Abstract
Background
Controversy exists about the safety of substituting generic anti-epileptic drugs (AEDs). Lamotrigine, the prototypical newer AED, is often used for psychiatric and neurological conditions other than epilepsy. The safety of generic substitution of lamotrigine in diverse populations of AED users is unclear.
Objective
The objective of this study was to evaluate potential associations between generic substitution of lamotrigine and adverse consequences in a population of diverse users of this drug.
Study Design
This study was a retrospective cohort-crossover design using state Medicaid claims data from July 2006 through June 2009.
Methods
Subjects were included in the cohort if they converted from brand to generic lamotrigine and had 2 years of lamotrigine use prior to conversion. The frequency of emergency department (ED) visits, hospitalizations and condition-specific ED visits or hospitalizations were recorded in the 60 days immediately following the conversion to generic lamotrigine, then compared with the incidence of the same events during a randomly selected time period indexed to one of the patient’s past refills of branded lamotrigine. Multivariate conditional logistic regression was used to quantify the association between generic conversion and health services utilization while controlling for changes in lamotrigine dose and concurrent drug use.
Results
Of the 616 unique subjects included in this analysis, epilepsy was the most common diagnosis (41%), followed by bipolar disorder (32%), pain (30%) and migraine (18%). Conversion to generic lamotrigine was not associated with a statistically significant increase in the odds of an ED visit (adjusted odds ratio [AOR] = 1.35; 95% confidence interval [CI] 0.92, 1.97), hospitalization (AOR = 1.21; 95% CI 0.60, 2.50) or condition-specific encounter (AOR 1.75; 95 CI 0.87, 3.51).
Conclusions
A statistically significant increase in ED visits, hospitalizations or condition-specific encounters was not observed following the switch from brand to generic lamotrigine, although a type II error cannot be ruled out.
Introduction
Promotion of generic prescription drugs is a major strategy used to manage the rising costs of medications.[1,2] The US FDA requires generic manufacturers to provide pharmacokinetic evidence that their generic product demonstrates plasma level profile characteristics very similar (i.e. bioequivalent) to the originator drug in order to be considered therapeutically equivalent.[3] Specifically, bioequivalence of two products is established when 90% confidence intervals (CI) for the means of the ratios of the maximum plasma drug concentration (Cmax) and the area under the drug plasma concentration-time curve (AUC) fall within 80–125%.[3] A review of 2070 studies from completed FDA applications for approved generics shows mean differences in Cmax and AUC are 4.4% (SD = 3.5) and 3.6% (SD = 2.9), respectively.[4] Clinical evidence suggests important differences between most branded and generic drugs are negligible.[5]
Significant controversy exists over generic substitution of antiepileptic drugs (AED).[6] Because small changes in AED plasma concentrations can impact seizure control as well as toxicity in patients with epilepsy, lingering concerns about true bioequivalence exists.[6–12] Many contend that even minor absorption and kinetic differences can elicit a breakthrough seizure in patients who are converted to a generic AED.[13] In response to these concerns, the American Academy of Neurology has issued a policy statement opposing generic substitution of AEDs for the treatment of epilepsy without physician approval.[14] However, the evidence supporting this policy and clinical assertion has been mixed and mostly based on anecdotal and observational epidemiological research.[15–18]
Propelled by the increase in prescribing for conditions other than epilepsy, newer AEDs such as gabapentin, lamotrigine and topiramate have become major expenses for healthcare payers.[19] In recent years many newer AEDs have lost patent protection, and opportunities for significant savings by payers and consumers exist.[20] Lamotrigine (brand name Lamictal®; GlaxoSmithKline, Research Triangle Park, NC, USA) is a newer AED that is FDA approved for the treatment of epilepsy and bipolar disorder. It also has gained significant off-label use for pain, migraine prophylaxis and fibromyalgia.[21,22] In July 2008, a generic competitor of Lamictal® was approved and released for marketing. While large economic incentives to develop and support policies permitting generic substitution exist, there is a critical need to evaluate the safety of such policies in diverse populations of AED users. To this end, we conducted a retrospective cohort-crossover study aimed at assessing potential adverse outcomes of generic substitution of lamotrigine for diverse indications among patients enrolled in the Oregon Medicaid program.
