Abstract
Fear conditioning and fear extinction are Pavlovian conditioning paradigms extensively used to study the mechanisms that underlie learning and memory formation. The neural circuits that mediate this learning are evolutionarily conserved, and seen in virtually all species from flies to humans. In mammals, the amygdala and medial prefrontal cortex are two structures that play a key role in the acquisition, consolidation and retrieval of fear memory, as well extinction of fear. These two regions have extensive bidirectional connections, and in recent years, the neural circuits that mediate fear learning and fear extinction are beginning to be elucidated. In this review, we provide an overview of our current understanding of the neural architecture within the amygdala and medial prefrontal cortex. We describe how sensory information is processed in these two structures and the neural circuits between them thought to mediate different aspects of fear learning. Finally, we discuss how changes in circuits within these structures may mediate fear responses following fear conditioning and extinction.
 |
Roger Marek (left), Cornelia Strobel, Tim Bredy and Pankaj Sah (right) all work at the Queensland Brain Institute in Brisbane, Australia. RM and CS have both just completed their PhD and TB and PS are group leaders in the institute. They share an interest in the mechanisms that underlie learning and memory formation in fear conditioning and extinction. Between the two labs they use a combination of electrophysiology, molecular and behavioural analysis. Their areas of interest are varied and this review comes from their common their interests in the roles of the prefrontal cortex and amygdala.
Fear is an evolutionarily conserved behavioural response that is essential for survival. Studies of fear have used the paradigm of classical fear conditioning in which an emotionally neutral stimulus, the conditioned stimulus (CS), such as a light or tone, is temporally paired with an aversive stimulus, the unconditioned stimulus (US), typically a footshock. Following a small number of pairings, subjects form an association between the CS and US, and learn to respond to the CS with an avoidance response, the conditioned response (CR), which is rapidly acquired and long lasting. However, subsequent presentations of the CS that are not paired with the US break this association, and lead to a gradual reduction of the CR through a process known as extinction. Since the first studies of Pavlov, it has been appreciated that extinction does not result from erasure of previous memory associated with the CS but is due, at least in part, to new learning (Pavlov, 1927). This idea rests on three key observations. First, the learnt fear response to the CS can reappear with the passage of time (spontaneous recovery). Secondly, the CR returns when the CS is presented in a context different from the one in which extinction training originally took place (renewal). Finally, unexpected delivery of the US following extinction can restore the response to the CS (reinstatement). Both renewal and reinstatement show that the CS retains its ability to drive the CR following extinction. Thus, although the original memory is still present, extinction training results in formation of a new memory trace that inhibits the response to the original CS. In effect, the subject has learnt that a previously aversive situation is no longer dangerous.
The neural circuitry engaged for evaluating and storing fear memories arose to enable animals to learn about, and respond rapidly to dangerous situations, and respond appropriately to changing contingencies. In both fear learning and extinction, the amygdala has emerged as a key structure that plays a central role in the acquisition and expression of learnt fear (Slotnick, 1973; Hitchcock & Davis, 1986; LeDoux et al. 1990). The amygdala is extensively interconnected with cortical and subcortical regions (McDonald, 1998; Sah et al. 2003) and, while structures such as the hippocampus can modulate aspects of fear learning(Maren, 2001), connections between the amygdala and medial prefrontal cortex (mPFC) are crucial for both fear conditioning and extinction (Sotres-Bayon & Quirk, 2010).
Although much of our understanding of fear conditioning comes from experimental work in animals, the roles of the amygdala and mPFC in fear learning and extinction have been validated in humans using functional magnetic resonance imaging. Thus, fear conditioning and subsequent presentations of fearful stimuli result in activation of the amygdala (Morris et al. 1996; LaBar et al. 1998), and prefrontal activity is seen during fear extinction (LaBar et al. 1998; Delgado et al. 2008). As a result, two lines of thought are driving the enormous interest in the neural circuits and molecular mechanisms that underlie fear conditioning and extinction. First, these behavioural paradigms are relatively simple to reproduce in the laboratory and they address fundamental issues regarding the mechanisms that underpin learning and memory formation (Johansen et al. 2011). Secondly, it is widely believed that the close correspondence between fear and human anxiety will provide insight into the biological nature of anxiety-related disorders and strategies to treat them. One such example is post-traumatic stress disorder. In this condition, stimuli that are clearly dangerous in one setting initially evoke an aversive response, but continue to be perceived as threatening even when circumstances change, thereby leading to inappropriate responses in conditions where a fear response is no longer appropriate (Milad & Quirk, 2012). The management of such disorders with procedures such as exposure therapy have their roots in fear extinction (McNally, 2007). Thus, an understanding of the biology that underpins fear learning and extinction will provide insight into the genesis of anxiety-related disorders, and the types of procedures that may help its treatment. In this review, we discuss the neuronal circuits within, and between the mPFC and amygdala and their roles in fear conditioning and extinction.
