Abstract
Whether hydrogen and methane gas produced during lactulose breath test (LBT) are associated with symptoms of irritable bowel syndrome (IBS) is not determined. We aimed to investigate whether hydrogen and methane on LBT are associated with IBS symptoms. Sixty-eight IBS patients meeting the Rome III criteria for IBS, and 55 healthy controls, underwent LBT. The IBS subjects recorded their customary gastrointestinal symptoms on a questionnaire using visual analogue scales. LBT positivity was defined to be above 20 ppm rise of hydrogen or 10 ppm rise of methane within 90 min. Gas amounts produced during LBT were determined by calculating area under the curve of hydrogen and methane excretion. Symptom severity scores were not different between the LBT (+) IBS and LBT (-) IBS subjects and also between methane producers and non-methane producers. Gas amounts produced during LBT were not associated with IBS symptoms, except a weak correlation between total gas amounts and a few IBS symptoms such as bloating (r = 0.324, P = 0.039), flatulence (r = 0.314, P = 0.046) and abdominal pain (r = 0.364, P = 0.018) only in LBT (+) IBS. In conclusion, hydrogen and methane gas on LBT are not useful for predicting the customary symptoms and subtypes of IBS.
Keywords: Irritable Bowel Syndrome, Lactulose Breath Test, Intestinal Gas, Hydrogen, Methane
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder characterized by bowel habit changes and abdominal pain or discomfort relieved by defecation (1). Patients with IBS complain of diverse GI symptoms such as diarrhea, constipation, abdominal pain, bloating, urgency, and flatulence. As there is no specific disease marker for IBS, it is diagnosed totally according to the standardized diagnostic criteria based on symptoms, the Rome criteria. Furthermore, its pathophysiology is multifactorial: visceral hypersensitivity, intestinal dysmotility, abnormal brain-gut axis, genetic factors and altered intestinal microbiota including small intestinal bacterial overgrowth (SIBO) (2).
Recently, SIBO has been advocated as a pathogenetic factor of IBS. It is defined as the presence of abnormally high numbers of bacteria or the growth of colon-type bacteria in the small intestine. Its predisposing factors include conditions with peristaltic impairment of small bowel or anatomical defects causing intestinal stasis. For the diagnosis of SIBO, invasive or non-invasive test can be used. Lactulose breath test (LBT) is one of the non-invasive tests and can detect hydrogen and methane gas produced by bacterial fermentation of unabsorbed intestinal lactulose and excreted in the breath. Studies using LBT reported that IBS patients have SIBO more frequently than healthy controls (HC), and administration of antibiotics improved IBS symptoms together with eradication of SIBO and normalization of breath test results (3, 4). However, LBT is not standardized as study results are different according to the diagnostic criteria applied to the study (5) and therefore, SIBO as a cause of IBS still remains a matter of debate.
Despite multifactorial pathophysiology including SIBO, gas-like symptoms including bloating are reported to be present in over 90% of IBS patients (6). Bloating could be explained by excessive amounts of intestinal gas. Intestinal gas is composed of predominantly N2 and O2 and includes variable concentrations of CO2, hydrogen and methane. Hydrogen is derived only from bacterial metabolism in the intestine. Excessive amount of hydrogen could be produced by fermentation of unabsorbed carbohydrates by the increased numbers of bacteria in the small and large intestines. It was demonstrated that maximal rate of hydrogen production and excretion was higher in IBS patients than controls and gas-like symptoms was improved by exclusion diet of gas-forming foods (7). Methane is produced through consumption of hydrogen by methane-producing bacteria, methanogens. Methane production in the intestine has recently reported to be associated with constipation. A direct inhibitory effect of the gas on intestinal transit has been suggested, based on in vivo and in vitro experiments (8), and reduction of postprandial serotonin levels has been suggested as a mediating mechanism (9). Measurement of exhaled methane has even been advocated as a diagnostic test for IBS-C (10). However, methanogens in IBS patients was reported to be significantly lower than healthy controls in a study comparing 345 IBS subjects with 254 matched healthy controls (11).
Therefore, it remains to be determined whether hydrogen or methane gas on LBT is associated with IBS symptoms and whether excessive amounts of hydrogen and methane produced during LBT are responsible for IBS symptoms including bloating. We aimed to investigate whether methane or hydrogen is associated with IBS subtypes and whether the amounts of hydrogen and methane gas correlates with customary GI symptoms in IBS patients. Therefore, we compared diverse IBS symptoms with LBT positivity for hydrogen and methane in IBS patients. We also compared the gas amounts of hydrogen and methane calculated by AUC on LBT with symptom severity scores of IBS.