Methods
The cohort was constructed using administrative claims data from the Oregon Medicaid program between July 2006 and June 2009 (3 years inclusive). Patients converting from branded to generic lamotrigine were considered for inclusion, and the first date a patient filled a prescription for generic lamotrigine was considered their conversion date. Subjects were selected for cohort inclusion if they had 2 or more years of sustained lamotrigine use. We required patients to be chronic users in order to ensure they had sufficient historical brand lamotrigine use for the crossover period. Sustained use was defined by calculating each subject’s proportion of days covered (i.e. interval between first and last fill date plus last day supply divided by the total days supply dispensed during the interval). We excluded subjects if they had any gap in therapy more than 31 days or had a proportion of days covered <80% during the study period. Subjects were required to maintain Medicaid enrolment for a minimum of 60 days following the conversion date. The control time period was indexed relative to a randomly selected branded lamotrigine fill (control index date) at least 120 days prior to generic conversion. We indexed the control period to a drug fill date to better mirror healthcare utilization that is purely associated with pharmacy filling. While most previous case-control studies used a 180-day exposure window, we selected a 60-day exposure window to be more compatible with the cohort-crossover design. The cohort-crossover and case-crossover studies are best suited for exposure outcome relationships that are transient and acute in nature.[23,24] We also conducted a sensitivity analysis that did not require subjects to have sustained use of lamotrigine to assess the robustness of our primary analysis. For this analysis, subjects were required to have at least one pharmacy or medical encounter claim in each quarter for 2 years prior to generic conversion. This criterion was applied to ensure subjects were enrolled and utilizing Medicaid members during the control period.
Adverse medical encounters in the 60 days following generic lamotrigine conversion were compared with 60 days following the randomly selected historical control period. Adverse medical encounters were defined as an emergency department (ED) visit, hospitalization, combined ED visit or hospitalization, epilepsy-related ED visit or hospitalization, or any ED visit or hospitalization with a diagnosis that might be treated with lamotrigine (condition-related). Encounters in the ED were identified as medical claims with the following Current Procedural Terminology codes: 99281–99285, 99288. Hospitalizations were defined using claims paid through a Diagnosis-Related Group code. We also identified ED encounters and hospitalizations for conditions possibly treated with lamotrigine. Specific diagnoses were identified using the International Classification of Diseases, Ninth Revision (ICD-9) coding. Encounters for epilepsy were defined using ICD-9 codes 345.xx. Other conditions where lamotrigine might be prescribed (both on and off-label) include bipolar disorder (296.0x, 296.1, 296.4x, 296.5x, 296.6x, 296.7x, 296.8x, 296.9x), neuropathic pain (350.xx, 357.2x, 250.6x), other types of pain such as back pain, fibromyalgia (724.x, 729.1x, 338.x), migraine and headache (346.x, 784.0x) and depression (296.2x, 296.3x, 311, 296.82, 296.9x, 309.0x, 309.1x).
While subjects served as their own comparator, we attempted to control for changes in co-morbidity over time by quantifying various patient features in the 60 days prior to both the conversion event and the random historical control span. We refer to these lead-up times as ‘baseline’ periods, with the ‘control baseline’ and the ‘conversion baseline’ referring to the 60 days prior to the control period and conversion period, respectively. Within these baseline periods, we quantified lamotrigine dose and concurrent medication usage. The average daily lamotrigine dose was computed for each baseline period using the drug strength field, day supply and quantities dispensed field (daily dose = (strength*quantity dispensed)/day supply). Other psychotropic medication use including anxiolytics (benzodiazepines and buspirone), other AEDs (carbamazepine, valproic acid, gabapentin, levetiracetam, oxcarbazepam, phenytoin, topiramate and pregabalin), antidepressants (older tricyclic antidepressants [TCAs], selective serotonin reuptake inhibitors [SSRIs] and selective noradrenaline (norepinephrine) inhibitors [SNRIs]), second-generation antipsychotics, mood stabilizers (lithium) and drugs for migraine treatment (triptans) were also quantified for the baseline periods. Finally, we quantified co-morbid conditions of the cohort during the baseline periods using the diagnosis codes outlined above. We did not determine incident diagnosis dates for these conditions and assumed patients were affected uniformly from cohort entrance to end.
The unit of analysis was the matched periods for each subject; thus paired analyses were used for all comparisons. Categorical data between the generic conversion period and the control period were compared using McNemar’s chi-square test. Continuous data were compared using paired Student’s t-test or Wilcoxon signed-rank test where appropriate. Conditional logistic regression models with a backwards stepwise algorithm (p-value to stay <0.05) were used to evaluate the association between cohort periods and the incidence of adverse outcomes of interest while statistically controlling for concurrent drug use and lamotrigine dose during the baseline period. Identical statistical procedures were performed on the expanded cohort for the sensitivity analysis. Data were managed using Microsoft3 Access (Microsoft Corporation, Redmond, WA, USA) and statistical analyses completed using SAS® (version 9.2; SAS Institute Inc., Cary, NC, USA). This study was approved by the Oregon State University Institutional Review Board and by the Oregon Department of Human Services.
