphorylated tau proteins in JNPL3 mice, independently of using phospho-Y-specific antibodies.
Fig. 4.
Identification of novel tyrosine phosphorylation sites on tau proteins. Immunoprecipitated tau proteins were digested with trypsin. The peptides produced were resolved by reverse-phase chromatography on line with nano-electrospray ionization mass spectrometry (MS) and analyzed as described in Materials and methods. (A) Two phospho-peptides containing phosphorylated tyrosine (Y) were identified. These phospho-peptides contain other known and unknown serine (S) phosphorylated sites. The amino acid number corresponds to its position in the longest tau isoform peptide sequence (2N4R). (B) The arrow on the MS spectrum points toward the doubly charged ion corresponding to phospho-peptide (2). (C) This precursor ion was then selected and subjected to fragmentation (MS2), generating b and y product ions that represent specific fragments used for identification of the peptide sequence and phosphorylation sites. The filled circle (●) denotes a doubly charged ion containing the neutral loss of 98 amu corresponding to the lost of the two phosphate groups attached to the serine residues on the phospho-peptide (2). One asterisk indicates b or y ions containing the neutral loss of the phosphate groups. Two asterisks denote a doubly charged b or y product ions.
3.5. Tyrosine-phosphorylated tau in AD brain
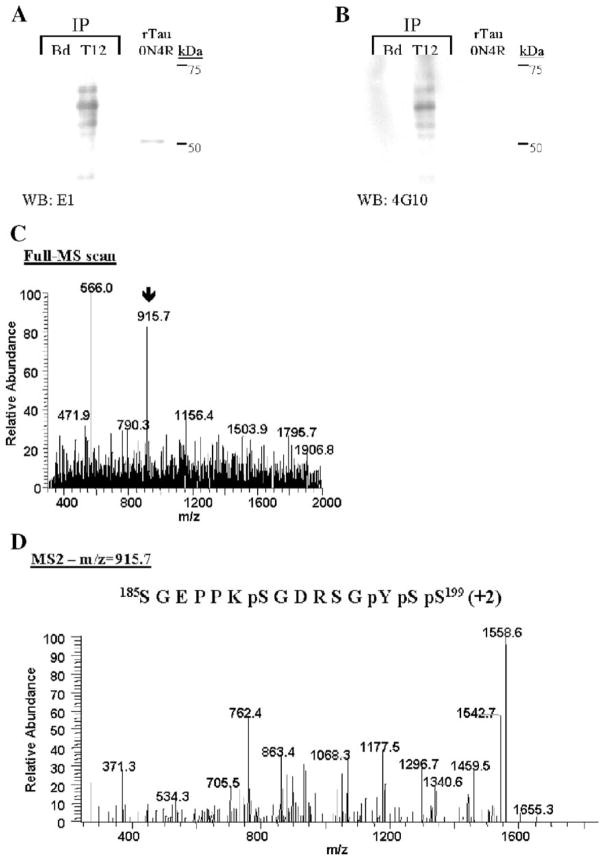
To determine if tau tyrosine phosphorylation observed in JNPL3 mice is conserved in humans, tau proteins were immunoprecipitated from the S1 fraction of AD brain using Tau12 antibodies. As control, protein A-sepharose beads alone (Fig. 5A, Bd) were incubated with brain extract to determine the specificity of Tau12 antibodies. The immunoprecipitated proteins were visualized by Western blot analysis. Tau12 immunoprecipitates in duplicate were resolved in the same gel. After transferring of proteins to nitrocellulose membrane, the membrane was cut in half and each half was probed individually with either E1 or 4G10 antibodies. Fig. 5A shows that Tau12 antibodies specifically immunoprecipitate tau proteins of apparent molecular weight 60 kDa, 64 kDa, 68 kDa (Fig. 5A, T12; WB: E1) and a weak band at 72 kDa (detected with longer exposure). This range of tau proteins represents different levels of tau phosphorylation characteristically observed in AD brain [11]. Tau proteins were not detected in the protein A-sepharose beads incubated with brain extract (Figs. 5A and B, Bd), indicating that immunoprecipitation of tau proteins is specific. The presence of tyrosine-phosphorylated tau in Tau12 immunoprecipitates from AD brain was determined by immunoblotting utilizing 4G10 antibodies (Fig. 5B, T12; WB: 4G10). The 4G10 antibodies recognized the same pattern of band detected with E1 antibodies (compare Figs. 5A and B). This result is consistent with that observed in tau proteins immunopurified from JNPL3 mice brain (Figs. 1C and 2A), confirming the presence of tyrosine-phosphorylated tau in AD brain.
Fig. 5.
Tyrosine-phosphorylated tau in AD brain. (A –B) AD brain S1 fraction was subjected to immunoprecipitation using Tau12 (T12) antibodies. The immunoprecipitated tau proteins were visualized by immunoblotting using either E1 antibody (A, WB: E1) or 4G10 (B, WB: 4G10). As negative control for immunoprecipitation, protein A-sepharose beads (Bd) were used. The 0N4R tau isoform was used as positive control for E1 immunoblotting. Immunoprecipitated tau proteins were digested with trypsin and analyzed by mass spectrometry as described in Materials and methods. (C) The arrow on the MS spectrum points toward the doubly charged ion corresponding to the identified phospho-peptide. (D) This doubly charged (+2) precursor ion was then selected and subjected to fragmentation (MS2), generating b and y product ions that represent specific fragments used for identification of the peptide sequence and phosphorylation sites depicted.
