Abstract
It has been persuasively shown in the last two decades that the development of heart failure is closely linked to distinct alterations in Ca2+ cycling. A crucial point in this respect is an increased spontaneous release of Ca2+ out of the sarcoplasmic reticulum during diastole via ryanodine receptors type 2 (RyR2). The consequence is a compromised sarcoplasmic reticulum Ca2+ storage capacity, which impairs systolic contractility and possibly diastolic cardiac function due to Ca2+ overload. Additionally, leaky RyR2 are more and more regarded to potently induce proarrhythmic triggers. Elimination of spontaneously released Ca2+ via RyR2 in diastole can cause a transient sarcolemmal inward current and hence delayed after depolarisations as substrate for cardiac arrhythmias. In this article, the pathological role and consequences of the SR Ca2+-leak and its regulation are reviewed with a main focus on protein kinase A and Ca2+-calmodulin-dependent kinase II. We summarise clinical consequences of “leaky RyR2” as well as possible therapeutic strategies in order to correct RyR2 dysfunction and discuss the significance of the available data.
Keywords: Heart failure, Ryanodine receptor, SR Ca2+ leak, PKA, CaMKII
Prevalence and socioeconomic relevance of heart failure
Heart failure is characterised by a progressive deterioration of cardiac function and represents a major public health burden. Despite a number of well-established therapies, the prevalence as well as the mortality of heart failure are still alarmingly high. Heart failure remains the most frequent reason for hospital admission of patients older than 65 years [1]. In the USA, the number of hospitalisations due to heart failure has more than tripled between 1979 and 2004 [2]. The over-all prevalence of heart failure is estimated to be about 2 % [3]. More than 10 % of people older than 80 years are affected, many of them are highly limited as to their physical capacities and thus do suffer significantly in everyday life. Furthermore, the prognosis of heart failure patients is still miserable. There are estimated survival rates of only 50 % 5 years and 10 % 10 years after diagnosis [4–6].
Aetiology and manifestations of heart failure
Huge efforts have been made to further elucidate underlying pathomechanisms and identify new targets for innovative therapies. The aetiologies of heart failure are various, stretching from coronary artery disease and heart valve dysfunctions to toxic influences on the myocardium or infectious diseases. Arterial hypertension could be identified as the most frequent risk factor in this context [7]. The common final path is an impairment of myocardial function. Importantly, systolic as well as diastolic function can be compromised independently leading to similar clinical manifestations. Current definitions of heart failure have taken this into account by distinguishing between heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). According to the guidelines of the American Heart Association, HFpEF is defined by symptomatic heart failure and a left ventricular ejection fraction of at least 50 % [8]. This entity has gained increasing attention in recent years as up to 50 % of heart failure patients can indeed be classified there [1, 9] and evidence-based medical therapy is still lacking.
On a histological and cellular level, heart failure could be linked to several alterations including fibrosis, inflammation and apoptosis. Furthermore, it has become more and more obvious that a fully functional excitation–contraction coupling process in the cardiomyocyte is fundamental for both, a normal systolic and diastolic function.
Excitation–contraction coupling and its regulation
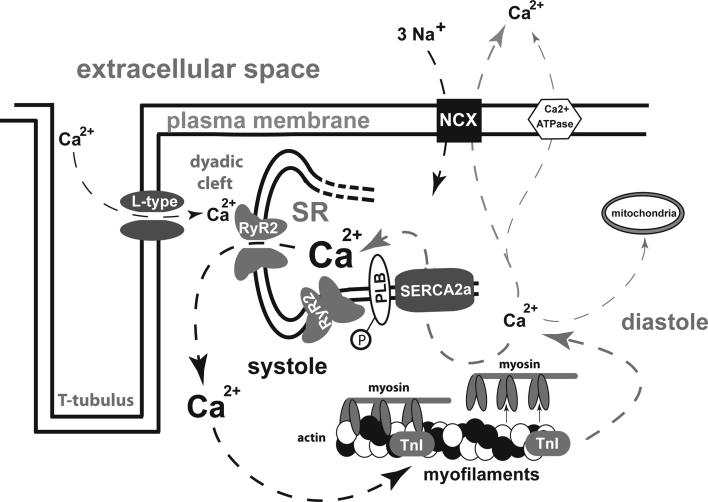
Physiological excitation–contraction coupling is the basis of a sound periodic succession of contraction and relaxation. It is characterised by a well-defined, triggered Ca2+ release out of the sarcoplasmic reticulum (SR) in systole followed by a quick Ca2+ elimination out of the cytosol to induce relaxation in diastole (Fig. 1).
Fig. 1.