MATERIALS AND METHODS
Study subjects
Subjects with IBS were recruited from our gastroenterology outpatient unit and through advertisements from October 2006 to October 2007. All IBS patients fulfilled the Rome III criteria for IBS (1). Exclusion criteria included conditions such as previous bowel resection, bowel adhesion, narcotics use, connective tissue diseases, diabetes, thyroid diseases, inflammatory bowel diseases or any organic GI diseases. Subjects with probiotics, antidepressants, or antibiotics use for the past three months were excluded. Of 75 IBS subjects recruited at first, we excluded 2 patients with probiotics users, 3 patients with antibiotics, one patient with fibromyalgia and one with thyroid disease, and thus 68 IBS subjects participated in the study. Also through paper advertisements in our hospital, we selected 55 healthy controls (HCs) after interviewing them to confirm that they had no GI symptoms or illnesses.
The sample size was determined using a statistical power of 80% and a type 1 error of 0.05. The number of IBS patients was determined by using the formula to calculate sample size in quantitative data in two groups based on the previous study to compare symptom severities of IBS (12), and the number of healthy controls was determined for the total enrollments to be over one hundred to follow a normal distribution.
Study design
All subjects came to the laboratory at our gastroenterology unit after an overnight fast. Before LBT, subjects were asked to fill out a symptom questionnaire for evaluating the severity of their lower GI symptoms such as abdominal pain, diarrhea, constipation, tenesmus, bloating, urgency, and flatulence, on a visual analogue scale ranging from 0 to 20, with 20 representing the extreme symptoms. The questionnaire was modified from the previous studies (13, 14).
Lactulose breath test
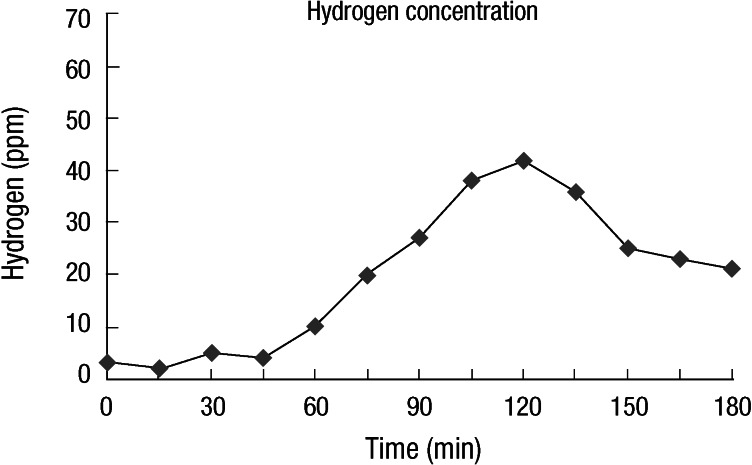
All subjects were asked to have carbohydrate-restricted diets one day before the test to minimize their basal hydrogen excretion. Smoking and physical exercises were not allowed 2 h prior to and during the test. Breath testing started after mouth washing with 20 mL of 1% chlorhexidine solution. After an initial baseline breath sample was collected, subjects ingested 15 mL of syrup containing 10 g lactulose (Duphalac®, Choong Wae Pharma Corporation, Seoul, Korea) with 200 mL of water. Endalveolar breath samples were obtained at 15 min intervals for 180 min with an Alveosampler (Quintron Instrument Co. Milwaukee, WI, USA). All breath samples were immediately analyzed to measure hydrogen and methane concentration in parts per million (ppm) using a QuinTron model SC gas chromatograph (QuinTron Instrument Co. Milwaukee, WI, USA). The measurements were plotted as a graph and interpreted (Fig. 1). LBT results were considered positive when breath hydrogen concentration rose over 20 ppm or methane over 10 ppm within 90 min (9, 15, 16). As for methane, subjects who excrete more than 1 ppm of methane during LBT were defined as methane producers, while those who excrete less than 1 ppm of methane as non-methane producers (10-12, 17). Cumulative hydrogen or methane production was quantified from the graph by calculating the area under the curve (AUC) of hydrogen or methane concentration over time (18). Total amounts of hydrogen plus methane were also calculated by adding the AUC of hydrogen to that of methane.
