Dear Editor
Wagoner et al.1, 2 suggested that Legalon-SIL (SIL), a commercially available intravenous preparation of silibinin, has an effect on HCV entry and cell-to-cell spread in vitro with only marginally suppression of HCV NS5B RNA-dependent RNA polymerase (RdRp) activity, a finding that is in contrast with Ahmed-Belkacem et al.3 findings. Three clinical studies have reported the viral response during SIL therapy4-6. The protocols were similar and consisted of daily injection of SIL for 7 days followed by Peg-IFN+ribavirin; however in5 ribavirin was administered before and during silibinin treatment. Viral decline after the initiation of SIL was monophasic until day 7 in the two case reports and in the majority of subjects in 4 (Fig. 1, red squares). Interestingly, a monophasic pattern of viral decline (Fig. 1, blue curves) was also observed in about half of patients (N=31) given 14 days of monotherapy with RG7128, a nucleoside HCV-RdRp inhibitor (manuscript in preparation), and in 3 subjects (N=5) in Le Pogam et al. (Fig. 1A in7). This monophasic decline is strikingly different from the biphasic viral decline typically observed in patients treated with protease inhibitors or (pegylated)interferon-α-based therapies8 (Fig 1, triangles). The fact that both SIL and RG7128 led to a monophasic HCV decline in some patients is interesting and tends to support Ahmed-Belkacem et al3 findings.
Figure 1.
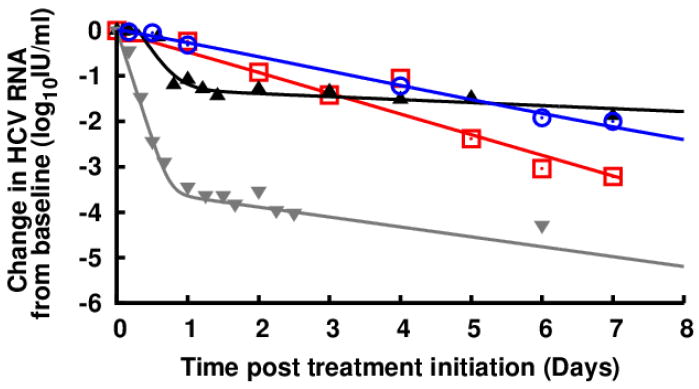
Representative serum HCV RNA decline from baseline during the first week of treatment with silibinin monotherapy6 (red squares), RG7128 1500-mg BID (blue circles; manuscript in preparation), daily 10MIU IFN9 (black triangles) and telaprevir+PegIFN8 (gray triangles). Solid lines were used to emphasize plausible phases of viral decline.
According to the standard HCV infection model9, a monophasic viral decline pattern results when viral infection is blocked, which tends to support the Wagoner et al.1, 2 findings. On the other hand, one can also predict a monophasic decline of virus if one assumes in the standard viral kinetic model a gradual reduction in viral production (unpublished observation), rather than an immediate high antiviral effectiveness in reducing viral production as it is the case with interferon-α or protease inhibitors. This gradual reduction in viral production could be related to drug pharmacokinetic and pharmacodynamic (PK/PD) properties. Such PK/PD properties leading to a progressive reduction in viral production could explain the similarity in the pattern of viral decline observed under treatment with SIL and RG7128 and possibly will shed light on why some patients treated with either of these two agents had a monophasic viral decline pattern.
In summary, to further investigate this controversy we suggest that PK/PD studies of SIL are needed to better understand the nature of the observed monophasic viral decline in treated patients. If SIL resistant strains can be identified, the nature of the resistance mutations would provide information about the MOA. If resistance mutations are found in the HCV polymerase it would favors an HCV-RdRp inhibitor mechanism, whereas if resistance mutations are in HCV E1/E2 it would support an entry inhibitor mechanism. Further in vitro experiments10 that include detailed kinetics of both intracellular and extracellular HCV RNA during treatment with SIL are likely to provide more insights into its MOAs against HCV.
Acknowledgments
This work was performed under the auspices of the U.S. Department of Energy under contract DE-AC52-06NA25396, and supported by NIH grants RR06555-19, P20-RR1875-6, AI065256-5 and AI28433-20, the National Science Foundation under Grant No. NSF PHY05-51164, and by the University of Illinois Walter Payton Liver Center GUILD.
Footnotes
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Wagoner J, Negash A, Kane OJ, et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 2010 Jun;51(6):1912–1921. doi: 10.1002/hep.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagoner J, Morishima C, Graf TN, et al. Differential In Vitro Effects of Intravenous versus Oral Formulations of Silibinin on the HCV Life Cycle and Inflammation. PLoS One. 2011;6(1):e16464. doi: 10.1371/journal.pone.0016464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed-Belkacem A, Ahnou N, Barbotte L, et al. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology. 2010 Mar;138(3):1112–1122. doi: 10.1053/j.gastro.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Ferenci P, Scherzer TM, Kerschner H, et al. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008 Nov;135(5):1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 5.Biermer M, Berg T. Rapid suppression of hepatitis C viremia induced by intravenous silibinin plus ribavirin. Gastroenterology. 2009 Jul;137(1):390–391. doi: 10.1053/j.gastro.2009.02.087. [DOI] [PubMed] [Google Scholar]
- 6.Payer BA, Reiberger T, Rutter K, et al. Successful HCV eradication and inhibition of HIV replication by intravenous silibinin in an HIV-HCV coinfected patient. J Clin Virol. 2010 Oct;49(2):131–133. doi: 10.1016/j.jcv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Le Pogam S, Seshaadri A, Ewing A, et al. RG7128 alone or in combination with pegylated interferon-alpha2a and ribavirin prevents hepatitis C virus (HCV) Replication and selection of resistant variants in HCV-infected patients. J Infect Dis. 2010 Nov 15;202(10):1510–1519. doi: 10.1086/656774. [DOI] [PubMed] [Google Scholar]
- 8.Guedj J, Rong L, Dahari H, Perelson AS. A perspective on modelling hepatitis C virus infection. J Viral Hepat. 2010 Aug 15;17(12):825–833. doi: 10.1111/j.1365-2893.2010.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998 Oct 2;282(5386):103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 10.Dahari H, Barretto N, Sainz B, Jr, Guedj J, Perelson AS, Uprichard SL. Modeling interferon-alpha mediated inhibition kinetics of intracellular and extracellular HCV RNA during HCV infection in vitro. J Hepatol. 2011;xx(Suppl):Sxx. [Google Scholar]