The amygdala
The amygdala is a temporal lobe structure divided into over 20 subnuclei with extensive internuclear connections (Sah et al. 2003; Pape & Pare, 2010). These subnuclei are commonly divided into three groups (Price et al. 1987; Sah et al. 2003): a deeper basolateral (BLA) group that includes the lateral nucleus, basal nucleus and accessory basal nucleus; a more superficial or cortical-like group that includes the cortical nuclei and nucleus of the lateral olfactory tract; and a centromedial group composed of the medial and central nuclei (CeA). Of these, the BLA is the primary sensory input zone of the amygdala, while the CeA is the primary output structure that initiates physiological responses of fear (Sah et al. 2003; Pape & Pare, 2010).
The BLA contains two types of neuron: glutamatergic principal neurons that form nearly 80% of the total cell population with the remaining being GABAergic interneurons (McDonald, 1982, 1992; McDonald & Mascagni, 2001; Spampanato et al. 2011). Principal neurons are large cells that resemble pyramidal neurons (Washburn & Moises, 1992; Faber et al. 2001). CS and US information arrives at the BLA as glutamatergic input to these cells from both cortical and subcortical regions (Farb & Ledoux, 1999; Sah et al. 2003; Lanuza et al. 2008). These afferents form classical dual component glutamatergic synapses (Mahanty & Sah, 1999; Weisskopf & LeDoux, 1999). Although the BLA does not have a laminar organization, principal neurons are not a single population, but can be separated into distinct populations by their firing properties (Washburn & Moises, 1992; Faber et al. 2001). As in other cortical-like areas (Ascoli et al. 2008), several families of interneurons are present within the BLA (McDonald & Mascagni, 2001; Spampanato et al. 2011). Interneurons also receive cortical and subcortical input (Smith et al. 1998, 2000) and make extensive local connections, providing both feed-forward and feedback inhibition within the BLA (Woodruff & Sah, 2007b; Ehrlich et al. 2009; Jasnow et al. 2009; Spampanato et al. 2011). Anatomical studies indicate that the nuclei within the BLA are extensively interconnected (Pitkänen et al. 1997) and incoming CS/US information is first processed in the BLA. Principal neurons in the BLA project to the CeA and downstream connections from this nucleus initiate the physiological responses of fear (Sah et al. 2003; Ehrlich et al. 2009).
While both cortical and thalamic inputs appear to innervate virtually all neurons in the BLA, a recent study has shown that distinct populations of neurons respond to CS stimulation following fear conditioning or extinction (Herry et al. 2008). This finding indicates that distinct populations of neuron can be engaged by fear learning and extinction and different neurons are driven by distinct inputs (see below). However, while there are different populations of neurons within the BLA, the anatomical and functional organization of inputs to these neurons, and their connectivity is largely not understood. Thus, whether principal neurons that can be separated based on their intrinsic firing properties, receive inputs from the same regions and make similar or different projections is not known. Similarly, interneurons within the BLA can be divided into distinct population based on their expression of particular markers and firing properties (Spampanato et al. 2011). Recent studies are beginning to reveal the intrinsic organization and roles of some of these interneuron families, showing that different types of interneuron make different types of local connections (Rainnie et al. 1991; Woodruff et al. 2006; Woodruff & Sah, 2007b,a; Jasnow et al. 2009). However, as with principal neurons, the organization of inputs to these interneurons, and the particular circuits in which they participate are little understood but is clearly required to develop models that explain CS/US processing in the amygdala.
In contrast to the BLA, the CeA is a striatal-like structure that contains GABAergic neurons (de Olmos et al. 1985), and is divided into lateral and medial divisions (Cassell et al. 1986). Projections from the BLA innervate the lateral CeA, which in turn is reciprocally connected with the medial CeA and outputs from the medial CeA to hypothalamic and brainstem nuclei then initiate fear responses (Pare & Duvarci, 2012). Neurons in both divisions of the CeA can also be separated into distinct populations based on their expression of peptides (Cassell et al. 1986) and intrinsic firing properties (Martina et al. 1999; Dumont et al. 2002; Lopez de Armentia & Sah, 2004). These neurons receive excitatory inputs from the BLA as well as from thalamic and cortical regions (Sah et al. 2003). Stimulation of BLA inputs to the CeA not only provides excitation to these cells (Lopez de Armentia & Sah, 2004) but is always accompanied by a disynaptic inhibitory response (Royer et al. 1999; Lopez de Armentia & Sah, 2004; Amano et al. 2010). This inhibition is provided by two distinct sources: first, a cluster of GABAergic neurons interposed between the BLA and CeA, the intercalated cells (ITC) (Millhouse, 1986) that receive inputs from the BLA and project to the CeA (Royer et al. 1999; Delaney & Sah, 2001). Secondly, neurons within the CeA, which are all GABAergic, are extensively interconnected providing strong local inhibition (Lopez de Armentia & Sah, 2004; Haubensak et al. 2010).
