Abstract
Parkinson’s disease (PD), the most common neurodegenerative movement disorder, is associated with selective degeneration of nigrostriatal dopamine neurons. While the underlying mechanisms contributing to neurodegeneration in PD appear to be multifactorial, mitochondrial impairment and oxidative stress are widely considered to be central to many forms of the disease. Whether oxidative stress is a cause or consequence of dopaminergic death, there is substantial evidence for oxidative stress in both human PD patients and in animal models of PD, especially using rotenone, a complex I inhibitor. There are many indices of oxidative stress, but this review covers the recent evidence for oxidative damage to nucleic acids, lipids and proteins in both the brain and peripheral tissues in human PD and in the rotenone model. Limitations of the existing literature and future perspectives are discussed. Understanding how each particular macromolecule is damaged by oxidative stress and the interplay of secondary damage to other biomolecules may help design better targets for treatment of PD.
Keywords: Parkinson’s disease, oxidative damage, macromolecules, rotenone
Oxidative damage: an overview
Free radicals are chemical species that contain one or more unpaired electrons in their outer orbit. Although free radicals may be very reactive, their reactivity varies depending on the particular species. Collectively, reactive oxygen species (ROS) include both oxygen radicals and non-radical derivatives of O2 that are oxidizing agents and/or are easily converted into radicals. Three biologically important ROS in mammalian cells are: superoxide (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (OH·, Fig. 1). Each plays a significant physiological role in the body; for instance, as part of the artillery of the immune system, O2− is involved in killing invading microorganisms [1], and H2O2 is an important signaling molecule.
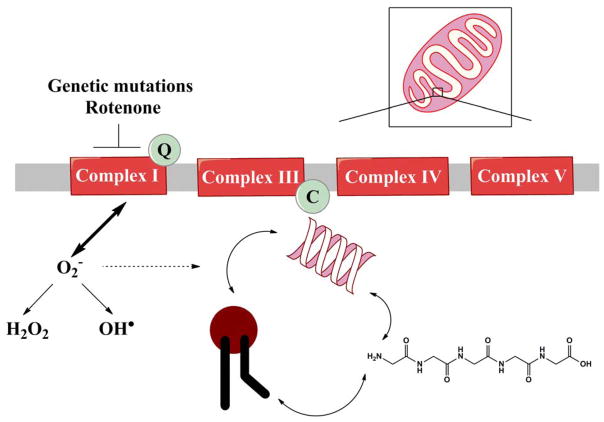
Fig 1. Mitochondrial dysfunction may lead to oxidative damage of macromolecules.
Genetic mutations or environmental factors inhibit complex I activity and/or result in mitochondrial impairment. Mitochondrial dysfunction produces ROS. The resultant ROS may damage macromolecules, including nucleic acids, lipids and proteins. An oxidized macromolecule can then damage another macromolecule, leading to a vicious cycle of oxidized products.
In eukaryotic cells, mitochondria are the major source of oxygen radicals, which are formed during oxidative adenosine-5′-triphosphate (ATP) production. The mitochondria use about 80% of the O2 we inhale [2–4] and during the course of oxidative phosphorylation, molecular O2 is reduced to H2O. However, there is normally also leakage of a very small proportion of electrons from the electron transport chain (ETC) directly to O2 to produce O2−. Of the O2 reduced in the mitochondria, 1–3% may form O2−; however this may be an overestimation [5]. The relative role of each ETC complex in forming O2− differs by tissue, yet complex I is a major source of O2− in the brain [6].
Despite the requirement for O2 to generate ATP in aerobes, oxidative toxicity/damage occurs even in ambient levels of O2. In a seminal paper in 1954, Denham Harman first proposed that oxygen radicals were responsible for the damaging effects of O2 and since then, the free radical theory of aging has expanded to include the mitochondrion’s role in the production of ROS [7]. Since superoxide can be toxic, nearly all organisms living in the presence of oxygen contain isoforms of the superoxide scavenging enzyme, superoxide dismutase, which catalyzes the dismutation of superoxide into O2 and H2O2 [6, 8]. Although H2O2 is not a free radical, it is a reactive oxygen species and it still may be dangerous because it can easily be converted, by interacting with Fe2+, into OH·, one of the most destructive free radicals. ROS may also be produced by monoamine oxidase in the outer mitochondrial membrane, as well as by specific Krebs cycle enzymes [6]. In general, ROS can either directly cause damage or participate in unwanted side reactions, resulting in cell damage.
An imbalance between the production of ROS and the ability to detoxify the reactive intermediates results in oxidative stress. Excessive ROS can damage all macromolecules, including nucleic acids, lipids and proteins, leading to an overall progressive decline in physiological function. Nucleic acids, both RNA and DNA, are subject to oxidative damage, with DNA damage occurring most readily at guanine bases. Free radical damage to fatty acids (known as lipid peroxidation), results in lipid degradation and cell membrane damage. ROS attack on proteins may be reversible or irreversible, often leading to either a loss function or protein aggregation. Oxidative damage has been characterized and implicated in aging [9], as well as in a variety of diseases such as cancer [10] and neurological disorders including Parkinson’s disease (PD) [11].
Direct measurement of ROS is often difficult due their short lifespan and there is not a practical method for ROS measurement in the living human brain. Thus, much of the current evidence for oxidative stress in neurological disease comes from measurement of oxidative modifications of various cellular components. In PD, there is considerable evidence for ROS-mediated damage in postmortem brain samples as well as in other tissues, even outside of the central nervous system. Moreover, animal models also provide convincing evidence for oxidative stress in PD. In fact, many of the current animal models of PD utilize oxidative stress as a means to selectively damage the nigrostriatal dopamine system in order to replicate the clinical, pathological and biochemical features of PD [12]. In this regard, fly, rat and non-human primate models using rotenone, a mitochondrial complex I inhibitor, replicate many of the core characteristics of PD and provide evidence for oxidative stress [12]. Here we will review the recent evidence for oxidative damage to nucleic acids, lipids and proteins in both the brain and peripheral tissues in human PD and in the rotenone model thereof.
Introduction to Parkinson’s disease
PD is the most common neurodegenerative movement disorder. A central pathological hallmark of PD is the selective loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc). These dopaminergic neurons are required for proper motor function, and their loss is associated with tremor, rigidity, bradykinesia and postural instability. To date, treatments are only symptomatic; they do not alter the inexorable progression of the disease – and PD patients continue to experience a higher mortality rate compared to the general population [13–15]. Even with expert treatment, PD patients typically deteriorate over time and endure considerable motor and cognitive disability in the years after diagnosis.
A second neuropathological hallmark of PD is the Lewy body (LB), which is a cytoplasmic spherical proteinaceous inclusion. LBs have been reported to contain various proteins including but not limited to alpha-synuclein, ubiquitin, parkin, and neurofilaments [16–18]. Whether LBs are neuroprotective or pathogenic in PD is controversial and is reviewed elsewhere [18]. The mechanisms by which alpha-synuclein and other proteins aggregate to form Lewy pathology are uncertain, but may involve oxidative modifications and/or cross-linking.
Historically, the neurodegeneration of PD was thought to be confined to dopaminergic cell loss in the SNpc; however, cell loss and neuropathology occur elsewhere, including in the locus coeruleus, raphe, nucleus basalis of Meynert, dorsal motor nucleus of the vagus, cerebral cortex, olfactory bulb and autonomic nervous system [19]. In addition, hippocampal and cortical neurodegeneration may contribute to dementia that is often associated with PD. It is likely that these nondopaminergic pathways are involved in the non-motor PD symptoms that often develop (reviewed in [18]).
Oxidative damage in human PD
The brain is particularly susceptible to oxidative damage due to its high levels of polyunsaturated fatty acids and relatively low antioxidant activity [20]. The majority of the resultant oxidative damage in PD has been found in the SN, as most of the research on underlying mechanisms of neurodegeneration in PD has focused on this brain region. However, mitochondrial dysfunction and complex I inhibition are not limited to the brain in PD, and oxidative damage has been reported in peripheral tissues of PD patients. While a wide variety of biological molecules undergo oxidative damage in PD, damage to the three classes of macromolecules will be reviewed below.
Oxidative damage to nucleic acids in human PD brain
ROS can damage nucleic acids, including DNA and RNA. DNA damage is any modification of DNA that alters its coding properties or interferes with normal metabolic function – and it is linked to many different types of diseases [21]. Unrepaired DNA damage may have major consequences for the cell, including genetic and protein instability. The presumed slow accumulation of oxidative DNA damage in both the mitochondrial and nuclear genomes can potentially trigger cell death. This type of damage would be particularly deleterious to neurons, which cannot rely on self-renewal through cell proliferation. Limited types of oxidative RNA damage have been reported, even though RNA should be at least equally subject to oxidative insults, if not more susceptible [22].
