Abstract
Cortical inhibition plays an important role in the processing of sensory information, and the enlargement of receptive fields by the in vivo application of GABAB receptor antagonists indicates that GABAB receptors mediate some of this cortical inhibition. Although there is evidence of postsynaptic GABAB receptors on cortical neurons, there is no evidence of GABAB receptors on thalamocortical terminals. Therefore, to determine if presynaptic GABAB receptors modulate the thalamic excitation of layer IV inhibitory neurons and excitatory neurons in layers II-III and IV of the somatosensory “barrel” cortex of mice we used a thalamocortical slice preparation and patch clamp electrophysiology. Stimulation of the ventrobasal thalamus elicited excitatory postsynaptic currents (EPSCs) in cortical neurons. Bath application of baclofen, a selective GABAB receptor agonist, reversibly decreased AMPA receptor-mediated and NMDA receptor-mediated EPSCs in inhibitory and excitatory neurons. The GABAB receptor antagonist, CGP 35348, reversed the inhibition produced by baclofen. Blocking the postsynaptic GABAB-mediated effects with a Cs+-based recording solution did not affect the inhibition, suggesting a presynaptic effect of baclofen. Baclofen reversibly increased the paired pulse ratio and the coefficient of variation, consistent with the presynaptic inhibition of glutamate release. Our results indicate that the presynaptic activation of GABAB receptors modulates thalamocortical excitation of inhibitory and excitatory neurons and provide another mechanism by which cortical inhibition can modulate the processing of sensory information.
Keywords: somatosensory, glutamate, interneurons, spiny stellate cells
INTRODUCTION
Our perception of the world around us is dependent on the cortical processing of somatosensory, visual, and auditory sensory input which is relayed from the thalamus to the cortex. Cortical inhibition, mediated by local GABAergic interneurons (Porter et al. 2001), plays a critical role in this processing by determining receptive field sizes and temporal interactions between adjoining receptive fields (Alloway et al. 1989; Dykes et al. 1984; Kyriazi et al. 1996b). In addition, cortical inhibition plays a role in activity-induced plasticity (Hensch et al. 1998; Knott et al. 2002; Micheva and Beaulieu 1995; Welker et al. 1989). For example in the rodent somatosensory system, repeated whisker stimulation induces a long-lasting increase in the number of inhibitory synapses in the corresponding cortical barrel (Knott et al. 2002) and in the enzyme glutamic acid decarboxylase which synthesizes GABA (Welker et al. 1989).
Cortical inhibition is mediated by the stimulation of GABAA receptors, which are ligand-gated chloride channels, and G protein-coupled GABAB receptors. Although many studies have focused on inhibition through GABAA receptors, several in vivo studies in the barrel cortex and other areas of the primary somatosensory cortex indicate that GABAB receptors also modulate the processing of thalamocortical inputs by regulating receptive field sizes and allowing for the temporal encoding of sensory inputs (Ajima et al. 1999; Chowdhury and Rasmusson 2002a, 2003a, 2002b; Kaneko and Hicks 1988; Kaneko and Hicks 1990). Mechanistically, this modulation could occur through the stimulation of presynaptic GABAB receptors on the thalamocortical axon terminals or postsynaptic GABAB receptors on the dendrites and cell bodies of the cortical neurons. GABAB receptors exist on many glutamatergic terminals where they decrease the release of glutamate by inhibiting voltage-dependent Ca2+ channels (VDCCs) and thus presynaptic calcium influx (Wu and Saggau 1995; Wu and Saggau 1997) and on dendrites and cell bodies where they activate inwardly rectifying K+ channels to cause inhibition (Luscher et al. 1997; Sodickson and Bean 1996). A previous study showed that GABAB receptor activation does not inhibit thalamocortical synapses onto layer II/III pyramidal neurons (Gil et al. 1997) suggesting that thalamocortical synapses are not presynaptically regulated by GABAB receptors. However, in the present study we demonstrate that presynaptic GABAB receptors exist at thalamocortical synapses onto inhibitory neurons in layer IV and excitatory neurons in layers II-III and IV of the mouse “barrel” cortex where they reduce the thalamic activation of cortical inhibition and excitation.
METHODS
Thalamocortical Slices
The Institutional Animal Care and Use Committee of the Ponce School of Medicine in compliance with National Institute of Health (NIH) guidelines for the care and use of laboratory animals (Publication DHHS NIH 86-23) approved all procedures involving animals. Mice (10–23 days postnatal) were anesthetized with halothane and decapitated under deep anesthesia. The brain was dissected out and put in ice cold artificial cerebral spinal fluid (ACSF) containing 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO4, 26 mM NaHCO3, 20 mM glucose and 2 mM CaCl2 and bubbled with 95% O2 and 5% CO2. 300 μm thick thalamocortical slices were obtained as previously described (Agmon and Connors 1991) with modifications (Porter et al. 2001) using a Vibratome 1000 Plus (Vibratome, St. Louis, MO). Slices were incubated at room temperature in ACSF for an hour prior to experiments. Most of the slices from animals greater than 18 days old were incubated with ACSF containing 10 μM MK-801 to block NMDA receptors.
Electrophysiology
Slices were transferred to a submersion recording chamber mounted on an upright E600FN microscope (Nikon Instruments, Melville, NY) and perfused at 2–3 mL/min with room temperature ACSF. Neurons were visualized with infrared video microscopy using a 40x water immersion objective. Neurons were tentatively identified as inhibitory or excitatory based on differences in somatic sizes. In layer IV excitatory neurons generally had smaller, rounder somas than inhibitory neurons. Whole cell recordings were done with glass pipettes of 3–5 MΩ filled with an internal solution consisting of 12 mM KCl, 140 mM KGluconate, 0.2 mM EGTA, 10 mM HEPES, 0.3 mM GTP and 0.4 mM ATP (pH 7.3, 285 mOsm) or 12 mM TEA-Cl, 140 mM CsGluconate, 0.2 mM Cs-EGTA, 10 mM HEPES, 0.1 mM spermine, 0.3 mM GTP and 0.4 mM ATP (pH 7.3, 285 mOsm). QX-314 (1 mM) was included in the Cs+ based internal solution for experiments done on layer II–III pyramidal neurons and experiments examining the effect of baclofen on NMDA receptor-mediated EPSCs. Using a patch clamp amplifier (MultiClamp 700A, Axon Instruments, Union City, CA) in current clamp mode, action potential discharges were elicited with the injection of current pulses to distinguish between inhibitory and excitatory cells as previously described (Porter et al. 2001). To record EPSCs, cells were held in voltage clamp mode at −60 mV and the ventrobasal nucleus of the thalamus was stimulated (25–125 μA of current; A-M Systems, Carlsborg, WA) with pairs of stimuli separated by a 50 ms interval with a unipolar tungsten electrode (WPI, Sarasota, FL) every 10–15 seconds to evoke EPSCs. Recordings were filtered at 4 kHz, digitized at 10 kHz, and saved to computer using pCLAMP8 (Axon Instruments, Union City, CA). Membrane potentials were not corrected for the junction potential. Recordings were not compensated for series resistance, but changes in series resistance were monitored throughout the recordings with a 500 ms prepulse in every sweep. Experiments in which the series resistance changed by more than 15% were eliminated from analysis. All experiments, except those in which NMDA receptor-mediated EPSCs were recorded, were done with 10 μM bicuculline and 100 μM DL-2-amino-5-phosphonopentanoic acid (AP5) in the bath to block GABAA and NMDA receptors, respectively. The remaining current was blocked by 6-cyano-7- nitroquinoxaline-2,3-dione (CNQX; n = 3) confirming that the recorded EPSCs are AMPA receptor-mediated. It is unlikely that the EPSCs were mediated by kainate receptors, since thalamocortical stimulation of kainite receptors generates EPSCs with much slower kinetics (Kidd and Isaac 1999).
