Abstract
The purpose of this study was to examine the association between the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and bone mineral density (BMD). Two large cohort studies were performed: the Dong-gu Study (3,621 men and 5,409 women) and the Namwon Study (3,703 men and 5,672 women). We assessed lumbar spine and femoral neck BMD by dual-energy X-ray absorptiometry. Genotypes were determined by real-time polymerase chain reaction. Multiple linear regression analysis was performed to evaluate the association between MTHFR C677T and BMD, adjusting for age, weight and height. The MTHFR C677T genotype frequencies for CC, CT, and TT genotypes were 34.5, 48.7, and 16.8%, respectively, in the Dong-gu Study and 33.6, 49.2, and 17.2%, respectively, in the Namwon Study. There are no significant differences between the MTHFR C677T genotype and the BMD at the lumbar spine and femoral neck in men or women in both cohorts.
Keywords: Bone Density; Methylenetetrahydrofolate Reductase; Polymorphism, Genetic
Hyperhomocysteinemia has been associated with bone mineral density (BMD) (1), increased bone turnover markers (1, 2), and increased hip fracture risk (2-4). A plausible mechanism for this is that homocysteine may interfere with the formation of collagen cross-links, thereby increasing bone fragility and susceptibility to fracture (5). Methylenetetrahydrofolate reductase (MTHFR) catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the carbon donor for methylation of homocysteine to methionine. The most extensively studied functional polymorphism in several enzymes in the folate metabolic pathway is MTHFR C677T. Individuals heterozygous (677CT) or homozygous (677TT) for this polymorphism have reduced in vitro enzyme activity, 65% and 30% that of the wild type (677CC), respectively, which can interfere with the methylation of homocysteine to methionine, possibly resulting in abnormal plasma homocysteine concentrations (6).
Many studies have investigated the association between MTHFR C677T polymorphism and BMD. However, the results of these studies have been inconsistent. Additionally, most prior research has used a limited number of participants, and few studies have been carried out in Asian populations. Therefore, we examined cross-sectional associations between MTHFR C677T polymorphism and BMD in two large independent cohorts from Korea.
The Dong-gu Study and Namwon Study are ongoing prospective studies designed to investigate the prevalence, incidence, and risk factors for chronic disease in urban and rural populations, respectively. Details of the study subjects and measurements have been published previously (7). The Dong-gu Study enrolled 9,260 subjects (3,711 men and 5,549 women) aged 50 yr and older from 2007 to 2010. Of those, 9,206 subjects underwent BMD measurement using a Lunar Prodigy bone densitometer (GE, Madison, WI, USA), and 9,056 subjects had both lumbar spine and femoral neck BMD data. After excluding 26 subjects without the MTHFR C677T genotype, 9,030 subjects (3,621 men and 5,409 women) were used for analyses. The Namwon Study enrolled 10,667 subjects (4,201 men and 6,466 women) in the baseline survey from 2004 to 2007 and 8,157 subjects (3,231 men and 4,926 women) were studied in a follow-up examination from October 2007 to February 2012. Six thousand one hundred and thirty-five subjects had BMD measurement in the baseline survey and 7,926 subjects at the follow-up survey. We either used the baseline survey data or the follow-up survey data if there was no BMD data in the baseline survey. Therefore, 9,780 subjects had a BMD data; 6,135 from baseline survey data and 3,645 from follow-up survey data. Of those, 9,440 subjects had both lumbar spine and femoral neck BMD data. After excluding 65 subjects without the MTHFR C677T, 9,375 subjects (3,703 men and 5,672 women) were used for analyses. These two studies were approved by the institutional review board of Chonnam National University Hospital (Dong-gu Study, IRB No. I-2008-05-056; Namwon Study, IRB No. I-2007-07-062), and informed consent was obtained from each subject.
Weight was measured to the nearest 0.1 kg while the subjects were dressed in light clothing. Height was measured to the nearest 0.1 cm in stocking feet. Participants' lumbar spine and femoral neck BMD were measured using a Lunar Prodigy bone densitometer (GE, Madison, WI, USA). The Dong-gu and Namwon studies both used the same machine and the same procedure. The lumbar spine BMD represents the average BMD over L1-L4. Daily phantom scans were performed each morning for proper quality control. All BMD scans were conducted using standardized procedures following the manufacturer's recommended protocols by well-trained examiners. All BMD scans were reviewed by one experienced investigator to ensure that the region of interest was defined properly and that scans with problems that might affect BMD. All unacceptable scans were reanalyzed, or rejected. We excluded vertebral scans with insufficient scanning of L1 or L4, metal implant, severe degenerative change, or compression fracture. Intrascanner reproducibility of repeated measurements was confirmed, with a coefficient of variation of less than 1%.
