Abstract
Intra-uterine growth restriction is an independent risk factor for adult psychiatric and cardiovascular diseases. In humans, intra-uterine growth restriction is associated with increased placental and fetal oxidative stress, as well as down-regulation of placental 11β-HSD (11β-hydroxysteroid dehydrogenase). Decreased placental 11β-HSD activity increases fetal exposure to maternal glucocorticoids, further increasing fetal oxidative stress. To explore the developmental origins of co-morbid hypertension and anxiety disorders, we increased fetal glucocorticoid exposure by administering the 11β-HSD inhibitor CBX (carbenoxolone; 12 mg · kg−1 of body weight · day−1) during the final week of murine gestation. We hypothesized that maternal antioxidant (tempol throughout pregnancy) would block glucocorticoid-programmed anxiety, vascular dysfunction and hypertension. Anxiety-related behaviour (conditioned fear) and the haemodynamic response to stress were measured in adult mice. Maternal CBX administration significantly increased conditioned fear responses of adult females. Among the offspring of CBX-injected dams, maternal tempol markedly attenuated the behavioural and cardiovascular responses to psychological stress. Compared with offspring of undisturbed dams, male offspring of dams that received daily third trimester saline injections had increased stress-evoked pressure responses that were blocked by maternal tempol. In contrast, tempol did not block CBX-induced aortic dysfunction in female mice (measured by myography and lucigenin-enhanced chemiluminescence). We conclude that maternal stress and exaggerated fetal glucocorticoid exposure enhance sex-specific stress responses, as well as alterations in aortic reactivity. Because concurrent tempol attenuated conditioned fear and stress reactivity even among the offspring of saline-injected dams, we speculate that antenatal stressors programme offspring stress reactivity in a cycle that may be broken by antenatal antioxidant therapy.
Keywords: antioxidant, cardiovascular response, carbenoxolone, fetal programming, 11β-hydroxysteroid dehydrogenase, stress
INTRODUCTION
Fetal glucocorticoid exposure alters brain development and programmes the development of adult hypertension [1–3]. Intriguingly, programmed hypertension is consistently demonstrated during restraint for tail cuff measurements, but not when baseline arterial pressures are recorded by unrestrained methods, including radiotelemetry [1,4–6]. Radiotelemetry studies have further shown that intra-uterine glucocorticoid exposure programmes an exaggerated hypertensive response to psychological stress, suggesting that increased stress reactivity may contribute to the tail cuff hypertension seen in other studies [5].
In addition to the programming of stress-induced hypertension, excessive fetal glucocorticoid exposure may increase the risk of developing anxiety disorders, including PTSD (post-traumatic stress disorder) [7,8]. PTSD is a mentally debilitating illness that carries an increased risk of co-morbid cardiovascular disease [9]. Although PTSD is a common disorder, most of those exposed to traumatic experiences do not develop PTSD, suggesting that PTSD susceptibility varies greatly between individuals [10]. As a prototypical example of this differential predisposition, there is a 4-fold higher incidence of PTSD in women [10]. Better knowledge of the genetic, environmental and neurobiological risk factors that contribute to PTSD susceptibility would lead to opportunities for prevention.
Intra-uterine growth restriction, an independent risk factor for adult psychiatric and cardiovascular diseases, is associated with down-regulation of placental 11β-HSD (11β-hydroxysteroid dehydrogenase) [4,11]. Down-regulation of 11β-HSD increases fetal exposure to maternal glucocorticoids [3,12] and may be an adaptive response to accelerate fetal maturation in the face of environmental adversity. To explore the developmental origins of hypertension, we previously administered the 11β-HSD inhibitor CBX (carbenoxolone) during the final week of murine gestation and demonstrated that maternal CBX increased adult blood pressures in male, but not female, offspring [13]. We further showed in a complementary maternal undernutrition model that programmed hypertension is associated with adult vascular dysfunction including exaggerated production of superoxide anion [13].
In addition to its association with exaggerated glucocorticoid exposure, intra-uterine growth restriction is associated with increased placental and fetal oxidative stress [14–16]. Although glucocorticoid excess is known to induce vascular superoxide production [17] and oxidative stress has been linked with programmed vascular dysfunction [18,19], these interactions have not been assessed from a developmental perspective. We speculated that maternal antioxidant therapy would block programmed hypertension. To further understand the mechanism contributing to programmed hypertension, we evaluated the effects of maternal tempol, a membrane-permeable SOD (superoxide dismutase) mimetic, on offspring anxiety-related behaviour and vascular reactivity. We hypothesized that maternal antioxidant therapy would attenuate the effects of maternal stress and exaggerated intra-uterine gluco-corticoid exposure on fear conditioning (a PTSD-like phenotype), stress-enhanced hypertension and aortic reactivity.
MATERIALS AND METHODS
Ethical approval
All procedures were approved by the University of Iowa Animal Care and Use Committee (protocol no. 0812259). The present investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the U.S. NIH (National Institutes of Health; NIH Publication No. 85–23, revised 1996).
Animal model
Adult C57Bl/6J mice (The Jackson Laboratory) were bred until discovery of a maternal plug. From embryonic day 12 to 19, dams were undisturbed or injected subcutaneously with saline (0.9% NaCl) or CBX (12.5 mg · kg−1 of body weight · day−1; Sigma). Dams received standard diet and either standard drinking water or drinking water supplemented with the antioxidant tempol (1 mmol/l; Sigma) throughout pregnancy (from detection of copulatory plug to delivery). Pups were neither cross-fostered nor culled until weaning on day 21 of life.