Baclofen, bicuculline, AP5, CNQX, and MK-801 were purchased from Sigma (St. Louis, MO). CGP 35348 was purchased from Tocris (Ellisville, MO).
Data analysis and statistics
Experiments were accepted for analysis if the effect of baclofen on the EPSCs reversed upon removal of the drug and there was no confounding di- or polysynaptic activity in the EPSCs. Data were analyzed using Clampfit (Axon Instruments, Union City, CA). To calculate the paired pulse ratio (PPR) for a given cell, the thalamus was stimulated with pairs of stimuli 50 ms apart and the mean amplitude of the EPSC generated by the second stimulus (EPSC2) was divided by the mean amplitude of the EPSC generated by the first stimulus (EPSC1). The average EPSCs were taken from ten consecutive traces including failures before, during, and after baclofen perfusion. The coefficient of variation (CV) was calculated from 10 consecutive traces including failures before, during, and after baclofen application as the standard deviation of the EPSC amplitude divided by the mean EPSC amplitude. The CV was corrected for changes in baseline noise by subtracting the variance of the baseline noise from the variance of the EPSC. Concentration response curves were generated with SigmaPlot (Aspire Software International, Leesburg, VA) and fitted with the equation, y = min + (max-min)/(1 + (x/EC50)Hillslope). The results presented were analyzed using the student’s t-test or ANOVA with Tukey’s post-hoc analysis where appropriate. Statistical significance was set at p < 0.05. Values are reported as the mean ± the standard error of the mean (S.E.M.).
Morphology
For post hoc morphological identification of inhibitory and excitatory cells 5 mM biocytin was included in the recording solution to label the neurons. Following the electrophysiological recordings, the slices were fixed and refrigerated overnight in 4% paraformaldehyde, and the labeled neurons were subsequently revealed with a standard advidin-biotin peroxidase procedure (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) as previously described (Porter et al. 2001). Images of the labeled neurons were taken with a cooled CCD camera (CoolSNAPcf, Roper Scientific, Trenton, NJ) using a 40x objective on an upright microscope (Olympus America, Melville, NY).
RESULTS
GABAB receptors reduce thalamocortical excitation of excitatory and inhibitory neurons in layer IV of the barrel cortex
Using the thalamocortical slice preparation (Agmon and Connors 1991), we examined the modulation of thalamic input onto inhibitory and excitatory cells in layer IV of the mouse barrel cortex by GABAB receptors in mice 10–23 days old. Inhibitory interneurons were distinguished from excitatory neurons by their action potential discharge patterns in response to injected depolarizing current pulses or post hoc morphology as previously described (Connors and Gutnick 1990; Porter et al. 2001). Twenty seven neurons were identified by both discharge pattern and morphology. In each case the morphology confirmed the classification based on the discharge pattern. Seventeen neurons were identified by action potential discharge pattern alone and 32 neurons were identified by morphology alone. To confirm that the studied EPSCs were of thalamocortical origin several different parameters were measured. The latency of the EPSCs was measured and the range and the coefficient of variation of the latencies were calculated. Previous studies have demonstrated that monosynaptic thalamocortical EPSCs exhibit latencies which vary by less than 1 ms (Agmon et al. 1996; Beierlein and Connors 2002) and coefficients of variation of less than 0.06 ms (Laurent et al. 2002). Consistent with this, the EPSCs in our study had latencies which varied by less than 1 ms and a coefficient of variation of 0.046 ± 0.002 (n = 97) identifying them as monosynaptic events. The latencies of the evoked EPSCs showed an age-dependent decline with an average latency from the beginning of the stimulation artifact of 6.09 ± 0.18 ms in slices from mice 10–13 postnatal days old (n = 47) and 3.37 ± 0.09 ms in slices from mice 16–23 postnatal days old (n = 50). These latencies are consistent with the latencies of thalamocortical EPSCs measured in slices from similarly aged animals (Agmon et al. 1996; Agmon and O’Dowd 1992; Crair and Malenka 1995; Laurent et al. 2002; Lu et al. 2001). It is unlikely that corticothalamic fibers activated antidromically from the thalamus contributed to the responses since corticothalamic EPSCs exhibit paired pulse facilitation (Beierlein and Connors 2002) and the EPSCs that we studied exhibited paired pulse depression (Fig. 5) like thalamocortical EPSCs examined in previous studies (Gibson et al. 1999; Gil et al. 1997; Porter et al. 2001).
FIG. 5.
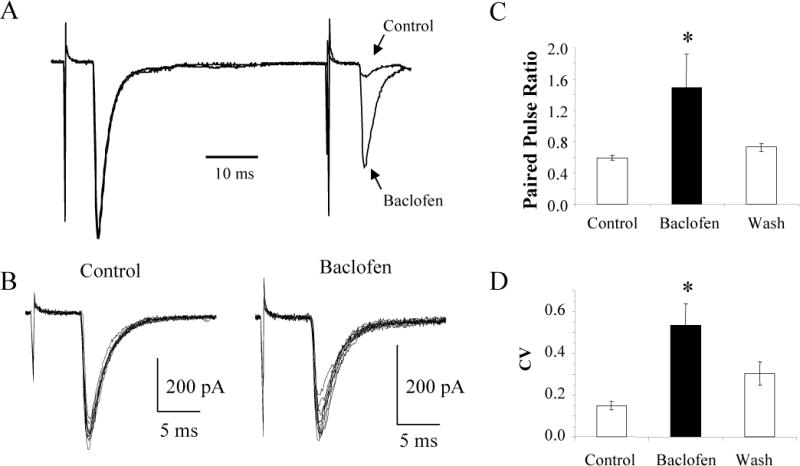
Baclofen increases the PPR and the CV of the thalamocortical EPSCs. A: Average EPSCs in response to paired stimuli taken before (Control) and during baclofen application to the neuron in Figure 1 scaled to equalize the first EPSCs. B: Overlays of 10 consecutive EPSCs under control conditions and during the application of baclofen showing that baclofen increased the CV. Data are from the same neuron as in A. C: The average PPR (n = 67) and CV (n = 30) before, during, and after (Wash) baclofen application. * p < 0.05.
