Abstract
Neurogenic pulmonary edema (NPE) leading to cardiopulmonary dysfunction is a potentially life-threatening complication in patients with central nervous system lesions. This case report describes a 28-yr woman with life-threatening fulminant NPE, which was refractory to conventional respiratory treatment, following the rupture of an aneurysm. She was treated successfully with extracorporeal membrane oxygenation (ECMO), although ECMO therapy is generally contraindicated in neurological injuries such as brain trauma and diseases that are likely to require surgical intervention. The success of this treatment suggests that ECMO therapy should not be withheld from patients with life-threatening fulminant NPE after subarachnoid hemorrhage.
Keywords: Extracorporeal Membrane Oxygenation, Pulmonary Edema, Subarachnoid Hemorrhage
INTRODUCTION
The fulminant form of neurogenic pulmonary edema (NPE) is a rare, life-threatening complication that can occur after the rupture of an intracranial aneurysm (1). Prompt neurosurgical interventions for the ruptured aneurysm and the increased intracranial pressure (ICP) are important in the management of NPE. However, neurosurgical interventions are possible only after the general treatment of pulmonary edema improves the respiratory condition of the patient with NPE.
We recently experienced a patient with life-threatening fulminant NPE, which was refractory to conventional respiratory treatment, following the rupture of an aneurysm. The patient was treated successfully by extracorporeal membrane oxygenation (ECMO).
CASE DESCRIPTION
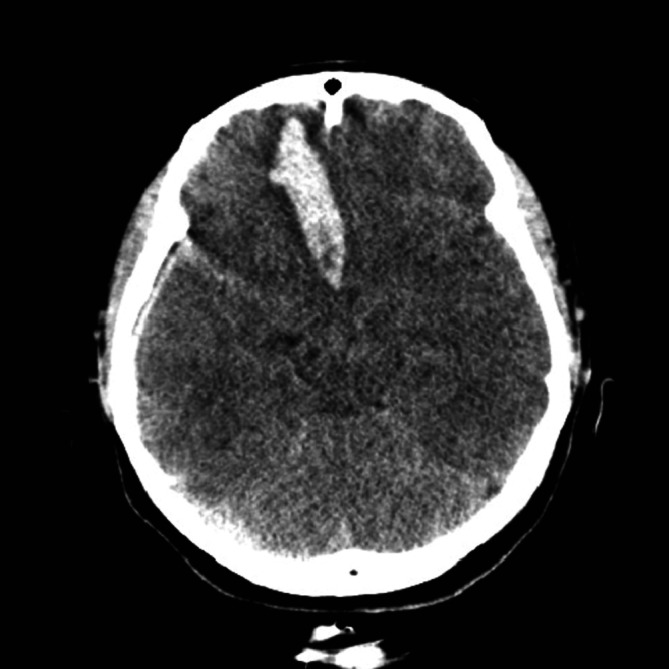
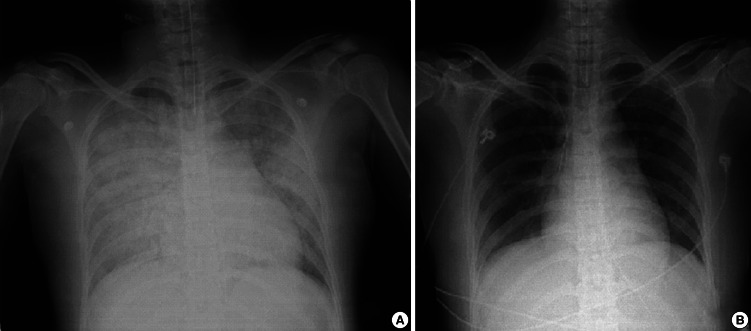
A 28-yr woman presented with sudden mental deterioration on September 13, 2011. Her mental status was stupor, but no other neurological deficit was observed. Her initial vital signs were as follows: blood pressure (BP) 80/50 mmHg, heart rate (HR) 128 beats/min, respiratory rate (RR) 24 breaths/min, and arterial blood gas analysis (ABGA) values of pH = 7.20, PaCO2 = 49, PaO2 = 31 mmHg, and oxygen saturation 41%. The patient was intubated immediately and transferred to the CT room. CT showed an acute hematoma with mass effect in the right frontal base, suggesting the rupture of an anterior communicating (ACom) artery aneurysm (Fig. 1). Additionally, ventricle and cisternal spaces were collapsed, indicating severe brain swelling and increased ICP. However, the most urgent problem was the respiratory condition of the patient, who did not respond to mechanical ventilation (CMV mode; FiO2, 100; tidal volume, 400 mL; respiratory rate, 20/min; PEEP, 8 mmHg). Repeated arterial blood gas analyses showed severe hypoxemia refractory to conventional treatment including mechanical ventilation with 1.0 of FiO2 and 8 mmHg of PEEP, and administration of furosemide (PO2, 32-44 mmHg; PCO2, 48-68 mmHg; O2 saturation, 37-41%). Pink froth percolated from the endotracheal tube and pulmonary edema was suspected. A chest X-ray confirmed severe pulmonary edema (Fig. 2A), but no abnormality in cardiac function was detected on echocardiography. NPE was diagnosed as the cause of the respiratory failure. In order to allow neurosurgical interventions for the ruptured aneurysm and ICP control, the life-threatening respiratory failure had to be improved. We chose ECMO as an option for respiratory support 3 hr after her visit, although its use had not been proven in cases with acute intracerebral hemorrhage.