Recent studies are beginning to reveal the intrinsic organization of the CeA. Thus, neurons in the medial CeA that project to different downstream targets, and mediate different physiological responses, are distinct populations of neurons that can be separated on electrophysiological as well as pharmacological grounds (Viviani et al. 2011). Moreover, recordings from the lateral CeA in vivo show that following fear conditioning some neurons increase their response to the CS, while others are inhibited (see below) suggesting that different cells within the CeA also receive different types of input. Finally, apart from the BLA, the CeA also receives inputs from a variety of cortical and subcortical regions (Sah et al. 2003). However, the anatomical organization of these inputs to the CeA is also largely unknown.
The amygdala in fear conditioning and extinction
A converging body of evidence has established that CS and US inputs converge on neurons within the amygdala (Sah et al. 2003; Pape & Pare, 2010). Blocking glutamatergic transmission within the BLA by infusion of non-NMDA receptor antagonists blocks fear conditioning, and post-learning infusions block expression of learnt fear (Falls et al. 1992; Kim et al. 1993). These pharmacological manipulations also block extinction (Kim et al. 1993), confirming that the BLA is an essential component of the neural circuit that mediates fear conditioning and extinction. In contrast, infusion of selective NMDA receptor antagonists into the amygdala blocks acquisition of fear conditioning and extinction but has no effect on previously learnt responses (Miserendino et al. 1990; Goosens & Maren, 2004). These results have led to the proposal that learning during both fear conditioning and extinction requires NMDA receptor-dependent plasticity within the BLA (Mayford et al. 2012). Consistent with this idea, inputs to BLA principal neurons can undergo different types of NMDA receptor-dependent plasticity (McKernan & Shinnick-Gallagher, 1997; Rogan et al. 1997; Weisskopf et al. 1999; Bissiere et al. 2003; Humeau et al. 2003; Rodrigues et al. 2004; Rumpel et al. 2005); however, as with many other forms of learning, how this plasticity is initiated during fear condition and extinction remains largely unknown (Sah et al. 2008).
Unit recordings in vivo have shown that during fear conditioning, a population of glutamatergic neurons in the BLA increase their firing rate in response to the CS (Quirk et al. 1995; Goosens et al. 2003) and have been called ‘fear neurons’ (Herry et al. 2008). Following extinction, these neurons reduce their response to the CS, and in addition, a different population of neurons, ‘extinction neurons’ now begin to respond to the CS (Herry et al. 2008; Amano et al. 2011). While it has not been directly demonstrated, it is thought that ‘fear neurons’ arise due to NMDA receptor-dependent plasticity of CS inputs to BLA neurons, engaged by CS-US conjunction during fear conditioning (Miserendino et al. 1990; Rogan et al. 1997). Similarly, as extinction is blocked by infusion of NMDA receptor antagonists into the BLA, ‘extinction neurons’ presumably arise because of plasticity at inputs to a different set of neurons during extinction training. In this scenario, the reduction in activity of ‘fear neurons’ with extinction may result from synaptic plasticity of CS input to interneurons, thus enhancing local inhibition (Mahanty & Sah, 1998; Ehrlich et al. 2009; Polepalli et al. 2010).
CS information is initially processed in the BLA and the physiological response to fear is driven by outputs from the medial CeA (Pape & Pare, 2010; Pare & Duvarci, 2012). Projections from the BLA enter the CeA at the level of the lateral division and unit studies have shown that following fear conditioning, the CS activates ‘ON neurons’ in the lateral CeA, and these cells inhibit a different population of tonically active ‘OFF neurons’ (Ciocchi et al. 2010; Haubensak et al. 2010). As OFF cells are GABAergic, and project to the medial CeA, the overall impact is disinhibition of the neurons in the medial CeA by the CS (Ciocchi et al. 2010; Haubensak et al. 2010). The resulting activity of medial CeA neurons evokes a fear response (Ciocchi et al. 2010). In extinction, there is a reduction in activity in ‘fear neurons’ and a new population of ‘extinction neurons’ become active (Herry et al. 2008). As described above, connections between the BLA and CeA also activate GABAergic ITC neurons that provide feed-forward inhibition to the CeA. These inputs also show NMDA receptor-dependent plasticity (Royer & Paré, 2002) and it has been proposed that following extinction, plasticity of these inputs results in an increase in disynaptic inhibition to the CeA, effectively reducing the activity of ON neurons, thus inhibiting the fear response (Amano et al. 2010). However, whether ‘extinction neurons’ in the BLA participate in this response is not known.
