Abstract
Persistent maladaptive memories that maintain drug seeking and are resistant to extinction are a hallmark of addiction. As such, disruption of memory reconsolidation after retrieval has received attention for its therapeutic potential. Unrestrained reconsolidation may have the opposite effect, leading to reiterative and cumulative strengthening of memory over long periods of time. Here we review the molecular mechanisms underlying reconsolidation of appetitive and drug-rewarded memories, and discuss how these findings contribute to our understanding of the nature of this process. Finally, we suggest that drug-induced alterations to signal transduction might lead to dysregulation of reconsolidation, causing enhancements of drug-related memory after retrieval, and significantly contribute to the compulsive drug seeking that is a core component of addiction.
Memory reconsolidation, the process by which memories are re-stabilized after retrieval, may have special relevance to addiction both for the treatment potential [1–4] and as a mechanism for maintaining and strengthening the cue-drug relationship over time. The study of reconsolidation was kick-started after a 30-year hiatus [5], by studies from Nader et al. [6], who described the disruption of Pavlovian fear memories by anisomycin administered after retrieval. This study demonstrated first, that consolidated memories could be “erased” after retrieval, and second, that mechanistically, this so-called “reconsolidation” process resembled the original consolidation in its requirement for protein synthesis.
Findings on reconsolidation come from a variety of memory tasks [7–11] and species [11–14] and have several important implications. First, if memories can be disrupted after retrieval, then this may have potential for treatment of persistent or exaggerated memory in disorders such as post-traumatic stress disorders and addiction. Second, given the similarities between the initial storage of memory and an active reconsolidation process after retrieval, it is possible that reconsolidation may not only maintain, but also update or strengthen memories after retrieval. This latter possibility may be a means by which memories become exaggerated over time, even without additional training trials (or exposures to the reinforcer) and thus may contribute to the aetiology of disorders such as PTSD and addiction.
Studies of reconsolidation have begun to describe the signal transduction and transcription events required for post-retrieval stabilization of memory. In this review, we will discuss these findings and how understanding the molecular networks required will inform our conceptualization of reconsolidation, its relationship with memory consolidation, and its potential role in both treatment and development of the compulsive drug seeking that is a core component of addiction.
How is reconsolidation studied?
The protocol for studying memory reconsolidation typically involves three phases: a training phase, a reactivation phase after which manipulations occur, and test (see Figure 1A). Memory reactivation is triggered by presentation of the conditioned stimulus (CS), without (and sometimes with) the occurrence of the unconditioned stimulus (US).
Figure 1.
How reconsolidation is studied. (a) Schematic of typical reconsolidation paradigms. Animals are trained on a Pavlovian memory task with one or many trials. At least one day after the end of training, memory reactivation is induced by re-exposure to the CS, at which time manipulations are applied. At least 24 hours after reactivation, animals undergo a test of responding to the CS. In (b) acquisition/consolidation, and (c) extinction, we infer an active process by observing a change in behavior (filled circles). Disruption of this process leads to no change in behavior (open circles). In contrast, (d) reconsolidation causes no observable change in behavior (filled circles). Evidence for an active process stems from changes in behavior as a consequence of manipulations (e.g., disruption, open circles).
In general, study of memory requires a change in behavior. For example, we attribute expression of a response to a previously reinforced cue to the formation and storage of a memory (Figure 1B). Similarly, a decrease in behavior after multiple exposures to previously conditioned cues is attributed to expression of extinction learning (Figure 1C). In contrast, reconsolidation, a mechanism that maintains memory after retrieval, should result in no behavioral change (Figure 1D). Without further evidence, however, it is difficult to attribute “no change” in behavior as evidence for an active process. In order to determine whether an active reconsolidation process occurs after memory retrieval, procedures aimed at disrupting or enhancing memory are used. Here, if manipulation after retrieval causes changes in behavior, then we can conclude there is an active process occurring at this time. Manipulations can range from behavioral [7,15,16] to pharmacological [6,17,18] to genetic [19,20], allowing for the dissection of roles of various proteins and gene products in stabilization of memory after retrieval. In addition to behavioral changes, imaging and quantitative methods are used to determine which signaling pathways are active after retrieval, providing further evidence for activation of a memory-storage related process.