The neuron shown in Figure 1 is an example of an identified layer IV inhibitory neuron from a P11 mouse. In response to depolarizing current pulses, it exhibited spikes with little or no frequency adaptation and large afterhyperpolarizing potentials (AHPs) with a fast repolarization (Fig. 1A), which is typical of cortical inhibitory neurons (Cauli et al. 1997; Kawaguchi and Kubota 1997; Porter et al. 1999). Post hoc morphological analysis demonstrates that this cell exhibits a nonpyramidal morphology with aspiny dendrites (Fig. 1B) confirming its electrophysiological classification as an inhibitory cortical neuron (Meinecke and Peters 1987; Ribak 1978). Furthermore, in this example the neuron forms putative synaptic contacts with an unlabeled cell body. Such perisomatic synapses are typical of inhibitory cortical basket cells (Kawaguchi and Kubota 1997; Somogyi et al. 1983). In the presence of bicuculline and AP5 to block GABAA and NMDA receptors, respectively, stimulation of the ventrobasal nucleus of the thalamus induced thalamocortical EPSCs in this neuron. Bath application of 10 μM baclofen, a selective GABAB receptor agonist, reversibly reduced the amplitude of the thalamocortical EPSCs by 40% (Fig. 1C, D) indicating that thalamic activation of cortical inhibitory circuits is modulated by GABAB receptors.
FIG. 1.
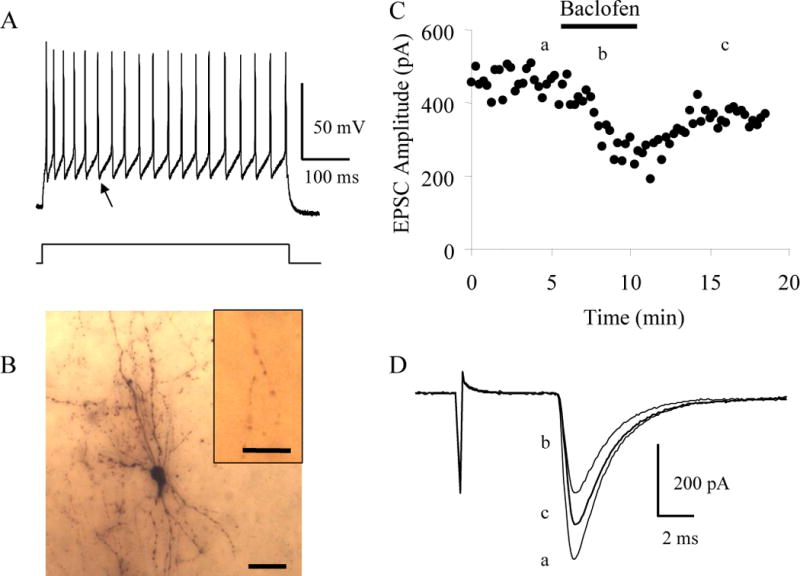
Baclofen inhibits thalamocortical EPSCs in inhibitory interneurons. A: The pattern of action potential discharge in response to a 320 pA current step in a P11 inhibitory interneuron layer IV cell distinguished by the rapid, deep AHP (arrow) and lack of adaptation of the action potentials. B: Image of the biocytin labeled inhibitory interneuron that exhibited the pattern of action potential discharge in A. Scale bar, 30 μm. Inset shows putative synaptic contacts made by this interneuron with the soma of an unlabeled neuron. Scale bar, 10 μm. C: Time course showing the effect of 10 μM baclofen on the evoked thalamocortical EPSCs in this interneuron. D: Averages of 10 consecutive EPSCs taken at the times indicated in C.
To test whether baclofen also modulates thalamocortical stimulation of excitatory layer IV neurons similar experiments were done on identified excitatory neurons. The layer IV cell from a P12 mouse shown in Figure 2 exhibited strongly adapting spikes and small and slowly repolarizing AHPs in response to injected current pulses (Fig. 2A) and a small cell body with thin radiating spiny dendrites (Fig. 2B), identifying it as an excitatory spiny stellate cell (Feldmeyer et al. 1999; Porter et al. 2001; Simons and Woolsey 1984). Bath application of 10 μM baclofen reduced the thalamocortical EPSCs recorded in this neuron by 68% (Fig. 2C, D) demonstrating that baclofen also modulates thalamic stimulation of cortical excitatory circuits. Within the population of neurons examined, the percent inhibition of thalamocortical EPSCs produced by baclofen in inhibitory interneurons (45 ± 8%; n = 12) did not statistically differ from that of excitatory spiny stellate cells (49 ± 6%; n = 13; t-test, p > 0.1; Fig. 2E).
FIG. 2.
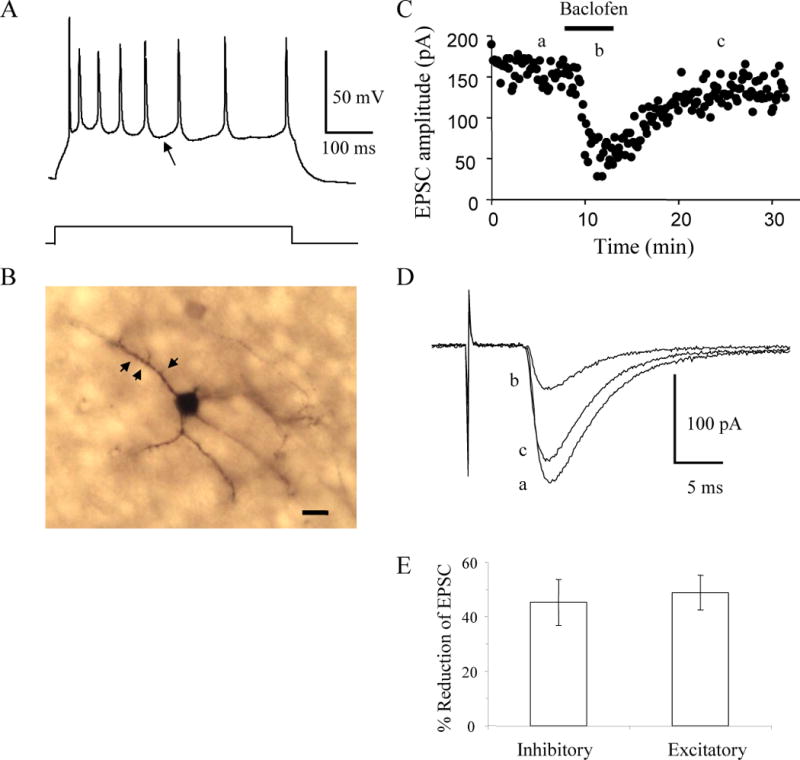
Baclofen inhibits thalamocortical EPSCs in excitatory layer IV neurons. A: The pattern of action potential discharge in response to an 80 pA current step in a P12 spiny stellate layer IV cell distinguished by the slow, shallow AHP (arrow) and significant adaptation. B: Image of the spiny stellate cell labeled with biocytin that exhibited the pattern of action potential discharges in A. Arrowheads point to visible spines. Scale bar, 10 μm. C: Time course showing the inhibition and recovery of evoked EPSCs in the cell in B produced by bath application of 10 μM baclofen. D: Averages of 10 consecutive EPSCs taken at the times indicated in C. E: Average % inhibition of thalamocortical EPSCs in inhibitory (n = 12) and excitatory (n = 13) layer IV neurons by 10 μM baclofen.