Results
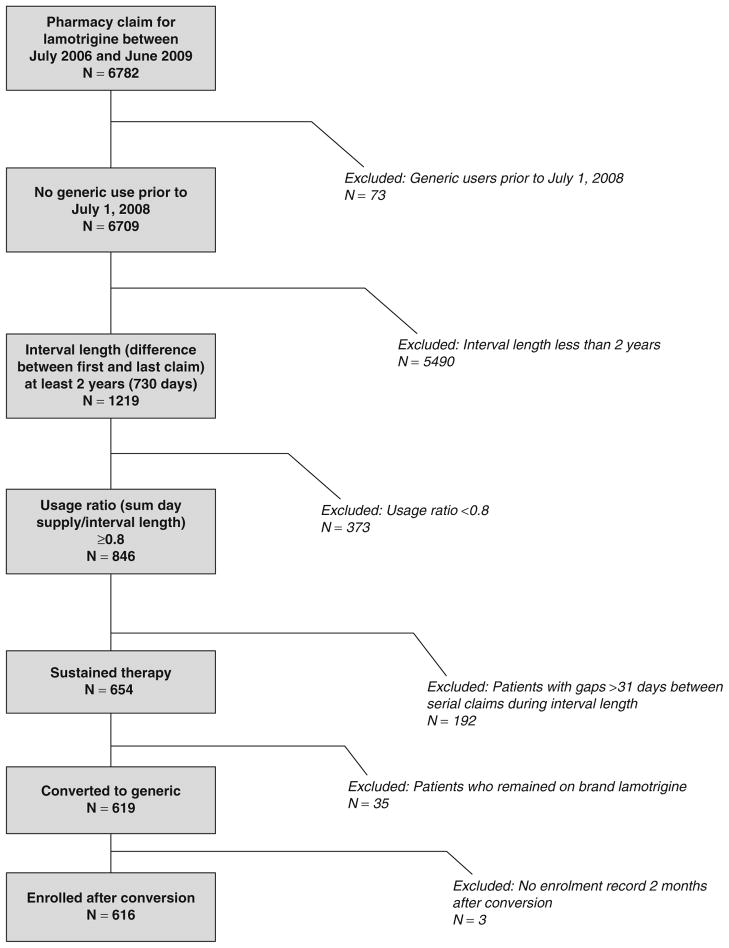
Between July 2006 and June 2009, 6782 patients had at least one prescription filled for lamotrigine. Of this group, 616 patients met the inclusion criteria and were included in the study. Figure 1 shows how subjects were eliminated from the study. Table I shows the demographic and clinical characteristics of the study sample and the underlying lamotrigine user population. While the study sample was similar to the entire lamotrigine user population with respect to age, sex and race, substantial differences in co-morbid psychiatric or neurological conditions were present. A diagnosis of epilepsy was more than twice as common in the study group relative to the entire user population (41% vs 18%). Conversely, the prevalence of bipolar disorder was twice as high in the entire population relative to the study sample (65% vs 32%). Diagnoses of depression (28% vs 16%), migraine (21% vs 18%) and non-neuropathic pain (34% vs 30%) were also more common in the underlying population compared with the study sample.
Fig. 1.
Study subject inclusion and exclusion criteria flow diagram.
Table I.
Study sample and source population demographics
| Characteristic | Study sample
|
Population
|
||
|---|---|---|---|---|
| N = 616
|
N = 6782
|
|||
| n | % | n | % | |
| Sex (female) | 406 | 65.9 | 5048 | 74.4 |
| Race | ||||
| White | 547 | 88.8 | 5765 | 85.0 |
| Hispanic | 41 | 6.7 | 632 | 9.3 |
| Native American | 16 | 2.6 | 154 | 2.3 |
| African-American | 10 | 1.6 | 182 | 2.7 |
| Other | 2 | 0.3 | 49 | 0.7 |
| Diagnoses | ||||
| Epilepsy | 255 | 41.4 | 1188 | 17.5 |
| Bipolar disorder | 196 | 31.8 | 4401 | 64.9 |
| Depression | 99 | 16.1 | 1916 | 28.3 |
| Migraine | 112 | 18.2 | 1406 | 20.7 |
| Neuropathic pain | 17 | 2.8 | 137 | 2.0 |
| Other pain | 182 | 29.5 | 2303 | 34.0 |
| Age (mean [SD], years) | 35.6 [15.5] | 34.37 [14.27] | ||
| Treatment length (mean [SD], months) | 32.8 [4.2] | 10.93 [12.19] | ||
The use of concurrent medicines was common but not statistically significantly different between conversion and control periods. Table II shows the use of related medicines in both baseline periods by class. Over 50% of study subjects had evidence of at least five or more concurrent medicines filled during the two baseline periods. The most common concurrent medications were antidepressants (51.3–51.8%), followed by anxiolytics (39.9–41.2%), antipsychotics (39.8–40.9%) and other AEDs (32.0–33.6%). While the absolute numbers were low, the use of mood stabilizers (i.e. lithium) was statistically significantly higher during the conversion period relative to the control period (5.5% vs 4.1%; p = 0.0027). The average daily dose of lamotrigine declined slightly from 282.7 mg to 281.3 mg from 60 days prior to generic conversion to the 60 days following conversion. The average daily lamotrigine dose increased slightly from the control baseline period (250.5 mg) to control observation period (259.2 mg). The net difference in this change was a 10.0 mg/day increase and was statistically significant.