Fear conditioning
At 4 months, mice were placed in a fear conditioning chamber (MED Associates). After 3 min, they were presented with a tone (80 dB, 20 s) that co-terminated with an electric foot-shock (0.5 mA, 1 s) for a total of five times at 1 min intervals [20]. The following day, context conditioning was tested by returning the mice to the original learning chamber in the absence of the electrical stimulus or auditory cue. The amount of time spent in a characteristic ‘freezing’ posture was recorded for 5 min. On the third day, cue conditioning was tested by placing the mice in a novel context and measuring the time spent ‘freezing’ during a 3 min re-presentation of the learned auditory cue (tone).
Telemetry recordings
At 6 months, radiotelemetry catheters (PA-C10; Data Sciences International) were implanted in the left carotid artery, as previously described [13]. After a 7 day recovery period, arterial pressures, heart rate and relative locomotor activity were sampled for 10 s every 5 min for 60 h (encompassing three dark cycles and two light cycles). At the end of the following light cycle, arterial waveforms were recorded at 2000 Hz during a 5 min phase of inactivity. Immediately after these baseline recordings, mice were transferred to a metabolic cage (Hatteras Instruments) to evoke a centrally mediated stress response [21,22]. The effects of psychological stress on blood pressure, heart rate and locomotor activity were assessed at the plateau phase of the stress response (40–60 min after initiation). For both control and CBX females, baseline haemodynamics (but not stress responses) have been previously reported for six mice [13]. The previously published baseline results are included to avoid biased interpretation of the primary end point (haemodynamic response to stress as a percentage of baseline). Mice weaned and maintained on a hypercaloric diet [45 kcal/g (where 1 kcal≈4.184 kJ), 41% of energy as fat; TD-06171, Harlan Teklad] contributed to baseline recordings (six female saline, six female CBX) and stress-evoked recordings (three male saline, three male CBX, six female saline, six female carbenolone). With our early results showing haemodynamic phenotypes in male but not female mice, follow-up studies investigated sex-specific effects of tempol on male haemodynamics and female aortic physiology.
Aortic reactivity
Female offspring never instrumented for blood pressure recording were killed at 6 months of age. The thoracic aorta was harvested and aortic segments were mounted on fixed pins within 5 ml myographs (Danish Myotechnology) [13]. Following equilibration with 4.5 mN resting tension, arteries were maximally constricted with 10−5 mol/l prostaglandin F2α (chosen over noradrenaline based on preliminary investigations showing it elicited more consistent preconstriction that was free of cyclic oscillations). After washing, vessels were preconstricted with consecutive additions of 10−6 mol/l prostaglandin F2α until the vessel achieved 50% of its maximal constriction. When stable constriction was achieved, increasing concentrations of acetylcholine (10−9–10−5 mol/l) or sodium nitroprusside (10−9– 10−5.5 mol/l) were added at fixed intervals. To isolate the effects of exogenous nitric oxide, the nitric oxide synthase inhibitor l-NNA (NG-nitro-l-arginine) was added to the myographs prior to the addition of sodium nitroprusside. Some arteries were pre-incubated in PEG [poly(ethylene glycol)] conjugates of SOD (50 units/ml) and catalase (250 units/ml) throughout the experiment. All compounds were acquired from Sigma.
Chemiluminescence
The aorta arch was harvested from the same non-instrumented female mice utilized for myography. Basal superoxide anion production was measured by lucigenin (25 μmol/l)-enhanced chemiluminescence, as previously described [13]. NADPH oxidase-dependent superoxide production was measured as the diphenyleneiodonium (10−4 mol/l)-inhibitable chemiluminescence measured after the addition of the enzyme substrate NADPH (10−4 mol/l). To further explore the relative importance of prenatal CBX and postnatal high-fat diet in the establishment of aortic superoxide production, additional mice were evaluated while receiving either the standard or high-fat diets.
Data analysis
All values are presented as means ± S.E.M. Results were compared by two-way ANOVA factoring for maternal injections and/or tempol administration. Aortic concentration–response curves were assessed by two-way repeated measures ANOVA. Post-hoc analysis (Holm–Sidak method) was performed if statistically significant differences were detected (P < 0.05). When a significant interaction was present between the programming stimulus (injections) and the intervention (tempol), Student’s unpaired t tests were utilized to evaluate for independent effects. In all cases, the a priori hypothesis that maternal CBX programmes adult phenotypes was assessed by one-way ANOVA. All analyses were performed using SigmaStat 3.0 (SPSS).
RESULTS
Fear conditioning
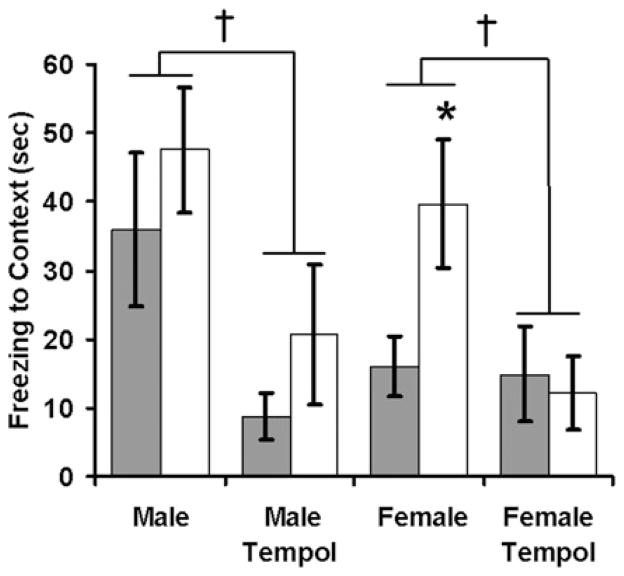
Maternal CBX or tempol had no significant effect on litter size, birth weight or sex distribution (results not shown). To test the effects of maternal CBX on offspring responses to psychological stress, we measured fear-related freezing behaviour during a 3-day fear-conditioning protocol. On the first day of testing, mice were presented with a series of tones that co-terminated with mild foot-shocks. Maternal CBX did not significantly alter the baseline responses to either the tone or shock (results not shown). Upon re-presentation of the conditioned stimuli, CBX-exposed female mice had increased context-based fear (Figure 1, main effect of CBX: F1,17 = 6.7, P = 0.02) and a trend towards increased cue-based fear (Figure 2, main effect of CBX: F1,17 = 3.28, P = 0.09). Overall, the offspring of tempol-treated dams had significantly decreased contextually conditioned freezing (Figure 1, main effect of tempol: F1,59 = 7.3, P = 0.009) and auditory-cue-conditioned freezing (Figure 2, main effect of tempol: F1,59 = 9.3, P = 0.003). In both cases, Holm–Sidak post hoc testing isolated the effect of tempol to CBX offspring (P = 0.02 for CBX–tempol compared with CBX–water), with no significant effects on control offspring (P = 0.16 for effect of tempol on context conditioned fear and P = 0.06 for effect of tempol on cue-conditioned fear).