Molecular Mechanisms of Reconsolidation
In the studies discussed here, reward-related Pavlovian tasks, including conditioned place preference and Pavlovian approached are used, with drug, food, or sucrose reinforcers. By detailing the similarities with other processes and specificity of activation in time, task, and brain region after retrieval, a picture of the mechanisms of reconsolidation in these paradigms has started to emerge. NMDA receptors play a crucial role in memory reconsolidation [18,21–28] as does CB1R [29,30], and changes in GABA activity [9,31]. Intracellular signaling molecules, including both PKA [17,32] and CREB [33] are also required for reconsolidation of reward related memories. In these procedures, retrieval of appetitive memory activates a number of signaling cascades, including the extracellular signal regulated kinase (ERK) [33,34], the immediate early gene Egr1 [22,35–39] and phosphorylation of the AMPA subunit GluR1 [34,40], CdK5 [41] and glycogen synthase 3 [42].
Given the promiscuous nature of many kinases, the outcome of their activity is determined by subcellular localization and other concurrent signaling events [43], it will be important to understand the pathways via which each molecule is acting. Indeed, the same kinase can exert very different effects depending on mechanism of activation, the subcellular compartment, and the availability and proximity of downstream effectors [44]. There is some evidence detailing the pathways of by which identified proteins exert their effects (Figure 2). For example, reconsolidation requires activation NMDA receptors and CaMKII, possibly via prevention of GluR1 phosphorylation [40]. The activation of ERK, PKA/CREB pathways are consistent with the receptors required for memory reconsolidation and the role of Egr1 [45]. PKA/CREB is downstream of several receptor types, including β-adrenergic receptors, which are also required for memory reconsolidation [24,46–51]. Similarly, ERK is triggered by NMDAR receptor activation. Furthermore, CDK5 modulates glutamatergic signaling [52] and may influence reconsolidation via NMDARs. The apparent similarities in molecular mechanisms underlying consolidation and reconsolidation suggest that the latter process plays a crucial role in the maintenance and long-term storage of memory. This suggests that enhancement of reconsolidation, like the enhancement of consolidation, may lead to strengthened memory [37,53–55].
Figure 2.
Mechanisms of reward-memory reconsolidation. Signaling molecules and possible pathways, underlying reconsolidation of appetitive memories. Solid symbols represent proteins with an identified role in reconsolidation. In some cases, the pathways have also been identified (solid arrows). Dashed arrows represent possible pathways by which kinases may mediate reconsolidation. Pale pink symbols represent interacting proteins that may define roles and pathways underlying reconsolidation.
(Abbreviations: pPKA phosphorylated protein kinase A pCaMKII phosphorylated calcium modulated kinase II; pERK phosphorylated extracellular regulated kinase; AKAP A kinase anchoring protein; pCREB phosphorylated Cre-response Element Binding Protein; CBP CREB Binding Protein; Egr1 early growth response protein 1 ; CDK cylin-dependent kinase 5; AC adenylyl cyclase; cAMP cyclic AMP; NMDAR N-methyl-D-aspartate receptor; GluA1 AMPA subunit Glutamate receptor 1; βAR beta-adrenergic receptor; Ca2+ Calcium)
This basic protocol has also been used to study enhancement of memory after retrieval of both fear and appetitive Pavlovian conditioning. In fear memories, activation of PKA after memory reactivation causes enhanced reconsolidation and increased fear [53]. Similarly, D-cycloserine enhances cocaine seeking after retrieval of drug-associated memories [37] possibly via increased levels of retrieval associated Egr1. In humans, repeated retrieval [56] and GABA receptor manipulations can also enhance memory [9]. That reconsolidation can be enhanced further supports the likelihood that this process acts to stabilize and store memories after retrieval. Furthermore, this finding suggests that in some circumstances, memories could continue to be strengthened after retrieval in the absence of further training trials. Environmental and genetic events that cause upregulation of mechanisms underlying reconsolidation might be one situation in which ongoing memory enhancement may occur.
There are several important caveats to this interpretation. It is noteworthy that inhibition of neither beta-adrenergic receptors [23,57,58] nor NMDARs [24] always disrupt reconsolidation. These differences may be due to route of administration (e.g., [57]), task specificity (e.g., [24]) or reinforcer (e.g., ethanol [58]). Additionally there is relatively little data on signaling molecules and pathways in reconsolidation of food, sucrose, and drug-reinforced tasks, thus it remains possible that the pattern of activation is very different from that of consolidation.
What is stabilized during reconsolidation?