Fig. 1.
Schematic drawing of lactulose breath tests indicating positivity for hydrogen.
Statistical analysis
For each of the three gas patterns (hydrogen, methane and total gas patterns) obtained in the LBT, the correlation with IBS symptoms was analyzed by the chi-square test or Fisher's exact test. Symptom severity scores and gas amounts in the IBS patients and HCs were compared by t-test. The relationship between AUCs of gas and symptom severity scores was analyzed by Pearson's correlation test. Data are reported as mean ± standard deviation and P values less than 0.05 are considered significant. Statistical analysis for gender adjustment was performed by SAS (ver 9.2).
Ethics statement
The study was approved by the institutional review board of the Hanyang University Hospital (IRB No. 2006-08-001). Written informed consent was obtained from all subjects prior to participation.
RESULTS
Subjects
A total of 68 IBS patients (32 males; mean age, 41.4 yr) and 55 HCs (31 males; 37.4 yr) underwent LBT. The demographic characteristics of the subjects showed no significant difference between the IBS patients and HCs (Table 1). Thirty five (51%) of the 68 IBS patients had IBS-D, 23 (34%) had IBS-C, and 10 (15%) had IBS-M.
Table 1.
Characteristics and LBT results in IBS patients and controls
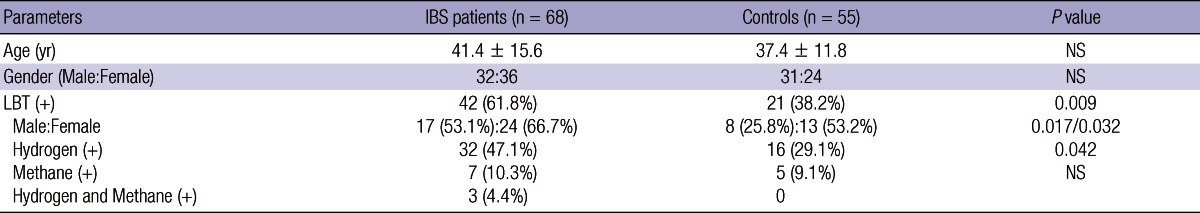
IBS, irritable bowel syndrome; LBT, lactulose breath test; NS, not significant.
LBT results
The IBS patients had a higher LBT positivity (including hydrogen [+] and methane [+]) than HCs (61.8%, 42/68 vs 38.2%, 21/55; P = 0.009) (Table 1). LBT positivity for hydrogen was also higher in the IBS patients than HCs (55.5%, 35/63 vs 29.1%, 16/55; P = 0.042). LBT positivity for methane was not different between the IBS group and HCs (14.7%, 10/68 vs 9.1%, 5/55; P = 0.344). The female patients with IBS had a higher LBT positivity than male patients (75.0% vs 47.2%; P = 0.017). LBT positivity was not different between the IBS subgroups (60.6%, 21/35 in IBS-D, 60.9%, 14/23 in IBS-C, and 70.0%, 7/10 in IBS-M).
Association between hydrogen or methane and IBS symptoms
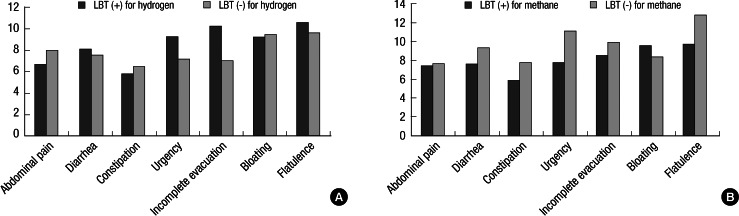
None of IBS symptoms were noted to be correlated with LBT-positivity. There were no significant differences in symptom severity scores between the LBT (+) and LBT (-) IBS patients (Fig. 2A and B). Methane producers were 29.4% (20/68) in the IBS patients and 27.3% (15/55) in the HCs, respectively, showing no significant difference. Mean symptom severity scores of diarrhea and constipation were 7.10 ± 6.71 vs 8.15 ± 6.07 (P = 0.532) and 6.90 ± 7.39 vs 5.79 ± 6.35 (P = 0.534) in the methane producers and non-methane producers, respectively (Table 2). Also, the LBT (+) IBS patients for methane did not differ from the LBT (-) IBS patients for methane in the symptom severity scores of constipation (7.70 ± 8.27 vs 5.84 ± 6.36, P = 0.419).