Mechanisms of excitation–contraction coupling in cardiomyocytes; arrows indicate Ca2+ shifts in systole (left) and diastole (right); L-type L-type Ca2+ channel; RyR2 ryanodine receptor type 2; SR sarcoplasmic reticulum; PLB phospholamban; TnI troponin I; NCX Na+–Ca2+-exchanger; SERCA2a sarcoplasmic endoplasmic reticulum Ca2+-ATPase 2a; P phosphate
During phase 0 of an action potential fast Na+-influx leads to a steep increase of the membrane potential increasing the probability that the voltage dependent L-type Ca2+ channels will open. Thereupon Ca2+-ions passively drawn by a concentration and electrochemical gradient enter the cytoplasm and maintain the plateau phase of the action potential. Inside the cell, they bind to ryanodine receptors type 2 (RyR2) that are located on the surface of the SR and trigger the release of an even bigger amount of Ca2+ out of the SR. This mechanism of Ca2+-induced Ca2+ release leads to a prominent increase in cytosolic Ca2+ that binds to troponin C (TnC) and thereby induces actin–myosin interaction. In diastole, Ca2+ has to be eliminated from the cytosol to allow relaxation of the myofilaments. This is mainly achieved by an active, energy-consuming Ca2+-reuptake into the SR via the sarcoplasmic reticulum Ca2+-ATPase type 2a (SERCA2a). A smaller amount of Ca2+ is extruded out of the cell via the Na+/Ca2+-exchanger (NCX), which is a facilitated diffusion in which the electrochemical potential gradients of Na+ and Ca2+ are the source of energy to drive the transport. NCX in its “forward” mode produces an electrical current because 3 Na+ are exchanged for 1 Ca2+. In human cardiomyocytes, the reuptake of Ca2+ into the SR makes up for approximately 70 % of the systolic Ca2+ depending on the heart rate [10]. Around 28 % are pumped out of the cell via NCX. The remaining 2 % are allotted to Ca2+ uptake into mitochondria and elimination via Ca2+-ATPases in the plasma membrane [11].
As the cyclic alterations of cytosolic Ca2+ concentrations make up the molecular trigger for cardiac contraction and relaxation, it is a matter of course that this system is elaborately regulated and highly adaptable to physical demands. SERCA2a activity is influenced by phospholamban (PLB) that is one of the major mediators of the cardiac contractility response upon β-adrenergic stimulation [12]. Inhibition of SERCA2a by PLB is dependent on the phosphorylation status of PLB and is most pronounced when PLB is unphosphorylated. Protein kinase A (PKA) as well as the Ca2+/calmodulin-dependent protein kinase IIδ (CaMKIIδ) is able to phosphorylate PLB at specific residues (serine 16 and threonine 17, respectively) and thereby abandon its inhibitory effect on SERCA2a. A second subcellular microdomain is the RyR2-complex that consists of several proteins and whose Ca2+ release capacity and diastolic closure can be regulated via phosphorylation by PKA and CaMKII [13–19] at Ser2809 and Ser2815, respectively. Furthermore, protein phosphatases 1 and 2a were found to regulate the phosphorylation status of several Ca2+ handling proteins [20].
Consequences of altered excitation–contraction coupling in heart failure: the diastolic leak
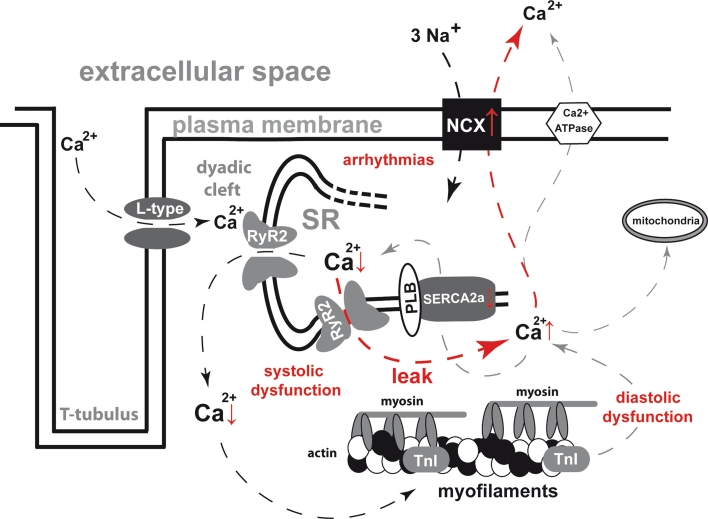
Several animal models as well as functional analyses of human tissue samples have persuasively shown that Ca2+ cycling is profoundly disturbed in heart failure (Fig. 2). The most distinctive aspect is a reduction of systolic Ca2+ transient amplitude, which is commonly accompanied by an increase of diastolic Ca2+ levels in the cytosol. Furthermore, the velocity of diastolic Ca2+ elimination is reduced [21], and the physiologically positive force-frequency relation is typically blunted and rather negative-shaped [10, 22]. These alterations resulting from the Ca2+ depletion of the SR in heart failure can be attributed to two distinct pathomechanisms with synergistic effects on SR Ca2+ load. On the one hand, the diastolic refill of the SR is compromised due to a decreased expression and activity of SERCA2a [10, 23, 24] and a reduction of PLB phosphorylation [25]. The regulation of the PLB-SERCA2a-interaction and the resulting new therapeutic options for the treatment of heart failure have recently been comprehensively reviewed [26].