In general, very little is known about DNA damage in PD. Specific types of oxidative DNA damage are reported to be elevated in the SN in PD, but are not restricted to this brain region [23–25]. In PD, the number of strand breaks in nuclear DNA is higher in the SN than in other brain areas, and there is evidence of alterations in DNA conformation and stability in the SN as well [25]. Alam and colleagues [23] reported that the sum of guanine damage products in nuclear DNA is not higher in PD brains relative to age-matched controls. Interestingly, however, if the guanine damaged products are evaluated separately, levels of 8-oxo-deoxyguanine (8-oxo-dG) are increased in the SN, frontal pole, and medial putamen, while FAPy guanine is reduced in almost every brain area in PD tissue when compared to the controls [23]. The mechanisms eliciting the selective rise in 8-oxo-dG and reduction in FAPy guanine are unknown, but the authors speculate that these results may not reflect a true rise or decline in damage products, but rather a shift in the chemical nature of guanine damage products due to more oxidizing conditions of the PD brain [23]. While these studies have been informative, there are limitations to the interpretation of the results. An artificially higher number of oxidative lesions may be associated with phenol chloroform extractions [26]. Further, the oxidative DNA damage observed in these studies was not ascribed to a particular cell type. In addition, mitochondrial DNA damage was not investigated [23, 25]. Such evaluation may be crucial, as mitochondrial DNA is reported to be more susceptible to oxidative damage than nuclear DNA [27].
To determine the sub-cellular distribution of DNA damage, Zhang and colleagues [24] used an immunohistochemical approach, with an antibody that recognizes both the DNA and RNA damaged products, 8-oxo-dG and 8-oxo-G, respectively. In age-matched controls, immunoreactivity is rarely observed, whereas it is increased in PD brains, particularly in the ventrolateral SN, as well as in additional midbrain regions and glial cells [24]. The reported immunoreactivity is restricted to the cytoplasm of the neuron, which suggests higher oxidatively damaged mitochondrial DNA and/or RNA [24]. These results are in contrast to previous results that show an increase of 8-oxo-dG with age in both nuclear and mitochondrial DNA from human postmortem brains [28].
This lesion, 8-oxo-dG, has been a particularly popular subject of study since it is an abundant, promutagenic lesion [29, 30] and there a variety of assays available for its measurement. While these features may render 8-oxo-dG lesions particularly harmful for replicating cells, this lesion type does not block transcription and represents a small fraction of total ROS-induced lesions [31]. Therefore, it is unlikely that this lesion itself is significantly interfering with cell metabolism in post-mitotic cells, but rather problems may arise with further oxidative DNA damage products, if it is left unrepaired. Furthermore, the current available data on levels of 8-oxo-dG are conflicting, probably due to technical problems involving sensitivity, background, sample preparation issues and an antibody with unclear specificity [26].
Overall, in all three studies [23–25], late stage PD cases were used, and therefore the increase in DNA damage was found in the remaining small fraction of surviving neurons (or perhaps glia), as it is likely that by this point, the vast majority of dopaminergic neurons have already degenerated. It is unclear whether DNA damage is responsible for neuronal loss or is an epiphenomenon of the disease in the surviving neurons—or both. Studies investigating the specific types of oxidative lesions, the proportion of nuclear vs. mitochondrial DNA damage, and levels in early PD cases will help further elucidate the role of DNA damage in PD. Furthermore, levels of oxidized RNA have not been evaluated in PD brains relative to controls, and will be important for further investigation, in order to shed light on whether or not the rise in oxidative DNA damage is specific to this nucleic acid.
Oxidative nucleic acid damage within and outside of the central nervous system
PD is increasingly being viewed as a systemic illness that affects tissues outside the brain and even outside the nervous system. Higher levels of oxidative damage to nucleic acids are found in cerebrospinal fluid (CSF) samples from PD patients [32, 33]. Damage to nucleic acid in PD has also been reported outside of the central nervous system (CNS). Lymphocytes are white blood cells that play a role in immune function. Lymphocytes are differentiated and post-mitotic; however, they can be renewed by stem cells. In the blood, the majority of nuclear and mitochondrial DNA originates from the lymphocytes. There is conflicting evidence regarding DNA damage levels in PD versus healthy control lymphocytes, with some studies indicating higher levels and others reporting no difference [34–38]. In one study, there is a gender bias - higher in females vs. males, with serum levels of nucleic acid oxidation higher overall in PD patients [32]. In this study, the specific cellular source of DNA or RNA was unclear. Mitochondrial DNA damage has not been evaluated in PD lymphocytes, platelets or CSF.
Two studies have analyzed 8-oxo-dG in urinary samples from PD patients and healthy controls and found opposing results. Halliwell’s group reported higher levels of 8-oxo-dG in early PD [39], while a different group demonstrated a rise in 8-oxo-dG with increasing severity of PD [40]. These differences might be attributable to the different methods used to assay 8-oxo-dG. In addition, the one study used samples collected from patients during hospitalization and it is unknown if their illness or treatment affected total 8-oxo-dG levels [40].
DNA repair in human PD
Maintaining the integrity of the genome is imperative for faithful replication of DNA, as well as for proper functioning of cellular metabolism; however, various endogenous and environmental agents can damage DNA. To protect against DNA damage, numerous mechanisms within the cell are devoted to DNA repair (reviewed in [41]). Expression of proteins involved in the repair of oxidative DNA damage, (OGG1, MUTY, and MTH1), is increased in dopaminergic neurons in the SN of PD patients [42–44]. Up-regulation of these DNA repair enzymes likely represents a compensatory response to oxidative DNA damage. Recently, an association between DNA repair variants and an increased PD risk was reported [45]. However, the role of DNA repair proteins in the development of PD is unclear and remains a promising avenue for investigation.
Mitochondrial DNA mutations in human PD
Damage to DNA, whether it is oxidative or not, may interfere with DNA replication and must to be repaired. If the damaged DNA is not repaired, this may result in a mutation. However, mutations may arise independently of damaged DNA through other mechanisms, and therefore mutational analysis is not necessarily an indirect measure of oxidative DNA damage. Levels of somatic mitochondrial DNA (mtDNA) mutations are modestly increased in late-stage PD patients [46–48]. Interestingly, larger increases in mtDNA mutations are reported in incidental LB disease (presumed to be pre-symptomatic PD) compared to late-stage PD or controls [49]. It is important to note that the robustness of mtDNA mutation analysis has now been questioned [50], and the functional consequences of the reported somatic mtDNA mutations are unclear.
Lipid peroxidation in human PD brain
Lipid peroxidation refers to the process by which lipids are oxidized leading to degradation. Polyunsaturated fatty acids (PUFAs) are the most prone to lipid peroxidation. After adipose tissue, the organ with the highest lipid content is the brain. Particularly enriched in the brain are the two PUFAs, arachidonic and docosahexaenoic acids, the components of which must be obtained from the diet [51]. Phosopholipid composition is dynamic throughout life; shorter PUFAs increase with age while longer PUFAs decrease with age [52]. Lipids in general are involved in membrane fluidity and permeability, can serve as an energy reservoir, and mediate inflammatory processes and apoptotic signals [53]. While specific lipid oxidation products can act as signaling molecules and exert protective cellular effects [54, 55], damaged or oxidized lipids may have deleterious effects on the aforementioned functions and lead to neuronal damage and ultimately death.
The rate of phospholipid membrane turnover in the SN is low, and may affect the ability of neurons to respond to stress [56]. Several markers of lipid peroxidation have been found to be significantly increased in PD brains. One particular marker of lipid peroxidation is malondialdehyde, (MDA), which largely results from peroxidation of PUFAs with more than two double bonds. The first report of lipid peroxidation in PD showed higher levels of MDA, although the overall levels of PUFAs were reduced in the SN from PD patients, as compared to other brain regions and control tissue [57]. The frontal cortex from PD patients exhibited higher levels of MDA [58, 59], and interestingly, lower levels of MDA were observed in the caudate nucleus and putamen [58]. However, the thiobarbituric assay to measure MDA levels is now in question, and these studies should be interpreted with caution [6].