Fig. 2.
(A) The comparison of symptom severity scores in lower gastrointestinal symptoms between IBS patients with LBT (+) and LBT (-) for hydrogen. There were no significant differences in the severity scores of IBS symptoms between the groups. (B) The comparison of symptom severity scores in lower gastrointestinal symptoms between IBS patients with LBT (+) and LBT (-) for methane. There were no significant differences in the severity scores of IBS symptoms between the groups.
Table 2.
Comparison of symptom severity between methane producers and non-methane producers in the IBS patients
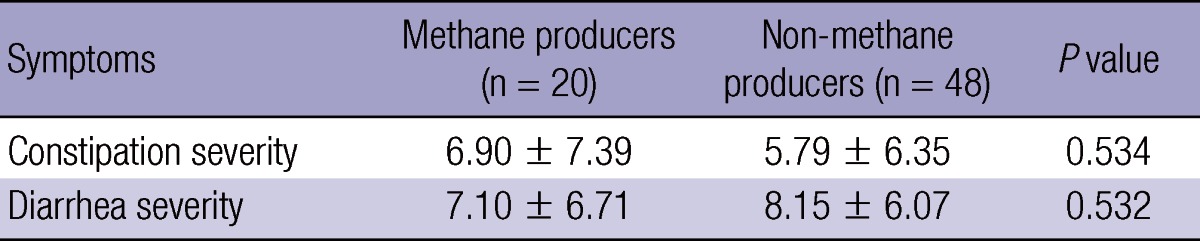
IBS, irritable bowel syndrome.
Hydrogen and methane gas amounts measured in the LBT and LBT results
Significantly greater amounts of hydrogen gas were produced in the LBT (+) IBS patients than in the LBT (-) IBS patients (5,218 ± 3,031 vs 2,126 ± 1,879; P < 0.001) and also in the LBT (+) HCs (5,218 ± 3,031 vs 3,367 ± 2,142; P = 0.007). Mean amounts of hydrogen excretion were not significantly different between the total IBS patients and HCs (4,035 ± 3,038 vs 3,154 ± 2,520; P = 0.087). Also, no difference was found in gas amounts between the LBT (+) and LBT (-) HCs (4,431 ± 2,247 vs 3,040 ± 2,147; P = 0.056). In terms of age, gender, or subtype of IBS, there were no significant differences in gas amounts of hydrogen.
Association between hydrogen and methane gas amounts and IBS symptoms
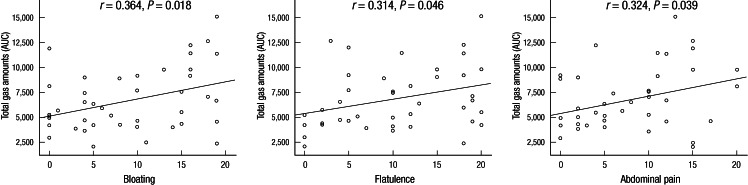
Symptom severity scores of any GI symptoms were not correlated with hydrogen gas amounts calculated by AUCs of hydrogen excretion over time in the LBT. Total gas amounts of hydrogen and methane were not associated with none of the IBS symptoms. Only in the LBT (+) IBS patients, there was a modest correlation between symptom severity scores for bloating (r = 0.364, P = 0.018), flatulence (r = 0.314, P = 0.046) and abdominal pain (r = 0.324, P = 0.039) and the AUCs of both hydrogen and methane (Fig. 3).
Fig. 3.
The correlation between symptom severity scores and gas amounts during LBT in the LBT (+) IBS patients. The severity scores of bloating, flatulence and abdominal pain were weakly correlated with gas amounts calculated by AUC in the LBT curve.
DISCUSSION
Our study shows that hydrogen and methane gas produced by intestinal fermentation of lactulose and excreted in the breath during LBT are not associated with any IBS symptoms including diarrhea and constipation. We also found that gas amounts of hydrogen and methane excreted during LBT are not associated with any GI symptoms of IBS. Therefore, hydrogen or methane gas in the LBT may not be useful in predicting symptom severity of IBS and not indicate the subtype of IBS which develop. Additionally, we observed that there is a gender difference in LBT results of IBS patients.