Evidence for altered behavioral responses after non-reinforced, reactivation trial is commonly interpreted as reflecting changes to the memory. However, it is not clear what aspect of the memory is modified. The original formulation of reconsolidation stated that disruptions of reconsolidation were disruptions of the CS-US association or the content of the memory [6]. There is evidence, however, that disruptions of reconsolidation primarily disrupt affective, rather than associative aspects of Pavlovian memories. Studies of fear conditioning using human subjects demonstrated that disruption of reconsolidation specifically abolished affective and sympathetic responses to the CS, without affecting associative or declarative memory [14,59]. The enhancement of memory after retrieval may similarly represent a change in the emotional/motivational components (Figure 3).
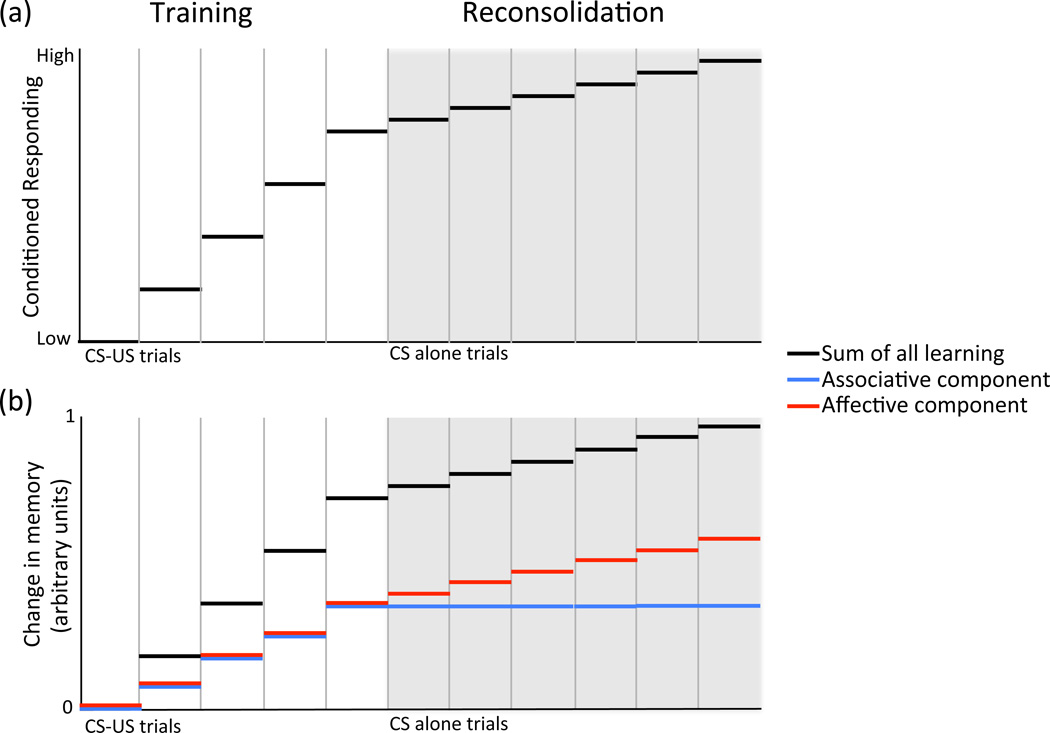
Figure 3.
Hypothetical enhancement of affective aspects of memory during reconsolidation. (a) Dysregulation of signaling mechanisms underlying reconsolidation may lead to the reiterative and cumulative enhancement of drug-associated memories. The conditioned response (CR) increases throughout training (CS-US trials). If strengthened by reconsolidation, CR will continue to increase after reactivation (CS alone) trials. (b). During training, both associative (blue) and affective (red) (e.g., emotion, motivation) components of memory increase with CS-US trials, and both contribute to increases in CR (black). During reconsolidation, it is possible that affective components are preferentially enhanced, leading to increased CR without increased associative strength. (Adapted from [76])
There is some evidence that affective aspects of memory, such as motivational and rewarding properties, can be experimentally distinguished from associative aspects, such as expectation and approach in animal models [18]. For example, Pavlovian to Instrumental Transfer (PIT) is believed to assess incentive motivational properties of the CS. Using these models, it appears that affective and motivational properties of the CS can be disrupted by memory reconsolidation [18,60]. Additional evidence suggests a distinction between the motivational components of drug-associated memories, which are disrupted after nucleus accumbens manipulations, and associative components which are mediated by the basolateral amygdala [39]. Many studies have shown parallel signaling changes in signaling in BLA and nucleus accumbens after retrieval, however these findings suggest that different brain regions may mediate differential aspects of memory during reconsolidation.