A battery of additional lipid peroxidation markers have also been found to be increased in PD. An early marker of lipid peroxidation, cholesterol lipid hydroperoxide, is increased 10-fold in PD SN compared to control subjects [60]. Isofurans, byproducts of arachidonic acid peroxidation, are specifically increased in both the SN of PD patients and in patients with LB disease, but are not elevated in other neurodegenerative diseases [61]. Acrolein is also higher in neuromelanin positive neurons in the SN of PD patients [62]. In addition, approximately one third of neuromelanin positive neurons labeled for acrolein in PD samples also co-localize with alpha-synuclein [62]. Recently it has been proposed that PUFAs may play a role in either stimulating or promoting α-synuclein aggregation [53].
4-Hydroxy-2-non-nenal (HNE) is a compound typically formed during the peroxidation of PUFAs with the double carbon bond six carbons from the methyl end of the chain. HNE is present in LBs in PD and in diffuse LB disease [63]. HNE adducts are detected in nigral neurons of PD patients, while these adducts are not as frequently observed in adjacent nuclei, glia or in nigral neurons of controls [64]. There is specificity to particular lipid peroxidation products, as not all are found in the PD brain. Levels of F2-isoprostanes (F2-iPs), which are chemically stable prostaglandin-like compounds formed from PUFAs with at least three carbon double bonds, were not elevated in PD [61, 65]. However, these studies only evaluated a small cohort of PD patients and a larger sample size is necessary to verify these results.
Peripheral lipid peroxidation in PD
There is strong evidence for lipid peroxidation in blood from PD patients. Plasma MDA levels are higher in PD patients than healthy controls [66]. Plasma levels of the oxidized lipid product, hydroxyeicosatetraenoic acid, are also elevated in PD patients [67]. In one study, serum MDA levels were higher in patients with PD relative to healthy controls [68], but this was not confirmed in two other studies [69, 70]. Higher levels of MDA were reported in PD leukocytes [71]. In PD patients, erythrocytes are more susceptible to lipid peroxidation [72], in addition to having higher MDA levels [71]. Two studies found higher plasma lipid peroxides in the PD group than in the control group [72, 73]. Plasma levels of 8,12-isoprostaneF2[alpha]-VI (iP), a type of F2-iP, are similar in PD subjects and controls, but iP urine levels are higher in PD, although there is substantial overlap with controls [74]. This was consistent with two additional studies that showed plasma F2-iPs levels are similar in the PD and control group [67, 75]. However, sample size may be crucial in detecting a significant difference among groups. When Halliwell’s group expanded the sample size of PD patients, they found that overall plasma F2-iPs levels did in fact increase, and that the highest levels are correlated with those with early PD [39].
There are limited studies investigating lipid peroxidation in the CSF of PD patients, but lipid peroxidation products are reported to be significantly elevated in the CSF of PD patients [76]. HNE is also significantly increased in the CSF of PD patients and the difference in HNE is greater in CSF than in serum [77]. In addition, MDA is increased in the CSF of newly diagnosed non-treated PD patients [78]. This result is in contrast, however, with a later study indicating that CSF levels of MDA are not correlated with PD status [79].
Using skin fibroblasts from PD patients, cholesterol biosynthetic capability was found to be lower compared to healthy controls [80]. Using primary fibroblasts cultures established from skin biopsies, those patients who were homozygous for the mutation G309D-PINK1 showed an increase in MDA levels relative to heterozygous carriers or controls [81]. As the technology to differentiate fibroblasts into neurons continues to progress, the utility of this model system will likely be used to further investigate mechanisms of PD [82].
Overall there is ample support for lipid peroxidation in both the brain and in the periphery in PD. Future studies may include investigation of cardiolipin, which is contained in the mitochondrial inner membrane and which may contribute to age-related decline in mitochondrial function [83]. Given the role of cardiolipin and its metabolites in apoptosis, cardiolipin as a target for oxidative damage seems likely [84]. Interestingly, cardiolipin forms a triple complex with cytochrome c and alpha-synuclein, which exerts a peroxidase activity and leads to aggregation of alpha-synuclein [85]. In addition, there has been renewed interested in the role of sphingolipids and PD as a result of the recent reports associating mutations in the glucocerebrosidase gene and the development of PD, even in heterozygrous carriers [86].
Protein oxidation in human PD
ROS can damage proteins in a reversible manner, such as methionine sulfoxide formation. Proteins can also be damaged by ROS in an irreversible fashion, for example amine groups on side chains of specific amino acid may be modified into carbonyls. Oxidative stress in the cell does not damage proteins universally. Certain proteins appear to be more vulnerable to oxidative damage. In addition, specific residues, such as cysteine or selenocysteine, are targeted for oxidation. Oxidative damage to proteins may alter expression levels and/or confer a toxic loss or gain of function. Moreover, oxidative damage to enzymes, receptors and antibodies may have significant functional consequences to the cell: oxidative protein damage may exert its effects on regulatory, metabolic, transport and signal transduction pathways that may impair crucial functions within the cell.
One of the most commonly used markers of protein oxidation is protein carbonyl content. An increase in protein carbonyls is found in PD-relevant brain regions compared to healthy controls [87]; however, the elevated levels of protein carbonyls are widespread and are not restricted to specific brain regions. Interestingly, protein carbonyls are not higher in incidental LB disease, suggesting that this is a phenotype that may progress with disease severity [87]. Another group confirmed that protein carbonyls are increased in the frontal cortex of PD patients relative to controls [59]. Recently, it was demonstrated that specific complex I subunits of PD frontal cortex showed increased protein carbonyl damage relative to controls [88].
While most studies have used brain homogenates and evaluated total protein carbonyl content, specific proteins have also been found to be oxidatively damaged in PD. Mutations in the DJ-1 gene lead to early onset familial PD [89]. Several studies have found DJ-1 to be more oxidized in PD brains [90, 91]. Furthermore, in the frontal cortex of idiopathic PD cases, there is evidence for DJ-1 cysteine and methionine oxidation [92]. Mutations in the uibiquitin carboxy-terminal hydrolase L1 (UCH-L1) gene have also been linked to PD [93]. Interestingly, carbonyl formation, and methionine and cysteine oxidation occur on UCH-L1 in idiopathic PD [94]. Iron dysregulation has also been associated with PD [95]. One key player in iron metabolism, transferrin, has increased oxidized thiols in SN of human PD patients [96].
Peripheral protein oxidation in PD
In peripheral tissues in PD, there is conflicting evidence for protein oxidation. One study found an increase in protein carbonyls in PD leukocytes [71], while another found no difference when compared to controls [97]. No differences in protein carbonyls are found in PD plasma [98]; yet this may be related to the clinical stage, as a different study found that protein carbonyls increased in later PD stages [71]. This same group also found an increase in protein carbonyls in PD erthyrocytes in later stage patients. Higher levels of oxidized DJ-1 (cysteine residue 106) in erthryocytes are found in untreated PD patients compared to controls or PD patients that had been treated [99].
Environmental risk factors associated with PD: Link to rotenone
PD is widely accepted as a multifactorial disease - with both genetic and environmental contributions. About 10% of PD cases are estimated to have a monogenic cause; the remaining cases are called “sporadic” or “idiopathic”, with unknown origin. In fact, epidemiological evidence from twin studies points to a small role of genetic factors in causing typical PD, especially after age 50 [100–105]. Although genetics have an important role in PD pathogenesis, other factors must be at play. Geographic variations in PD distribution within and among countries support environmental risk factors [106–109].
Many environmental risk factors have been implicated in PD [110]. Factors that may enhance or decrease PD susceptibility, include smoking, well water consumption, exercise, head trauma and infectious agents [111–116]. Exposure to metals has also been postulated to be a potential environmental risk factor for PD [117–120]. Occupational exposure to manganese has been suggested to contribute to PD susceptibility, but this remains controversial [121–124]. One of the primary classes of environmental agents associated with PD is pesticides, which include fumigants, fungicides, herbicides, insecticides, and rodenticides [125, 126]. The chronic use of certain herbicides is associated with PD [113, 117, 127], with increased risk applied to certain pesticides [128]. These pesticides may be used for residential purposes, in fish management and in commercial agriculture. In addition, residence in a rural environment and presumed exposure to pesticides may be related to an elevated risk of PD [129]. Several commercial pesticides are known to inhibit complex I of the ETC [130, 131], but their role in causing parkinsonism is unknown. Of note, there are many types of naturally-occurring complex I inhibitors that presumably contaminate our food, water and air [130].
One such pesticide that inhibits complex I is rotenone. Rotenone is a prototypical example of how an exogenous toxin can mimic clinical and pathological features of PD in an animal model [132, 133]. Reports that rotenone could reproduce many features of PD in rats led to follow-up epidemiological studies to investigate the role of human exposure to rotenone as a risk factor for PD development. Although the number of cases of exposure to rotenone was low, two small studies found the risk of developing PD elevated [134, 135]. However, in a follow-up rigorous, case-control study, the examination of 31 pesticides revealed two that increased the risk of developing PD, one of which is rotenone [136].