The medial prefrontal cortex
The mPFC is a neocortical structure and is cytoarchitectonically divided into four distinct regions from dorsal to ventral: medial precentral cortex, anterior cingulate cortex, prelimbic and infralimbic prefrontal cortex (Heidbreder & Groenewegen, 2003). Most neocortical regions, particularly sensory areas, are divided into six distinct layers (I–VI), with layer VI being the deepest layer and layer I near the pial surface. In the sensory cortex, cells in layers II/III and layers V/VI contain pyramidal neurons that have apical dendrites that project to layer I, whereas layer IV is largely devoid of pyramidal neurons and is the primary target for sensory input from the thalamus (Miller et al. 2001). The rodent prefrontal cortex appears to have well defined layers I, II/III and V/VI; however, the existence of discrete layer IV is not clear (Van De Werd et al. 2010).
The neuronal organization within the mPFC mirrors those seen in other parts of the neocortex. Pyramidal cells are located in layers II/III and layers V/VI (Yang et al. 1996), and in acute brain slices, these neurons show a range of firing properties (Wang et al. 2006) similar to those described for other neocortical regions (Connors & Gutnick, 1990). The mPFC also contains a variety of types of interneuron (Van De Werd et al. 2010), with the expected distribution of interneuronal markers (Markram et al. 2004). Intrinsic circuits within sensory cortical regions have been extensively studied both in vitro and in vivo (Thomson et al. 2002; Holmgren et al. 2003; Brown & Hestrin, 2009). In contrast, little is known about the connections within and between the mPFC areas. For intrinsic connections, one study in ferrets has shown connections between layer V pyramidal neurons in the mPFC (Wang et al. 2006); however, in this study the different divisions of the mPFC were not separated. Moreover, the presence of, or types of connections between other cell types within the mPFC are currently not known. Thus, the intrinsic circuits within the mPFC are largely unexplored. Both retrograde (Hoover & Vertes, 2007) and anterograde (Jones et al. 2005) tracer injections have shown connections between the infralimbic prefrontal cortex (ILPFC) and prelimbic prefrontal cortex (PLPFC). Moreover, extracellular recordings in vitro show that local field potentials in the ILPFC have higher frequency components than those in the PLPFC (van Aerde et al. 2008), and this difference is abolished by disconnecting. These findings suggest that connections between these two regions may have physiological consequences; however, the organization and nature of these connections is not currently understood.
The medial prefrontal cortex in fear conditioning and extinction
As described above, learning during fear conditioning as well as extinction requires activity in the amygdala (Falls et al. 1992). However, it was recognized early that ablation of the mPFC results in a deficit in extinction memory (Morgan et al. 1993) suggesting that the mPFC is required for consolidation of extinction. Subsequent stimulation and inactivation studies of the mPFC have established that this region is involved in both fear conditioning and its extinction (Burgos-Robles et al. 2007; Corcoran & Quirk, 2007; Laurent & Westbrook, 2008; Sotres-Bayon & Quirk, 2010), with the ILPFC and PLPFC having distinct roles (Burgos-Robles et al. 2007). While the amygdala plays a central role in acquisition and expression of learnt fear, the PLPFC has been implicated in modulating both consolidation and recall of fear memory (Maren & Quirk, 2004; Quirk & Mueller, 2008). Thus, for example, inactivation of the PLPFC with tetrodotoxin after fear acquisition results in reduced fear responses (Corcoran & Quirk, 2007). Similarly, the ILPFC does not appear to have a significant role in extinction learning, but is required for consolidation of extinction memory, and extinction is significantly blunted when ILPFC activity is silenced (Laurent & Westbrook, 2009) but enhanced if the ILPFC is stimulated during extinction training (Vidal-Gonzalez et al. 2006).
In support of inactivation studies, electrophysiological recordings from neurons in the ILPFC have found that following extinction training, neurons in the ILPFC show enhanced responses to the CS (Milad & Quirk, 2002). Moreover, infusion of NMDAR antagonists into the ILPFC, either before or immediately after extinction training impaired extinction learning (Burgos-Robles et al. 2007). Together, these results suggest that consolidation of extinction memory involves NMDA receptor-dependent plasticity within the ILPFC.