It has also been proposed that a primary role of reconsolidation in memory is the updating of old memories [10,61]. For example, sensory and emotional information about the CS and contextual cues might be incorporated into the original memory trace. This hypothesis is yet to be examined in detail, however, there is some evidence that memories only become labile after retrieval if some new information is present [62]. In line with this, some studies have shown when the outcome of CS presentation is predictable, either because the CS was followed by a US at test [63] or because subjects were explicitly instructed that the US would not be presented after the CS [64] then reconsolidation is not induced. Other studies, however, have demonstrated disruption of reconsolidation when a training trial is used as reactivation [65]. Additionally, non-associative memories, such as object recognition, are subject to reconsolidation [66], and in these tasks reactivation is independent of prediction. It remains possible that there is a requirement for new information without a requirement for a prediction error. Rather, the presentation of a CS in a new context (as is standard in cued-fear conditioning), or changing the number of trials, may be sufficient “new information” to trigger reconsolidation. How – and under what conditions – new information is required for destabilization of the original memory and reconsolidation after retrieval is a subject of ongoing study. Nonetheless, whether such updating of memories is due to reconsolidation, where the original memory trace is changed, or consolidation, where a new memory is linked to existing trace, remains controversial [67].
Here, a clearer understanding of the molecular mechanisms required for reconsolidation will allow for a cleaner dissection of which aspects of memory are becoming labile after retrieval, whether affective or associative processes are primarily targeted during manipulation of reconsolidation, and whether incorporation of new information more closely resembles reconsolidation or consolidation.
Role of reconsolidation in addiction
Enhancement of memory after retrieval supports the hypothesis that reconsolidation is a real, specific process that maintains, strengthens, and possibly updates memory. These findings also raise the possibility that dysfunctional reconsolidation processes might also be involved in the aetiology of disorders including addiction. For example, repeated drug use and chronic alterations to signal transduction may cause ongoing strengthening of memory. Here, continuing to strengthen memories after retrieval, repeated over long periods of time, could lead to strong, intrusive memories that are resistant to extinction.
There is some support for this hypothesis. Post-retrieval cocaine also enhances reward-related memories [68], and chronic exposure to psychostimulants increases reward-related learning, via cyclic AMP (cAMP) second messenger pathways [69,70], and ΔFosB [71]. Given the similarities of PKA/CREB signaling in consolidation and reconsolidation [33], these long-lasting drug-induced adaptations may mediate ongoing strengthening of drug related memories and thereby compulsive, extinction-resistant memories.
Reconsolidation may also play a role in incubation of craving [72]. Here, ERK plays a critical role in both incubation [73] and reconsolidation of drug-related memory [33,34]. Currently the relation of incubation to reconsolidation is unclear, however, it remains possible that incubation reflects the enhancement of memory after spontaneous (non cued) retrieval of drug-related memory [74]. Finally, there is some evidence that memories can be enhanced after retrieval in humans [75], further supporting the possibility that dysregulation of cellular and molecular mechanisms may lead to ongoing strengthening of memory and potentially cause dysfunctional emotional and behavioral responses, a hallmark of several psychiatric disorders.
In considering how and why some memories become abnormally strong and others do not, it may be important to distinguish the role of reconsolidation in normal memories versus pathological memories. Under normal conditions, reconsolidation may act to update and maintain memories. In contrast, under altered conditions due to acute or chronic drug use, stress, or genetic predisposition, reconsolidation may act to enhance memories [76] contributing to persistent drug-related memories. The possibility that chronic drug use may cause abnormal, rigid memories may also explain the lack of behavioral and cognitive flexibility, impaired plasticity, and resistance to extinction observed in addiction [1,2].
Molecular mechanisms may provide a framework for understanding reconsolidation
Elucidating the molecular mechanisms of reconsolidation is an important step towards understanding the long-term storage and ongoing maintenance of memory. Thus far, our limited understanding of the mechanisms underlying memory reconsolidation has generated some insight into the nature of this process. The apparent similarity of signaling underlying reconsolidation and consolidation suggests that there is also a functional concordance of these mechanisms. That is, reconsolidation is likely required for the ongoing storage – or possibly updating – of memory. Most research has focused on the possibility that maladaptive memories can be disrupted after retrieval, and the possibility for intervention [1,2,15,16]. Alternatively, dysregulation of reconsolidation may lead to strengthening of memory leading to abnormal behavioral and emotional responses.