The rotenone model of PD
The rotenone model of PD was developed in an effort to experimentally model in vivo nigrostriatal dopamineric degeneration, LB formation, a systemic complex I defect, and the potential relevance of pesticide exposure to PD [132]. The pesticide rotenone, a member of the rotenoids, is derived from the roots or leaves of certain plant species. Rotenone shares a common mode of action with the pro-parkinsonian toxin, 1-methyl-4-phenylpyridinium (MPP+), in that it is a specific inhibitor of mitochondrial complex I. In addition, it is able to readily cross biological membranes, including those of the blood brain barrier, due to its hydrophobic nature, thereby gaining access to all organs quickly after exposure [137]. Unlike MPP+, however, rotenone does not depend on specific transporters for access into the cell. Therefore, rotenone is well suited for in vivo experimentation to investigate systemic mitochondrial complex I inhibition.
The rotenone model reproduces PD pathology and behavior
Rotenone was first used to model PD by injecting a high concentration directly into the brain and was found to kill dopaminergic neurons [138]. Others followed up this study by administering rotenone systemically at very high doses, but this resulted in peripheral toxicity and non-specific brain lesions [139]. However, when rotenone was used at lower doses (2–3mg/kg/day), that approximate the complex I inhibition found in platelets from PD patients, highly selective nigrostriatal degeneration was observed [132]. In addition to selective degeneration of nigrostriatal dopamine neurons, alpha-synuclein inclusions similar to LBs were seen in surviving dopaminergic neurons [132]. Additionally, the animals were hypokinetic and had postural instability [132]. Because rotenone freely enters all cells, the high susceptibility of DA neurons to rotenone in this study suggested that dopaminergic neurons are preferentially sensitive to complex I inhibition. In fact, using a radioreceptor assay, the highest concentration of rotenone in the brain was estimated to be 30 nM [140]. Strikingly, the concentration and degree of inhibition of complex I was uniform in both the brain and other organs as well [140]. Most importantly, for the first time, the rotenone model provided a proof of concept: systemic mitochondrial dysfunction can cause selective nigrostriatal degeneration.
In addition to selective nigrostriatal degeneration, the rotenone model accurately recapitulated many other features of human PD including anatomical, neurochemical, behavioral and neuropathological characteristics. In vitro studies have shown that chronic rotenone exposure leads to the accumulation and aggregation of both alpha-synuclein and ubiquitin, and caspase-activated cell death [141]. Alpha-synuclein and polyubiquitin containing LBs and Lewy neurites have also been observed in vivo [132, 133]. The ubiquitin-proteasome system is also found to be impaired [142, 143]. Iron accumulation [96], gastrointestinal impairment [144, 145], L-DOPA and apomorphine-responsive behavioral deficits [133, 146, 147], retinal disease [148–151], and sleep disturbances, which are typical of human PD, are also recapitulated in the rotenone model [152].
Mechanism of rotenone action
As an inhibitor of mitochondrial respiration, it might be anticipated that rotenone would exert toxicity by decreasing ATP synthesis (bioenergetic crisis); however, it appears instead that toxicity in the rotenone model is via oxidative stress [141, 153]. Rotenone specifically binds complex I with an affinity of 10–20 nM [140] and in so doing, reduces electron flow to ubiquinone. Upstream of the rotenone binding pocket, there is a site of electron leak in complex I [154] and, as a result, there are more free electrons available to react with molecular oxygen to produce O2−. The generation of O2−, and further ROS can damage complex I itself, as well as other complexes of the ETC, especially those containing iron-sulfur clusters [152]. ROS may also damage other proteins in the mitochondria, as well as other macromolecules. Therefore, it is thought that the primary mechanism by which chronic rotenone exposure leads to degeneration of dopaminergic neurons is by cumulative oxidative damage.
It should be noted that rotenone may have effects other than complex I inhibition, and it has been claimed that rotenone-induced microtubule disruption (rather than ETC inhibition) may account for its toxicity in dopamine neurons [154–156]. It was also recently suggested that rotenone and other complex I inhibitors, do not require complex I inhibition for dopaminergic neuronal death [157]. However, these reports do not seem compatible with results from the Greenamyre lab or the literature: To determine the molecular site of action of rotenone, cells were transfected with the rotenone-insensitive single-subunit NADH dehydrogenase of Saccharomyces cerevisiae (NDI1), which incorporates into the mammalian ETC and acts as a “replacement” for endogenous complex I. In response to rotenone, NDI1-transfected cells did not show mitochondrial impairment, oxidative damage, or death, demonstrating that these effects of rotenone were caused by specific interactions at complex I [158]; similar results have been obtained in in vivo systems [159, 160]. Thus, the preponderance of evidence suggests that rotenone acts via specific inhibition of complex I and that chronic oxidative stress plays a more important role than ATP depletion in this model of PD.
Oxidative nucleic acid damage in the brain and periphery in the rotenone model
Similar to human PD, very little is known regarding nucleic acid damage in the rotenone model. Multiple in vitro studies with cell lines using an antibody to 8-oxo-dG and 8-oxo-G, found an increase in nuclear staining following an acute high concentration of rotenone [161, 162] or a chronic low concentration [141, 163]. Additional types of DNA damage, including strand breaks were increased following rotenone treatment in cell lines [162, 163]. However, rotenone’s effect on levels of nucleic acid damage may be influenced by the stage of the cell cycle, and therefore it will be important to pursue further studies in post-mitotic neurons to better determine relevance for PD. However, one study using an in vivo rotenone model demonstrated an increase in 8-oxo-dG or 8-oxo-G staining in SN neurons [159]. Lastly, while it has been demonstrated that mitochondrial mutations and rotenone treatment further exacerbate respiratory dysfunction [164], it has not been tested whether rotenone alone can induce mutations.
One advantage to the rotenone model is that complex I inhibition is not restricted to the SN, but is systemic. Therefore, the rotenone model is an ideal platform to study oxidative damage in peripheral tissues. Currently, there have been no studies utilizing this beneficial aspect to the model. However, ex vivo treatment of human whole blood and peripheral lymphocytes with rotenone highlighted cell cycle dependent cytotoxicity in addition to an increase in chromosomal aberrations and strand breaks [165]. Therefore, one avenue for future investigations in the rotenone model may include determining nucleic acid oxidative damage in blood and lymphocytes.
DNA repair in rotenone model
Greene and colleagues [166] were interested in gene pathways affected by a chronic low concentration of rotenone in human neural cells. Gene sets related to DNA damage and repair were up-regulated after four weeks of rotenone treatment [166]. In addition, specific genes involved in the DNA damage response were elevated only after one week of rotenone treatment. While the interpretation of these studies is limited by the fact that these studies were performed in replicating cells and not in differentiated or post-mitotic neurons, DNA repair pathways are likely functionally relevant following rotenone exposure. In addition, effects of DNA damage may be cumulative, given that the DNA damage sensing and repair gene responses are augmented with progressive rotenone exposure.
Lipid Peroxidation in rotenone model
In vitro and in vivo rotenone models consistently demonstrate an increase in lipid peroxidation. In dopaminergic neuroblastoma cells, rotenone treatment induces higher lipid peroxidation products, as determined by the loss of cis-parinaric acid fluorescence [167]. PC-12 cells or human skin fibroblasts incubated for three days with rotenone showed increased levels of lipid peroxidation using a C11-BODIPY581/591 dye [161, 168]; yet the precise lipid peroxidation products indicated by this particular dye are unclear. Higher levels of HNE adducts in retinal cell cultures are observed in both neurons and glial cells after rotenone treatment, although the neuronal population is more sensitive to rotenone toxicity [169]. In isolated forebrain mitochondria, MDA production induced by rotenone was greater in the presence of calcium [170]. Several studies found that MDA levels are increased in midbrain, cerebellum, and striatum of rotenone treated animals compared to vehicle treated animals [171–176]. There may be a temporal development of MDA in the brain, as MDA levels increased first in the striatum and later in cortex in an in vivo rotenone model [177]. Interestingly, lipid peroxidation is enhanced in rotenone treated ceruloplasmin, (a copper-binding ferroxidase), deficient mice [178].
Rotenone model: protein oxidation
Studies using the rotenone model provide convincing evidence of protein oxidation. Overall, evaluating total protein, rotenone induces protein carbonyls in neuroblastoma cell lines [179, 180]. In a similar cell line, SK-N-MC cells, either short-term or chronic exposure to rotenone increases protein carbonyls in the insoluble fraction [141, 158]. These results are also replicated using postnatal midbrain organotypic slices [181]. Additionally, rotenone treated rats exhibit a three-fold increase in protein carbonyl levels in striatal tissue as compared to the vehicle group [176].