The consolidation of memory requires gene transcription and protein synthesis (Lubin et al. 2011). In support of the role of mPFC in the consolidation of memory for fear extinction, much evidence exists for the necessity of protein synthesis and gene transcription within the mPFC during the establishment of long-lasting fear extinction memories (Santini et al. 2004; Mamiya et al. 2009). Furthermore, the formation of fear extinction memory as well as long-term potentiation within the ILPFC is modulated by epigenetic mechanisms. Inhibition of the histone acetyltransferase p300 in the ILPFC leads to enhanced fear extinction memory and long-term potentiation (Marek et al. 2011). This effect is most likely mediated by augmenting the activity of the repressor protein Yin Yang 1 (YY1), therefore enhancing overall transcriptional processes to increase protein synthesis (Marek et al. 2011). In contrast, reduced activity of the histone acetyltransferase p300/CBP-associated factor (PCAF) in the ILPFC leads to impaired fear-related extinction memory and long-term potentiation (Wei et al. 2012). This effect is mediated by the role of PCAF as a transcriptional co-activator of the repressive transcription factor ATF4. PCAF-mediated recruitment of ATF4 to the promoter of the immediate-early gene zif268 within the ILPFC, transiently represses its activity at the time of retrieval to allow the formation of a new extinction memory (Wei et al. 2012). Together, these findings show that fear extinction requires long-term synaptic changes with the ILPFC that are mediated initially by a variety of mechanisms, including NMDA receptor-dependent plasticity and epigenetic processes within neurons of the ILPFC. Physiologically, the end result is enhanced activation of mPFC neurons by repeated, non-reinforced, exposures to the CS.
The amygdala and medial prefrontal cortex: partners in the fear circuit
As described above, the amygdala is a key structure in the acquisition and expression of fear conditioning and extinction, while the mPFC does not play a role during acquisition, but is required for expression of learnt fear and consolidation of extinction memory. Tracer injections have shown extensive projections from the mPFC to the amygdala. These studies show that injections into the ILPFC lead to extensive labelling in the lateral amygdala as well as intermediate capsule, a region between the BLA and CeA (McDonald et al. 1996; McDonald, 1998). In contrast, injection of tracer into the PLPFC shows terminals largely limited to the basal nucleus (McDonald et al. 1996; McDonald, 1998). In return, the mPFC receives afferents from the amygdala as well as a number of cortical and subcortical regions (Conde et al. 1995). Both the ILPFC and PLPFC receive projections from the BLA (Hoover & Vertes, 2007) although the exact target of these connections is not known.
Together, these findings indicate that interactions between the amygdala and mPFC during fear learning and extinction are likely mediated by reciprocal synaptic connections between them (Quirk & Mueller, 2008; Sotres-Bayon & Quirk, 2010) (Fig. 1). Unit recordings in vivo show that within the BLA, both ‘fear neurons’ and ‘extinction neurons’ have connections with the mPFC, with ‘fear neurons’ only sending projections to the mPFC while ‘extinction neurons’ appear to be reciprocally connected to the mPFC (Herry et al. 2008). The neuronal targets of these connections within the mPFC are not yet known; however, these data provide strong support for an active role of the mPFC in both fear conditioning and extinction. As described above, neurons in the BLA are not a homogeneous population but contain different types of both principal neurons and interneurons. The projection patterns of mPFC inputs to the BLA, the cells they innervate, and the functional consequences of the different projections are still to be characterized, but future studies will no doubt delineate how these circuits interact during fear learning and extinction.
Figure 1. Schematic drawing of the inter- and intraconnectivity of the three major regions involved in fear conditioning and extinction.