Table II.
Concurrent medication use and lamotrigine dosing in conversion and control period (n = 1092)
| Characteristic | Conversion period
|
Control period
|
p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Concurrent drug therapy | |||||
| Anxiolytics | 246 | 39.9 | 254 | 41.2 | 0.3524 |
| Antidepressants | 319 | 51.8 | 316 | 51.3 | 0.6547 |
| Antiepileptic drugs | 207 | 33.6 | 197 | 32.0 | 0.1573 |
| Antipsychotics | 245 | 39.8 | 252 | 40.9 | 0.2858 |
| Mood stabilizers | 34 | 5.5 | 25 | 4.1 | 0.0027 |
| Triptans | 14 | 2.3 | 13 | 2.1 | 0.7055 |
| >5 concurrent prescriptions | 309 | 50.2 | 317 | 51.5 | 0.4927 |
| Mean prescription fill count [SD] | 7.44 [7.2] | 7.74 [7.5] | 0.9635 | ||
| Mean lamotrigine daily dose [SD] | |||||
| Baseline | 282.7 [199.9] | 250.5 [162.6] | |||
| Observation | 281.3 [170.1] | 259.2 [164.4] | |||
| Change in dose | −1.4 | 114.9 | 8.7 | 40.2 | |
| Difference in changes | 10.0 | 121.7 | <0.0001 | ||
Table III shows the results of our univariate analysis and conditional multivariate logistic regression models. The risk of all ED encounters following generic conversion was 15% compared with 13% in the control period (adjusted odds ratio = 1.35; 95% CI 0.92, 1.97). The rate of all-cause hospitalizations was 2.9% following generic conversion and 2.4% in the control period (adjusted odds ratio = 1.21; 95% CI 0.60, 2.46). Conversion to generic lamotrigine was not associated with a statistically significant increase in the risk of an epilepsy-related ED or hospitalization (adjusted odds ratio = 1.25; 95% CI 0.49, 3.17) or an ED or hospitalization for any condition possibly treated with lamotrigine (adjusted odds ratio = 1.75; 95% CI 0.87, 3.51). When we stratified our analysis by patients with or without epilepsy, similar estimates of associations were observed.
Table III.
Univariate and multivariate analyses of adverse events in conversion and control period (n = 1092)
| Adverse event | Conversion period
|
Control period
|
p-Value | Adjusted odds ratio | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| All-cause ED | 93 | 15.1 | 80 | 13.0 | 0.23 | 1.35 | 0.92, 1.97 | 0.13 |
| All-cause hospital | 18 | 2.9 | 15 | 2.4 | 0.59 | 1.21 | 0.60, 2.46 | 0.59 |
| All-cause ED/hospital | 98 | 15.9 | 89 | 14.4 | 0.41 | 1.16 | 0.81, 1.67 | 0.41 |
| Epilepsy ED/hospital | 12 | 1.9 | 10 | 1.6 | 0.64 | 1.25 | 0.49, 3.17 | 0.64 |
| Condition-related ED/hospital | 27 | 4.4 | 19 | 3.1 | 0.21 | 1.75 | 0.87, 3.51 | 0.12 |
| All-cause ED/hospital | ||||||||
| Epilepsy (n = 255) | 46 | 18.0 | 44 | 17.3 | 0.80 | 1.07 | 0.64, 1.81 | 0.79 |
| Non-epilepsy (n = 361) | 52 | 14.4 | 45 | 12.5 | 0.38 | 1.68 | 0.95, 2.98 | 0.08 |
ED = emergency department.
Results from our sensitivity analysis in a less restrictive population of 1092 lamotrigine users during the same period were similar to primary analysis. Adjusted odds ratio for all-cause ED encounters or hospitalizations in the expanded study sample was 1.18 (95% CI 0.92, 1.53) compared with 1.16 (95% CI 0.81, 1.67) in the primary sample. The results of this expanded analysis are shown in the Appendix.