Many outstanding questions about the nature and role of reconsolidation remain. For example, what aspects of memory are targeted by reconsolidation? There is some evidence that disruption of reconsolidation in fear memories preferentially disrupt emotional responses, while leaving associative aspects of the memory intact. Other studies, however, suggest that declarative components of memory can also be disrupted. Initial evidence suggests the molecular and regional specificity of these processes. Careful separation of the behavioral, neural circuitry, and molecular mechanisms underlying disparate facets of reconsolidation will be required to fully determine which aspects of memory are targeted by reconsolidation. A related question is whether reconsolidation – or consolidation – is responsible for updating memory. To address this controversy, it will be important to determine if retrieval leading to reconsolidation requires the same cells as initially activated or that are responsible for memory storage. Here, overlapping populations would suggest an updating mechanism for reconsolidation, whereas distinctive populations may suggest a new learning process.
Addressing both of these questions will require delineation the signaling pathways and molecular networks activated during reconsolidation, and comparison with how they relate to those underlying consolidation. Although the field is only beginning to understand what individual proteins are involved in these processes, a more comprehensive view will provide targets for intervention, and a novel insight into how dysfunctional signaling networks can lead to maladaptive memories, emotions, and responses.
At this stage, the majority of studies have utilized inhibitors of receptors, kinases, and IEGs to examine mechanisms underlying reconsolidation of appetitive and drug-related memories. These findings have implicated several pathways and a number of proteins in memory reconsolidation. Importantly, in addition to suggesting utility for disruption of reconsolidation for addiction [1,2,16], these findings support the perspective that drugs of abuse might alter normal memory maintenance. Thereby, enhancements of signaling pathways due to exposure to drugs of abuse, other environmental effects such as stress, or genetic predisposition may play a significant role in the reiterative and cumulative strengthening of memories, and persistent behavioral and emotional responses in disorders including PTSD and addiction.
Highlights.
Reconsolidation functions to maintain or update memories after retrieval
Reconsolidation may primarily mediate emotional or motivational aspects of memory
Mechanisms of reconsolidation significantly overlap with those of consolidation
Drug exposure alters signal transduction and may enhance reconsolidation
Dysregulated reconsolidation may thereby contribute to the aetiology of addiction
Acknowledgements
This work was funded in part by National Institute of Drug Abuse DA015222 (JRT) and National Institute of Mental Health MH093459 (NCT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2750-9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiology of learning and memory. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorg BA. Reconsolidation of drug memories. Learning & Memory. 2012;36:1400–1417. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. European Journal of Neuroscience. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- 5.Misanin JR, Miller RR, Lewis DJ. Retrograde Amnesia Produced by Electroconvulsive Shock after Reactivation of a Consolidated Memory Trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 6.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval : Article : Nature. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 8.Bozon B, Davis S, Laroche S. A Requirement for the Immediate Early Gene zif268 in Reconsolidation of Recognition Memory after Retrieval. Neuron. 2003;40:695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- 9. Rodríguez MLC, Campos J, Forcato C, Leiguarda R, Maldonado H, Molina VA, Pedreira ME. Enhancing a declarative memory in humans: The effect of clonazepam on reconsolidation. Neuropharmacology. 2013;64:432–442. doi: 10.1016/j.neuropharm.2012.06.059. These authors use a cue-response verbal task to demonstrate that the benzodiazepine clozapam can enhance memory when taken in concert with memory retrieval. This evidence is the first demonstration of enhanced memory reconsolidation due to pharmacological manipulation in humans, and suggests a role for GABAA receptors in memory reconsolidation.
- 10.Jones B, Bukoski E, Nadel L, Fellous JM. Remaking memories: Reconsolidation updates positively motivated spatial memory in rats. Learning & Memory. 2012;19:91–98. doi: 10.1101/lm.023408.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai D, Pearce K, Chen S, Glanzman DL. Reconsolidation of Long-Term Memory in Aplysia. Current Biology. 2012;22:1–6. doi: 10.1016/j.cub.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaczer L, Klappenbach M, Maldonado H. Dissecting mechanisms of reconsolidation: octopamine reveals differences between appetitive and aversive memories in the crab Chasmagnathus. Learning & Memory. 2011;34:1170–1178. doi: 10.1111/j.1460-9568.2011.07830.x. [DOI] [PubMed] [Google Scholar]
- 13.Rose JK, Rankin CH. Blocking memory reconsolidation reverses memory-associated changes in glutamate receptor expression. The Journal of Neuroscience. 2006;26:11582–11587. doi: 10.1523/JNEUROSCI.2049-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nature Neuroscience. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 15.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, et al. A Memory Retrieval-Extinction Procedure to Prevent Drug Craving and Relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. These authors demonstrate that extinction performed in the window of memory lability after retrieval causes a persistent disruption of cue-induced responding for drugs in rats. Remarkably, the authors used the same procedure in heroin addicts and demonstrate persistent disruption of cue-induced craving compared with addicts exposed to extinction alone. This is an important demonstration of the treatment efficacy of disrupted reconsolidation in drug addicts.