Fig. 2.
Deteriorations of excitation–contraction coupling in heart failure; arrows indicate Ca2+ shifts in systole (left) and diastole (right); diastolic Ca2+ leak is highlighted in red; changes in protein function and ion concentrations are indicated by vertical red arrows; ↑ increase; ↓ decrease; L-type L-type Ca2+ channel; RyR2 ryanodine receptor type 2; SR sarcoplasmic reticulum; PLB phospholamban; TnI troponin I; NCX Na+–Ca2+-exchanger; SERCA2a sarcoplasmic endoplasmic reticulum Ca2+-ATPase 2a; P phosphate
On the other hand, there is an increased diastolic Ca2+ loss out of the SR via substantially altered RyR2. It has been widely shown in different animal models as well as in human tissue that Ca2+ can leave the SR during diastole via numerous short and tightly localised Ca2+ release events, the so-called Ca2+ sparks. A Ca2+ spark is generated from a limited number of clustered RyR2s that form a Ca2+ release unit. The spontaneous opening of one receptor can consecutively trigger the activation of neighbouring RyR2s of the same Ca2+ release unit via Ca2+-induced Ca2+ release (CICR) in the dyadic cleft. This locally restricted Ca2+ release is accompanied by a local decrease of SR Ca2+ content in the corresponding areas, the so-called Ca2+ blinks [27], and was shown to terminate when the local SR Ca2+ content reaches a threshold of approximately 60 % of the diastolic SR Ca2+ concentration [28]. The stability of RyR2-closure is severely compromised in heart failure leading to an increased diastolic open probability and thus an increased diastolic leak. Furthermore, the Ca2+ ions, once released, are partly removed from the cytosol via the NCX, which was found to be overexpressed in heart failure [10, 24, 29]. Extruding two positive electric charges (1 Ca2+ ion) in exchange for three (3 Na+ ions) leads to a depolarisation of the cell membrane and can trigger delayed after depolarisations [30]. Thus, as simple as the physiological task of the RyR2 complex to open quickly and widely in systole and to close tightly and densely in diastole might appear as far-reaching are the cellular consequences once it is disturbed. A leaky RyR2 could possibly be linked to all major drawbacks of heart failure: impaired systolic force generation, compromised passive diastolic relaxation and a disposition for arrhythmias. Therefore, huge efforts have been made in the last two decades to further elucidate mechanisms of RyR2 regulation in different physiological states as well as in different cardiac pathologies [13–15, 17, 18, 27, 31–37].
The ryanodine receptor type 2: the gatekeeper of Ca2+ storage
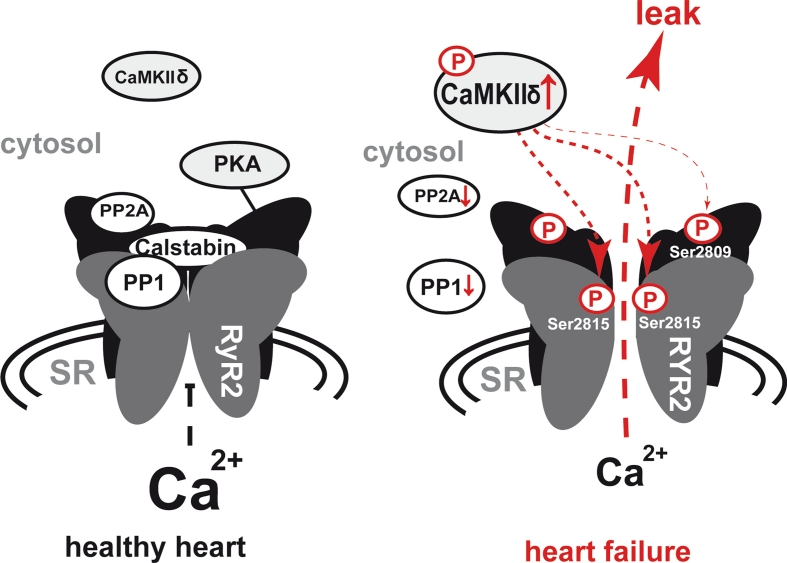
Ryanodine receptors are the largest known ion channels. In the heart, ryanodine receptor type 2 is the predominantly expressed isoform. It is a huge macromolecular protein exceeding a molecular weight of 2 MDa. Structural analysis have shown that RyRs are mushroom-shaped assemblies of four identical subunits each comprising approximately 5,000 amino acids [38]. This core part of the RyR2-complex then serves as a molecular scaffold for further proteins that attach to the receptor and regulate its function. Calsequestrin, junctin, triadin and FK506-binding protein, 12.6 kDa, (FKBP12.6) were shown to associate with the RyR2 complex [39–42] (Fig. 3). Calsequestrin is linked to the RyR2 complex via junctin and triadin [43] and acts as a Ca2+ buffer thus influencing RyR2 behaviour. Furthermore, RyR2 function is crucially linked to its phosphorylation status, which can be seen as the net effect of a steady competition between two major cellular kinases, PKA and CaMKII, and two protein phosphatases, PP1 and PP2a, all of which are closely associated with the receptor [44]. This close proximity enables dynamic changes of the phosphorylation status upon subcellular signals [44]. The relative phosphorylation of the receptor complex influences its Ca2+ sensitivity and thus the diastolic open probability as well as the systolic Ca2+ release capacity [14, 45] by inducing conformational changes of the RyR2 complex. The RyR2 complex therefore acts as a molecular switchboard, transducing cellular signals to the Ca2+ release pore and thus adapting Ca2+ release kinetics to cellular demands.