Discussion
In this observational cohort-crossover study, we were unable to find evidence of a statistically significant increase in adverse events following conversion to generic lamotrigine. While several 95% CIs included estimates of up to a 3-fold increase in odds of adverse encounters and cannot be altogether discounted, the point estimates suggest that any additional risk is low or modest. This study consisted of patients with sustained Medicaid enrolment and chronic lamotrigine use. Because of the co-morbidity and disability inherent in such a cohort, it is likely that these individuals would be the most sensitive to small changes in formulation following generic substitution. Within the larger lamotrigine-using population during this period, nearly two-thirds of patients had a diagnosis of bipolar disorder, a condition where dosing is typically not as high and therapeutic drug monitoring not usually recommended.[25] Less than half of the cohort in this study had a diagnosis of epilepsy. Stratified analyses of patients with epilepsy were consistent with the overall findings. Results from our sensitivity analysis were similar to the primary study sample.
A qualitative and quantitative systematic review of the evidence comparing generic with branded AEDs by Kesselheim et al.[15] has been recently conducted. In their synthesis of both randomized and observational studies, conflicting evidence emerged about risks of conversion-related adverse events. The observational studies included in this review generally demonstrated significant safety problems associated with generic AEDs. Case-control studies by Rascati et al.,[26] Zachry et al.[27] and Hansen et al.[28] all demonstrate an association between seizure-related events and generic AED conversion with odds ratios ranging from 1.57 to 1.81. However, another case-control study with arguably better co-morbidity adjustment did not find a statistically significant increase in risk associated with generic conversion (adjusted odds ratio = 1.08; 95% CI 0.91, 1.3).[29] Observational cohort studies provide similar conflicting evidence. LeLorier et al.[30,31] and Duh et al.[32] conducted a series of studies that investigated the impact of generic substitution of AEDs, specifically lamotrigine and topiramate, on healthcare utilization and costs among those with epilepsy. In their analyses, subjects who switched to a generic AED were more likely to switch back to the brand compared with non-AED generic switchers. Additionally, periods of generic AED use were associated with increased healthcare utilization. A similar observation in a broader class of AEDs among those with epilepsy has been put forth by Labiner et al.[33] However, work by Erickson et al.[34] failed to demonstrate that switching from branded lamotrigine or divalproex was associated with an increased risk of ED use or hospitalization. A major limitation of existing pharmacoepidemiology research is that patients and providers may have preconceived negative connotations about the effectiveness of generic AEDs that results in anxiety or stress that can trigger a seizure.[11] The so-called ‘nocebo effect’ may also contribute to patients being more self-aware of adverse effects or other symptoms such as changes in seizure activity. In contrast, data from prospective crossover trials, while limited, have yet to demonstrate conclusively a loss of seizure control following conversion from a branded AED to its generic.[15] Among 16 randomized trials evaluated, the evidence was equivocal about the superiority of branded versus generic AEDs, although many of these trials were small and involved older AEDs such as carbamazepine. Another limitation of the existing AED literature is that it is primarily confined to populations with epilepsy while most newer AEDs are used for other psychiatric and neurological conditions ranging from bipolar disease to migraine prophylaxis and neuropathic pain.[21,22]
Our study is consistent in approach and findings to a recent study by Gagne et al.[35] who used pharmacy and medical data from British Columbia, Canada to evaluate the association between generic switching and refilling of AEDs and seizure events. In their case-crossover study, refilling the same AED (from the same manufacturer) in and of itself was associated with a 2-fold increase (odds ratio = 2.31; 95% CI 1.56, 3.44) in the odds of having a seizure among subjects with epilepsy. After controlling for the risk inherent with refilling an AED medication, these investigators found that switching to a different manufacturer was not associated with a statistically significant increase in the risk of seizure events (odds ratio 1.19; 95% CI 0.35, 3.99).
The retrospective cohort-crossover study is analogous to both an observational case-crossover study as well as prospective crossover designs. The case-crossover study was initially developed for the purpose of avoiding many of the biases inherent in selection of controls.[23] Rather than selecting traditional controls, cases serve as their own control at a different time point.[24] The design is best suited to evaluate the association between transient exposures and acute outcomes while controlling for confounders, known and unknown, that are relatively stable over time. However, like all observational studies, the cohort-crossover study is limited by lack of randomization and blinding. Non-statistically significant, yet positive associations between generic conversion and utilization observed in our study may reflect this potential for bias.