In order to corroborate the incidence of tau tyrosine phosphorylation in humans, Tau12-immunoprecipitated tau proteins from AD brains were subjected to trypsin digestion followed by mass spectrometric analysis of tryptic peptides. A doubly charged peptide of m/z equal to 195.7 was detected (Fig. 5C). Subsequent fragmentation and analyses of this peptide (Fig. 5D) revealed that it contains four phosphorylated residues. Based on the longest 2N4R tau isoform amino acid sequences, this peptide was phosphorylated at serines 191 (S191), 198 (S198), and 199 (S199) and tyrosine 197 (Y197). Tau phosphorylation at S198 and S199 has been previously reported [1], but S191 and Y197 are novel tau phosphorylation sites. The identification of phosphorylated Y197 is consistent with the result obtained in JNPL3 mice. Together, these results indicate that phosphorylation of tau proteins at Y197 is an event conserved in both transgenic JNPL3 mice and AD brain.
4. Discussion
Our studies provide strong evidence for the tyrosine phosphorylation of tau proteins in the course of developing tauopathy in JNPL3 mice. Such phosphorylation was provoked by the expression of P301L tau mutant in JNPL3 mice and not by tau overexpression per se, since phosphotyrosine immunoreactive tau proteins were not detected in JN25 mice. We found that the abundance of tau tyrosine phosphorylation increases in an age-dependent manner, and tyrosine-phosphorylated tau was detected only when animals began to show signs of tau aggregation [13,16]. In addition, we found that such event takes place concurrently with phosphorylation of serine and threonine residues S202/T205 and S396/S404, which are known to be differentially phosphorylated in tauopathy. More importantly, a portion of the tyrosine-phosphorylated tau exhibited sarkosyl insolubility and distributed in neuronal cell bodies in a pattern consistent with that of abnormal tau aggregates. Collectively, these results indicate that tyrosine phosphorylation may be as important as serine and threonine phosphorylation in facilitating tau assembly and/or stabilizing aggregated tau.
Based on our mass spectrometric studies it is evident that tau proteins in JNPL3 mice are phosphorylated at Y197 and Y394, and in AD brains are phosphorylated at Y197. It is worth emphasizing that tyrosine phosphorylation (Y394) of a tau peptide (GAEIVYKSPVVSGD) corresponding to amino acid sequence 389–402 has been reported to provoke more conformational changes than that achieved with phosphorylation at S396 [5]. Although the impact of tyrosine phosphorylation on the conformation of full-length tau awaits further investigations, we are tempted to speculate that tyrosine phosphorylation of tau is as important as that of serine and threonine phosphorylation in provoking aberrant conformational changes considered to be important for formation of tau inclusions. The importance of tau phosphorylation in its assembly is supported by a recent study in which incubation of rat brain extracts with recombinant tau (0.5 mg/ml), mutant tau in particular, was shown to result in tau filament assembly [3]. However, whether tyrosine residues were phosphorylated was not determined in that study.
Studies of autopsy brains have demonstrated that several serine–threonine kinases may have a role in abnormal tau phosphorylation in human tauopathy. No information is available regarding the kinases involved in abnormal tyrosine phosphorylation of tau in human tauopathy. In their studies of COS7 cells co-transfected with tau and fyn, Lee et al. demonstrated the association of tau proteins with fyn tyrosine kinase and tyrosine phosphorylation of tau at Y18 [10,12]. Furthermore, Williamson et al. showed that treatment of primary rat and human cortical neurons with Aβ peptides promotes a transient activation of various signaling proteins including fyn, and tyrosine phosphorylation of neuronal proteins, such as tau [19]. These reports have suggested that fyn kinase may mediate tau tyrosine phosphorylation at Y18 and Y29 and have a role in neurodegeneration [10,12,19]. We did not detect Y18 and Y29 phosphorylation by mass spectrometry. However, it is possible that detection of Y18 and Y29 could be compromised by intrinsic experimental constrains in mass spectrometric analyses of tryptic- and phospho-peptides [15,17,18]. For example, it is possible that tryptic peptides containing Y18 and Y29 are less ionizable than other peptides, since they were not found in our search of unphosphorylated peptides. Thus, we cannot rule out the presence of phosphorylated Y18 and Y29 based on mass spectrometry. In JNPL3 mice, we did not uncover the phosphorylation of Y18 (anti-pY18; [12]) and Y29 (anti-pY29; [19]) by Western blotting of total lysates and Tau12-immunopurified preparations from S1 and P3 fractions (data not shown). Furthermore, we did not detect a differential increase in expression or co-localization of fyn kinase and intraneuronal tau aggregates, or detect tyrosine-phosphorylated fyn kinase in brain extracts (data not shown). It is possible that a substantial phosphorylation of Y18 and/or Y29 occurs primarily at later stages of neurofibrillary tangle formation than those observed in the JNPL3 we examined. In this regard, it has been reported that antibodies to tau peptides phosphorylated at Y18 (aa 12–24) recognize fewer tangles in AD brain than those unphosphorylated tau peptides (aa 19–46), and that compact fibrillary tangles, but not early diffuse tangles, display phosphorylated Y18 immunoreactivity [8].