Multiple studies have also revealed an increase in thiol oxidation with rotenone treatment. Overall, thiol oxidation is increased in DA neurons when compared to both vehicle treated and cortical neurons [182]. With acute exposures, rotenone induces a multiphasic response, with alternating cycles of oxidation and reduction. Thiol oxidation of DA neurons in living zebrafish larvae is strikingly similar to results in rat primary ventral midbrain cultures [182]. In rats, under basal conditions, DA neurons are more oxidized than neurons in the VTA or cortical regions [182]. Moreover, in rats or monkeys treated with rotenone to endpoint, DA neurons show an overall increase in thiol oxidation [96].
Similar to human studies, few groups have investigated oxidation to single proteins in PD models. There are two examples however using the rotenone model. In SK-N-MC cells, the mitochondrial form of thioredoxin is oxidized with rotenone treatment [183]. Furthermore, consistent with the human transferrin protein, the rat tranferrin protein is subject to thiol oxidation [96].
Conclusions
Human PD samples from both brain and peripheral tissues provide much evidence for oxidative damage to all categories of macromolecules. While only direct oxidative damage to nucleic acids, lipids and protein were covered within this review, many other indices of oxidative stress are also found in human PD. The role of oxidative stress in the etiology of PD is unclear; however, numerous animal studies, including the rotenone model, confirm the human postmortem data and suggest that oxidative damage plays a significant role in pathogenesis.
Antioxidants have been proposed as treatments for PD but, as yet, these strategies have had limited success in clinical trials. One theory is that by the time of diagnosis it is too late to save the remaining neurons and efforts should be made to improve early diagnosis—this would allow administration of antioxidants well before substantial oxidative damage has occurred. In addition, many of the tested antioxidants have different abilities to access sub-cellular compartments and may not be in the required location to exert maximal protective effects. While there are likely numerous factors contributing to the failure of such trials, perhaps currently tested antioxidant strategies do not adequately take into account the complexity of the sequence and types of damage that occur in this disease or the specific molecular targets of oxidative damage. Thiol oxidation may initially be reversible, and it may lead to adaptive or deleterious signaling mechanisms. Reversal of the oxidation may seem like a rational approach, but it may prevent the adaptive signaling and paradoxically worsen the situation. Moreover, oxidation of one macromolecule likely influences damage to others (Fig. 1). For instance, damage to proteins can occur directly from ROS, or by attack from the end-products of lipid peroxidation. Understanding the sequence of events, downstream mechanisms, how each macromolecule is impacted by oxidative stress, and the interplay of secondary damage to other biomolecules may lead to design of better antioxidants or identification of new targets to alter the course of this devastating illness.
Highlights.
Oxidative stress is a key player in Parkinson’s disease
Oxidative damage to nucleic acids, proteins and lipids is found in Parkinson’s disease
Rotenone model recapitulates macromolecule oxidative damage found in Parkinson’s disease
Acknowledgments
We thank Terina Martinez and Evan Howlett for carefully editing the manuscript. Drs. Sanders and Greenamyre are supported by research grants from the NIEHS and NINDS (NIH) and by the American Parkinson Disease Association Center for Advanced Research at the University of Pittsburgh.
Abbreviations
- O2−
superoxide
- H2O2
hydrogen peroxide
- OH·
hydroxyl radical
- ATP
adenosine-5′ triphosphate
- ROS
reactive oxygen species
- ETC
electron transport chain
- PD
Parkinson’s disease
- SN
substantia nigra pars compacta
- DA
dopamine
- LB
Lewy body
- L-DOPA
levodopa L 3,4dihydroxyphenylalanine
- VTA
ventral tegmental area
- PUFAs
polyunsaturated fatty acids
- CSF
cerebrospinal fluid
- CNS
central nervous system
- MDA
malondialdehyde
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mtDNA
mitochondrial DNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B. Phagocyte-derived reactive species: salvation or suicide? Trends Biochem Sci. 2006;31(9):509–15. doi: 10.1016/j.tibs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Chloroplast Metabolism. Oxford University Press; 1984. [Google Scholar]
- 3.Halestrap A. Biochemistry: a pore way to die. Nature. 2005;434(7033):578–9. doi: 10.1038/434578a. [DOI] [PubMed] [Google Scholar]
- 4.Babcock GT. How oxygen is activated and reduced in respiration. Proc Natl Acad Sci U S A. 1999;96(23):12971–3. doi: 10.1073/pnas.96.23.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St-Pierre J, et al. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277(47):44784–90. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell BaG, JMC . Free Radicals in Biology and Medicine. 4. Oxford University Press; 2007. [Google Scholar]
- 7.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 8.Horowitz MP, Greenamyre JT. Gene-environment interactions in Parkinson’s disease: the importance of animal modeling. Clin Pharmacol Ther. 2010;88(4):467–74. doi: 10.1038/clpt.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haigis MC, Yankner BA. The aging stress response. Mol Cell. 40(2):333–44. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 11.Shukla V, Mishra SK, Pant HC. Oxidative stress in neurodegeneration. Adv Pharmacol Sci. 2011:572634. doi: 10.1155/2011/572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez TN, Greenamyre JT. Toxin models of mitochondrial dysfunction in Parkinson’s disease. Antioxid Redox Signal. 2012;16(9):920–34. doi: 10.1089/ars.2011.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hely MA, et al. The Sydney Multicentre Study of Parkinson’s disease: a report on the first 3 years. J Neurol Neurosurg Psychiatry. 1989;52(3):324–8. doi: 10.1136/jnnp.52.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy G, et al. The association of incident dementia with mortality in PD. Neurology. 2002;59(11):1708–13. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- 15.Morgante L, et al. Parkinson disease survival: a population-based study. Arch Neurol. 2000;57(4):507–12. doi: 10.1001/archneur.57.4.507. [DOI] [PubMed] [Google Scholar]
- 16.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55(3):259–72. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Spillantini MG, et al. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95(11):6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jellinger KA. Formation and development of Lewy pathology: a critical update. J Neurol. 2009;256(Suppl 3):270–9. doi: 10.1007/s00415-009-5243-y. [DOI] [PubMed] [Google Scholar]
- 19.Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson’s disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- 20.Mariani E, et al. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827(1):65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567(1):1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Nunomura A, et al. Oxidative Damage to RNA in Aging and Neurodegenerative Disorders. Neurotox Res. doi: 10.1007/s12640-012-9331-x. [DOI] [PubMed] [Google Scholar]
- 23.Alam ZI, et al. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69(3):1196–203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, et al. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154(5):1423–9. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegde ML, et al. Studies on genomic DNA topology and stability in brain regions of Parkinson’s disease. Arch Biochem Biophys. 2006;449(1–2):143–56. doi: 10.1016/j.abb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Maynard S, et al. Mitochondrial base excision repair assays. Methods. 51(4):416–25. doi: 10.1016/j.ymeth.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988;85(17):6465–7. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mecocci P, et al. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34(4):609–16. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 29.Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res. 2003;531(1–2):231–51. doi: 10.1016/j.mrfmmm.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Michaels ML, Miller JH. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) J Bacteriol. 1992;174(20):6321–5. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tornaletti S. DNA repair in mammalian cells: Transcription-coupled DNA repair: directing your effort where it’s most needed. Cell Mol Life Sci. 2009;66(6):1010–20. doi: 10.1007/s00018-009-8738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi A, et al. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol Dis. 2002;9(2):244–8. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 33.Abe T, et al. Alteration of 8-hydroxyguanosine concentrations in the cerebrospinal fluid and serum from patients with Parkinson’s disease. Neurosci Lett. 2003;336(2):105–8. doi: 10.1016/s0304-3940(02)01259-4. [DOI] [PubMed] [Google Scholar]