Our study has several other limitations that need to be acknowledged. This study is based on administrative pharmacy and medical databases not intended for research. Both cohort-defining exposure and outcome variables are subject to misclassification. While pharmacy data are typically recognized as a good approximation of medication use, medical encounter data do not always reflect true clinical outcomes and co-morbidity.[36] Because the analysis was based on administrative data it seems likely that any mis-classification would be random and bias towards the null hypothesis. We employed an observational analogue of the crossover trial design in an attempt to control for differences in co-morbidity between distinct cohorts of generic switchers and non-switchers. It is possible that over time an individual’s disease severity may increase, thus increasing an individual’s risk for an adverse event. This would result in the conversion period being associated with a higher frequency of medical encounters, thus biasing towards an increased odds ratio. Furthermore, because subjects were followed for 2 years through generic conversion, the selected cohort by definition did not experience a fatal adverse event during the control period, or was not lost to follow-up for any other reason. This artificial construct would likely bias towards increased co-morbidity and associated utilization in the distal observation period. Our primary findings were not statistically significant and could be explained by a type II error. The 95% CIs for several estimates of association include up to a 3-fold increase in risk for an adverse event. While the merits of post hoc power analyses are debatable, we estimate our study had power sufficient to detect a 55% increase in the odds of an ED encounter (1−β = 0.8; control period event rate = 0.13).[37] Our sensitivity analysis using less restrictive enrolment criteria would have had power to detect a 37% increase in the odds of an ED encounter (1−β = 0.8; control period event rate = 0.16). These estimates of risk increase are similar to those found in other observational studies (odds ratios ~1.8).[15] Finally, our results may have limited generalizability due to the Medicaid population from which the cohort was derived. Because patients enrolled in Medicaid, especially those with prolonged enrolment, are typically comprised of poor, elderly or disabled individuals, our findings may be less applicable to other less severely ill populations (e.g. employee-sponsored healthcare plans, Medicare).
Conclusions
In summary, our results contrast with case-control studies by Zachry et al.,[26] Rascati et al.[27] and Hansen et al.[28] who observed a statistically significant association between seizure events and a switch in AED formulation. While the precise reason for this discrepancy is unclear, our findings, especially when taken in context with those of Gagne et al.,[35] suggest that conversion to generic lamotrigine is not associated with a statistically significant increase in adverse outcomes. The data from Gagne et al.[35] suggest the act of refilling the same formulation of an AED may predispose or portend a loss of seizure control. However, because our CIs were wide, we cannot exclude the possibility that generic conversion may lead to unintended harm. Despite these limitations, our results offer greater generalizability for patients who are taking this drug for conditions other than epilepsy.
Acknowledgments
This study was unfunded.
Appendix
Expanded sample sensitivity analysis
Table A.
Study sample and source population demographics
| Characteristic | Study sample
|
Population
|
||
|---|---|---|---|---|
| N = 1092
|
N = 6782
|
|||
| n | % | n | % | |
| Sex (female) | 771 | 70.6 | 5048 | 74.4 |
| Race | ||||
| White | 977 | 89.5 | 5765 | 85.0 |
| Hispanic | 66 | 6.0 | 632 | 9.3 |
| Native American | 25 | 2.3 | 154 | 2.3 |
| African-American | 17 | 1.6 | 182 | 2.7 |
| Other | 7 | 0.6 | 49 | 0.7 |
| Diagnoses | ||||
| Epilepsy | 341 | 31.2 | 1188 | 17.5 |
| Bipolar disorder | 425 | 38.9 | 4401 | 64.9 |
| Depression | 191 | 17.5 | 1916 | 28.3 |
| Migraine | 200 | 18.3 | 1406 | 20.7 |
| Neuropathic pain | 38 | 3.5 | 137 | 2.0 |
| Other pain | 357 | 32.7 | 2303 | 34.0 |
| Age (mean [SD], years) | 36.0 [14.8] | 34.37 [14.27] | ||
| Treatment length (mean [SD], months) | 30.7 [6.9] | 10.93 [12.19] | ||
Table B.
Concurrent medication use and lamotrigine dosing in conversion and control period (n = 1092)
| Characteristic | Conversion period
|
Control period
|
p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Concurrent drug therapy | |||||
| Anxiolytics | 455 | 41.7 | 435 | 39.8 | 0.1025 |
| Antidepressants | 596 | 54.6 | 610 | 55.9 | 0.1975 |
| Antiepileptic drugs | 298 | 27.3 | 307 | 28.1 | 0.3173 |
| Antipsychotics | 440 | 40.3 | 442 | 40.5 | 0.8415 |
| Mood stabilizers | 57 | 5.2 | 52 | 4.8 | 0.3532 |
| Triptans | 34 | 3.1 | 29 | 2.7 | 0.2752 |
| Mean prescription fill count [SD] | 7.22 [6.9] | 7.59 [7.4] | 0.0707 | ||
| Count with >5 concurrent prescriptions | 516 | 47.3 | 554 | 50.7 | 0.0142 |
| Mean lamotrigine daily dose [SD] | |||||
| Baseline | 255.8 [171.8] | 224.1 [146.6] | |||
| Observation | 256.8 [152.7] | 234.3 [147.4] | |||
| Change in dose | −1.0 [90.4] | −10.2 [45.2] | |||
| Difference in changes | 9.2 [103.0] | <0.0001 | |||
Table C.