Fig. 3.
Development of an increased diastolic Ca2+ leak in heart failure; normal diastolic ryanodine receptor 2 (RyR2) closure (left) vs. diastolic SR Ca2+ leak due to spontaneous opening of a hyperphosphorylated RyR2 complex (right); the four subunits of the RyR2 complex are illustrated in grey and black; the diastolic leak is highlighted in red; relevant phosphorylation sites at the RyR2-protein are indicated; changes in protein function are indicated by vertical red arrows; ↑ increase; ↓ decrease; SR sarcoplasmic reticulum; PP1 protein phosphatase 1; PP2A protein phosphatase 2A; CaMKIIδ Ca2+ calmodulin depending kinase IIδ; PKA protein kinase A; P phosphate
Mechanisms of RyR2 dysregulation in heart failure
The mechanisms of RyR2 regulation have been subject of intense research in the last years due to their pathologic relevance. It has become undoubtedly clear that phosphorylation of the receptor protein is a key event in this context. The RyR2 complex thereby was found to be one of the first proteins that undergo phosphorylation upon beta-adrenergic stimulation [19]. As early as in 1991, it was described that RyR2 can be phosphorylated by CaMKII in canine cardiomyocytes, which leads to an activation of this Ca2+-release channel [19]. Furthermore, it could be demonstrated shortly after by a different group that the phosphorylation of RyR2 receptors in rat cardiomyocytes upon beta-adrenergic stimulation was PKA dependent [46]. From that time on, both kinases were intensively studied as to their impact on the RyR2 in different animal models as well as human heart tissue—with controversial results up to date.
The role of PKA in the development of an increased SR Ca2+-leak
It was described by Marks and colleagues that PKA phosphorylation of the RyR2 leads to a dissociation of FKBP12.6 from the channel and consecutively to an increased Ca2+ sensitivity and compromised diastolic RyR2 closure [14]. Some years later, the same group proposed Ser2808 as the only functional PKA phosphorylation site on the RyR2 by showing that immunoprecipitated RyR2 from S2808A mutant mice could not be phosphorylated by PKA [16]. In contrast to their wild-type littermates these S2808A mutant mice did not show any PKA phosphorylation of RyR2 after isoproterenol treatment in vivo. Furthermore, these mice exhibited significantly improved cardiac function compared to wild type after myocardial infarction and were thus protected from maladaptive remodelling. Marks and colleagues also found a hyperphosphorylation of RyR2 at S2809 in overt human heart failure [16]. On account of these findings, a hyperphosphorylation of RyR2 by PKA had been proposed as central mechanism in the pathogenesis of heart failure. The scenario of persistent PKA activation in heart failure due to chronic activation of the sympathetic nervous system also fits to clinical observations, as most heart failure patients do have elevated catecholamine levels. Beta-adrenergic receptors have been reported to be downregulated [47]. Other groups, however, contradicted the data on the functional relevance of PKA-dependent RyR2 hyperphosphorylation. It could be shown in mouse ventricular myocytes that PKA phosphorylation of the RyR2 did not significantly affect diastolic SR Ca2+ leak [48]. Furthermore, another group rather found an intact beta-adrenergic response and an unmodified progression towards heart failure in mice with the same genetic ablation of the major PKA phosphorylation site (S2808A) [49]. In this study, the authors only found slight modifications of single-channel activity in Ser2808-mutated mice that allowed for normal Ca2+ transients and unaltered overall cellular function. Obvious protection against maladaptive remodelling after TAC was absent. Moreover, they did not find a significant impact of RyR-S2808A mutation on the diastolic SR Ca2+ leak and showed that RyR2-S2808A mutants could still be phosphorylated by PKA, which might be attributed to another phosphorylation site at S2830. The authors further concluded that genetic ablation of one single phosphorylation site might lead to conformational changes and shift kinase activity to other, physiologically not equally relevant sites that in turn make up for the missing phosphorylation site. Furthermore, different amounts of phosphatases included in the isolated RyR2 complexes could, at least partially, explain the contradictory results of different works in the field. Another group just recently exposed RyR-S2808A-mutated mice to myocardial infarction. They, however, could not find any differences compared with wild-type littermates, neither as to global cardiac function and calcium cycling parameters after myocardial infarction nor as to infarct size [50].