- 34.Oli RG, et al. No increased chromosomal damage in L-DOPA-treated patients with Parkinson’s disease: a pilot study. J Neural Transm. 2010;117(6):737–46. doi: 10.1007/s00702-010-0401-z. [DOI] [PubMed] [Google Scholar]
- 35.Cornetta T, et al. Levodopa therapy reduces DNA damage in peripheral blood cells of patients with Parkinson’s disease. Cell Biol Toxicol. 2009;25(4):321–30. doi: 10.1007/s10565-008-9086-6. [DOI] [PubMed] [Google Scholar]
- 36.Migliore L, et al. Oxidative damage and cytogenetic analysis in leukocytes of Parkinson’s disease patients. Neurology. 2002;58(12):1809–15. doi: 10.1212/wnl.58.12.1809. [DOI] [PubMed] [Google Scholar]
- 37.Migliore L, et al. Chromosome and oxidative damage biomarkers in lymphocytes of Parkinson’s disease patients. Int J Hyg Environ Health. 2001;204(1):61–6. doi: 10.1078/1438-4639-00074. [DOI] [PubMed] [Google Scholar]
- 38.Petrozzi L, et al. Cytogenetic analysis oxidative damage in lymphocytes of Parkinson’s disease patients. Neurol Sci. 2001;22(1):83–4. doi: 10.1007/s100720170058. [DOI] [PubMed] [Google Scholar]
- 39.Seet RC, et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic Biol Med. 2010;48(4):560–6. doi: 10.1016/j.freeradbiomed.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Sato S, Mizuno Y, Hattori N. Urinary 8-hydroxydeoxyguanosine levels as a biomarker for progression of Parkinson disease. Neurology. 2005;64(6):1081–3. doi: 10.1212/01.WNL.0000154597.24838.6B. [DOI] [PubMed] [Google Scholar]
- 41.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger . DNA Repair and Mutagenesis. 2. ASM Press; 2006. [Google Scholar]
- 42.Shimura-Miura H, et al. Increased 8-oxo-dGTPase in the mitochondria of substantia nigral neurons in Parkinson’s disease. Ann Neurol. 1999;46(6):920–4. [PubMed] [Google Scholar]
- 43.Arai T, et al. Up-regulation of hMUTYH, a DNA repair enzyme, in the mitochondria of substantia nigra in Parkinson’s disease. Acta Neuropathol. 2006;112(2):139–45. doi: 10.1007/s00401-006-0081-9. [DOI] [PubMed] [Google Scholar]
- 44.Fukae J, et al. Expression of 8-oxoguanine DNA glycosylase (OGG1) in Parkinson’s disease and related neurodegenerative disorders. Acta Neuropathol. 2005;109(3):256–62. doi: 10.1007/s00401-004-0937-9. [DOI] [PubMed] [Google Scholar]
- 45.Gencer M, et al. DNA repair genes in Parkinson’s disease. Genet Test Mol Biomarkers. 16(6):504–7. doi: 10.1089/gtmb.2011.0252. [DOI] [PubMed] [Google Scholar]
- 46.Elstner M, et al. Neuromelanin, neurotransmitter status and brainstem location determine the differential vulnerability of catecholaminergic neurons to mitochondrial DNA deletions. Mol Brain. 2011;4(1):43. doi: 10.1186/1756-6606-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bender A, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38(5):515–7. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 48.Kraytsberg Y, et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38(5):518–20. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 49.Lin MT, et al. Somatic mitochondrial DNA mutations in early parkinson and incidental lewy body disease. Ann Neurol. 71(6):850–4. doi: 10.1002/ana.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vermulst M, Wanagat J, Loeb LA. On mitochondria, mutations, and methodology. Cell Metab. 2009;10(6):437. doi: 10.1016/j.cmet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Chen CT, et al. Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):85–91. doi: 10.1016/j.plefa.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968;9(5):570–9. [PubMed] [Google Scholar]
- 53.Ruiperez V, Darios F, Davletov B. Alpha-synuclein, lipids and Parkinson’s disease. Prog Lipid Res. 2010;49(4):420–8. doi: 10.1016/j.plipres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Bochkov VN, et al. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419(6902):77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 55.Roberts LJ, 2nd, Fessel JP. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem Phys Lipids. 2004;128(1–2):173–86. doi: 10.1016/j.chemphyslip.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 56.Ross BM, et al. Low activity of key phospholipid catabolic and anabolic enzymes in human substantia nigra: possible implications for Parkinson’s disease. Neuroscience. 1998;83(3):791–8. doi: 10.1016/s0306-4522(97)00454-5. [DOI] [PubMed] [Google Scholar]
- 57.Dexter DT, et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem. 1989;52(2):381–9. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 58.Mythri RB, et al. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem Res. 2011;36(8):1452–63. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- 59.Navarro A, et al. Human brain cortex: mitochondrial oxidative damage and adaptive response in Parkinson disease and in dementia with Lewy bodies. Free Radic Biol Med. 2009;46(12):1574–80. doi: 10.1016/j.freeradbiomed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Dexter DT, et al. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord. 1994;9(1):92–7. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- 61.Fessel JP, et al. Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson’s disease and with dementia with Lewy body disease. J Neurochem. 2003;85(3):645–50. doi: 10.1046/j.1471-4159.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 62.Shamoto-Nagai M, et al. In parkinsonian substantia nigra, alpha-synuclein is modified by acrolein, a lipid-peroxidation product, and accumulates in the dopamine neurons with inhibition of proteasome activity. J Neural Transm. 2007;114(12):1559–67. doi: 10.1007/s00702-007-0789-2. [DOI] [PubMed] [Google Scholar]
- 63.Castellani RJ, et al. Hydroxynonenal adducts indicate a role for lipid peroxidation in neocortical and brainstem Lewy bodies in humans. Neurosci Lett. 2002;319(1):25–8. doi: 10.1016/s0304-3940(01)02514-9. [DOI] [PubMed] [Google Scholar]
- 64.Yoritaka A, et al. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93(7):2696–701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pratico D, et al. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12(15):1777–83. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 66.!!! INVALID CITATION !!!
- 67.Lee CY, et al. Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and Parkinson’s disease patients: cautions in the use of biomarkers of oxidative stress. Antioxid Redox Signal. 2009;11(3):407–20. doi: 10.1089/ars.2008.2179. [DOI] [PubMed] [Google Scholar]
- 68.Kalra J, et al. Oxygen free radical producing activity of polymorphonuclear leukocytes in patients with Parkinson’s disease. Mol Cell Biochem. 1992;112(2):181–6. doi: 10.1007/BF00227575. [DOI] [PubMed] [Google Scholar]
- 69.Molina JA, et al. Serum lipid peroxides in patients with Parkinson’s disease. Neurosci Lett. 1992;136(2):137–40. doi: 10.1016/0304-3940(92)90033-4. [DOI] [PubMed] [Google Scholar]
- 70.Ahlskog JE, et al. No evidence for systemic oxidant stress in Parkinson’s or Alzheimer’s disease. Mov Disord. 1995;10(5):566–73. doi: 10.1002/mds.870100507. [DOI] [PubMed] [Google Scholar]
- 71.Cristalli DO, et al. Peripheral markers in neurodegenerative patients and their first-degree relatives. J Neurol Sci. 2012;314(1–2):48–56. doi: 10.1016/j.jns.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Kilinc A, et al. Increased erythrocyte susceptibility to lipid peroxidation in human Parkinson’s disease. Neurosci Lett. 1988;87(3):307–10. doi: 10.1016/0304-3940(88)90467-3. [DOI] [PubMed] [Google Scholar]
- 73.Agil A, et al. Plasma lipid peroxidation in sporadic Parkinson’s disease. Role of the L-dopa. J Neurol Sci. 2006;240(1–2):31–6. doi: 10.1016/j.jns.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 74.Connolly J, et al. F2 isoprostane levels in plasma and urine do not support increased lipid peroxidation in cognitively impaired Parkinson disease patients. Cogn Behav Neurol. 2008;21(2):83–6. doi: 10.1097/WNN.0b013e31817995e7. [DOI] [PubMed] [Google Scholar]
- 75.Irizarry MC, et al. Plasma F2A isoprostane levels in Alzheimer’s and Parkinson’s disease. Neurodegener Dis. 2007;4(6):403–5. doi: 10.1159/000107699. [DOI] [PubMed] [Google Scholar]
- 76.Boll MC, et al. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res. 2008;33(9):1717–23. doi: 10.1007/s11064-008-9610-3. [DOI] [PubMed] [Google Scholar]
- 77.Selley ML. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic Biol Med. 1998;25(2):169–74. doi: 10.1016/s0891-5849(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 78.Ilic TV, et al. Oxidative stress indicators are elevated in de novo Parkinson’s disease patients. Funct Neurol. 1999;14(3):141–7. [PubMed] [Google Scholar]
- 79.Shukla R, et al. Nitrite and malondialdehyde content in cerebrospinal fluid of patients with Parkinson’s disease. Int J Neurosci. 2006;116(12):1391–402. doi: 10.1080/00207450500513989. [DOI] [PubMed] [Google Scholar]
- 80.Musanti R, et al. Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson disease. Biochem Med Metab Biol. 1993;49(2):133–42. doi: 10.1006/bmmb.1993.1016. [DOI] [PubMed] [Google Scholar]
- 81.Hoepken HH, et al. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis. 2007;25(2):401–11. doi: 10.1016/j.nbd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 82.Cooper O, Hallett P, Isacson O. Using stem cells and iPS cells to discover new treatments for Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S14–6. doi: 10.1016/S1353-8020(11)70007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paradies G, et al. Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: role of cardiolipin. FEBS Lett. 1997;406(1–2):136–8. doi: 10.1016/s0014-5793(97)00264-0. [DOI] [PubMed] [Google Scholar]