Figure 1. Maternal antioxidant therapy attenuates conditioned fear and blocks the programming effects of CBX.
One day after training mice to associate a specific context with an aversive stimulus, they were returned to the conditioned context. Fearful behaviour (time spent freezing) was measured in adult offspring of dams that received either saline (solid bars) or CBX (open bars) during pregnancy. n = 13 male saline, n = 10 male CBX, n = 6 male saline–tempol, n = 6 male CBX–tempol, n = 14 female saline, n = 5 female CBX, n = 5 female saline–tempol, n = 4 female CBX–tempol. *P < 0.05 CBX compared with saline; †P < 0.01 tempol compared with no tempol.
Figure 2. Maternal antioxidant therapy attenuates conditioned fear in mice programmed by exaggerated fetal glucocorticoid exposure.
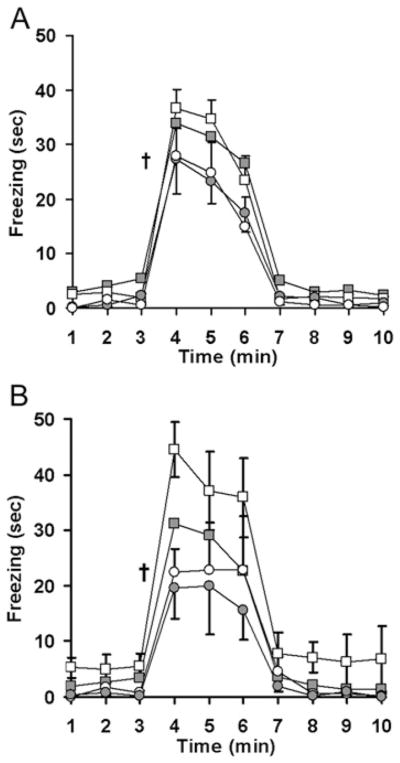
Mice trained to associate an auditory cue with an aversive stimulus were placed in a masked context for 3 min prior to a 3 min exposure to the conditioned cue. Fearful behaviour (freezing) was measured in adult male (A) and female (B) offspring of dams that received either saline (solid symbols) or CBX (open symbols) along with either water (squares) or the antioxidant tempol (circles) during pregnancy. n = 13 male saline, n = 10 male CBX, n = 6 male saline–tempol, n = 6 male CBX–tempol, n = 14 female saline, n = 5 female CBX, n = 5 female saline–tempol, n = 4 female CBX–tempol; †P < 0.01 tempol compared with no tempol.
Radiotelemetry
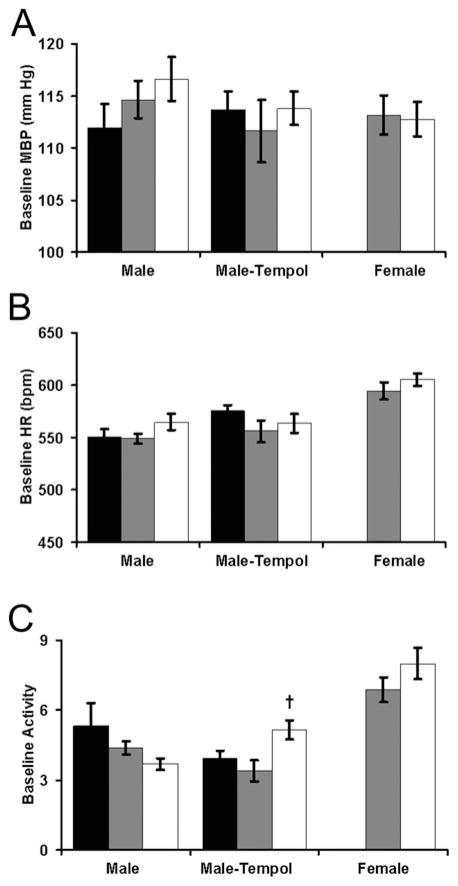
We next explored the effects of fetal glucocorticoid exposure and antioxidant intervention on adult cardiovascular responses to psychological stress. Maternal saline or CBX injections did not independently alter baseline blood pressure, heart rate or locomotor activity (Figure 3). For males, baseline locomotor activity was influenced by a significant interaction between the injections and antioxidant therapy (F2,31 = 4.1, P = 0.03). By planned contrast testing (Student’s t test), maternal tempol increased the activity of CBX-exposed mice (P = 0.03 for CBX–tempol compared with CBX–water).
Figure 3. Antenatal saline, CBX and tempol do not significantly alter baseline haemodynamics.