To confirm that baclofen’s effect was mediated through GABAB receptors, a selective GABAB receptor antagonist CGP 35348 (Olpe et al. 1990) was used. As shown in Figure 3, bath application of 5 μM baclofen reduced the thalamocortical EPSC recorded in the layer IV interneuron by 47%. In the continued presence of baclofen the subsequent application of 100 μM CGP 35348 reversed the EPSC reduction to 90% of control values. In the four neurons tested CGP 35348 reversed the baclofen-mediated reduction to 88 ± 4 % of control values (Fig. 3C) indicating that the inhibition required the stimulation of GABAB receptors.
FIG. 3.
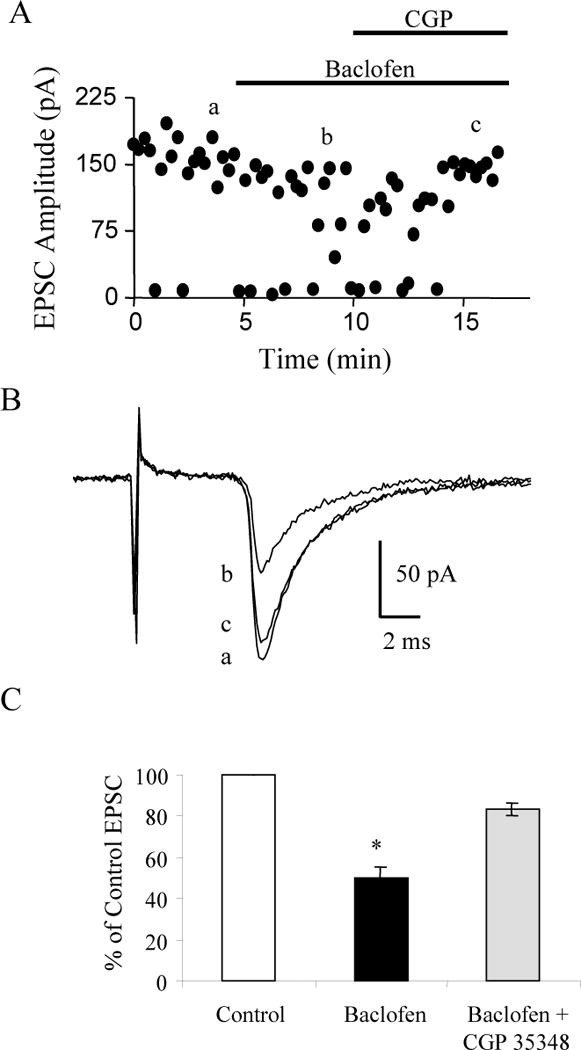
GABAB receptors mediate the reduction produced by baclofen. A: Time course showing the reduction and increase in failure rate of evoked EPSCs by bath application of 5 μM baclofen and the reversal of baclofen’s effect by co-application of 100 μM CGP 35348 in a P12 interneuron. B: Average EPSCs corresponding to the average of 10 traces taken at the times indicated in A. C: The average EPSC expressed as a percent of the control EPSC in 4 cells. * p < 0.05.
GABAB receptors modulate thalamic excitation of cortical neurons presynaptically
The effect of baclofen could be due to stimulation of either presynaptic or postsynaptic GABAB receptors. Presynaptic GABAB receptors inhibit VDCCs to decrease the release of neurotransmitter (Wu and Saggau 1995; Wu and Saggau 1997), while postsynaptic GABAB receptors generally activate inwardly rectifying K+ channels causing hyperpolarization (Luscher et al. 1997; Sodickson and Bean 1996). Within the population of neurons examined, baclofen did not significantly affect the holding current or the membrane resistance (n = 24; ANOVA, p > 0.05); however, in a few neurons there was a decrease in the holding current and the input resistance consistent with the postsynaptic activation of GABAB receptors (Fig 4A). These results suggest that baclofen’s effect was not mediated postsynaptically. To further determine whether a postsynaptic effect of baclofen produced the reduction in EPSC amplitude we used a Cs+-based recording solution to inhibit the K+ currents activated by GABAB receptors (Jarolimek et al. 1994; Morishita and Sastry 1995). As shown in the example in Figure 4B with Cs+ in the patch pipette baclofen reduced thalamocortical EPSCs without changing the holding current or the input resistance. Baclofen did not affect the holding current or membrane resistance of the cells recorded with intracellular Cs+ (n = 32; ANOVA; p > 0.05). In the population studied, 10 μM baclofen reduced thalamocortical EPSCs in inhibitory interneurons recorded in the presence of intracellular Cs+ by 54 ± 3% (n = 20) and in excitatory neurons by 49 ± 5% (n = 17). As in experiments without Cs+ in the internal solution, the inhibition observed in both cell types was not statistically different (p > 0.1, t-test), so the data from both cell types was grouped for the remaining analyses. As shown in Fig. 4C there was no difference between the percent inhibition produced by baclofen with Cs+ in the internal solution (52 ± 3%; n = 37) and without Cs+ (47 ± 5%; n = 25; p > 0.1, t-test) suggesting that presynaptic GABAB receptors mediate the reduction in thalamocortical EPSC amplitudes.
FIG. 4.