- 84.Pope S, Land JM, Heales SJ. Oxidative stress and mitochondrial dysfunction in neurodegeneration; cardiolipin a critical target? Biochim Biophys Acta. 2008;1777(7–8):794–9. doi: 10.1016/j.bbabio.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 85.Bayir H, et al. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome C: protection against apoptosis versus delayed oxidative stress in Parkinson disease. J Biol Chem. 2009;284(23):15951–69. doi: 10.1074/jbc.M900418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almeida Mdo R. Glucocerebrosidase involvement in Parkinson disease and other synucleinopathies. Front Neurol. 3:65. doi: 10.3389/fneur.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alam ZI, et al. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997;69(3):1326–9. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 88.Keeney PM, et al. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26(19):5256–64. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–9. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 90.Bandopadhyay R, et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127(Pt 2):420–30. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 91.Blackinton J, et al. Post-transcriptional regulation of mRNA associated with DJ-1 in sporadic Parkinson disease. Neurosci Lett. 2009;452(1):8–11. doi: 10.1016/j.neulet.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choi J, et al. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281(16):10816–24. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–8. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 94.Choi J, et al. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem. 2004;279(13):13256–64. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 95.Horowitz MP, Greenamyre JT. Mitochondrial iron metabolism and its role in neurodegeneration. J Alzheimers Dis. 20(Suppl 2):S551–68. doi: 10.3233/JAD-2010-100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mastroberardino PG, et al. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson’s disease. Neurobiol Dis. 2009;34(3):417–31. doi: 10.1016/j.nbd.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watfa G, et al. Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. J Nutr Health Aging. 2011;15(4):277–81. doi: 10.1007/s12603-010-0275-7. [DOI] [PubMed] [Google Scholar]
- 98.Baillet A, et al. The role of oxidative stress in amyotrophic lateral sclerosis and Parkinson’s disease. Neurochem Res. 2010;35(10):1530–7. doi: 10.1007/s11064-010-0212-5. [DOI] [PubMed] [Google Scholar]
- 99.Saito Y, et al. Preparation and application of monoclonal antibodies against oxidized DJ-1. Significant elevation of oxidized DJ-1 in erythrocytes of early-stage Parkinson disease patients. Neurosci Lett. 2009;465(1):1–5. doi: 10.1016/j.neulet.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 100.Tanner CM, et al. Parkinson disease in twins: an etiologic study. JAMA. 1999;281(4):341–6. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 101.Wirdefeldt K, et al. Complete ascertainment of Parkinson disease in the Swedish Twin Registry. Neurobiol Aging. 2008;29(12):1765–73. doi: 10.1016/j.neurobiolaging.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wirdefeldt K, et al. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63(2):305–11. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- 103.Lin MT, Simon DK. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2005;64(5):932. doi: 10.1212/wnl.64.5.932. author reply 932. [DOI] [PubMed] [Google Scholar]
- 104.Kosel S, et al. Parkinson disease: analysis of mitochondrial DNA in monozygotic twins. Neurogenetics. 2000;2(4):227–30. doi: 10.1007/pl00022973. [DOI] [PubMed] [Google Scholar]
- 105.Vieregge P, et al. Parkinson’s disease in twins. Neurology. 1992;42(8):1453–61. doi: 10.1212/wnl.42.8.1453. [DOI] [PubMed] [Google Scholar]
- 106.Rosati G, et al. The risk of Parkinson disease in Mediterranean people. Neurology. 1980;30(3):250–5. doi: 10.1212/wnl.30.3.250. [DOI] [PubMed] [Google Scholar]
- 107.Zhang ZX, Roman GC. Worldwide occurrence of Parkinson’s disease: an updated review. Neuroepidemiology. 1993;12(4):195–208. doi: 10.1159/000110318. [DOI] [PubMed] [Google Scholar]
- 108.Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov Disord. 2003;18(1):19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- 109.Ashok PP, et al. Epidemiology of Parkinson’s disease in Benghazi, North-East Libya. Clin Neurol Neurosurg. 1986;88(2):109–13. doi: 10.1016/s0303-8467(86)80005-1. [DOI] [PubMed] [Google Scholar]
- 110.Cannon JR, Greenamyre JT. Gene-environment interactions in Parkinson’s disease: Specific evidence in humans and mammalian models. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tanner CM, et al. Smoking and Parkinson’s disease in twins. Neurology. 2002;58(4):581–8. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- 112.Wirdefeldt K, et al. Risk and protective factors for Parkinson’s disease: a study in Swedish twins. Ann Neurol. 2005;57(1):27–33. doi: 10.1002/ana.20307. [DOI] [PubMed] [Google Scholar]
- 113.Hertzman C, et al. Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am J Ind Med. 1990;17(3):349–55. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- 114.Gorell JM, et al. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50(5):1346–50. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- 115.Hubble JP, et al. Risk factors for Parkinson’s disease. Neurology. 1993;43(9):1693–7. doi: 10.1212/wnl.43.9.1693. [DOI] [PubMed] [Google Scholar]
- 116.Hernan MA, et al. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52(3):276–84. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 117.Liou HH, et al. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48(6):1583–8. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- 118.Stephenson J. Exposure to home pesticides linked to Parkinson disease. JAMA. 2000;283(23):3055–6. doi: 10.1001/jama.283.23.3055. [DOI] [PubMed] [Google Scholar]
- 119.Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurol Clin. 1996;14(2):317–35. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gorell JM, et al. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology. 1999;20(2–3):239–47. [PubMed] [Google Scholar]
- 121.Gorell JM, et al. Occupational metal exposures and the risk of Parkinson’s disease. Neuroepidemiology. 1999;18(6):303–8. doi: 10.1159/000026225. [DOI] [PubMed] [Google Scholar]
- 122.Racette BA, et al. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56(1):8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- 123.Galvani P, Fumagalli P, Santagostino A. Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur J Pharmacol. 1995;293(4):377–83. doi: 10.1016/0926-6917(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 124.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20(2–3):445–53. [PubMed] [Google Scholar]
- 125.Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29(6):322–9. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dick FD. Parkinson’s disease and pesticide exposures. Br Med Bull. 2006;79–80:219–31. doi: 10.1093/bmb/ldl018. [DOI] [PubMed] [Google Scholar]
- 127.Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42(7):1328–35. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- 128.Kamel F, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165(4):364–74. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 129.Tanner CM. Epidemiology of Parkinson’s disease. Neurol Clin. 1992;10(2):317–29. doi: 10.1016/S0733-8619(18)30212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Greenamyre JT, et al. Complex I and Parkinson’s disease. IUBMB Life. 2001;52(3–5):135–41. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- 131.Lummen P. Complex I inhibitors as insecticides and acaricides. Biochim Biophys Acta. 1998;1364(2):287–96. doi: 10.1016/s0005-2728(98)00034-6. [DOI] [PubMed] [Google Scholar]
- 132.Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3(12):1301–6. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 133.Cannon JR, et al. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis. 2009;34(2):279–90. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hancock DB, et al. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dhillon AS, et al. Pesticide/environmental exposures and Parkinson’s disease in East Texas. J Agromedicine. 2008;13(1):37–48. doi: 10.1080/10599240801986215. [DOI] [PubMed] [Google Scholar]
- 136.Tanner CM, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866–72. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Talpade DJ, et al. In vivo labeling of mitochondrial complex I (NADH:ubiquinone oxidoreductase) in rat brain using [(3)H]dihydrorotenone. J Neurochem. 2000;75(6):2611–21. doi: 10.1046/j.1471-4159.2000.0752611.x. [DOI] [PubMed] [Google Scholar]
- 138.Heikkila RE, et al. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci Lett. 1985;62(3):389–94. doi: 10.1016/0304-3940(85)90580-4. [DOI] [PubMed] [Google Scholar]