Univariate and multivariate analyses of adverse events in conversion and control period (n = 1092)
| Adverse event | Conversion period
|
Control period
|
p-Value | Odds ratio | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| All-cause ED | 188 | 17.2 | 171 | 15.7 | 0.249 | 1.170 | 0.896, 1528 | 0.2490 |
| All-cause hospital | 37 | 3.4 | 34 | 3.1 | 0.7055 | 1.100 | 0.671, 1.804 | 0.7056 |
| All-cause ED/hospital | 203 | 18.6 | 183 | 16.8 | 0.1948 | 1.183 | 0.917, 1.527 | 0.1954 |
| Epilepsy ED/hospital | 16 | 1.5 | 9 | 0.8 | 0.0896 | 2.400 | 0.846, 6.812 | 0.1000 |
| Indication-related ED/hospital | 44 | 4.0 | 36 | 3.3 | 0.3017 | 1.308 | 0.785, 2.179 | 0.3031 |
| All-cause ED/hospital | ||||||||
| Epilepsy (n = 341) | 64 | 18.8 | 55 | 16.1 | 0.2855 | 1.290 | 0.807, 2.062 | 0.2868 |
| Non-epilepsy (n = 751) | 139 | 18.5 | 128 | 17.0 | 0.3947 | 1.141 | 0.842, 1.546 | 0.3950 |
ED = emergency department.
Footnotes
The authors do not have any conflicts of interest to disclose.
References
- 1.Haas JS, Phillips KA, Gerstenberger EP, et al. Potential savings from substituting generic drugs for brand-name drugs: Medical Expenditure Panel Survey, 1997–2000. Ann Intern Med. 2005;142:891–7. doi: 10.7326/0003-4819-142-11-200506070-00006. [DOI] [PubMed] [Google Scholar]
- 2.Shrank WH, Choudhry NK, Liberman JN, et al. The use of generic drugs in prevention of chronic disease is far more cost-effective than thought, and may save money. Health Aff (Millwood) 2011;30:1351–7. doi: 10.1377/hlthaff.2010.0431. [DOI] [PubMed] [Google Scholar]
- 3.Meredith P. Bioequivalence and other unresolved issues in generic drug substitution. Clin Ther. 2003;25:2875–90. doi: 10.1016/s0149-2918(03)80340-5. [DOI] [PubMed] [Google Scholar]
- 4.Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bio-equivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583–97. doi: 10.1345/aph.1M141. [DOI] [PubMed] [Google Scholar]
- 5.Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease. JAMA. 2008;300:2514–26. doi: 10.1001/jama.2008.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besag FMC. Generic antiepileptic drugs and increased health care utilization. Neurology. 2010;74:1562–3. doi: 10.1212/WNL.0b013e3181e1d4b4. [DOI] [PubMed] [Google Scholar]
- 7.Kesselheim AS. The backlash against bioequivalence and the interchangeability of brand-name and generic drugs. CMAJ. 2011;183:1350–1. doi: 10.1503/cmaj.110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bialer M, Midha KK. Generic products of antiepileptic drugs: a perspective on bioequivalence and interchangeability. Epilepsia. 2010;51:941–50. doi: 10.1111/j.1528-1167.2010.02573.x. [DOI] [PubMed] [Google Scholar]
- 9.Mintzer S. Brand spanking? The presumptive risks of generic antiepileptic drugs. Epilepsy Curr. 2011;11:54–5. doi: 10.5698/1535-7511-11.2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg MJ. What’s the problem with generic antiepileptic drugs? A call to action. Neurology. 2007;68:1245–6. doi: 10.1212/01.wnl.0000262876.37269.8b. [DOI] [PubMed] [Google Scholar]
- 11.Privitera MD. Generic antiepileptic drugs: current controversies and future directions. Epilepsy Curr. 2008;8:113–7. doi: 10.1111/j.1535-7511.2008.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore N, Berdai D, Begaud B. Are generic drugs really inferior medicines? Clin Pharmacol Ther. 2010;88:302–4. doi: 10.1038/clpt.2010.168. [DOI] [PubMed] [Google Scholar]
- 13.Krauss GL, Caffo B, Chang Y-T, et al. Assessing bioequivalence of generic antiepilepsy drugs. Ann Neurol. 2011;70:221–8. doi: 10.1002/ana.22452. [DOI] [PubMed] [Google Scholar]
- 14.Liow K, Barkley GL, Pollard JR, et al. Position statement on the coverage of anticonvulsant drugs for the treatment of epilepsy. Neurology. 2007;68:1249–50. doi: 10.1212/01.wnl.0000259400.30539.cc. [DOI] [PubMed] [Google Scholar]
- 15.Kesselheim AS, Stedman MR, Bubrick EJ, et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs. 2010;70:605–21. doi: 10.2165/10898530-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada M, Welty TE. Generic substitution of antiepileptic drugs: a systematic review of prospective and retrospective studies. Ann Pharmacother. 2011;45:1406–15. doi: 10.1345/aph.1Q349. [DOI] [PubMed] [Google Scholar]