FKBP12.6: a key player of RyR stabilisation or just a side show
Several studies strongly support the hypothesis that phosphorylation of RyR2 at Ser2808 by PKA causes the RyR2-associated protein FKBP12.6 to dissociate from the receptor with the result of an increased propensity for diastolic SR Ca2+ release [14, 51, 52]. However, these observations were challenged by several groups. Xiao et al. [53] examined the binding behaviour of FKBP12.6 with unphosphorylated and phosphorylated RyR2 proteins and did not find significant differences. Complete phosphorylation of Ser2808 by exogenous PKA did not disrupt FKBP12.6-RyR2 complexes and a S2808D mutated receptor that corresponds to a constitutively phosphorylated state of Ser2808 and thus mimics a constitutive phosphorylation also retained the ability to bind FKBP12.6 [53]. Another group examined the effect of a sticky FKBP12.6 mutant on the development of heart failure [54]. They generated mice expressing a FKBP12.6 (D37S) mutant that binds with greater stability to the RyR2. These mice showed increased SR Ca2+ load but did not exhibit any attenuation of cardiac functional decline after aortic banding. Another aspect that argues against an important, physiologically relevant FKBP12.6-mediated RyR2 regulation is the small amount of FKBP12.6 normally existing in rat and mouse myocytes [55]. FKBP12.6 was found to be numerically far less abundant in these cells than RyR2-receptors. This in turn means that the bulk of RyR2 proteins physiologically already lacks FKBP12.6 and might explain why some groups did not see any obvious effect of physiologically expressed FKBP12.6 on RyR2 gating while studies using animals overexpressing FKBP12.6 showed relevant effects an SR Ca2+ leak and SR Ca2+ content [56, 57].
Whereas the activating effect of PKA on L-type Ca2+ channels [58] and SERCA2a via PLB phosphorylation [59] is indisputable, its direct impact on RyR2 function and the role of FKBP12.6 dissociation is still not finally elucidated. The discrepancies of different studies in this field are difficult to explain. The varying outcomes in different mouse models harbouring the same RyRS2808A mutation might, at least partially, be attributed to different genetic backgrounds of the mouse lines used. Furthermore, the potency of cardiomyocytes to compensate artificially introduced defects of Ca2+ cycling cannot be overestimated. It has been shown that mice with a conditional knockout of the NCX could escape death by an adaptive reduction of Ca2+ influx through L-type Ca2+ channels instead of upregulation of Ca2+ efflux mechanisms [60]. This, however, means in turn that the validity of data from genetically altered animal models and their applicability for the explanation of pathomechanisms in human disease have to be handled with care. Eventually, functional data in human tissue will be needed to resolve this issue.
The role of CaMKII for the development of an increased SR Ca2+ leak
There is an increasing body of knowledge concerning the crucial role of CaMKII for modulation of RyR2 function. Different sequence analysis revealed that there are up to four serine and two threonine residues at the RyR2 that can be phosphorylated by CaMKII. The first report was published by Witcher et al. [19], showing a direct phosphorylation of the myocardial RyR2 at Ser2809 by CaMKII. However, site-directed mutagenesis experiments showed that CaMKII binding at RyR2 might not be at Ser2809, which is supposed to be the PKA-dependent phosphorylation site, but at Ser2815 [13]. CaMKII-dependent RyR2 phosphorylation thereby resulted in a more pronounced RyR2 activation than PKA-dependent RyR2 phosphorylation. Additionally, this group clearly showed that CaMKII-dependent RyR2 phosphorylation increased the open probability of the cardiac RyR2 in lipid bilayer experiments. Some years later, it was also shown by Huke et al. [36] that the phosphorylation of RyR2 at Ser2814 by CaMKII rather than PKA-dependent phosphorylation at Ser2808 is crucial for RyR2 function. However, there are also reports that suggested a reduced SR Ca2+ leak due to CaMKII phosphorylation of the cardiac RyR2 [61].
After the discovery of RyR2 phosphorylation by CaMKII, several other groups confirmed the pathologically relevant impact of CaMKII on RyR2 function in animal models as well as in human disease [15, 17, 18, 31, 34, 62]. A transgenic mouse line overexpressing the CaMKIIδc exhibited severe alterations of Ca2+ cycling and dilated heart failure [15, 34]. An increased diastolic Ca2+ leak in these mice lead to a severe depletion of SR Ca2+ storage and reduced systolic Ca2+ transients [15, 34].