- 139.Ferrante RJ, et al. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Res. 1997;753(1):157–62. doi: 10.1016/s0006-8993(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 140.Higgins DS, Jr, Greenamyre JT. [3H]dihydrorotenone binding to NADH: ubiquinone reductase (complex I) of the electron transport chain: an autoradiographic study. J Neurosci. 1996;16(12):3807–16. doi: 10.1523/JNEUROSCI.16-12-03807.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sherer TB, et al. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22(16):7006–15. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Betarbet R, et al. Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22(2):404–20. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 143.Wang XF, et al. Inhibitory effects of pesticides on proteasome activity: implication in Parkinson’s disease. Neurobiol Dis. 2006;23(1):198–205. doi: 10.1016/j.nbd.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 144.Drolet RE, et al. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol Dis. 2009;36(1):96–102. doi: 10.1016/j.nbd.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 145.Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp Neurol. 2009;218(1):154–61. doi: 10.1016/j.expneurol.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sindhu KM, et al. Rats with unilateral median forebrain bundle, but not striatal or nigral, lesions by the neurotoxins MPP+ or rotenone display differential sensitivity to amphetamine and apomorphine. Pharmacol Biochem Behav. 2006;84(2):321–9. doi: 10.1016/j.pbb.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 147.Alam M, Schmidt WJ. L-DOPA reverses the hypokinetic behaviour and rigidity in rotenone-treated rats. Behav Brain Res. 2004;153(2):439–46. doi: 10.1016/j.bbr.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 148.Rojas JC, Saavedra JA, Gonzalez-Lima F. Neuroprotective effects of memantine in a mouse model of retinal degeneration induced by rotenone. Brain Res. 2008;1215:208–17. doi: 10.1016/j.brainres.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Biehlmaier O, Alam M, Schmidt WJ. A rat model of Parkinsonism shows depletion of dopamine in the retina. Neurochem Int. 2007;50(1):189–95. doi: 10.1016/j.neuint.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 150.Zhang X, Rojas JC, Gonzalez-Lima F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox Res. 2006;9(1):47–57. doi: 10.1007/BF03033307. [DOI] [PubMed] [Google Scholar]
- 151.Zhang X, Jones D, Gonzalez-Lima F. Mouse model of optic neuropathy caused by mitochondrial complex I dysfunction. Neurosci Lett. 2002;326(2):97–100. doi: 10.1016/s0304-3940(02)00327-0. [DOI] [PubMed] [Google Scholar]
- 152.Garcia-Garcia F, et al. Sleep disturbances in the rotenone animal model of Parkinson disease. Brain Res. 2005;1042(2):160–8. doi: 10.1016/j.brainres.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 153.Panov A, et al. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005;280(51):42026–35. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 154.Feng J. Microtubule: a common target for parkin and Parkinson’s disease toxins. Neuroscientist. 2006;12(6):469–76. doi: 10.1177/1073858406293853. [DOI] [PubMed] [Google Scholar]
- 155.Jiang Q, Yan Z, Feng J. Neurotrophic factors stabilize microtubules and protect against rotenone toxicity on dopaminergic neurons. J Biol Chem. 2006;281(39):29391–400. doi: 10.1074/jbc.M602740200. [DOI] [PubMed] [Google Scholar]
- 156.Ren Y, et al. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem. 2005;280(40):34105–12. doi: 10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- 157.Choi WS, et al. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105(39):15136–41. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sherer TB, et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23(34):10756–64. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marella M, et al. Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson’s disease. PLoS One. 2008;3(1):e1433. doi: 10.1371/journal.pone.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sterky FH, et al. Altered dopamine metabolism and increased vulnerability to MPTP in mice with partial deficiency of mitochondrial complex I in dopamine neurons. Hum Mol Genet. 2012;21(5):1078–89. doi: 10.1093/hmg/ddr537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Seo BB, et al. The single subunit NADH dehydrogenase reduces generation of reactive oxygen species from complex I. FEBS Lett. 2006;580(26):6105–8. doi: 10.1016/j.febslet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 162.Swarnkar S, et al. Rotenone-induced apoptosis and role of calcium: a study on Neuro-2a cells. Arch Toxicol. 2012 doi: 10.1007/s00204-012-0853-z. [DOI] [PubMed] [Google Scholar]
- 163.Jia H, et al. Synergistic anti-Parkinsonism activity of high doses of B vitamins in a chronic cellular model. Neurobiol Aging. 2010;31(4):636–46. doi: 10.1016/j.neurobiolaging.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 164.Porcelli AM, et al. Respiratory complex I dysfunction due to mitochondrial DNA mutations shifts the voltage threshold for opening of the permeability transition pore toward resting levels. J Biol Chem. 2009;284(4):2045–52. doi: 10.1074/jbc.M807321200. [DOI] [PubMed] [Google Scholar]
- 165.de Lima PD, et al. Genotoxic effects of rotenone on cultured lymphocytes. Genet Mol Res. 2005;4(4):822–31. [PubMed] [Google Scholar]
- 166.Greene JG, Greenamyre JT, Dingledine R. Sequential and concerted gene expression changes in a chronic in vitro model of parkinsonism. Neuroscience. 2008;152(1):198–207. doi: 10.1016/j.neuroscience.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Molina-Jimenez MF, et al. Neuroprotective effect of fraxetin and myricetin against rotenone-induced apoptosis in neuroblastoma cells. Brain Res. 2004;1009(1–2):9–16. doi: 10.1016/j.brainres.2004.02.065. [DOI] [PubMed] [Google Scholar]
- 168.Koopman WJ, et al. Inhibition of complex I of the electron transport chain causes O2−· - mediated mitochondrial outgrowth. Am J Physiol Cell Physiol. 2005;288(6):C1440–50. doi: 10.1152/ajpcell.00607.2004. [DOI] [PubMed] [Google Scholar]
- 169.Beretta S, et al. Partial mitochondrial complex I inhibition induces oxidative damage and perturbs glutamate transport in primary retinal cultures. Relevance to Leber Hereditary Optic Neuropathy (LHON) Neurobiol Dis. 2006;24(2):308–17. doi: 10.1016/j.nbd.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 170.Sousa SC, et al. Ca2+-induced oxidative stress in brain mitochondria treated with the respiratory chain inhibitor rotenone. FEBS Lett. 2003;543(1–3):179–83. doi: 10.1016/s0014-5793(03)00421-6. [DOI] [PubMed] [Google Scholar]
- 171.Swarnkar S, et al. Rotenone induced neurotoxicity in rat brain areas: a histopathological study. Neurosci Lett. 501(3):123–7. doi: 10.1016/j.neulet.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 172.Verma R, Nehru B. Effect of centrophenoxine against rotenone-induced oxidative stress in an animal model of Parkinson’s disease. Neurochem Int. 2009;55(6):369–75. doi: 10.1016/j.neuint.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 173.Nehru B, et al. Behavioral alterations in rotenone model of Parkinson’s disease: attenuation by co-treatment of centrophenoxine. Brain Res. 2008;1201:122–7. doi: 10.1016/j.brainres.2008.01.074. [DOI] [PubMed] [Google Scholar]
- 174.Kaur H, Chauhan S, Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem Res. 36(8):1435–43. doi: 10.1007/s11064-011-0469-3. [DOI] [PubMed] [Google Scholar]
- 175.Sanchez-Reus MI, et al. Standardized Hypericum perforatum reduces oxidative stress and increases gene expression of antioxidant enzymes on rotenone-exposed rats. Neuropharmacology. 2007;52(2):606–16. doi: 10.1016/j.neuropharm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 176.Zaitone SA, Abo-Elmatty DM, Shaalan AA. Acetyl-L-carnitine and alpha-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson’s disease therapy. Pharmacol Biochem Behav. 100(3):347–60. doi: 10.1016/j.pbb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 177.Bashkatova V, et al. Chronic administration of rotenone increases levels of nitric oxide and lipid peroxidation products in rat brain. Exp Neurol. 2004;186(2):235–41. doi: 10.1016/j.expneurol.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 178.Kaneko K, et al. Increased vulnerability to rotenone-induced neurotoxicity in ceruloplasmin-deficient mice. Neurosci Lett. 2008;446(1):56–8. doi: 10.1016/j.neulet.2008.08.089. [DOI] [PubMed] [Google Scholar]
- 179.Hajieva P, et al. Novel imine antioxidants at low nanomolar concentrations protect dopaminergic cells from oxidative neurotoxicity. J Neurochem. 2009;110(1):118–32. doi: 10.1111/j.1471-4159.2009.06114.x. [DOI] [PubMed] [Google Scholar]
- 180.Filomeni G, et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson’s disease. Neurobiol Aging. 2012;33(4):767–85. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 181.Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134(1):109–18. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 182.Horowitz MP, et al. Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults. Antioxid Redox Signal. 15(4):855–71. doi: 10.1089/ars.2010.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ramachandiran S, et al. Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicol Sci. 2007;95(1):163–71. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]