Mean blood pressure (MBP, A), heart rate (HR, B) and relative locomotor activity (C) were assessed in adult offspring of dams that were either undisturbed during pregnancy (black bars) or received daily third trimester injections of saline (grey bars) or CBX (open bars). These baseline recordings were obtained every 5 min for 60 h. n = 6 male undisturbed, n = 6 male saline, n = 6 male CBX, n = 5 male undisturbed–tempol, n = 6 male saline–tempol, n = 8 male CBX–tempol, n = 12 female saline (n = 6 weaned to a hypercaloric diet), n = 12 female CBX (n = 6 weaned to a hypercaloric diet); †P < 0.05 CBX–tempol compared with CBX–water.
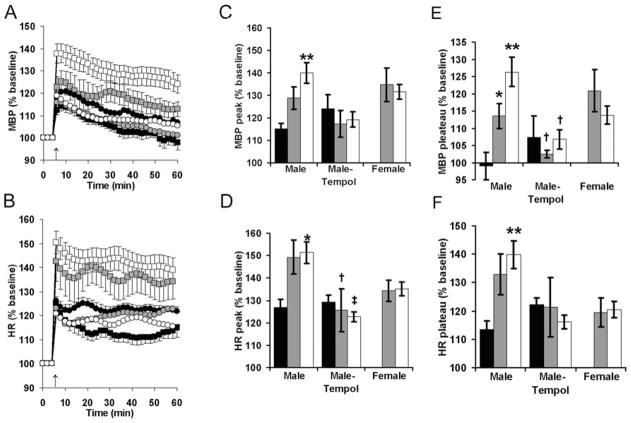
When first placed in a metabolic cage to elicit psychological stress, CBX-exposed male offspring had exaggerated pressure responses (Figure 4C, P = 0.008 compared with undisturbed) and tachycardic responses (Figure 4D, P = 0.013 compared with undisturbed). Similar trends were seen in the male offspring of saline-injected dams, but these did not approach statistical significance (P = 0.12 and P = 0.09 respectively). Overall, tempol decreased the peak heart rate response to placement in the metabolic cage (Figure 4D, main effect of tempol: F1,38 = 8.1, P = 0.007). Holm–Sidak post hoc testing identified significant effects of tempol on the male offspring of saline-injected dams (P = 0.02) and CBX-injected dams (P = 0.003), but not the male offspring of undisturbed dams (P = 0.83).
Figure 4. Maternal antioxidant attenuates programmed cardiovascular responses to psychological stress.
Mean blood pressure (MBP) (A, C and E) and heart rate (HR) (B, D and F) were recorded 5 min before and 60 min after placement in a metabolic cage for adult offspring of dams that were either undisturbed during pregnancy (black) or received daily third trimester injections of saline (grey) or CBX (white). In (A and B) only male results are presented, with offspring of dams receiving standard drinking water denoted by squares and offspring of dams receiving tempol during pregnancy denoted by circles. n = 5 male undisturbed, n = 9 male saline (n = 3 weaned to a hypercaloric diet), n = 13 male CBX (n = 3 weaned to a hypercaloric diet), n = 5 male undisturbed–tempol, n = 6 male saline–tempol, n = 6 male CBX–tempol, n = 12 female saline (n = 6 weaned to a hypercaloric diet), n = 11 female CBX (n = 6 weaned to a hypercaloric diet); *P < 0.05 or **P < 0.01 compared with undisturbed; †P < 0.05 or ‡P < 0.01 compared with no tempol.
At 30–40 min after placement in the metabolic cage, blood pressures and heart rates plateau (Figures 4A and 4B). During this plateau phase, male offspring blood pressure was influenced by maternal injection status, as well as an interaction between injection status and tempol administration (Figure 4E, main effect of injections: F2,38 = 4.1, P = 0.03; interaction: F2,38 = 4.0, P = 0.03). On post-hoc testing, male offspring blood pressures were increased by maternal saline and CBX injections (P = 0.04 and P = 0.003 compared with undisturbed offspring), and maternal tempol decreased the blood pressures of the male offspring of saline-injected and CBX-injected mice (P = 0.04 for saline–tempol compared with saline–water; P = 0.01 for CBX–tempol compared with CBX–water). Similarly, maternal saline or CBX injection increased male offspring heart rates during placement in the metabolic cage (Figure 4F, P = 0.11 and P = 0.009 compared with undisturbed offspring), but tempol did not have a significant effect on stress tachycardia (overall effect tempol: F1,38 = 2.3, P = 0.14; interaction between injections and tempol: F2,38 = 2.5, P = 0.10). Compared with the female offspring of saline-injected dams, female offspring of CBX-injected dams did not have any significant alteration in baseline or stress-evoked haemodynamics (Figures 3 and 4 respectively). There were no differences in the arterial pressures or heart rates of mice weaned to the regular diet (4 kcal/g, 6% of energy as fat, 7013; Harlan Teklad) or the hypercaloric diet (e.g. female mean blood pressures: 113 ± 2 mmHg compared with 112 ± 2 mmHg; male stress-evoked heart rate plateau: 137 ± 5% compared with 137 ± 10% baseline).