The identity of kinases involved in the in vivo phosphorylation of tau at Y197 and Y394 in the JNPL3 tauopathy mouse model remains unknown. Further investigations are necessary to determine the signaling pathway(s) involved in tyrosine phosphorylation of tau proteins and its impact on the development of tau-induced neurodegeneration.
Acknowledgments
This study was supported, in part, by Robert and Clarice Smith Fellowship in Neurodegenerative Diseases and Stroke (to I.E.V.), Ruth L. Kirschstein National Research Service Award F32NS047930 (to I.E.V.), National Institutes of Health Grant AG17216 (to S.-H.Y), and Mayo Clinic Foundation. We thank Dr. Peter Davies for providing the phospho-tau-specific antibodies CP13 and PHF-1 and Dr. Lester I. Binder for the Tau12 antibodies. We are also grateful to Drs. Li-Wen Ko and Michael DeTure for critical reading of the manuscript.
References
- 1.Bueé L, Bussière T, Bueé-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 2.Cui L, Blanchard RK, Cousins RJ. The permissive effect of zinc deficiency on uroguanylin and inducible nitric oxide synthase gene upregulation in rat intestine induced by interleukin 1aplha is rapidly reversed by zinc repletion. J Nutr. 2003;133:51– 56. doi: 10.1093/jn/133.1.51. [DOI] [PubMed] [Google Scholar]
- 3.del A, Alonso C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34873– 34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- 4.DeTure M, Ko LW, Easson C, Yen SH. Tau assembly in inducible transfectants expressing wild-type of FTDP-17 tau. Am J Pathol. 2002;161:1711–1721. doi: 10.1016/S0002-9440(10)64448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabian H, Otvos L, Szendrei GI, Lang E, Mantsch HH. Tyrosine-versus serine-phosphorylation leads to conformational changes in a synthetic tau peptide. J Biomol Struct Dyn. 1994;12:573– 579. doi: 10.1080/07391102.1994.10508760. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacryl-amide gel electrophoresis. Proc Natl Acad Sci. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horiguchi T, Uryu K, Giasson BI, Ischiropoulos H, LightFoot R, Bellmann C, Richter-Landsberg C, Lee VM, Trojanowski JQ. Nitration of tau proteins is linked to neurodegeneration in tauopathies. Am J Pathol. 2003;163:1021– 1031. doi: 10.1016/S0002-9440(10)63462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz PM, Patterson KR, Guillozetongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI. Early N-terminal changes and caspase-6 cleavag of tau in Alzheimer’s disease. J Neurosci. 2004;24:7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko LW, Ko EC, Nacharaju P, Liu WK, Chang E, Kenessey A, Yen SH. An immunochemical study on tau glycation in paired helical filaments. Brain Res. 1999;830:301– 313. doi: 10.1016/s0006-8993(99)01415-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111:3167– 3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- 11.Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121– 1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 12.Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J Neurosci. 2004;24:2304– 2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis J, McGowan E, Rockwood J, Melrose H, Nachajura P, Slegtenhorst MV, Gwinn-Hardy K, Murphy MP, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Zaidi T, Iqbal, Grundke-Iqbal I, Merkle RK, Gong C-X. Role of glycosylation in hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett. 2002;512:101–106. doi: 10.1016/s0014-5793(02)02228-7. [DOI] [PubMed] [Google Scholar]
- 15.MacCoss MJ, Wu CC, Yates JR., III Probability-based validation of protein identification using a modified SEQUEST algorithm. Anal Chem. 2002;74:5593– 5599. doi: 10.1021/ac025826t. [DOI] [PubMed] [Google Scholar]
- 16.Sahara N, Lewis J, DeTure M, McGowan E, Dickson DW, Hutton M, Yen SH. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498– 1508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- 17.Schweppe RE, Haydon CE, Lewis TS, Resing KA, Ahn NG. The characterization of protein post-translational modifications by mass spectrometry. Acc Chem Res. 2003;36:453–461. doi: 10.1021/ar020143l. [DOI] [PubMed] [Google Scholar]
- 18.Wenner BR, Lynn BC. Factors that affect ion trap data-dependent MS/MS in proteomics. J Am Soc Mass Spectrom. 2004;15:150–157. doi: 10.1016/j.jasms.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Williamson R, Scales T, Clark BR, Gibb G, Reynolds CH, Kellie S, Bird IN, Varndell IM, Sheppard PW, Everall I, Anderton BH. Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-β peptide exposure: involvement of src family protein kinases. J Neurosci. 2002;22:10–20. doi: 10.1523/JNEUROSCI.22-01-00010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]