- 17.Desmarais JE, Beauclair L, Margolese HC. Switching from brand-name to generic psychotropic medications: a literature review. CNS Neurosci Ther. 2011;17:750–60. doi: 10.1111/j.1755-5949.2010.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makus KG, McCormick J. Identification of adverse reactions that can occur on substitution of generic for branded lamotrigine in patients with epilepsy. Clin Ther. 2007;29:334–41. doi: 10.1016/j.clinthera.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Savica R, Beghi E, Mazzaglia G, et al. Prescribing patterns of antiepileptic drugs in Italy: a nationwide population-based study in the years 2000–2005. Eur J Neurol. 2007;14:1317–21. doi: 10.1111/j.1468-1331.2007.01970.x. [DOI] [PubMed] [Google Scholar]
- 20.Heaney DC, Sander JW. Antiepileptic drugs: generic versus branded treatments. Lancet Neurol. 2007;6:465–8. doi: 10.1016/S1474-4422(07)70105-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Deshpande AD, Jiang R, et al. An epidemiological investigation of off-label anticonvulsant drug use in the Georgia Medicaid population. Pharmacoepidemiol Drug Saf. 2005;14:629–38. doi: 10.1002/pds.1051. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67:972–82. doi: 10.4088/jcp.v67n0615. [DOI] [PubMed] [Google Scholar]
- 23.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 24.Maclure M, Mittleman MA. Should we use a case-crossover design? Ann Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith DR, Wagstaff AJ, Ibbotson T, et al. Spotlight on lamotrigine in bipolar disorder. CNS Drugs. 2004;18:63–7. doi: 10.2165/00023210-200418010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Rascati KL, Richards KM, Johnsrud MT, et al. Effects of antiepileptic drug substitutions on epileptic events requiring acute care. Pharmacotherapy. 2009;29:769–74. doi: 10.1592/phco.29.7.769. [DOI] [PubMed] [Google Scholar]
- 27.Zachry WM, III, Doan QD, Clewell JD, et al. Case-control analysis of ambulance, emergency room, or inpatient hospital events for epilepsy and antiepileptic drug formulation changes. Epilepsia. 2009;50:493–500. doi: 10.1111/j.1528-1167.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 28.Hansen RN, Campbell JD, Sullivan SD. Association between antiepileptic drug switching and epilepsy-related events. Epilepsy Behav. 2009;15:481–5. doi: 10.1016/j.yebeh.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Devine ST, Weisbart E, Barron J, et al. Acute epilepsy exacerbations in patients switched between A-rated antiepileptic drugs. Curr Med Res Opin. 2010;26:455–63. doi: 10.1185/03007990903488704. [DOI] [PubMed] [Google Scholar]
- 30.LeLorier J, Sheng Duh M, Emmanuel Paradis P, et al. Economic impact of generic substitution of lamotrigine: projected costs in the US using findings in a Canadian setting. Curr Med Res Opin. 2008;24:1069–81. doi: 10.1185/030079908x280572. [DOI] [PubMed] [Google Scholar]
- 31.LeLorier J, Duh MS, Paradis PE, et al. Clinical consequences of generic substitution of lamotrigine for patients with epilepsy. Neurology. 2008;70:2179–86. doi: 10.1212/01.wnl.0000313154.55518.25. [DOI] [PubMed] [Google Scholar]
- 32.Duh MS, Paradis PE, Latremouille-Viau D, et al. The risks and costs of multiple-generic substitution of topiramate. Neurology. 2009;72:2122–9. doi: 10.1212/WNL.0b013e3181aa5300. [DOI] [PubMed] [Google Scholar]
- 33.Labiner DM, Paradis PE, Manjunath R, et al. Generic antiepileptic drugs and associated medical resource utilization in the United States. Neurology. 2010;74:1566–74. doi: 10.1212/WNL.0b013e3181df091b. [DOI] [PubMed] [Google Scholar]
- 34.Erickson SC, Le L, Ramsey SD, et al. Clinical and pharmacy utilization outcomes with brand to generic antiepileptic switches in patients with epilepsy. Epilepsia. 2011;52:1365–71. doi: 10.1111/j.1528-1167.2011.03130.x. [DOI] [PubMed] [Google Scholar]
- 35.Gagne JJ, Avorn J, Shrank WH, et al. Refilling and switching of antiepileptic drugs and seizure-related events. Clin Pharmacol Ther. 2010;88:347–53. doi: 10.1038/clpt.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clinl Epidemiol. 2005;58:323–37. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Levine M, Ensom MH. Post hoc power analysis: an idea whose time has passed? Pharmacotherapy. 2001;21:405–9. doi: 10.1592/phco.21.5.405.34503. [DOI] [PubMed] [Google Scholar]