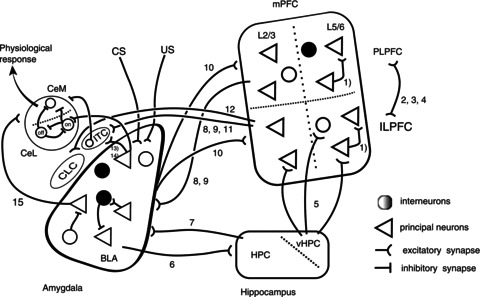
Fear behaviour is a complex phenomenon which primarily involves connections between the amygdala, hippocampus and prefrontal cortex. The known connections between the amygdala, hippocampus and medial prefrontal cortex are shown schematically. Triangles representing principal neurons and interneurons are shown as black or white circles, indicating the wide variety of these neurons. The simplified medial prefrontal cortex (mPFC) shows L2/3 and L5/6 of the prelimbic prefrontal cortex (PLPFC) and the infralimbic prefrontal cortex (ILPFC). The hippocampus (HPC) is lumped together as the HPC, except for the ventral hippocampal region (vHPC). The amygdala is divided into the input regions, the basolateral amygdala (BLA) where inputs from conditioned (CS) and unconditioned stimulus (US) converge. The output zone, the central amygdala is divided into three regions: the central capsular (CLC); central lateral (CeL); and central medial. Two types of recently identified neurons in the CeL are shown, which respond differently to CS stimulation. The numbers refer to the following citations. (1) Wang et al. 2006; (2) Hoover & Vertes, 2007; (3) Vertes, 2004; (4) Van Aerde et al. 2008; (5) Parent et al. 2009; (6) Pikkarainen et al. 1999; (7) Kishi et al. 2006; (8) Mcdonald et al. 1996; (9) Mcdonald, 1998; (10) Condéet al. 1995; (11) Pinto & Sesack, 2008; (12) Pinard et al. 2012; (13) Royer et al. 1999; (14) Delaney & Sah, 2001; (15) Smith & Paré, 1994.
Fear conditioning results in activation of a population of neurons in the BLA thought to drive neurons in the lateral CeA, resulting in disinhibition of neurons in the medial CeA. In this scenario, following extinction, BLA fear neurons show a reduction in response to the CS (Herry et al. 2008), indicating that physiological changes within the BLA are required during extinction (Fig. 2). However, a second mechanism of extinction has also been proposed. Between the BLA and CeA, the ITC neurons form a cluster of GABAergic neurons that mediate feed-forward inhibition that inhibits output of the CeA (Royer et al. 1999). Several lines of evidence indicate that following extinction training, ITC neurons are driven by mPFC projections, inhibiting the output of the CeA and providing a second mechanism of extinction. First, anterograde tracer injections in both primates (Freedman et al. 2000) and rodents (McDonald et al. 1996; Pinto & Sesack, 2008) have shown terminal projections near the ITC clusters. Moreover in vivo recordings have shown that ITC neurons can be driven by ILPFC stimulation (Amano et al. 2012). Secondly, ITC neurons show enhanced cFOS expression following extinction, suggesting their activity during extinction. Thirdly, selective lesions of ITC neurons result in deficits in extinction (Likhtik et al. 2008). Finally, stimulation of the mPFC results in a reduction in BLA evoked output from the CeA (Quirk et al. 2003). Together, these results have led to a model in which activation of ITC neurons by a combination of input from the BLA and ILPC inhibits output CeA neurons following extinction, training, thereby reducing the response to the CS (Likhtik et al. 2008; Ehrlich et al. 2009; Amano et al. 2010; Pare & Duvarci, 2012). However, a recent publication has suggested that afferents from the ILPFC do not, in fact, innervate ITC neurons (Pinard et al. 2012). The reason for differences between this recent report and previous studies are currently not clear.
Figure 2. Amygdala–medial prefrontal cortex networks engaged during resting conditions, during fear learning, following consolidation and extinction.

Different activity states of fear-related structures during fear learning and extinction evokes a distinct pattern of synaptic activity. In control conditions, fear expression is absent and activity in the medial central nucleus (CeM) neurons is low. During the acquisition of fear conditioning, conditioned (CS) and unconditioned stimulus (US) information converges on neurons in the basolateral group (BLA), leading to potentiation of CS inputs to neurons in the BLA. In turn, CeL ‘on’ cells are activated with ultimate disinhibition of CeM output neurons and an enhanced fear response. In the consolidation phase of fear conditioning, hippocampal (HPC) and prelimbic prefrontal cortex (PLPFC) activity increases and is thought to add context dependency (HPC) as well as to increase the basolateral amygdala activity for stronger fear expression. Extinction leads to suppression of the fear response through an additional pathway that suppresses the fear circuit. PLPFC activity not only decreases, but infralimbic prefrontal cortex (ILPFC) activity increases, which is thought to directly activate intercalated neurons (ITCs). This in turn leads to feed-forward inhibition of CeM output neurons and the suppression of the fear response. Interneurons are represented as black and white circles, indicating the large variety of interneurons, whereas pyramidal cells are shown as triangles. The strength of connections is represented by the width of the connecting lines with wider lines representing stronger connections. The activity of specific neuronal types is indicated as action potential spikes next to the individual regions, with two spikes representing low activity and multiple spikes representing increased activity.