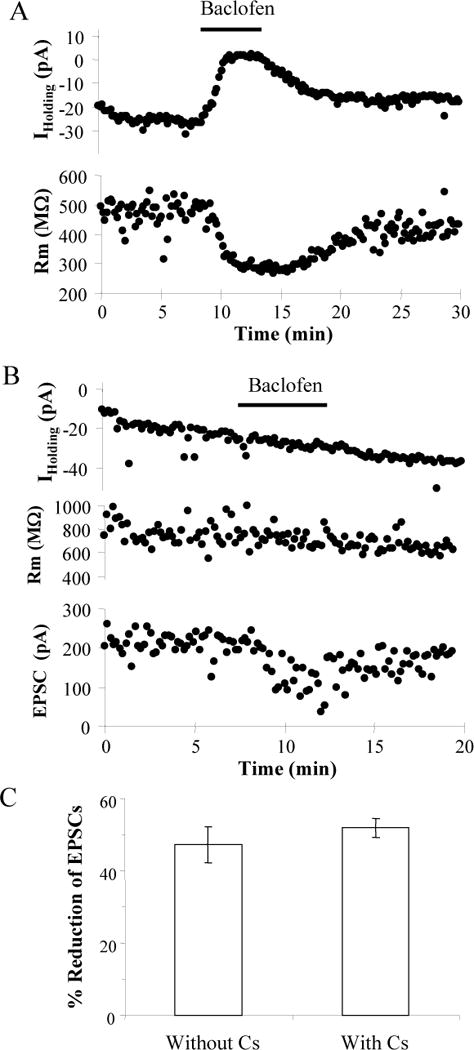
Postsynaptic Cs+ does not block Baclofen’s effect. A: Time course of changes in holding current and input resistance (Rm) in response to 10 μM baclofen recorded without intracellular Cs+ in the neuron shown in Fig. 2. B: Time course of experiment on an inhibitory neuron recorded with intracellular Cs+ showing that 10 μM baclofen reduced the EPSC without changing the holding current or Rm. C: Comparison of the average reduction of thalamocortical EPSCs by 10 μM baclofen with (n = 37) or without (n = 25) Cs+ in the recording pipette.
To further determine whether baclofen was working pre- or postsynaptically we examined baclofen’s effect on the PPR and the CV of the EPSCs. Manipulations that decrease the probability of neurotransmitter release produce an increase in the PPR and in the CV of the EPSCs (Ohana and Sakmann 1998; Zucker 1989). Typical of thalamocortical synapses (Amitai 2001; Gil and Amitai 1996), paired stimuli evoked strongly depressing EPSCs (Fig. 5A). Scaling the average traces taken before and during the perfusion of baclofen, so that the first EPSC is the same amplitude in both traces, indicates that baclofen increased the PPR from 0.08 to 0.59. An overlay of the 10 consecutive traces used to generate the average traces demonstrates that baclofen also increased the CV from 0.07 to 0.14 (Fig. 5B). The combined data from the experiments done with and without Cs+ in the internal solution indicate that baclofen reversibly increased the PPR from 0.59 ± 0.03 to 1.49 ± 0.42 (n = 67; ANOVA, p < 0.05) and the CV from 0.15 ± 0.02 to 0.53 ± 0.10 (n = 30; ANOVA, p < 0.05; Fig. 5C–D). A monoexponential function fit to the decay of EPSC1 gave time constants of 6.36 ± 0.48 ms in control conditions and 6.10 ± 0.52 ms in the presence of baclofen for the excitatory neurons (n = 14). For the inhibitory neurons (n = 15) the decay time constants were 3.28 ± 0.35 ms and 2.98 ± 0.39 ms in the absence and presence of baclofen, respectively. Therefore, baclofen did not affect the kinetics of the EPSCs suggesting that the postsynaptic AMPA receptors were not modified by the application of baclofen (p > 0.05 for both excitatory and inhibitory neurons). Together these results are most consistent with the presynaptic inhibition of glutamate release by baclofen.
Although the hyperpolarization of thalamocortical projecting neurons by baclofen could reduce the number of thalamocortical neurons which discharge in response to the stimulation and thus reduce the resultant EPSCs recorded in the cortical neurons, our results are unlikely to be caused by this mechanism since a general inhibition of the thalamocortical neurons should equally affect both the first EPSC and the second EPSC and should; therefore, not affect the PPR of the EPSCs. Furthermore, baclofen reduced thalamocortical EPSCs evoked by stimulating the internal capsule (66 ± 10%, n = 3) which contains only axons (data not shown).
GABAB receptors modulate thalamocortical inputs onto layer II–III pyramidal cells
Since previous results indicated that thalamocortical EPSCs onto layer II–III pyramidal neurons were not modulated by baclofen, our results raised the possibility that thalamocortical synapses in layer IV could be selectively modulated by baclofen. To directly test this possibility, we examined the effects of baclofen on thalamocortical EPSCs onto layer II–III pyramidal neurons from mice 14 to 20 days postnatal. In response to injected current pulses, layer II–III pyramidal neurons exhibited strongly adapting spikes and small and slowly repolarizing AHPs (Fig. 6A) similar to the excitatory spiny stellate neurons. Post hoc morphological analysis confirmed that the six recorded neurons were layer II–III pyramidal neurons with prominent apical dendrites and dendrites (Fig. 6B). After recording the response to injected current, the neuron in Figure 6 was repatched with an internal solution containing both Cs+ and 1 mM QX-314 to block postsynaptic GABAB receptor-mediated effects. Stimulation of the thalamus evoked EPSCs in the presence of bicuculline and AP5. Consistent with thalamocortical EPSCs the evoked currents exhibited paired pulse depression with a paired pulse ratio of 0.49 ± 0.15 (Fig. 6E; n = 6) and latencies that varied by less than 1 ms. The application of 10 μM baclofen reversibly reduced the thalamocortical EPSCs recorded in the layer II–III pyramidal neurons (n = 6) by an average of 74 ± 9% (Fig. 6C–F). Given that corticothalamic EPSCs exhibit paired pulse facilitation (Beierlein and Connors 2002), our results are most consistent with GABAB receptor-mediated inhibition of thalamocortical synaptic transmission.
FIG. 6.