Hydrogen or methane produced by intestinal fermentation of lactulose does not seem to contribute to any IBS symptoms. Our data showed that none of the IBS symptoms assessed by symptom severity questionnaire were correlated with LBT positivity for hydrogen or methane. It was previously known that hydrogen or methane produced by intestinal bacterial fermentation is inert to the intestine except for its distending effect. However, studies have recently reported that methane production is associated with IBS-C. The quantity of methane produced during LBT was described to be proportional to the severity of constipation in IBS (12). The effect of methane on the intestine was shown to slow small intestinal transit by augmenting small bowel contractility in both animal and human studies (8). However, in the present study, constipation severity scores of IBS patients with LBT (+) for methane did not differ from those with LBT (-) for methane. Furthermore, the proportions of methane producers did not differ between the IBS patients and HCs and between IBS-C and IBS-D. Also, symptom severity scores for constipation in methane producers were similar to those in non-methane producers.
Regarding methane on constipation in IBS, there is a discrepancy between other studies and ours. A possible explanation is that subjects in our study may have reported constipation severity differently from those of other studies; the symptom of constipation is variable in expression among patients. Our questionnaire did not discern the symptom of constipation as such. However, in Western studies, investigators usually considered the symptom of constipation only in view of stool frequency and consistency (10, 12, 15), but our subjects might consider excessive straining, hard stool, digital evacuation, and incomplete evacuation sensation as constipation, even except infrequent defecation. On the other hand, what methane is claimed to be associated with constipation by slowing GI transit means that methane seems to have a neurotransmitter function on the GI tract (8). However, to our knowledge, no evidence exists that there is a receptor for methane in the GI tract. Furthermore, many studies demonstrating the association between methane and constipation have a confounding bias because they did not exclude patients with colonic diverticulosis that is a condition known to be associated with breath methane excretion (19).
Taken together, we could speculate that neither hydrogen nor methane after lactulose ingestion has any specific effect on the development and aggravation of constipation or diarrhea in IBS and that LBT positivity for hydrogen or methane does not indicate the subtype of IBS.
The present study results suggest that gas amounts of hydrogen and methane excreted during LBT are not associated with IBS symptoms. The severity scores of customary IBS symptoms assessed by symptom questionnaire were not associated with total amounts of hydrogen and methane calculated by AUCs in the LBT. These results also indicate that amounts of hydrogen or methane produced after lactulose fermentation are not associated with gas-like symptoms such as bloating in IBS. Bloating was previously suggested to be due to excessive amounts of gas on the intestine. It was also shown that intestinal gas measured by plain abdominal radiography was greater in IBS patients than HCs (20). Subgroup analysis in our results revealed a weak correlation between symptoms of bloating, flatulence and abdominal pain and total gas amounts of hydrogen and methane. That means IBS patients with severe degree of customary symptoms such as bloating, flatulence or abdominal pain may have greater amounts of gas production after lactulose ingestion than those with mild symptoms. However, the correlations were very weak, just reached statistical significance, and furthermore, observed only in the LBT (+) IBS patients. This association confined to the LBT (+) IBS patients may be due to our measurements of gas amounts for only 180 min, and not until the gas levels have normalized or recovered to the baseline. LBT (-) subjects may excrete the same amount of gas as LBT (+) subjects, only later than 180 min. Furthermore, it was reported that 24-hr excretion of hydrogen measured by indirect calorimetry was greater in IBS than HC, but no significant difference in breath hydrogen excretion for 3 hr (21). Taken together, our results have a limitation to supporting the idea that hydrogen and methane gas amounts produced during LBT have an association with gas-like IBS symptoms.
The gas amounts excreted during LBT can be explained in a way by increased production due to bacterial fermentation of lactulose in the small intestine, i.e. SIBO. In diagnosing SIBO, various invasive and non-invasive tests such as direct culture methods and breath tests using glucose have been used, but there have been many problems. In direct culture methods, intestinal aspirates are technically limited to the proximal portion of the small intestine, and in glucose breath tests, glucose may be absorbed in the proximal small intestine instead of being fermented by intestinal bacteria. Therefore, both methods may underestimate SIBO, i.e. high false negative rate. As for LBT, study results are variable according to a diagnostic criterion of LBT used (5). In the present study, the 90 min criterion for LBT positivity was used because the mean orocecal transit time (OCTT) measured by LBT is known to be approximately 90 min, although lactulose tend to accelerate OCTT. In the double peak criterion, there have been concerns regarding subjectiveness of its interpretations and high interobserver variability. The criterion > 20 ppm by 180 min might overestimate the prevalence of SIBO by counting subjects fermenting lactulose in their colon.