- 17.Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR. Reconsolidation of a Cocaine-Associated Stimulus Requires Amygdalar Protein Kinase A. Journal of Neuroscience. 2010;30:4401–4407. doi: 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JLC, Everitt BJ. Reactivation-dependent amnesia in Pavlovian approach and instrumental transfer. Learning & Memory. 2008;15:597–602. doi: 10.1101/lm.1029808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tronson NC, Wiseman SL, Neve RL, Nestler EJ, Olausson P, Taylor JR. Distinctive roles for amygdalar CREB in reconsolidation and extinction of fear memory. Learning & Memory. 2012;19:178–181. doi: 10.1101/lm.025783.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. PLOS One. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- 21.Sadler R, Herzig V, Schmidt WJ. Repeated treatment with the NMDA antagonist MK-801 disrupts reconsolidation of memory for amphetamine-conditioned place preference. Behavioral Pharmacology. 2007;18:699–703. doi: 10.1097/FBP.0b013e3282effb81. [DOI] [PubMed] [Google Scholar]
- 22.Milton AL, Lee JLC, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. The Journal of Neuroscience. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milton AL, Schramm MJW, Wawrzynski JR, Gore F, Oikonomou-Mpegeti F, Wang NQ, Samuel D, Economidou D, Everitt BJ. Antagonism at NMDA receptors, but not β-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology. 2011;219:751–761. doi: 10.1007/s00213-011-2399-9. [DOI] [PubMed] [Google Scholar]
- 24.Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learning & Memory. 2008;15:857–865. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Li Y, Gao J, Sui N. Differential effect of NMDA receptor antagonist in the nucleus accumbens on reconsolidation of morphine -related positive and aversive memory in rats. European Journal of Pharmacology. 2012;674:321–326. doi: 10.1016/j.ejphar.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Lee JLC, Everitt BJ. Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiology of learning and memory. 2008;90:147–154. doi: 10.1016/j.nlm.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, Spanagel R. Cue-induced alcohol-seeking behaviour is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology. 2009;205:389–397. doi: 10.1007/s00213-009-1544-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhai H, Wu P, Chen S, Li F, Liu Y, Lu L. Effects of scopolamine and ketamine on reconsolidation of morphine conditioned place preference in rats. Behavioural Pharmacology. 2008;19:211–216. doi: 10.1097/FBP.0b013e3282fe88a0. [DOI] [PubMed] [Google Scholar]
- 29.Yu L-L, Wang X-Y, Zhao M, Liu Y, Li Y-Q, Li F-Q, Wang X, Xue Y-X, Lu L. Effects of cannabinoid CB1 receptor antagonist rimonabant in consolidation and reconsolidation of methamphetamine reward memory in mice. Psychopharmacology. 2009;204:203–211. doi: 10.1007/s00213-008-1450-y. [DOI] [PubMed] [Google Scholar]
- 30.Fang Q, Li F-Q, Li Y-Q, Xue Y-X, He Y-Y, Liu J-F, Lu L, Wang J-S. Cannabinoid CB1 receptor antagonist rimonabant disrupts nicotine reward-associated memory in rats. Learning & Memory. 2011;99:738–742. doi: 10.1016/j.pbb.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Heinrichs SC, Leite-Morris KA, Carey RJ, Kaplan GB. Baclofen enhances extinction of opiate conditioned place preference. Behavioral Brain Research. 2009;207:353–359. doi: 10.1016/j.bbr.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 32. Taubenfeld SM, Muravieva EV, Garcia-Osta A, Alberini CM. Disrupting the memory of places induced by drugs of abuse weakens motivational withdrawal in a context-dependent manner. Proceedings of the National Academy of Sciences. 2010;107:12345–12350. doi: 10.1073/pnas.1003152107. This paper demonstrates that the motivational component of drug-associated places can be modified by manipulations of reconsolidation. It is less clear, however, whether the entire memory or just the motivational aspects were affected.