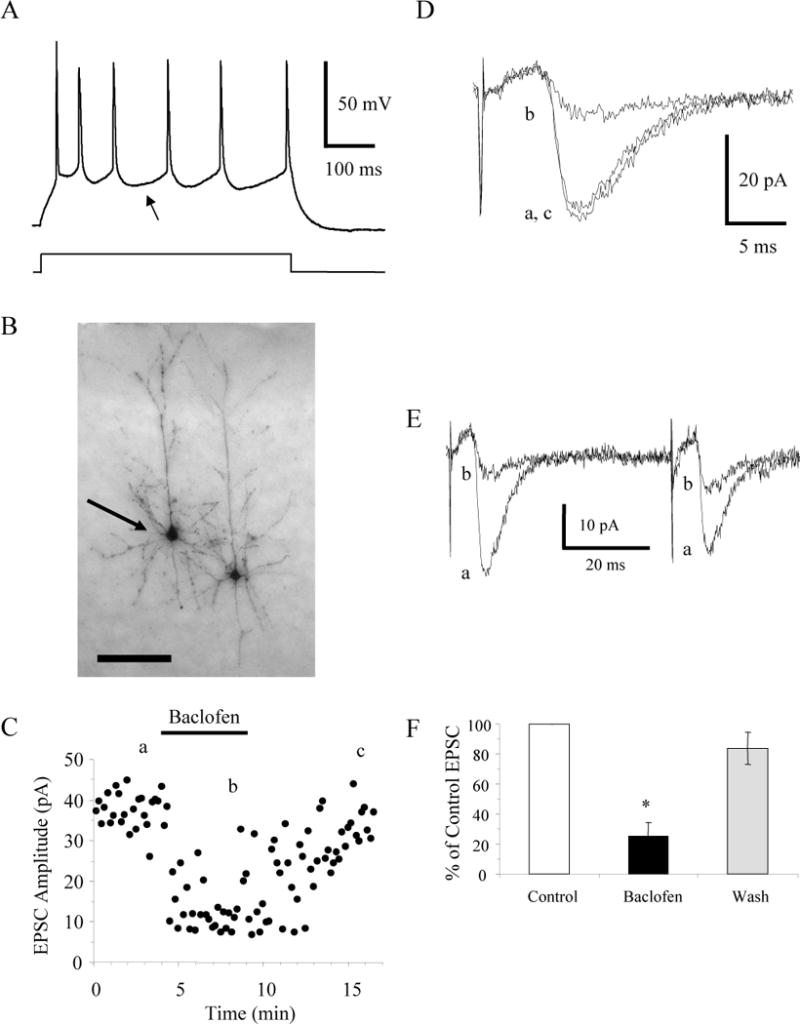
Baclofen inhibits thalamocortical EPSCs in layer II–III pyramidal neurons. A: The pattern of action potential discharge in response to a current step in a P14 layer II–III pyramidal cell is distinguished by the slow, shallow AHP (arrow) and significant adaptation. B: Image of the pyramidal cell (arrow) labeled with biocytin that exhibited the pattern of action potential discharges in A. Scale bar, 100 μm. C: Time course showing the inhibition and recovery of evoked EPSCs in this pyramidal cell produced by bath application of 10 μM baclofen. D: Averages of 10 consecutive EPSCs taken at the times indicated in C. E: Averages of 10 consecutive EPSCs taken at the times indicated in C showing that these inputs exhibit paired pulse depression. F: The average EPSC expressed as a percent of the control EPSC in layer II–III pyramidal neurons (n = 6) before, during, and after (Wash) application of 10 μM baclofen. * p < 0.05.
Therefore, our results are contrary to the previous results (Gil et al. 1997). Since the previous study had shown that intracortical inputs were modulated by baclofen, we needed to confirm that we were recording monosynaptic thalamocortical synaptic currents. Based on the fact that the latency of the evoked EPSCs varied by less than 1 ms suggests that they are indeed monosynaptic (Agmon et al. 1996; Beierlein and Connors 2002). To further confirm that monosynaptic EPSCs evoked by stimulating the thalamus are modulated by baclofen, we examined the effect of baclofen on NMDA receptor-mediated synaptic currents. Slices were perfused with 10 μM bicuculline and 10 μM CNQX to essentially block synaptic transmission by blocking GABAA and AMPA/kainate receptors, respectively. The thalamus was stimulated and NMDA receptor-mediated synaptic currents were recorded in cortical neurons voltage-clamped at + 60 mV to remove the Mg2+ block of the NMDA receptors. Under these conditions all synaptic transmission except for the monosynaptic input to the recorded neuron should be blocked. Application of 10 μM baclofen reversibly reduced the NMDA receptor-mediated synaptic currents recorded under these conditions by 73 ± 10% (n = 4; 2 layer IV inhibitory neurons and 2 layer II–III pyramidal neurons; Fig 7A–C). These experiments further confirm that monosynaptic inputs coming from the thalamus are modulated by GABAB receptors.
FIG. 7.
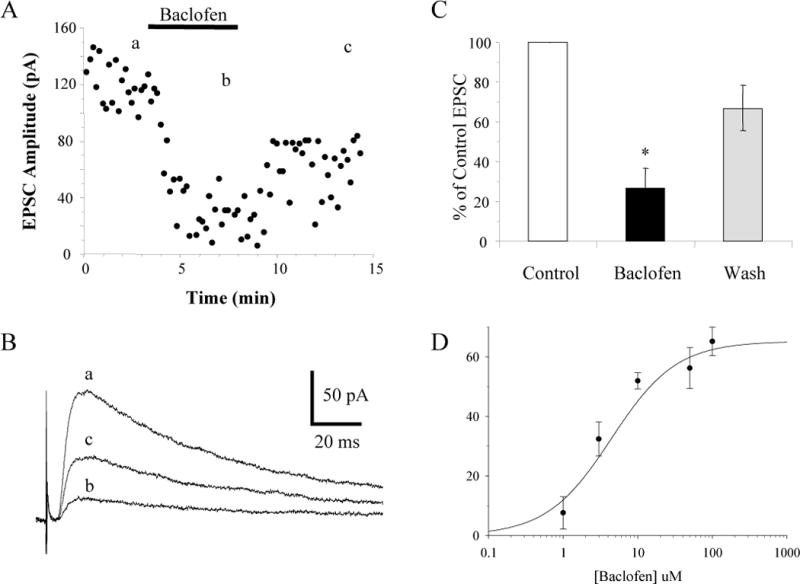
Baclofen inhibits NMDA receptor-mediated thalamocortical EPSCs. A: Time course showing the inhibition and recovery of EPSCs produced by bath application of 10 μM baclofen that were measured at + 60 mV in the presence of bicuculline and CNQX in a morphologically-identified layer IV inhibitory neuron. B: Averages of 10 consecutive EPSCs taken at the times indicated in A. C: The average NMDA receptor-mediated thalamocortical EPSC expressed as a percent of the control EPSC in cortical neurons (n = 4) before, during, and after (Wash) application of 10 μM baclofen. * p < 0.05. D: Concentration-response relationship of baclofen-mediated inhibition of thalamocortical EPSCs. All cells were recorded with intracellular Cs+ in the pipette.
Since the previous article (Gil et al. 1997) used 1 μM baclofen and we were using 10 μM baclofen, we decided to test the concentration-dependency of the baclofen-mediated inhibition of thalamocortical EPSCs. A concentration-response curve generated by applying different concentrations of baclofen gave an EC50 of 4.4 μM with an average inhibition of only 8 ± 5% produced by 1 μM baclofen (Fig. 7D). These results are consistent with the EC50 of baclofen-mediated inhibition of hippocampal EPSCs (Lei and McBain 2003; Wu and Saggau 1995). Therefore the apparent discrepancy between our results and the previous study can be accounted for by the difference in the concentrations of baclofen.