Conclusions
Fear conditioning and extinction are two well-preserved learning paradigms present in all mammalian species and involve the storage, consolidation and retrieval of a memory trace. It is widely believed that unravelling the mechanisms that underlie these behaviours will provide a more detailed understanding of learning and memory formation in the mammalian brain. The functional similarity between fear and anxiety disorders, and the fact that extinction recapitulates treatment strategies for these disorders, suggests that understanding the mechanisms that underpin these behaviours will lead to the development of treatments for human anxiety-related disorders. The neural circuits that mediate these two behaviours and the synaptic, biochemical and genetic changes that accompany them are beginning to be understood; however, there are clearly many gaps in our understanding.
While the amygdala and its intrinsic components have been placed at the centre of circuits mediating fear behaviour, it has become evident that the bed nucleus of the stria terminals (BNST), another subcortical structure, may also play a major role. The BNST and the central amygdala have very similar cell types (McDonald, 1983) and share many afferent and efferent connections (Alheid et al. 1995). Thus, the BNST receives inputs from the basolateral amygdala (Walker et al. 2009) and prefrontal cortex (Radley et al. 2009), and projects to many of the same brainstem structures as the central amygdala. Moreover, a converging body of evidence suggests that while outputs from the central amygdala are involved in the phasic components of the fear response, the BNST mediates the sustained components (Walker et al. 2009). As clinical anxiety appears to be more akin to a sustained fear response, these findings raise the possibility that while the immediate response to threats are mediated via the CeA, sustained responses, as seen in anxiety and stress, may be mediated via the BNST. However, as compared to the CeA, the neural circuits within the BNST and the properties and roles of its afferent and efferent connections are little understood. The rapid development of imaging and optogenetic techniques to probe these circuits will no doubt guide a rapid understanding of these very interesting behaviours.
Acknowledgments
This work for funded by grants from the National Health and Medical Research Council of Australia (T.W.B., P.S.) and the Australian Research Council (T.W.B., P.S.).
References
- Alheid GF, de Olmos J, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. California: Academic Press; 1995. pp. 495–578. [Google Scholar]
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Duvarci S, Popa D, Pare D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Amir A, Goswami S, Pare D. Morphology, PKCdelta expression, and synaptic responsiveness of different types of rat central lateral amygdala neurons. J Neurophysiol. 2012 doi: 10.1152/jn.00514.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Brown SP, Hestrin S. Cell-type identity: a key to unlocking the function of neocortical circuits. Curr Opin Neurobiol. 2009;19:415–421. doi: 10.1016/j.conb.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–103. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos J, Hardy H, Heimer L. Amygdala. In: Paxinos G, editor. The Rat Nervous System. Academic Press; 1985. pp. 317–223. , Australia, Sydney. [Google Scholar]
- Delaney AJ, Sah P. Pathway-specific targeting of GABA(A) receptor subtypes to somatic and dendritic synapses in the central amygdala. J Neurophysiol. 2001;86:717–723. doi: 10.1152/jn.2001.86.2.717. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Martina M, Samson RD, Drolet G, Paré D. Physiological properties of central amygdala neurons: species differences. Eur J Neurosci. 2002;15:544–552. doi: 10.1046/j.0953-816x.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Faber ESL, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J Neurophysiol. 2001;85:714–723. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb CR, Ledoux JE. Afferents from rat temporal cortex synapse on lateral amygdala neurons that express NMDA and AMPA receptors. Synapse. 1999;33:218–229. doi: 10.1002/(SICI)1098-2396(19990901)33:3<218::AID-SYN6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditional fear and associative spike firing in the lateral amygdala. Eur J Neurosci. 2004;20:537–548. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Hobin JA, Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias. Neuron. 2003;40:1013–1022. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Ressler KJ, Hammack SE, Chhatwal JP, Rainnie DG. Distinct subtypes of cholecystokinin (CCK)-containing interneurons of the basolateral amygdala identified using a CCK promoter-specific lentivirus. J Neurophysiol. 2009;101:1494–1506. doi: 10.1152/jn.91149.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience. 2005;133:193–207. doi: 10.1016/j.neuroscience.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Kim M, Campeau S, Falls WA, Davis M. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behav Neural Biol. 1993;59:5–8. doi: 10.1016/0163-1047(93)91075-x. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Moncho-Bogani J, Ledoux JE. Unconditioned stimulus pathways to the amygdala: effects of lesions of the posterior intralaminar thalamus on foot-shock-induced c-Fos expression in the subdivisions of the lateral amygdala. Neuroscience. 2008;155:959–968. doi: 10.1016/j.neuroscience.