Aortic reactivity
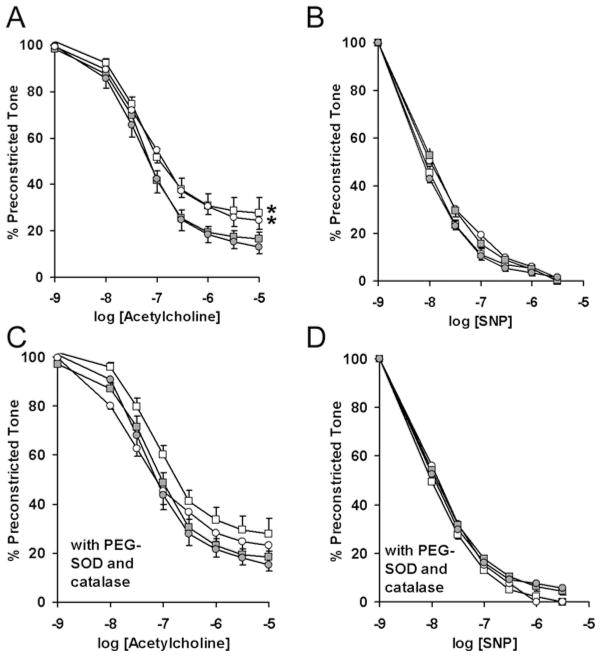
Because CBX-exposed female mice are normotensive, they provided an opportunity to test the effects of maternal CBX and tempol on aortic vasomotor function. We tested the hypotheses that (i) maternal CBX programmes primary (blood pressure-independent) alterations in vascular function and (ii) concurrent tempol blocks the programming of aortic reactivity. Prostaglandin F2α induced preconstriction did not differ between the groups (results not shown). Endothelium-dependent vasodilation to acetylcholine was significantly decreased in aorta from CBX offspring, independent of maternal tempol administration (Figure 5A, main effect of CBX: F1,203 = 7.7, P = 0.01; main effect of tempol: F1,203 = 0.19, P = 0.66). After pre-incubation in the antioxidants PEG–SOD and PEG–catalase, the effect of maternal CBX on aortic reactivity was no longer significant (Figure 5B, F1,203 = 2.0, P = 0.17). Maternal CBX or tempol did not significantly alter aortic responses to the endothelium-independent vasodilator sodium nitroprusside (Figures 5C and 5D).
Figure 5. Maternal antioxidant does not correct glucocorticoid-programmed endothelial dysfunction.
Aortic dilation to acetylcholine (A) or sodium nitroprusside (SNP) (B) was evaluated for female offspring of dams that received injections of either saline or CBX in addition to treatment with either tempol or water. Additional aortic rings were incubated in a combination of PEG-conjugated catalase and SOD prior to the addition of the vasodilators (C and D). Responses were compared by repeated measures ANOVA after the addition of cumulative concentrations of the vasodilator. n = 11 saline (■), n = 7 CBX (□), n = 8 saline–tempol (●), n = 6 CBX–tempol (○). *P < 0.05 CBX compared with the control.
These results suggested that programmed endothelial dysfunction may be mediated by a persistent increase in intravascular oxidative stress. By lucigenin-enhanced chemiluminescence, baseline aortic superoxide production was low and not influenced by maternal CBX or tempol administration (initial 7 min of Figure 6A). NADPH stimulated a dramatic increase in aortic superoxide production (subsequent 7 min of Figure 6A) that was significantly greater than from aorta of CBX offspring (Figure 6B, main effect of CBX: F1,39 = 8.4, P = 0.006, Holm–Sidak: P = 0.04 within water and P = 0.051 within tempol). This programmed increase in aortic superoxide production was independent of maternal tempol (P = 0.37 for the main effect of tempol and P = 0.84 for an interaction between tempol and CBX). To better understand the relative importance of CBX in the programming of aortic superoxide production, both male and female mice were evaluated after having been weaned to either a standard or hypercaloric diet (Figure 7). Once again there was an overall effect of CBX (P = 0.03), with subgroup analysis showing a significant increase in female mice (P = 0.04), but not in male mice (P = 0.40). This effect was independent of postnatal diet (P = 0.12 for regular diet compared with high-fat diet). In summary, there is converging evidence from myography and chemiluminescence showing maternal CBX programmes alterations in aortic physiology that are not blocked by maternal tempol administration.
Figure 6. Maternal antioxidant therapy does not correct glucocorticoid-programmed aortic superoxide production.
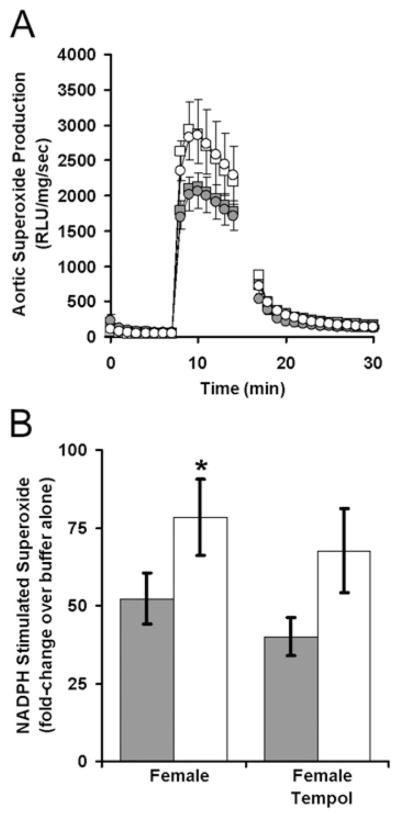
Superoxide production was measured by lucigenin-enhanced chemiluminescence among aorta obtained from female offspring of dams that received CBX and/or tempol during pregnancy. After the measurement of relative light unit (RLU) production during 7 min incubation in a buffer, superoxide was measured for 7 min after the addition of NADPH and for another 14 min after the addition of diphenyleneiodonium (A). NADPH-stimulated superoxide production was compared in the offspring of dams that received saline or CBX during pregnancy (B). n = 13 saline (■), n = 7 CBX (□), n = 11 saline–tempol (●), n = 11 CBX–tempol (○). *P < 0.05 CBX compared with the control.
Figure 7. Antenatal CBX consistently increases aortic superoxide production among adult female offspring.