DISCUSSION
Our main finding is that GABAB receptors on thalamocortical terminals modulate thalamic excitation of cortical neurons in layers II-III and IV. This is an important finding since it provides another mechanism whereby cortical inhibition can modulate the processing of sensory information and perhaps even plasticity, and it is the first demonstration that afferent fibers in the cortex can be modulated by GABAB receptors. Since baclofen equally inhibited thalamocortical EPSCs onto excitatory and inhibitory neurons the targeting of GABAB receptors to thalamocortical synapses is independent of the type of postsynaptic cell. The reduction of thalamic stimulation of cortical excitatory and inhibitory circuits suggests that GABAB receptors play a prominent role in the modulation of sensory input.
Our experiments demonstrate the existence of GABAB receptors presynaptically on the thalamocortical terminals. Stimulation of postsynaptic GABAB receptors does not mediate the inhibition of thalamocortical EPSCs produced by baclofen, since blocking the postsynaptic effects of baclofen with intracellular Cs+ did not decrease the baclofen-mediated inhibition of evoked EPSCs in either the excitatory or the inhibitory neurons. Furthermore, with and without Cs+ in the pipette, baclofen induced an increase in both the PPR and the CV of the EPSCs which are indicative of a reduction in neurotransmitter release (Ohana and Sakmann 1998; Zucker 1989). The lack of effect of intracellular Cs+ together with the increase in PPR and CV suggest that the stimulation of GABAB receptors on presynaptic thalamocortical terminals produces the reduction of EPSCs. Presynaptic GABAB receptors have been shown to reduce the release of glutamate from a variety of different synapses (Batchelor and Garthwaite 1992; Chen and Regehr 2003; Gaiarsa et al. 1995; Isaacson 1998; Kombian et al. 1996; Lei and McBain 2003; Morishita and Sastry 1995; Nisenbaum et al. 1992; Wu and Saggau 1995; Yamada et al. 1999) including intracortical glutamatergic synapses in the cerebral cortex (Chu and Hablitz 2003; Fukuda et al. 1993; Gil et al. 1997; Zilberter et al. 1999).
The results of present study represent the first demonstration that afferents within the cortex can be modulated by presynaptic GABAB receptors. Previous studies have demonstrated that perforant path synapses in the hippocampus (Colbert and Levy 1992) and afferent fibers in the olfactory cortex (Tang and Hasselmo 1994) are not modulated by GABAB receptors. Since baclofen does modulate the intrinsic fibers of both of these brain regions, it has been proposed that GABAB receptors are selectively targeted to the intracortical synaptic terminals (Hasselmo 1995). Based on our results, the models of how GABAB receptors regulate the processing of incoming afferent signals should be modified to include the inhibition of sensory afferents in the primary somatosensory cortex. The structural similarities between the olfactory cortex and the hippocampus (Tang and Hasselmo 1994) suggest the possibility that GABAB receptors may be selectively targeted to intrinsic fibers only in cortical areas which lack the six layer structure of the primary somatosensory cortex. However, the selective modulation of intrinsic fibers in the primary somatosensory cortex may still occur since the intracortical fibers (Gil et al. 1997) were inhibited by lower concentrations of baclofen than the thalamocortical fibers.
In conclusion, our results demonstrate that glutamate release from thalamocortical synapses onto both inhibitory layer IV neurons and excitatory neurons in layers II-III and IV is regulated by the activity of presynaptic GABAB receptors. This mechanism could account for the in vivo observations that indicate that GABAB receptors are involved in the processing of sensory information in the primary somatosensory cortex (Kaneko and Hicks 1988; Kaneko and Hicks 1990). A reduction in glutamate release from thalamocortical terminals would preferentially suppress weak and longer latency inputs leading to a reduction in receptive field sizes (Chowdhury and Rasmusson 2002a, b; Kyriazi et al. 1996a). For example the prior stimulation of an adjacent whisker inhibits the cortical response to the principle whisker (Simons 1985; Simons and Carvell 1989). A recent study indicates that the stimulation of adjacent whiskers reduces the thalamocortical EPSP by a mechanism other than hyperpolarization or shunting inhibition (Higley and Contreras 2003). The activation of presynaptic GABAB receptors by the stimulation of the adjacent whisker could explain these findings, since the presynaptic inhibition of glutamate release would cause a reduction in the EPSP without changing the membrane potential or input resistance of the cortical neuron. Consistent with this hypothesis, blocking GABAB receptors reduces adjacent whisker-mediated inhibition (Ajima et al. 1999; Chowdhury and Rasmusson 2003).
Acknowledgments
We are very grateful to Aric Agmon, Etienne Audinat, Franck Debarbieux, and Gregory Quirk for their many useful comments on an earlier version of the manuscript. The National Institutes of Health Grant S06 GM08239 supported this work. Statistical support for this project was provided by the Epidemiology and Biostatistics Core Program of the Ponce School of Medicine which is funded by grant number 2G12RR03050-19 from NIH.