Genomic DNA was extracted from peripheral blood with an AccuPrep Genomic DNA Extraction Kit (Bioneer, Seoul, Korea) or a QIAamp DNA Mini Kit (Qiagen, Inc., Chatsworth, CA, USA) according to the manufacturer's protocol. Genotyping by realtime polymerase chain reaction (PCR) was performed by allelic discrimination using dual-labeled probes containing locked nucleic acids (LNA) in a real-time PCR assay. PCR primers and LNA probes were designed and synthesized by Integrated DNA Technologies (IDT) (Coralville City, IA, USA). Our MTHFR genotyping method has been reported previously (8).
Data are presented as mean ± standard deviation (SD) or percentage for categorical variables. The MTHFR C677T was categorized into three groups. Analysis of variance was used to compare baseline characteristics across MTHFR C677T groups. All analyses were stratified by sex. Multiple linear regression analysis was performed to evaluate the association of MTHFR C677T with lumbar spine and femoral neck BMD, adjusting for age, weight and height. Hardy-Weinberg equilibrium was tested by use of a chi-square goodness of fit test. Statistical analyses were performed using SPSS version 20.0 (IBM SPSS, Chicago, IL, USA). Statistical significance was set at P < 0.05.
The MTHFR C677T genotype frequencies were consistent with Hardy.Weinberg equilibrium in both the Dong-gu Study and the Namwon Study (P = 0.64, P = 0.27, respectively). The MTHFR C677T genotype frequencies for CC, CT, and TT were 34.5, 48.7, and 16.8%, respectively, in the Dong-gu Study, and 33.6, 49.2, and 17.2%, respectively, in the Namwon Study.
The overall mean age of the Namwon cohort (63.0 ± 8.1 yr) was lower than that of the Dong-gu cohort (65.1 ± 8.2 yr). No significant difference in age, weight, and height was found among MTHFR C676T genotypes in either men or women in both cohorts, except for a significant difference in age in women of Namwon cohort. No association between MTHFR C677T and BMD at the lumbar spine and femoral neck was found in men or women in both cohorts after adjusting for age, weight and height (Table 1).
Table 1.
Association between bone mineral density and MTHFR C677T genotype
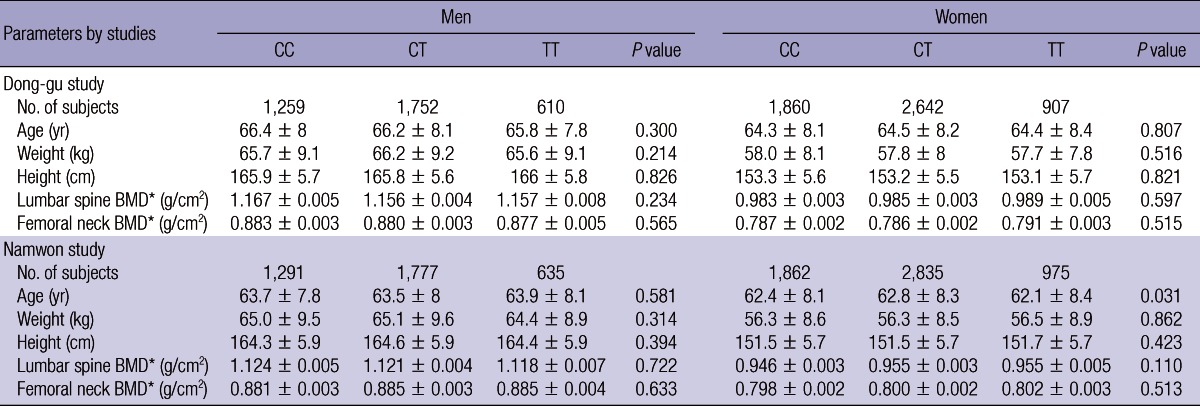
Data are mean ± standard deviation. *Data are adjusted mean ± standard error, adjusted for age, weight and height. BMD, bone mineral density.
In these two large population-based cohort studies, we found no association between the MTHFR C677T polymorphism and BMD in either men or women. To our knowledge, this is the largest population-based study to date examining the association of MTHFR C677T polymorphism and BMD.