- 33.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Valjent E, Corbille A-G, Bertran-Gonzalez J, Herve D, Girault J-A. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. PNAS. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JLC, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. The Journal of Neuroscience. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JLC, Di Ciano P, Thomas KL, Everitt BJ. Disrupting Reconsolidation of Drug Memories Reduces Cocaine-Seeking Behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Lee JLC, Gardner RJ, Butler VJ, Everitt BJ. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learning & Memory. 2009;16:82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellemans KGC, Everitt BJ, Lee JLC. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. The Journal of Neuroscience. 2006;26:12694–12699. doi: 10.1523/JNEUROSCI.3101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theberge FRM, Milton AL, Belin D, Lee JLC, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learning & Memory. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakurai S, Yu L, Tan S-E. Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behavioral Pharmacology. 2007;18:497–506. doi: 10.1097/FBP.0b013e3282ee7b62. [DOI] [PubMed] [Google Scholar]
- 41.Li F-Q, Xue Y-X, Wang J-S, Fang Q, Li Y-Q, Zhu W-L, He Y-Y, Liu J-F, Xue L-F, Shaham Y, et al. Basolateral Amygdala Cdk5 Activity Mediates Consolidation and Reconsolidation of Memories for Cocaine Cues. The Journal of Neuroscience. 2010;30:10351–10359. doi: 10.1523/JNEUROSCI.2112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura T, Yamashita S, Nakao S, Park J-M, Murayama M, Mizoroki T, Yoshiike Y, Sahara N, Takashima A. GSK-3β Is Required for Memory Reconsolidation in Adult Brain. PLOS One. 2008;3:e3540. doi: 10.1371/journal.pone.0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant SGN, O'Dell TJ. Multiprotein complex signaling and the plasticity problem. Current opinion in neurobiology. 2001;11:363–368. doi: 10.1016/s0959-4388(00)00220-8. [DOI] [PubMed] [Google Scholar]
- 44.Shalin SC, Hernandez CM, Dougherty MK, Morrison DK, Sweatt JD. Kinase Suppressor of Ras1 Compartmentalizes Hippocampal Signal Transduction and Subserves Synaptic Plasticity and Memory Formation. Neuron. 2006;50:765–779. doi: 10.1016/j.neuron.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 45.Machado HB, Vician LJ, Herschman HR. The MAPK pathway is required for depolarization-induced “promiscuous” immediate-early gene expression but not for depolarization-restricted immediate-early gene expression in neurons. Journal of neuroscience research. 2008;86:593–602. doi: 10.1002/jnr.21529. [DOI] [PubMed] [Google Scholar]
- 46.Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuropharmacology and Neurotoxicology. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- 47.Milton AL, Lee JLC, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on -adrenergic receptors. Learning & Memory. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- 48.Fricks-Gleason AN, Marshall JF. Post-retrieval -adrenergic receptor blockade: Effects on extinction and reconsolidation of cocaine-cue memories. Learning & Memory. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou S-J, Xue L-F, Wang X-Y, Jiang W-G, Xue Y-X, Liu J-F, He Y-Y, Luo Y-X, Lu L. NMDA receptor glycine modulatory site in the ventral tegmental area regulates the acquisition, retrieval, and reconsolidation of cocaine reward memory. Psychopharmacology. 2011;221:79–89. doi: 10.1007/s00213-011-2551-6. [DOI] [PubMed] [Google Scholar]
- 50.Alaghband Y, Marshall JF. Common influences of non-competitive NMDA receptor antagonists on the consolidation and reconsolidation of cocaine-cue memory. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2793-y. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Achterberg EJM, Trezza V, Vanderschuren LJMJ. β-Adrenoreceptor Stimulation Mediates Reconsolidation of Social Reward-Related Memories. PLOS One. 2012;7:e39639. doi: 10.1371/journal.pone.0039639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chergui K, Svenningsson P, Greengard P. Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. PNAS. 2004;101:2191–2196. doi: 10.1073/pnas.0308652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nature Neuroscience. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- 54.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 55. Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. The Journal of Neuroscience. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. This study demonstrates that although young memories are strengthened after retrieval, older memories undergo extinction in the same retrieval/test scenario. This might be an important difference between "normal" and "dysfunctional" memories, where the latter never undergo a switch to enable extinction and continue to gain strength after retrieval.
- 56. Forcato C, Rodríguez MLC, Pedreira ME. Repeated labilization-reconsolidation processes strengthen declarative memory in humans. PLOS One. 2011;6:e23305. doi: 10.1371/journal.pone.0023305. These authors demonstrate that brief presentation of a previously conditioned cue after recent retrieval causes increases in memory strength. This enhancement of memory due to retrieval without additional manipulations provides information about the role of reconsolidation in normal memory.