References
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Agmon A, Hollrigel G, O’Dowd DK. Functional GABAergic synaptic connection in neonatal mouse barrel cortex. J Neurosci. 1996;16:4684–4695. doi: 10.1523/JNEUROSCI.16-15-04684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A, O’Dowd DK. NMDA receptor-mediated currents are prominent in the thalamocortical synaptic response before maturation of inhibition. J Neurophysiol. 1992;68:345–349. doi: 10.1152/jn.1992.68.1.345. [DOI] [PubMed] [Google Scholar]
- Ajima A, Matsuda Y, Ohki K, Kim DS, Tanaka S. GABA-mediated representation of temporal information in rat barrel cortex. Neuroreport. 1999;10:1973–1979. doi: 10.1097/00001756-199906230-00033. [DOI] [PubMed] [Google Scholar]
- Alloway KD, Rosenthal P, Burton H. Quantitative measurements of receptive field changes during antagonism of GABAergic transmission in primary somatosensory cortex of cats. ExpBrain Res. 1989;78:514–532. doi: 10.1007/BF00230239. [DOI] [PubMed] [Google Scholar]
- Amitai Y. Thalamocortical synaptic connections: efficacy, modulation, inhibition and plasticity. Rev Neurosci. 2001;12:159–173. doi: 10.1515/revneuro.2001.12.2.159. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Garthwaite J. GABAB Receptors in the Parallel Fibre Pathway of Rat Cerebellum. Eur J Neurosci. 1992;4:1059–1064. doi: 10.1111/j.1460-9568.1992.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Connors BW. Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. J Neurophysiol. 2002;88:1924–1932. doi: 10.1152/jn.2002.88.4.1924. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Presynaptic modulation of the retinogeniculate synapse. J Neurosci. 2003;23:3130–3135. doi: 10.1523/JNEUROSCI.23-08-03130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Comparison of receptive field expansion produced by GABA(B) and GABA(A) receptor antagonists in raccoon primary somatosensory cortex. Exp Brain Res. 2002a;144:114–121. doi: 10.1007/s00221-002-1035-7. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Corticocortical inhibition of peripheral inputs within primary somatosensory cortex: the role of GABA(A) and GABA(B) receptors. J Neurophysiol. 2003;90:851–856. doi: 10.1152/jn.01059.2002. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Effect of GABAB receptor blockade on receptive fields of raccoon somatosensory cortical neurons during reorganization. Exp Brain Res. 2002b;145:150–157. doi: 10.1007/s00221-002-1130-9. [DOI] [PubMed] [Google Scholar]
- Chu Z, Hablitz JJ. GABA(B) receptor-mediated heterosynaptic depression of excitatory synaptic transmission in rat frontal neocortex. Brain Res. 2003;959:39–49. doi: 10.1016/s0006-8993(02)03720-4. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Levy WB. Electrophysiological and pharmacological characterization of perforant path synapses in CA1: mediation by glutamate receptors. J Neurophysiol. 1992;68:1–8. doi: 10.1152/jn.1992.68.1.1. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons [see comments] Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Dykes RW, Landry P, Metherate R, Hicks TP. Functional role of GABA in cat primary somatosensory cortex: shaping receptive fields of cortical neurons. J Neurophysiol. 1984;52:1066–1093. doi: 10.1152/jn.1984.52.6.1066. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. J Physiol. 1999;521(Pt 1):169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Mody I, Prince DA. Differential ontogenesis of presynaptic and postsynaptic GABAB inhibition in rat somatosensory cortex. J Neurophysiol. 1993;70:448–452. doi: 10.1152/jn.1993.70.1.448. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Tseeb V, Ben-Ari Y. Postnatal development of pre- and postsynaptic GABAB-mediated inhibitions in the CA3 hippocampal region of the rat. J Neurophysiol. 1995;73:246–255. doi: 10.1152/jn.1995.73.1.246. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gil Z, Amitai Y. Properties of convergent thalamocortical and intracortical synaptic potentials in single neurons of neocortex. J Neurosci. 1996;16:6567–6578. doi: 10.1523/JNEUROSCI.16-20-06567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Nonlinear integration of sensory responses in the rat barrel cortex: an intracellular study in vivo. J Neurosci. 2003;23:10190–10200. doi: 10.1523/JNEUROSCI.23-32-10190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. GABAB receptor-mediated modulation of presynaptic currents and excitatory transmission at a fast central synapse. J Neurophysiol. 1998;80:1571–1576. doi: 10.1152/jn.1998.80.3.1571. [DOI] [PubMed] [Google Scholar]
- Jarolimek W, Bijak M, Misgeld U. Differences in the Cs block of baclofen and 4-aminopyridine induced potassium currents of guinea pig CA3 neurons in vitro. Synapse. 1994;18:169–177. doi: 10.1002/syn.890180302. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Hicks TP. Baclofen and gamma-aminobutyric acid differentially suppress the cutaneous responsiveness of primary somatosensory cortical neurones. Brain Res. 1988;443:360–366. doi: 10.1016/0006-8993(88)91634-4. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Hicks TP. GABA(B)-related activity involved in synaptic processing of somatosensory information in S1 cortex of the anaesthetized cat. Br J Pharmacol. 1990;100:689–698. doi: 10.1111/j.1476-5381.1990.tb14077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Zidichouski JA, Pittman QJ. GABAB receptors presynaptically modulate excitatory synaptic transmission in the rat supraoptic nucleus in vitro. J Neurophysiol. 1996;76:1166–1179. doi: 10.1152/jn.1996.76.2.1166. [DOI] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Effects of baclofen and phaclofen on receptive field properties of rat whisker barrel neurons. Brain Res. 1996a;712:325–328. doi: 10.1016/0006-8993(95)01562-0. [DOI] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Quantitative effects of GABA and bicuculline methiodide on receptive field properties of neurons in real and simulated whisker barrels. J Neurophysiol. 1996b;75:547–560. doi: 10.1152/jn.1996.75.2.547. [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. GABA B receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol. 2003;546:439–453. doi: 10.1113/jphysiol.2002.034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K + channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Meinecke DL, Peters A. GABA immunoreactive neurons in rat visual cortex. J Comp Neurol. 1987;261:388–404. doi: 10.1002/cne.902610305. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. An anatomical substrate for experience-dependent plasticity of the rat barrel field cortex. Proc Natl Acad Sci U S A. 1995;92:11834–11838. doi: 10.1073/pnas.92.25.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Sastry BR. Pharmacological characterization of pre- and postsynaptic GABAB receptors in the deep nuclei of rat cerebellar slices. Neuroscience. 1995;68:1127–1137. doi: 10.1016/0306-4522(95)00206-x. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Berger TW, Grace AA. Presynaptic modulation by GABAB receptors of glutamatergic excitation and GABAergic inhibition of neostriatal neurons. J Neurophysiol. 1992;67:477–481. doi: 10.1152/jn.1992.67.2.477. [DOI] [PubMed] [Google Scholar]
- Ohana O, Sakmann B. Transmitter release modulation in nerve terminals of rat neocortical pyramidal cells by intracellular calcium buffers. J Physiol. 1998;513:135–148. doi: 10.1111/j.1469-7793.1998.135by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe HR, Karlsson G, Pozza MF, Brugger F, Steinmann M, Van Riezen H, Fagg G, Hall RG, Froestl W, Bittiger H. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990;187:27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci. 1999;19:5228–5235. doi: 10.1523/JNEUROSCI.19-13-05228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE. Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J Neurocytol. 1978;7:461–478. doi: 10.1007/BF01173991. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Temporal and spatial integration in the rat SI vibrissa cortex. J Neurophysiol. 1985;54:615–635. doi: 10.1152/jn.1985.54.3.615. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol. 1984;230:119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Kisvarday ZF, Martin KA, Whitteridge D. Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience. 1983;10:261–294. doi: 10.1016/0306-4522(83)90133-1. [DOI] [PubMed] [Google Scholar]
- Tang AC, Hasselmo ME. Selective suppression of intrinsic but not afferent fiber synaptic transmission by baclofen in the piriform (olfactory) cortex. Brain Res. 1994;659:75–81. doi: 10.1016/0006-8993(94)90865-6. [DOI] [PubMed] [Google Scholar]
- Welker E, Soriano E, Dorfl J, Van der Loos H. Plasticity in the barrel cortex of the adult mouse: transient increase of GAD-immunoreactivity following sensory stimulation. ExpBrain Res. 1989;78:659–664. doi: 10.1007/BF00230256. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2 + influx. J Physiol (Lond) 1995;485:649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Yamada J, Saitow F, Satake S, Kiyohara T, Konishi S. GABA(B) receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology. 1999;38:1743–1753. doi: 10.1016/s0028-3908(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Zilberter Y, Kaiser KM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]