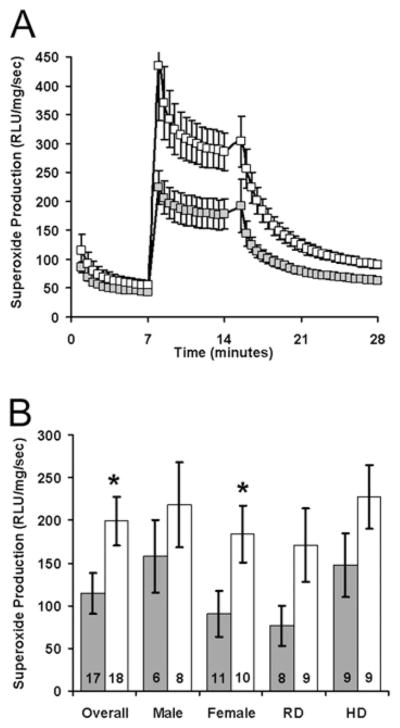
Superoxide production was measured by lucigenin-enhanced chemiluminescence among aorta obtained from adult offspring of dams that received either saline (■) or CBX (□) during pregnancy. These mice were weaned to either regular diet (RD) or hypercaloric diet (HD) until tissue collection. Relative light unit (RLU) production was measured for 7 min with only lucigenin and NADPH, then for 7 min with the aortic segment added, and finally for 14 min after the addition of diphenyleneiodonium (A). NADPH-augmented, diphenyleneiodonium-inhibitable superoxide production was determined by subtracting superoxide production at 28 min from that at 14 min (B). The numbers at the base of the bars represent aggregate numbers. n = 6 female saline–RD, n = 4 female CBX–RD, n = 5 female saline–HD, n = 6 female CBX–HD, n = 2 male saline–RD, n = 5 male CBX–RD, n = 4 male saline–HD and n = 3 male CBX–HD; *P < 0.05 CBX compared with the control.
DISCUSSION
Human studies and animal models have shown that diverse maternal environmental stressors alter adult offspring responses to psychological stress, often in a sex-specific fashion [3–5,23–25]. Accentuated maternal stress responses may ultimately underlie a trans-generational susceptibility to psychological stress [26,27]. Although placental 11β-HSD inactivates maternal glucocorticoids and protects the developing fetus from excessive steroid exposure, 11β-HSD activity is down-regulated during maternal undernutrition or fetal growth restriction [4,28–30]. To model this process, we induced 11β-HSD inactivation through administration of CBX during the final week of murine gestation.
A novel finding of the present study is that maternal CBX exposure increases conditioned fear responses in female offspring. This sex-specific programming is consistent with a higher incidence of PTSD in women [10]. Context-conditioned fear was most influenced, suggesting that maternal CBX administration may affect adult hippocampal more than amygdala function. Notably, exaggerated glucocorticoid exposure during critical developmental windows has been shown to reduce hippocampal neurogenesis and reduce adult hippocampal neuronal numbers [31,32]. In addition to cognitive effects, reduced hippocampal volumes are a key marker of increased PTSD susceptibility [33]. Further studies are necessary to further explore hippocampal structural and functional correlates in the context of antenatal stressors and antioxidant interventions. As suggested by the findings in related models, epigenetic alterations of hippocampal glucocorticoid receptors may contribute to the persistence of these phenotypes. Further studies will be necessary to delineate the ontogeny of the observed changes.
The striking effects of maternal antioxidant therapy on offspring behavioural and cardiovascular responses to psychological stress have not been previously described. In addition to negating the programming of conditioned fear by CBX, maternal tempol attenuated stress-evoked haemodynamic responses. This raises the possibility that prenatal stress and exaggerated transplacental glucocorticoid exposure may be sufficient to programme behavioural alterations in the offspring and that these may be mediated by oxidative stress. This speculation is supported by results showing that prenatal stress enhances fetal corticosterone exposure [23] and selectively increases hippocampal oxidative stress in female offspring [34]. Although further studies are necessary to localize the protective effects of maternal tempol administration within the placenta or fetus, a complementary maternal protein restriction model has demonstrated that direct tempol administration to prehypertensive 3-week-old rats blocks the development of programmed hypertension [35].
In contrast with the female predisposition to PTSD, diverse animal models have demonstrated a sexually dimorphic male predisposition towards programmed hypertension [36–39]. Although the causes of this sexual dimorphism have not been fully elucidated, previous studies have demonstrated that the protection of females from programmed hypertension is lost with ovariectomy or aging [37–39]. Our results support this gender-specific programming of stress-evoked hypertension and extend our mechanistic understanding in linking prenatal oxidative stress with the programming of multiple psychological-stress-induced phenotypes, including fear and hypertension. Despite the lack of effect of postnatal diet on adult haemodynamics or aortic superoxide production, the inclusion of mice weaned to a hypercaloric diet may have confounded the results while simultaneously enhancing the study’s generalizability. It is noteworthy that CBX increased aortic superoxide production independent of the fat content of the postnatal diet.
A potential role of persistently enhanced superoxide production in programmed cardiovascular disease was assessed by lucigenin-enhanced chemiluminescence as well as aortic reactivity in the presence of catalase and SOD. Although high concentrations of lucigenin are known to induce artifactual superoxide levels through redox cycling, we specifically chose a concentration (25 μM) below the threshold described for redox cycling in cellular systems (50 μM). The use of 25 μM lucigenin in these studies may have led to higher superoxide detection than would be seen with lower concentrations, but recent studies have shown a linear relationship between superoxide detection and lucigenin concentrations ranging from 5 to 250 μM [40]. In female offspring, combined prenatal and acute antioxidant exposure normalized the impairment in endothelium-dependent vasodilatation induced by maternal CBX, although neither treatment alone had a significant effect. These results compliment a growing body of literature demonstrating impaired endothelial function and increased vascular superoxide production after maternal undernutrition or fetal growth restriction [41–44].