Since Miyao et al. (9) first reported the association of MTHFR C677T polymorphism with BMD in postmenopausal Japanese women in 2000, many studies have investigated the association between MTHFR C677T polymorphism and BMD. Several studies have demonstrated a relationship between MTHFR C677T polymorphism and BMD (9-11). However, other studies, including two large cohort studies, the Rotterdam Study and the Hordaland Homocysteine Study, have reported no association between MTHFR C677T and BMD (12-15). In the present study, we also found no association between MTHFR C677T and BMD. Recently, one meta-analysis suggested that this polymorphism was modestly associated with BMD of the lumbar spine, femoral neck, total hip, and total body. However, many studies included in the meta-analysis had small sample sizes. Additionally, this meta-analysis did not include the Hordaland Homocysteine Study, one of the largest studies showing no relationship between MTHFR C677T polymorphism and BMD (13). We cannot fully explain these discrepancies in the results. One possible explanation may be the differences in nutrient intake across the populations studied. Genetic and environmental factors can interact to influence BMD. Two studies found that the association between the MTHFR C677T polymorphism and BMD depended on the plasma folate status (16) and dietary intake of riboflavin (17). Further studies are needed to examine the gene-nutrient interaction.
The mechanism linking MTHFR C677T polymorphism to BMD could be explained by differences in homocysteine levels according to the MTHFR C677T genotype. In vitro studies suggest that high homocysteine levels interfere with the formation of collagen cross-links (18) and affect both osteoclasts and osteoblasts (19). Many studies have reported that mild to moderate hyperhomocysteinemia is a risk factor for low BMD (1, 13) and osteoporotic fractures (2-4). If we can assume that the present study is well powered to detect small effects of homocysteine on BMD, the absence of an association between MTHFR C677T and BMD suggests that the association between homocysteine and BMD may be biased by confounding and/or reverse causation (20).
Strengths of this study are its very large sample size and adequate statistical power. Nevertheless, our study has several limitations. First, some studies suggest that MTHFR C677T might be a risk factor for fracture, but we did not have enough incident cases to evaluate this association due to relatively short follow-up period in these cohorts. Second, we did not evaluate the gene-environment interaction, which may modify the association between C677T polymorphism and BMD. Third, we did not evaluate the association between MTHFR C677T polymorphism and homocysteine and between homocysteine and BMD. Finally, we did not exclude subjects with secondary osteoporosis because we did not have information to distinguish between primary and secondary osteoporosis.
In conclusion, we found no association between MTHFR C677T and BMD in two large cohorts from Korea. Although further studies are needed to examine the gene.nutrient interaction, our data do not support the hypothesis that MTHFR C677T is associated with BMD.
Footnotes
This study was supported by Research Institute of Medical Sciences, Chonnam National University. This study was partially supported by the Health Promotion Fund, Ministry of Health&Welfare, Republic of Korea (07-27). This study was also partially supported by the Korea Centers for Disease Control and Prevention (2004-347-6111-213, 2005-347-2400-2440-215, 2006-347-2400-2440-215 and 20070308455-00).
The authors have no conflicts of interest to disclose.