Fig. 1.
Computed tomographic scan showing a hematoma with mass effect in the right frontal base, suggesting rupture of an anterior communicating artery aneurysm.
Fig. 2.
Thoracic radiography: (A) severe pulmonary edema is evident, (B) the pulmonary edema had improved by the last day of extracorporeal membrane oxygenation treatment.
ECMO application and cerebral angiography
The patient was transferred to the angiography room. After infusion of heparin (3000 u), a 21 Fr venous cannula (DLP; Medtronic Inc., Minneapolis, MN, USA) was placed in the right atrium, and a 24 Fr venous cannula (RMI; Edwards Lifesciences LLC, Irvine, CA, USA) was placed in the inferior vena cava with fluoroscopy, both via femoral veins using the Seldinger method. Venovenous ECMO was initiated at a flow rate of 4.5 L/min and cerebral angiography was performed using a catheter. No abnormality in cardiac function was detected on fluoroscopy, and a ruptured aneurysm (8.5 mm in diameter) in the ACom artery was confirmed by cerebral angiography.
Aneurysm clipping and decompressive craniectomy for ICP control
The patient was transferred to the operating room. The ACom artery aneurysm was clipped successfully via a left large frontotemporal craniectomy. Because severe brain edema was noted, a right large frontotemporal craniectomy was also performed, and the hematoma in the right frontal base was removed. A duroplasty was performed at the bilateral frontotemporal duras, and a probe for ICP monitoring (Spiegelberg GmbH & Co. KG, Hamburg, Germany) was applied.
Postoperative course
To control the increased ICP resulting from the severe brain edema, hypothermia (target temperature, 34℃) was established using ECMO, and barbiturate coma therapy and osmotic therapy (target serum osmolality, 310-320 mOsm/L) were also initiated. Cerebral perfusion pressure was maintained adequately (> 70 mmHg), based on blood pressure and ICP monitoring. NPE improved (Fig. 2B), and ECMO was removed 7 days after application. Neurological status also recovered fully by 1 month after surgical clipping, and a cranioplasty was performed using autologous bone flaps. She returned to her normal life, and Glasgow outcome scale and modified Rankin scale scores were 5 and 0, respectively, at 3 months after treatment.
DISCUSSION
NPE is characterized by an increase in interstitial and alveolar lung fluid, occurring as a direct consequence of an acute central nervous system injury, as in patients with acute increases in intracranial pressure (ICP) (2, 3). Frequent causes of NPE are traumatic head injury and aneurysmal subarachnoid hemorrhage (SAH) (2, 4). Fulminant NPE that is refractory to conventional respiratory treatment is always potentially life-threatening, and mortality from fulminant NPE is between 60% and 100% (1).
We experienced a patient with fulminant NPE following an aneurysmal SAH. Management of NPE is critical for improving outcomes after SAH. ECMO can be a last-resort treatment for patients with severe acute respiratory failure, in whom mechanical ventilation and adjunctive therapies such as inhaled nitric oxide cannot provide adequate gas exchange (5). However, neurological injuries, including brain trauma and diseases that are likely to require surgical intervention, are generally considered contraindications for ECMO therapy because the need for therapeutic anticoagulation increases the risk for bleeding (6, 7).
A recent study reported that initially heparin-free ECMO can be a safe and effective rescue treatment for severe trauma patients with coexisting bleeding shock (8). A previous study at our hospital showed that nafamostat mesilate, which has been used widely as an anticoagulant for hemodialysis patients with a tendency to bleed, can be used as an alternative anticoagulant to heparin in order to reduce bleeding complications during ECMO (9). In the present case, ECMO was used successfully with nafamostat mesilate as the anticoagulant, and neurosurgical operations were performed under ECMO therapy.
To our knowledge, this is the first report of successful neurosurgery under ECMO treatment in a patient with fulminant NPE after the rupture of an intracranial aneurysm. Following SAH, ICP is frequently elevated, usually in immediate response to the aneurysm rupture (10). Elevated ICP post-SAH is associated with worse outcomes, particularly when ICP does not respond to treatment (11).