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol. 2004;92:1285–1294. doi: 10.1152/jn.00211.2004. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Gupta S, Parrish RR, Grissom NM, Davis RL. Epigenetic mechanisms: critical contributors to long-term memory formation. Neuroscientist. 2011;17:616–632. doi: 10.1177/1073858411386967. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Excitatory synaptic inputs to pyramidal neurons of the lateral amygdala. Eur J Neurosci. 1999;11:1217–1222. doi: 10.1046/j.1460-9568.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Coelho CM, Sullivan RK, Baker-Andresen D, Li X, Ratnu V, Dudley KJ, Meyers D, Mukherjee C, Cole PA, Sah P, Bredy TW. Paradoxical enhancement of fear extinction memory and synaptic plasticity by inhibition of the histone acetyltransferase p300. J Neurosci. 2011;31:7486–7491. doi: 10.1523/JNEUROSCI.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Pare D. Physiological properties of central medial and central lateral amygdala neurons. J Neurophysiol. 1999;82:1843–1854. doi: 10.1152/jn.1999.82.4.1843. [DOI] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005751. DOI: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: A Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the bed nucleus of the stria terminalis: a Golgi study in the rat. Brain Res Bull. 1983;10:111–120. doi: 10.1016/0361-9230(83)90082-5. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York: Wiley Liss; 1992. pp. 67–96. [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Brain Res. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Pinto DJ, Simons DJ. Processing in layer 4 of the neocortical circuit: new insights from visual and somatosensory cortex. Curr Opin Neurobiol. 2001;11:488–497. doi: 10.1016/s0959-4388(00)00239-7. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MA, Wang L, Su J, Netoff T, Yuan L-L. Identification of the Hippocampal Input to Medial Prefrontal Cortex In Vitro. Cereb. Cortex. 2009;20:393–403. doi: 10.1093/cercor/bhp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. New York: Dover; 1927. [Google Scholar]
- Pinard CR, Mascagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience. 2012;205:112–124. doi: 10.1016/j.neuroscience.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Sesack SR. Ultrastructural analysis of prefrontal cortical inputs to the rat amygdala: spatial relationships to presumed dopamine axons and D1 and D2 receptors. Brain Struct Funct. 2008;213:159–175. doi: 10.1007/s00429-008-0180-6. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Polepalli JS, Sullivan RK, Yanagawa Y, Sah P. A specific class of interneuron mediates inhibitory plasticity in the lateral amygdala. J Neurosci. 2010;30:14619–14629. doi: 10.1523/JNEUROSCI.3252-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The Limbic Region. II: The Amygdaloid Complex. Elsevier Science; 1987. [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdaloid neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Schinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol. 1991;66:999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Royer S, Paré D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF, Luthi A. Fear conditioning and long-term potentiation in the amygdala: what really is the connection. Ann N Y Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM. Fear behaviour and passive avoidance deficits in mice with amygdala lesions. Physiol Behav. 1973;11:717–720. doi: 10.1016/0031-9384(73)90258-8. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labelling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol. 1994;342:232–248. doi: 10.1002/cne.903420207. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Cat intraamygdaloid inhibitory network: ultrastructural organization of parvalbumin-immunoreactive elements. J Comp Neurol. 1998;391:164–179. doi: 10.1002/(sici)1096-9861(19980209)391:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology. 2011;60:765–773. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- van Aerde KI, Heistek TS, Mansvelder HD. Prelimbic and infralimbic prefrontal cortex interact during fast network oscillations. PLoS ONE. 2008;3:e2725. doi: 10.1371/journal.pone.0002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Werd HJ, Rajkowska G, Evers P, Uylings HB. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct. 2010;214:339–353. doi: 10.1007/s00429-010-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Coelho CM, Li X, Marek R, Yan S, Anderson S, Meyers D, Mukherjee C, Sbardella G, Castellano S, Milite C, Rotili D, Mai A, Cole PA, Sah P, Kobor MS, Bredy TW. p300/CBP-associated factor selectively regulates the extinction of conditioned fear. J Neurosci. 2012;32:11930–11941. doi: 10.1523/JNEUROSCI.0178-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, LeDoux JE. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. J Neurophysiol. 1999;81:930–934. doi: 10.1152/jn.1999.81.2.930. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol. 2007a;98:2956–2961. doi: 10.1152/jn.00739.2007. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007b;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Monyer H, Sah P. GABAergic excitation in the basolateral amygdala. J Neurosci. 2006;26:11881–11887. doi: 10.1523/JNEUROSCI.3389-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layers V-VI principal pyramidal cells in rat prefrontal cortex in vitro. J Neurosci. 1996;16:1904–1921. doi: 10.1523/JNEUROSCI.16-05-01904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