Whether the gas detected in the LBT would have been produced in the small intestine or in the colon could not be determined in our study design. It may depend on intestinal transit time, particularly OCTT. In subjects with rapid OCTT, lactulose are exposed to intestinal bacteria for shorter time periods than those with slow OCTT. In subjects with very slow OCTT, lactulose fermentation in the intestine would continue over 3 hr. Also, a significant discrepancy was identified between gas production in the intestine and gas excretion into the breath (7). That is because all the factors such as the diffusivity of a gas, partial pressure difference between lumen and blood, and the exposure of the gas to the mucosal surface may affect the excretion rate of hydrogen and methane into the breath. Apart from hydrogen and methane, metabolism of other intestinal gases including N2, O2, and CO2 should be considered. On the other hand, a gas infusion study by using nasojejunal tube showed that IBS patients with bloating had slower gas transit than controls (22), attributing impaired handling of intestinal gas to bloating (23). Other possible explanations include visceral hypersensitivity to normal amount of intestinal gas (24), abnormally weak abdominal musculature intolerable to gaseous distension (25), and psychosomatic factors.
As for LBT results, gender effect on IBS was also found in the present study. Our data showed that the female IBS patients had a significantly higher prevalence of LBT positivity than male patients. Gender effects on IBS have been also noted not only in the prevalence, pathophysiology, and symptomatology of IBS but also the response to treatment (26, 27). According to a recent report, female IBS patients may have different mucosal infiltration by immune cells from male IBS patients (28). It may be postulated, considering that intestinal microbiota is responsible for mucosal immune infiltration, that altered intestinal microbiota has a potential contribution to gender differences in IBS, consistently with our data, although it was not evaluated in the study.
Several limitations to this study should be considered including a limitation of small sample size. First, we enrolled healthy controls with no GI symptoms but general population usually has symptoms of bloating and flatulence. Therefore, it might be needed to investigate the correlation of LBT results and gas-like symptoms in IBS patients and healthy population with bloating and flatulence but not meeting the Rome III criteria of IBS, although a study reported that similar gas production caused symptoms only in IBS patients, not in HC (7). Second, LBT results may be affected by a variety of factors including alcohol consumption and smoking history as well as prokinetics. We had to manipulate them in order to accurately interpret our data, but unfortunately we did not collect these variables. Third, the questionnaire used was modified from that of previous studies (13, 14) because only lower GI symptoms of IBS were needed for comparing with LBT results. More reliable data could have been obtained through a validated questionnaire. The validated ones in severity of IBS do not include all of the lower GI symptoms of IBS, although they may include HRQOL, psychosocial factors, and health-care utilization behavior. Forth, gastric acid barrier normally plays one of the protective roles for bacterial overgrowth in the GI tract (29). Therefore, acid suppressants including proton pump inhibitor (PPI) may predispose subjects to SIBO (30) and gas amount in the LBT could be altered in PPI users. Therefore, our study should have excluded PPI users at enrollment. However, the inclusion of PPI users in our study may not have affected results, considering a recent report that 46.2% of 106 patients taking PPIs were positive for SIBO, which is not significantly different from 56.3% positives among non-PPI users (31). On the other hand, this study also reported that methane detection was lower in PPI users than non-PPI users, which suggests to pay attention to interpretation of our data.