- 57.Wu Y, Li Y, Yang X, Sui N. Differential effect of beta-adrenergic receptor antagonism in basolateral amygdala on reconsolidation of aversive and appetitive memories associated with morphine in rats. Learning & Memory. 2012 doi: 10.1111/j.1369-1600.2012.00443.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 58.Font L, Cunningham CL. Post-retrieval propranolol treatment does not modulate reconsolidation or extinction of ethanol-induced conditioned place preference. Learning & Memory. 2012;101:222–230. doi: 10.1016/j.pbb.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soeter M, Kindt M. Dissociating response systems: Erasing fear from memory. Neurobiology of learning and memory. 2010;94:30–41. doi: 10.1016/j.nlm.2010.03.004. In this study, the authors demonstrate that propranolol acts to disrupt the physiological and emotional aspects of fear conditioning in humans, however, the associative or declarative aspects of memory, measured by expectation of shock during cue presentations, remained intact. This is the first study to delineate this distinction between disrupted memory and attenuated emotional response.
- 60.Wang SH, Ostlund SB, Nader K, Balleine BW. Consolidation and reconsolidation of incentive learning in the amygdala. The Journal of Neuroscience. 2005;25:830–835. doi: 10.1523/JNEUROSCI.4716-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez-Ortiz CJ, Bermudez-Rattoni F. Memory Reconsolidation or Updating Consolidation? In: Bermudez-Rattoni F, editor. Neural Plasticity and Memory: From Genes to brain Imaging. Boca Raton (FL): CRC Press; 2007. [PubMed] [Google Scholar]
- 62.Suárez LD, Smal L, Delorenzi A. Updating contextual information during consolidation as result of a new memory trace. Neurobiology of learning and memory. 2010;93:561–571. doi: 10.1016/j.nlm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Pedreira ME. Mismatch Between What Is Expected and What Actually Occurs Triggers Memory Reconsolidation or Extinction. Learning & Memory. 2004;11:579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Learning & Memory. 2012;97:338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Duvarci S, Nader K. Characterization of fear memory reconsolidation. The Journal of Neuroscience. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis S, Renaudineau S, Poirier R, Poucet B, Save E, Laroche S. The Formation and Stability of Recognition Memory: What Happens Upon Recall? Frontiers in Behavioral Neuroscience. 2010;4 doi: 10.3389/fnbeh.2010.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dudai Y. The Restless Engram: Consolidations Never End. Learning & Memory. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez WA, Rodriguez SB, Phillips MY, Martinez JJ. Post-reactivation cocaine administration facilitates later acquisition of an avoidance response in rats. Behavioral Brain Research. 1993;59:125–129. doi: 10.1016/0166-4328(93)90158-m. [DOI] [PubMed] [Google Scholar]
- 69.Jentsch JD, Olausson P, Nestler EJ, Taylor JR. Stimulation of protein kinase a activity in the rat amygdala enhances reward-related learning. Biological Psychiatry. 2002;52:111–118. doi: 10.1016/s0006-3223(02)01358-6. [DOI] [PubMed] [Google Scholar]
- 70.Lynch WJ, Kiraly DD, Caldarone BJ, Picciotto MR, Taylor JR. Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology. 2006;191:263–271. doi: 10.1007/s00213-006-0656-0. [DOI] [PubMed] [Google Scholar]
- 71.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. delta-FosB in the Nucleus Accumbens Regulates Food-Reinforced Instrumental Behavior and Motivation. Journal of Neuroscience. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends in Neurosciences. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nature Neuroscience. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 74.Sara SJ. Reactivation, Retrieval, Replay and Reconsolidation in and Out of Sleep: Connecting the Dots. Frontiers in Behavioral Neuroscience. 2010;4 doi: 10.3389/fnbeh.2010.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Javadi AH, Cheng P. Transcranial direct current stimulation (tDCS) enhances reconsolidation of long-term memory. Brain Stimulation. 2012;5:231–241. doi: 10.1016/j.brs.2012.10.007. This study used anodal tDCS to specifically in the left dorsolateral prefrontal cortex (dlPFC). Previous work by this group has implicated dlPFC in long term memory. Here the authors demonstrate that brain region specific manipulations can alter (in this case enhance) memory reconsolidation in humans.
- 76.Corlett PR, Krystal JH, Taylor JR, Fletcher PC. Why do delusions persist? Frontiers in Human Neuroscience. 2009;3 doi: 10.3389/neuro.09.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]