In the present case, increased ICP was managed using mannitol, barbiturate coma, and mild hypothermia (34℃). In particular, mild hypothermia may serve as an effective management option for elevated ICP that is refractory to conventional therapy (12). Hypothermia may be induced by surface cooling or an intravascular cooling catheter. Sahuquillo et al. (13) reported that intravascular cooling methods appear to be feasible and effective for patients with refractory high ICP. In this regard, ECMO itself can be used as an intravascular cooling system, because it has a temperature control system.
Thus, hypothermia therapy is another benefit of using ECMO in a fulminant pulmonary edema patient with refractory elevated ICH after SAH. Ahrens et al. (1) reported a patient in a condition similar to that of our patient, but their patient expired because they could not control both life-threatening conditions. In the present case, the patient successfully underwent neurosurgery and recovered from both life-threatening conditions with the use of ECMO.
In conclusion, we suggest that ECMO therapy should be considered in patients with life-threatening fulminant pulmonary edema after SAH. In cases similar to our patient, ECMO therapy makes it possible to perform neurosurgery and can be used to control ICP via hypothermia. Physicians should be aware of this management option as it may save lives.
References
- 1.Ahrens J, Capelle HH, Przemeck M. Neurogenic pulmonary edema in a fatal case of subarachnoid hemorrhage. J Clin Anesth. 2008;20:129–132. doi: 10.1016/j.jclinane.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Baumann A, Audibert G, McDonnell J, Mertes PM. Neurogenic pulmonary edema. Acta Anaesthesiol Scand. 2007;51:447–455. doi: 10.1111/j.1399-6576.2007.01276.x. [DOI] [PubMed] [Google Scholar]
- 3.Carlson RW, Schaeffer RC, Jr, Michaels SG, Weil MH. Pulmonary edema following intracranial hemorrhage. Chest. 1979;75:731–734. doi: 10.1378/chest.75.6.731. [DOI] [PubMed] [Google Scholar]
- 4.Fein IA, Rackow EC. Neurogenic pulmonary edema. Chest. 1982;81:318–320. doi: 10.1378/chest.81.3.318. [DOI] [PubMed] [Google Scholar]
- 5.Lindstrom SJ, Pellegrino VA, Butt WW. Extracorporeal membrane oxygenation. Med J Aust. 2009;191:178–182. doi: 10.5694/j.1326-5377.2009.tb02735.x. [DOI] [PubMed] [Google Scholar]
- 6.McManus ML, Kevy SV, Bower LK, Hickey PR. Coagulation factor deficiencies during initiation of extracorporeal membrane oxygenation. J Pediatr. 1995;126:900–904. doi: 10.1016/s0022-3476(95)70205-9. [DOI] [PubMed] [Google Scholar]
- 7.Michaels AJ, Schriener RJ, Kolla S, Awad SS, Rich PB, Reickert C, Younger J, Hirschl RB, Bartlett RH. Extracorporeal life support in pulmonary failure after trauma. J Trauma. 1999;46:638–645. doi: 10.1097/00005373-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Arlt M, Philipp A, Voelkel S, Rupprecht L, Mueller T, Hilker M, Graf BM, Schmid C. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation. 2010;81:804–809. doi: 10.1016/j.resuscitation.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Han SJ, Kim HS, Kim KI, Whang SM, Hong KS, Lee WK, Lee SH. Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation. J Korean Med Sci. 2011;26:945–950. doi: 10.3346/jkms.2011.26.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nornes H. The role of intracranial pressure in the arrest of hemorrhage in patients with ruptured intracranial aneurysm. J Neurosurg. 1973;39:226–234. doi: 10.3171/jns.1973.39.2.0226. [DOI] [PubMed] [Google Scholar]
- 11.Heuer GG, Smith MJ, Elliott JP, Winn HR, LeRoux PD. Relationship between intracranial pressure and other clinical variables in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:408–416. doi: 10.3171/jns.2004.101.3.0408. [DOI] [PubMed] [Google Scholar]
- 12.Shiozaki T, Sugimoto H, Taneda M, Yoshida H, Iwai A, Yoshioka T, Sugimoto T. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg. 1993;79:363–368. doi: 10.3171/jns.1993.79.3.0363. [DOI] [PubMed] [Google Scholar]
- 13.Sahuquillo J, Pérez-Bárcena J, Biestro A, Zavala E, Merino MA, Vilalta A, Poca MA, Garnacho A, Adalia R, Homar J, et al. Intravascular cooling for rapid induction of moderate hypothermia in severely head-injured patients: results of a multicenter study (IntraCool) Intensive Care Med. 2009;35:890–898. doi: 10.1007/s00134-008-1357-4. [DOI] [PubMed] [Google Scholar]