Consistent with our present results, the endothelial dysfunction seen in femoral arteries isolated from neonatal dexamethasone-exposed rats is not altered by concurrent antioxidant therapy [45]. Our results support these studies showing that programming stimuli elicit blood-pressure-independent endothelial dysfunction that may be mediated by an imbalance between reactive oxygen and reactive nitrogen species. The role of RAS (renin–angiotensin system) activation in this pathway is an area of ongoing investigation. The lack of blood pressure phenotype in CBX females suggests that these vascular alterations are a primary consequence of maternal CBX administration, although they may not play a causative role in the programming of hypertension. Alternatively, the aortic myography may not model the resistance vasculature, and our results do not exclude a role for alterations in resistance arteries in programmed hypertension.
The use of maternal CBX administration to increase fetal exposure to maternal glucocorticoids replicates the decrease in 11β-HSD seen during maternal undernutrition [4,28,29]. It is important to note that, in addition to inhibiting placental 11β-HSD, maternal CBX blocks related dehydrogenases and disrupts gap junctions at high concentrations [46]. Each of these alterations may influence maternal, placental or fetal physiology. To overcome these limitations, others have induced programmed phenotypes by administering synthetic glucocorticoids that are not 11β-HSD substrates [1,5,7]. It is reassuring that exogenous glucocorticoid administration, endogenous glucocorticoid exposure (through 11β-HSD inhibition) and natural intra-uterine growth restriction induce similar adult phenotypes [1–5].
Our results demonstrate that the intra-uterine environment and antenatal interventions influence phenotypic responses to postnatal psychological stress. We have identified novel programmed phenotypes that support an important role of altered neuroregulation in the establishment of adult disease susceptibility. We speculate that intra-uterine glucocorticoid exposure alters central blood pressure and behavioural regulation, and intra-uterine oxidative stress plays a key role in the establishment of adult cardiovascular and behavioural responses to stress. Moreover, these results suggest that antioxidant administration, targeted to pregnancies complicated by maternal stress or fetal growth restriction, could help prevent the emergence of psychiatric disease-and stress-evoked hypertension. Because antioxidant exposure may have both beneficial and detrimental effects based on the end point assessed and the type or severity of prenatal stress, further studies (including biomarkers of oxidative stress) are needed to better understand the role of maternal, placental and fetal oxidative stress in the programming of adult physiology.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health [grant numbers HD050359, MH085724, HL04495, HL62483 and HL102659]; the Department of Veteran’s Affairs; and a McKnight Neuroscience of Brain Disorders Award. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Abbreviations
- CBX
carbenoxolone
- 11β-HSD
11β-hydroxysteroid dehydrogenase
- PEG
poly(ethylene glycol)
- PTSD
post-traumatic stress disorder
- SOD
superoxide dismutase
Footnotes
AUTHOR CONTRIBUTION
Robert Roghair, John Wemmie, Thomas Scholz, Fred Lamb and Jeffrey Segar conceived the study and designed the experiments; Robert Roghair and Kenneth Volk collected the data; and Robert Roghair and John Wemmie analysed the data. All authors contributed to drafting and revising the paper, and approved the final version.
References
- 1.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 2.O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- 3.Welberg LA, Seckl JR, Holmes MC. Inhibition of 11β-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 4.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 5.O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone ‘programmes’ hypotension, but stress-induced hypertension in adult offspring. J Endocrinol. 2008;196:343–352. doi: 10.1677/JOE-07-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonkiss J, Trzcińska M, Galler JR, Ruiz-Opazo N, Herrera VL. Prenatal malnutrition-induced changes in blood pressure: dissociation of stress and nonstress responses using radiotelemetry. Hypertension. 1998;32:108–114. doi: 10.1161/01.hyp.32.1.108. [DOI] [PubMed] [Google Scholar]
- 7.de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, Wolfe-Coote S, Meaney MJ, Levitt NS, Seckl JR. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic–pituitary–adrenal axis function. J Clin Invest. 2007;117:1058–1067. doi: 10.1172/JCI30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seckl JR, Meaney MJ. Glucocorticoid ‘programming’ and PTSD risk. Ann NY Acad Sci. 2006;1071:351–378. doi: 10.1196/annals.1364.027. [DOI] [PubMed] [Google Scholar]
- 9.Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behav Med. 2009;34:125–132. doi: 10.3200/BMED.34.4.125-132. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 11.Dy J, Guan H, Sampath-Kumar R, Richardson BS, Yang K. Placental 11β-hydroxysteroid dehydrogenase type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth restriction: evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta. 2008;29:193–200. doi: 10.1016/j.placenta.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Staud F, Mazancová K, Miksík I, Pávek P, Fendrich Z, Pácha J. Corticosterone transfer and metabolism in the dually perfused rat placenta: effect of 11β-hydroxysteroid dehydrogenase type 2. Placenta. 2006;27:171–180. doi: 10.1016/j.placenta.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Roghair RD, Segar JL, Volk KA, Chapleau MW, Dallas LM, Sorenson AR, Scholz TD, Lamb FS. Vascular nitric oxide and superoxide anion contribute to sex-specific programmed cardiovascular physiology in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R651–R662. doi: 10.1152/ajpregu.90756.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Invest. 2007;64:187–192. doi: 10.1159/000106488. [DOI] [PubMed] [Google Scholar]