Furthermore, Ai et al. [32] could show in 2005 that the enhanced SR Ca2+ leak in isolated cardiomyocytes from a rabbit heart failure model could be reduced by CaMKII inhibition but not by PKA inhibition. Curran et al. [63] proposed that even the inotropic response upon beta-adrenergic receptor stimulation is caused by CaMKII. The fact that CaMKII was shown to be activated by autophosphorylation as well as oxidation [64, 65] enables a vicious circle to set in during conditions of diastolic Ca2+ overload and shortage of energy supply in heart failure leading to chronic CaMKII activation. These findings could recently be confirmed for human heart failure by our group [17]. CaMKII expression and phosphorylation were shown to be increased in left and right ventricular tissue probes of human heart failure. Inhibition of CaMKII yielded a reduction of SR Ca2+ leak in human cardiomyocytes isolated from failing hearts and improved SR Ca2+ storage. Furthermore, positive inotropic effects could be detected in twitching muscle preparations after CaMKII inhibition during increasing stimulation frequencies. These data provide compelling evidence for an important pathological role of CaMKII in heart failure.
There is also increasing evidence for the role of the CaMKII-induced diastolic Ca2+ leak in the development of arrhythmias. Isolated cardiomyocytes from mice overexpressing CaMKIIδc [15] showed an increased incidence of early after-depolarisations compared with wild type leading to an increased number of potentially arrhythmic spontaneous action potentials [18]. These proarrhythmic events could be further increased by isoproterenol treatment and were significantly reduced by CaMKII inhibition [18]. A recent study could link the increased incidence of arrhythmias in ischaemic canine heart tissue to a CaMKII-related shortening of the Ca2+ signalling refractoriness [66]. It could further be shown that the progression of human heart failure is associated with a continuous increase in RyR2-mediated Ca2+ leak, which is paralleled by an increased incidence of diastolic spontaneous Ca2+ waves [33]. The aetiology underlying the development of heart failure might also play an important role regarding the cellular pathomechanisms involved. A recent study showed that RyR2 is only hyperphosphorylated at the CaMKII-dependent site Ser2815 in non-ischaemic, but not in ischaemic heart failure [67]. Accordingly, mice harbouring a mutation at Ser2814 (S2814A) were protected from afterload-induced heart failure after trans-aortic constriction but not from ischaemic heart failure after myocardial infarction. More studies will be needed to resolve this issue. Other studies directly linked the SR Ca2+ leak and an increased CaMKII activity to atrial fibrillation (AF), the most common sustained cardiac arrhythmia [68, 69]. It could be shown that CaMKII expression and its phosphorylation are significantly increased in right atria of AF patients compared with patients in stable sinus rhythm, which was accompanied by an increased RyR2 phosphorylation at the CaMKII-specific site Ser2814 [68, 69]. Diastolic Ca2+ levels were found to be increased as the result of an increased diastolic Ca2+ leak. Both findings could be normalised by CaMKII inhibition [68]. These data could be nicely confirmed by a recent study [70], which also showed that the SR Ca2+ leak could be reduced in AF cells by CaMKII inhibition whereas PKA inhibition did not yield relevant effects on the SR Ca2+ leak again stressing the role of CaMKII under pathophysiological conditions as compared to PKA.
RyR2 stabilisers and CaMKII inhibitors
The use of CaMKII inhibitors in patients is significantly hindered by the ubiquitous expression of this enzyme in different organs. Currently available CaMKII inhibitors cannot be administered to patients due to unspecific effects and pharmacodynamic problems (e.g. oral application of peptide inhibitors). Major hurdles as to organ-specific attribution still have to be overcome. Gene therapy-based approaches might, however, be feasible in the near future. Additionally, several compounds directly leading to a stabilisation of diastolic RyR2 closure have already been developed. The major challenge thereby is to identify compounds that exert a beneficial effect on the diastolic leak without compromising systolic RyR2 opening and Ca2+ release. One of the first compounds that combined both features was JTV519. It was shown to improve diastolic and systolic function in isolated human failing myocardium but also had unspecific effects on other ion channels and a narrow therapeutic range with negative inotropic effects at higher concentrations [71–73]. A newer and more specific drug called S44121 has been developed and is currently being evaluated in a phase 2 multicentre clinical study (ISRCTN reg. number 14227980).
Conclusion
Taken together, the RyR2-dependent SR Ca2+ leak has emerged as a pivot in the development of cardiac arrhythmias as well as heart failure under various conditions and therefore represents a promising toehold for future therapies. Whereas the direct contribution of PKA to the development of an increased SR Ca2+ leak is still controversial, the crucial role of CaMKII in this context seems to be more established. There are convincing data from animal models as well as human tissue showing that an increased CaMKII activity in cardiac pathologies contributes to the manifestation of pump failure and arrhythmias and that CaMKII inhibition can yield beneficial effects. Thus, to our current knowledge, the diastolic SR Ca2+ leak can be addressed by two different approaches: direct stabilisation of the leaky RyR2 via suitable new compounds (RyR stabilisers) on the one hand and reduction of its hyperphosphorylation by CaMKII inhibition on the other hand. The clinical implications for both approaches would be various, stretching from arrhythmias to systolic pump failure as well as diastolic dysfunction.