In conclusion, methane and hydrogen on the LBT is not associated with specific symptoms in IBS patients. Furthermore, hydrogen and methane gas amounts calculated by AUC in the LBT are not associated with IBS symptoms. Our results indicate that hydrogen and methane in the LBT are not useful for predicting customary IBS symptoms and the form of IBS which develop. In order to understand symptomatology and pathophysiology of IBS, further Studies are needed for investigating other gases and their metabolism as well as hydrogen and methane.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson RM., Jr Normal bacterial populations of the intestine and their relation to intestinal function. N Engl J Med. 1964;270:1050–1056. doi: 10.1056/NEJM196405142702007. [DOI] [PubMed] [Google Scholar]
- 3.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 4.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 5.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 7.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–1189. doi: 10.1016/s0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 8.Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, Park S, Kong Y, Conklin J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089–G1095. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 9.Pimentel M, Kong Y, Park S. IBS subjects with methane on lactulose breath test have lower postprandial serotonin levels than subjects with hydrogen. Dig Dis Sci. 2004;49:84–87. doi: 10.1023/b:ddas.0000011607.24171.c0. [DOI] [PubMed] [Google Scholar]
- 10.Hwang L, Low K, Khoshini R, Melmed G, Sahakian A, Makhani M, Pokkunuri V, Pimentel M. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci. 2010;55:398–403. doi: 10.1007/s10620-009-0778-4. [DOI] [PubMed] [Google Scholar]
- 11.Rana SV, Sharma S, Sinha SK, Kaur H, Sikander A, Singh K. Incidence of predominant methanogenic flora in irritable bowel syndrome patients and apparently healthy controls from North India. Dig Dis Sci. 2009;54:132–135. doi: 10.1007/s10620-008-0315-x. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837–841. doi: 10.1111/j.1572-0241.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel B, Strickland A, Naliboff BD, Mayer EA, Chang L. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol. 2008;103:2536–2543. doi: 10.1111/j.1572-0241.2008.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 15.Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48:86–92. doi: 10.1023/a:1021738515885. [DOI] [PubMed] [Google Scholar]
- 16.Joseph F, Jr, Rosenberg AJ. Breath hydrogen testing: diseased versus normal patients. J Pediatr Gastroenterol Nutr. 1988;7:787–788. doi: 10.1097/00005176-198809000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Levitt MD, Furne JK, Kuskowski M, Ruddy J. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol. 2006;4:123–129. doi: 10.1016/j.cgh.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Kotler DP, Holt PR, Rosensweig NS. Modification of the breath hydrogen test: increased sensitivity for the detection of carbohydrate malabsorption. J Lab Clin Med. 1982;100:798–805. [PubMed] [Google Scholar]
- 19.Weaver GA, Krause JA, Miller TL, Wolin MJ. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986;27:698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koide A, Yamaguchi T, Odaka T, Koyama H, Tsuyuguchi T, Kitahara H, Ohto M, Saisho H. Quantitative analysis of bowel gas using plain abdominal radiograph in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1735–1741. doi: 10.1111/j.1572-0241.2000.02189.x. [DOI] [PubMed] [Google Scholar]
- 21.Sen S, Dear KL, King TS, Elia M, Hunter JO. Evaluation of hydrogen excretion after lactulose administration as a screening test for causes of irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2002;14:753–756. doi: 10.1097/00042737-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Salvioli B, Serra J, Azpiroz F, Lorenzo C, Aguade S, Castell J, Malagelada JR. Origin of gas retention and symptoms in patients with bloating. Gastroenterology. 2005;128:574–579. doi: 10.1053/j.gastro.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–19. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galati JS, McKee DP, Quigley EM. Response to intraluminal gas in irritable bowel syndrome: motility versus perception. Dig Dis Sci. 1995;40:1381–1387. doi: 10.1007/BF02065555. [DOI] [PubMed] [Google Scholar]
- 25.Tremolaterra F, Villoria A, Azpiroz F, Serra J, Aguadé S, Malagelada JR. Impaired viscerosomatic reflexes and abdominal-wall dystony associated with bloating. Gastroenterology. 2006;130:1062–1068. doi: 10.1053/j.gastro.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Kim JH, Sung IK, Park HS, Jin CJ, Choe WH, Kwon SY, Lee CH, Choi KW. Irritable bowel syndrome is more common in women regardless of the menstrual phase: a Rome II-based survey. J Korean Med Sci. 2007;22:851–854. doi: 10.3346/jkms.2007.22.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 29.Armbrecht U, Bosaeus I, Gillberg R, Seeberg S, Stockbruegger R. Hydrogen (H2) breath test and gastric bacteria in acid-secreting subjects and in achlorhydric and postgastrectomy patients before and after antimicrobial treatment. Scand J Gastroenterol. 1985;20:805–813. doi: 10.3109/00365528509088827. [DOI] [PubMed] [Google Scholar]
- 30.Spiegel BM, Chey WD, Chang L. Bacterial overgrowth and irritable bowel syndrome: unifying hypothesis or a spurious consequence of proton pump inhibitors? Am J Gastroenterol. 2008;103:2972–2976. doi: 10.1111/j.1572-0241.2008.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law D, Pimentel M. Proton pump inhibitor therapy does not affect hydrogen production on lactulose breath test in subjects with IBS. Dig Dis Sci. 2010;55:2302–2308. doi: 10.1007/s10620-009-1010-2. [DOI] [PubMed] [Google Scholar]