- 15.Hracsko Z, Orvos H, Novak Z, Pal A, Varga IS. Evaluation of oxidative stress markers in neonates with intra-uterine growth retardation. Redox Rep. 2008;13:11–16. doi: 10.1179/135100008X259097. [DOI] [PubMed] [Google Scholar]
- 16.Saker M, Soulimane Mokhtari N, Merzouk SA, Merzouk H, Belarbi B, Narce M. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur J Obstet Gynecol Reprod Biol. 2008;141:95–99. doi: 10.1016/j.ejogrb.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, Matsumoto T. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res. 2003;92:81–87. doi: 10.1161/01.res.0000050588.35034.3c. [DOI] [PubMed] [Google Scholar]
- 18.Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Lê NL, Pladys P, Gauthier C, Lahaie I, Abran D, et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1236–R1245. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- 19.Franco MC, Dantas AP, Akamine EH, Kawamoto EM, Fortes ZB, Scavone C, Tostes RC, Carvalho MH, Nigro D. Enhanced oxidative stress as a potential mechanism underlying the programming of hypertension in utero. J Cardiovasc Pharmacol. 2002;40:501–509. doi: 10.1097/00005344-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amico JA, Cai HM, Vollmer RR. Corticosterone release in oxytocin gene deletion mice following exposure to psychogenic versus non-psychogenic stress. Neurosci Lett. 2008;442:262–266. doi: 10.1016/j.neulet.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoppe CC, Moritz KM, Fitzgerald SM, Bertram JF, Evans RG. Transient hypertension and sustained tachycardia in mice housed individually in metabolism cages. Physiol Res. 2009;58:69–75. doi: 10.33549/physiolres.931390. [DOI] [PubMed] [Google Scholar]
- 23.Darnaudéry M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Jones A, Godfrey KM, Wood P, Osmond C, Goulden P, Phillips DI. Fetal growth and the adrenocortical response to psychological stress. J Clin Endocrinol Metab. 2006;91:1868–1871. doi: 10.1210/jc.2005-2077. [DOI] [PubMed] [Google Scholar]
- 25.Pyhälä R, Räikkönen K, Feldt K, Andersson S, Hovi P, Eriksson JG, Järvenpää AL, Kajantie E. Blood pressure responses to psychosocial stress in young adults with very low birth weight: Helsinki study of very low birth weight adults. Pediatrics. 2009;123:731–734. doi: 10.1542/peds.2008-0277. [DOI] [PubMed] [Google Scholar]
- 26.Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35:686–693. doi: 10.1016/j.psyneuen.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yehuda R, Bell A, Bierer LM, Schmeidler J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. J Psychiatr Res. 2008;42:1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langley-Evans SC, Phillips GJ, Benediktsson R. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-β-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 30.Bogdarina I, Haase A, Langley-Evans S, Clark AJ. Glucocorticoid effects on the programming of AT1b angiotensin receptor gene methylation and expression in the rat. PLoS ONE. 2010;5:e9237. doi: 10.1371/journal.pone.0009237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II: effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- 32.Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794:199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- 33.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song L, Zheng J, Li H, Jia N, Suo Z, Cai Q, Bai Z, Cheng D, Zhu Z. Prenatal stress causes oxidative damage to mitochondrial DNA in hippocampus of offspring rats. Neurochem Res. 2009;34:739–745. doi: 10.1007/s11064-008-9838-y. [DOI] [PubMed] [Google Scholar]
- 35.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68:2180–2188. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 36.Hermann GM, Miller RL, Erkonen GE, Dallas LM, Hsu E, Zhu V, Roghair RD. Neonatal catch up growth increases diabetes susceptibility but improves behavioral and cardiovascular outcomes of low birth weight male mice. Pediatr Res. 2009;66:53–58. doi: 10.1203/PDR.0b013e3181a7c5fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musha Y, Itoh S, Hanson MA, Kinoshita K. Does estrogen affect the development of abnormal vascular function in offspring of rats fed a low-protein diet in pregnancy? Pediatr Res. 2006;59:784–789. doi: 10.1203/01.pdr.0000219126.78372.c8. [DOI] [PubMed] [Google Scholar]
- 38.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janiszewski M, Souza HP, Liu X, Pedro MA, Zweier JL, Laurindo FR. Overestimation of NADH-driven vascular oxidase activity due to lucigenin artifacts. Free Radical Biol Med. 2002;32:446–453. doi: 10.1016/s0891-5849(01)00828-0. [DOI] [PubMed] [Google Scholar]
- 41.Franco MC, Akamine EH, Rebouças N, Carvalho MH, Tostes RC, Nigro D, Fortes ZB. Long-term effects of intrauterine malnutrition on vascular function in female offspring: implications of oxidative stress. Life Sci. 2007;80:709–715. doi: 10.1016/j.lfs.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Goodfellow J, Bellamy MF, Gorman ST, Brownlee M, Ramsey MW, Lewis MJ, Davies DP, Henderson AH. Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res. 1998;40:600–606. doi: 10.1016/s0008-6363(98)00197-7. [DOI] [PubMed] [Google Scholar]
- 43.Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103:1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- 44.Martin H, Hu J, Gennser G, Norman M. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birth weight. Circulation. 2000;102:2739–2744. doi: 10.1161/01.cir.102.22.2739. [DOI] [PubMed] [Google Scholar]
- 45.Herrera EA, Verkerk MM, Derks JB, Giussani DA. Antioxidant treatment alters peripheral vascular dysfunction induced by postnatal glucocorticoid therapy in rats. PLoS ONE. 2010;5:e9250. doi: 10.1371/journal.pone.0009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaytor AT, Marsh WL, Hutcheson IR, Griffith TM. Comparison of glycyrrhetinic acid isoforms and carbenoxolone as inhibitors of EDHF-type relaxations mediated via gap junctions. Endothelium. 2000;7:265–278. doi: 10.3109/10623320009072213. [DOI] [PubMed] [Google Scholar]