References
- 1.Gerdhem P, Ivaska KK, Isaksson A, Pettersson K, Väänänen HK, Obrant KJ, Akesson K. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. J Bone Miner Res. 2007;22:127–134. doi: 10.1359/jbmr.061003. [DOI] [PubMed] [Google Scholar]
- 2.Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Meyer HE, Tell GS. Plasma homocysteine, folate, and vitamin B 12 and the risk of hip fracture: the hordaland homocysteine study. J Bone Miner Res. 2007;22:747–756. doi: 10.1359/jbmr.070210. [DOI] [PubMed] [Google Scholar]
- 3.McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med. 2004;350:2042–2049. doi: 10.1056/NEJMoa032739. [DOI] [PubMed] [Google Scholar]
- 4.Van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J, de Groot LC, Hofman A, Witteman JC, van Leeuwen JP, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 5.Whiting SJ, Draper HH. Effect of a chronic acid load as sulfate or sulfur amino acids on bone metabolism in adult rats. J Nutr. 1981;111:1721–1726. doi: 10.1093/jn/111.10.1721. [DOI] [PubMed] [Google Scholar]
- 6.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 7.Kweon SS, Shin MH, Jeong SK, Nam HS, Lee YH, Park KS, Ryu SY, Choi SW, Kim BH, Rhee JA, et al. Cohort profile: the Namwon Study and the Dong-gu Study. Int J Epidemiol. 2013 doi: 10.1093/ije/dys244. doi: 10.1093/ije/dys244. [DOI] [PubMed] [Google Scholar]
- 8.Cui LH, Shin MH, Kweon SS, Kim HN, Song HR, Piao JM, Choi JS, Shim HJ, Hwang JE, Kim HR, et al. Methylenetetrahydrofolate reductase C677T polymorphism in patients with gastric and colorectal cancer in a Korean population. BMC Cancer. 2010;10:236. doi: 10.1186/1471-2407-10-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyao M, Morita H, Hosoi T, Kurihara H, Inoue S, Hoshino S, Shiraki M, Yazaki Y, Ouchi Y. Association of methylenetetrahydrofolate reductase (MTHFR) polymorphism with bone mineral density in postmenopausal Japanese women. Calcif Tissue Int. 2000;66:190–194. doi: 10.1007/s002230010038. [DOI] [PubMed] [Google Scholar]
- 10.Abrahamsen B, Madsen JS, Tofteng CL, Stilgren L, Bladbjerg EM, Kristensen SR, Brixen K, Mosekilde L. A common methylenetetrahydrofolate reductase (C677T) polymorphism is associated with low bone mineral density and increased fracture incidence after menopause: longitudinal data from the Danish osteoporosis prevention study. J Bone Miner Res. 2003;18:723–729. doi: 10.1359/jbmr.2003.18.4.723. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsen B, Jørgensen HL, Nielsen TL, Andersen M, Haug E, Schwarz P, Hagen C, Brixen K. MTHFR c.677C>T polymorphism as an independent predictor of peak bone mass in Danish men: results from the Odense Androgen Study. Bone. 2006;38:215–219. doi: 10.1016/j.bone.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Lau EM, Woo J. Methylenetetrahydrofolate reductase polymorphism (MTHFR C677T) and bone mineral density in Chinese men and women. Bone. 2004;35:1369–1374. doi: 10.1016/j.bone.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Drevon CA, Gjessing HK, Tell GS. Plasma total homocysteine level and bone mineral density: the Hordaland Homocysteine Study. Arch Intern Med. 2006;166:88–94. doi: 10.1001/archinte.166.1.88. [DOI] [PubMed] [Google Scholar]
- 14.Hong X, Hsu YH, Terwedow H, Tang G, Liu X, Jiang S, Xu X, Xu X. Association of the methylenetetrahydrofolate reductase C677T polymorphism and fracture risk in Chinese postmenopausal women. Bone. 2007;40:737–742. doi: 10.1016/j.bone.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazdanpanah N, Uitterlinden AG, Zillikens MC, Jhamai M, Rivadeneira F, Hofman A, de Jonge R, Lindemans J, Pols HA, van Meurs JB. Low dietary riboflavin but not folate predicts increased fracture risk in postmenopausal women homozygous for the MTHFR 677 T allele. J Bone Miner Res. 2008;23:86–94. doi: 10.1359/jbmr.070812. [DOI] [PubMed] [Google Scholar]
- 16.McLean RR, Karasik D, Selhub J, Tucker KL, Ordovas JM, Russo GT, Cupples LA, Jacques PF, Kiel DP. Association of a common polymorphism in the methylenetetrahydrofolate reductase (MTHFR) gene with bone phenotypes depends on plasma folate status. J Bone Miner Res. 2004;19:410–418. doi: 10.1359/JBMR.0301261. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald HM, McGuigan FE, Fraser WD, New SA, Ralston SH, Reid DM. Methylenetetrahydrofolate reductase polymorphism interacts with riboflavin intake to influence bone mineral density. Bone. 2004;35:957–964. doi: 10.1016/j.bone.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Lubec B, Fang-Kircher S, Lubec T, Blom HJ, Boers GH. Evidence for McKusick's hypothesis of deficient collagen cross-linking in patients with homocystinuria. Biochim Biophys Acta. 1996;1315:159–162. doi: 10.1016/0925-4439(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 19.Koh JM, Lee YS, Kim YS, Kim DJ, Kim HH, Park JY, Lee KU, Kim GS. Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. J Bone Miner Res. 2006;21:1003–1011. doi: 10.1359/jbmr.060406. [DOI] [PubMed] [Google Scholar]
- 20.Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]