Acknowledgments
Dr. Sossalla was funded by the Research Program, Faculty of Medicine, Georg-August-University Göttingen. Dr. Maier is funded by the Deutsche Forschungsgemeinschaft (DFG) through the Clinical Research group KFO155 (MA 1982/2-2) and a Heisenberg grant (MA 1982/4-1), as well as by the Fondation Leducq Award to the Alliance for CaMK Signalling in Heart Disease. Dr. Sossalla and Dr. Maier are also funded by the DFG through the SFB 1002.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Roger VL. The heart failure epidemic. Int J Environ Res Public Health. 2010;7:1807–1830. doi: 10.3390/ijerph7041807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 3.Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the echocardiographic heart of England screening study: a population based study. Lancet. 2001;358:439–444. doi: 10.1016/S0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]
- 4.Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, et al. The prognosis of heart failure in the general population: the Rotterdam study. Eur Heart J. 2001;22:1318–1327. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 5.MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, et al. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation. 2000;102:1126–1131. doi: 10.1161/01.CIR.102.10.1126. [DOI] [PubMed] [Google Scholar]
- 6.Cowie MR, Wood DA, Coats AJ, Thompson SG, Suresh V, Poole-Wilson PA, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart. 2000;83:505–510. doi: 10.1136/heart.83.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case–control study. Am J Med. 2009;122:1023–1028. doi: 10.1016/j.amjmed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JG, Havranek EP, et al. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: a report of the American College of Cardiology/American heart association task force on clinical data standards (writing committee to develop heart failure clinical data standards): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Failure Society of America. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 9.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 10.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.RES.85.1.38. [DOI] [PubMed] [Google Scholar]
- 11.Neef S, Maier LS. Remodeling of excitation-contraction coupling in the heart: inhibition of sarcoplasmic reticulum Ca(2+) leak as a novel therapeutic approach. Curr Heart Fail Rep. 2007;4:11–17. doi: 10.1007/s11897-007-0020-7. [DOI] [PubMed] [Google Scholar]
- 12.Mattiazzi A, Kranias EG (2011) CaMKII Regulation of phospholamban and SR Ca2+ load. Heart Rhythm 8(5):784–787 [DOI] [PMC free article] [PubMed]
- 13.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 14.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 15.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 16.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 18.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, et al. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 20.Wittkopper K, Dobrev D, Eschenhagen T, El-Armouche A. Phosphatase-1 inhibitor-1 in physiological and pathological beta-adrenoceptor signalling. Cardiovasc Res. 2011;91:392–401. doi: 10.1093/cvr/cvr058. [DOI] [PubMed] [Google Scholar]
- 21.Schlotthauer K, Schattmann J, Bers DM, Maier LS, Schutt U, Minami K, et al. Frequency-dependent changes in contribution of SR Ca2+ to Ca2+ transients in failing human myocardium assessed with ryanodine. J Mol Cell Cardiol. 1998;30:1285–1294. doi: 10.1006/jmcc.1998.0690. [DOI] [PubMed] [Google Scholar]
- 22.Pieske B, Sutterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, et al. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest. 1996;98:764–776. doi: 10.1172/JCI118849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding SE, Jones SM, O’Gara P, del Monte F, Vescovo G, Poole-Wilson PA. Isolated ventricular myocytes from failing and non-failing human heart; the relation of age and clinical status of patients to isoproterenol response. J Mol Cell Cardiol. 1992;24:549–564. doi: 10.1016/0022-2828(92)91843-T. [DOI] [PubMed] [Google Scholar]
- 24.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–289. doi: 10.1016/S0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 25.Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol. 1999;31:479–491. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 26.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brochet DX, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci USA. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res. 2008;103:e105–e115. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, et al. Relationship between Na + -Ca2+ -exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.CIR.99.5.641. [DOI] [PubMed] [Google Scholar]
- 30.Bers DM. Excitation-contraction coupling and cardiac contractile force. Dordrecht: Kluwer Academic Publishers; 2001. [Google Scholar]
- 31.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, et al. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res. 2006;98:235–244. doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 32.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 33.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, et al. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 35.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huke S, Bers DM. Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376:80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacherer M, Sedej S, Wakula P, Wallner M, Vos MA, Kockskamper J, et al. (2012) JTV519 (K201) Reduces sarcoplasmic reticulum Ca(2+) leak and improves diastolic function in vitro in ouabain-induced cellular Ca(2+) overload in murine and human non-failing myocardium. Br J Pharmacol. doi:10.1111/j.1476-5381.2012.01995.x [DOI] [PMC free article] [PubMed]
- 38.Serysheva II. Structural insights into excitation-contraction coupling by electron cryomicroscopy. Biochemistry (Mosc) 2004;69:1226–1232. doi: 10.1007/s10541-005-0068-5. [DOI] [PubMed] [Google Scholar]
- 39.Bers DM, Li L, Satoh H, McCall E. Factors that control sarcoplasmic reticulum calcium release in intact ventricular myocytes. Ann N Y Acad Sci. 1998;853:157–177. doi: 10.1111/j.1749-6632.1998.tb08264.x. [DOI] [PubMed] [Google Scholar]
- 40.Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 41.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo W, Campbell KP. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J Biol Chem. 1995;270:9027–9030. doi: 10.1074/jbc.270.16.9027. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- 44.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida A, Takahashi M, Imagawa T, Shigekawa M, Takisawa H, Nakamura T. Phosphorylation of ryanodine receptors in rat myocytes during beta-adrenergic stimulation. J Biochem. 1992;111:186–190. doi: 10.1093/oxfordjournals.jbchem.a123735. [DOI] [PubMed] [Google Scholar]
- 47.Bristow MR. Myocardial beta-adrenergic receptor downregulation in heart failure. Int J Cardiol. 1984;5:648–652. doi: 10.1016/0167-5273(84)90179-7. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- 49.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, et al. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Makarewich CA, Kubo H, Wang W, Duran JM, Li Y, et al. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ Res. 2012;110:831–840. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang F, Shan J, Reiken S, Wehrens XH, Marks AR. Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci USA. 2006;103:3456–3461. doi: 10.1073/pnas.0511282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellison GM, Torella D, Karakikes I, Purushothaman S, Curcio A, Gasparri C, et al. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem. 2007;282:11397–11409. doi: 10.1074/jbc.M607391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao B, Sutherland C, Walsh MP, Chen SR. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+ -release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6) Circ Res. 2004;94:487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 54.Seidler T, Teucher N, Hellenkamp K, Unsold B, Grebe C, Kramps P, et al. Limitations of FKBP12.6-directed treatment strategies for maladaptive cardiac remodeling and heart failure. J Mol Cell Cardiol. 2011;50:33–42. doi: 10.1016/j.yjmcc.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, et al. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res. 2010;106:1743–1752. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loughrey CM, Seidler T, Miller SL, Prestle J, MacEachern KE, Reynolds DF, et al. Over-expression of FK506-binding protein FKBP12.6 alters excitation-contraction coupling in adult rabbit cardiomyocytes. J Physiol. 2004;556:919–934. doi: 10.1113/jphysiol.2003.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gellen B, Fernandez-Velasco M, Briec F, Vinet L, LeQuang K, Rouet-Benzineb P, et al. Conditional FKBP12.6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation-contraction coupling. Circulation. 2008;117:1778–1786. doi: 10.1161/CIRCULATIONAHA.107.731893. [DOI] [PubMed] [Google Scholar]
- 58.Sculptoreanu A, Rotman E, Takahashi M, Scheuer T, Catterall WA. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel alpha 1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1993;90:10135–10139. doi: 10.1073/pnas.90.21.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujii J, Maruyama K, Tada M, MacLennan DH. Expression and site-specific mutagenesis of phospholamban. Studies of residues involved in phosphorylation and pentamer formation. J Biol Chem. 1989;264:12950–12955. [PubMed] [Google Scholar]
- 60.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, et al. Functional adult myocardium in the absence of Na + -Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res. 2004;95:604–611. doi: 10.1161/01.RES.0000142316.08250.68. [DOI] [PubMed] [Google Scholar]
- 61.Yang D, Zhu WZ, Xiao B, Brochet DX, Chen SR, Lakatta EG, et al. Ca2+/calmodulin kinase II-dependent phosphorylation of ryanodine receptors suppresses Ca2+ sparks and Ca2+ waves in cardiac myocytes. Circ Res. 2007;100:399–407. doi: 10.1161/01.RES.0000258022.13090.55. [DOI] [PubMed] [Google Scholar]
- 62.Toischer K, Rokita AG, Unsold B, Zhu W, Kararigas G, Sossalla S, et al. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 64.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belevych AE, Terentyev D, Terentyeva R, Ho HT, Gyorke I, Bonilla IM, et al. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res. 2012;110:569–577. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, Dealmeida A, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 69.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I et al. (2012) Enhanced sarcoplasmic reticulum Ca2+ -leak and increased Na+ -Ca2+ -exchanger function underlie delayed after depolarizations in patients with chronic atrial fibrillation. Circulation 125(17):2059–2070 [DOI] [PMC free article] [PubMed]
- 71.Toischer K, Lehnart SE, Tenderich G, Milting H, Korfer R, Schmitto JD, et al. K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res Cardiol. 2010;105:279–287. doi: 10.1007/s00395-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakaya H, Furusawa Y, Ogura T, Tamagawa M, Uemura H. Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea-pig hearts. Br J Pharmacol. 2000;131:1363–1372. doi: 10.1038/sj.bjp.0703713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H. Effects of a novel cardioprotective drug, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol. 1999;79:275–281. doi: 10.1254/jjp.79.275. [DOI] [PubMed] [Google Scholar]