Abstract
Extensive in vitro data and modeling studies suggest that intrinsic properties of medial entorhinal cortex (MEC) neurons contribute to the spiking behaviour of functional cell types of MEC neurons, such as grid cells, recorded in behaving animals. It remains unclear, however, how intrinsic properties of MEC neurons influence cellular dynamics in intact networks in vivo. In order to begin to bridge the gap between electrophysiological data sets from brain slices and behaving animals, in the present study we performed intracellular recordings using sharp electrodes in urethane-anaesthetized rats to elucidate the cellular dynamics of MEC neurons in vivo. We focused on the h-current-dependent sag potential during hyperpolarizing current steps, subthreshold resonance in response to oscillatory frequency sweeps (chirp stimuli), persistent spiking in response to brief depolarizing inputs and the relationship between firing frequency and input (f–I curve), each of which is sensitive to cholinergic modulation in vitro. Consistent with data from in vitro studies, cholinergic activation by systemic application of the acetylcholinesterase inhibitor, physostigmine, resulted in decreased sag amplitude, increased sag time constant and a decrease of the peak resonance frequency. The f–I curve was also modulated by physostigmine in many neurons, but persistent spiking was not observed in any of our recordings, even when picrotoxin, a GABAA blocker, was included in the internal solution of the recording pipette to reduce possible effects of network inhibition. These results suggest that intrinsic oscillatory and rate-coding mechanisms, but not intrinsic bistability, are significantly modulated by acetylcholine in the intact entorhinal network.
Key points
Medial entorhinal cortex neurons show special intrinsic properties in vitro, which might be important for contributing to functional cell properties, such as grid cell firing.
Both intrinsic properties in slices of medial entorhinal cortex and grid cell activity in vivo are affected by cholinergic activation, but the relationships between these effects are unknown.
Using intracellular recording, we show that intrinsic properties including sag amplitude, sag time constant and resonance frequency are affected by cholinergic activation in vivo, and these results are consistent with in vitro studies.
Furthermore, we show that the relationship between firing frequency and input current is also changed by cholinergic activation in our in vivo recordings.
These results suggest the importance of cholinergic influences on the intrinsic properties of medial entorhinal neurons, and help us understand how this influence contributes to mechanisms of spatial memory and the cause of memory impairment.
Introduction
The medial entorhinal cortex (MEC) acts as the gateway for information flowing into and out of the hippocampus (Witter et al. 1989; van Strien et al. 2009). The MEC plays an important role in episodic memory, based on impairments of memory caused by lesions of the entorhinal cortex (EC) in rats (Steffenach et al. 2005) and primates (Leonard et al. 1995). Numerous extracellular recordings from EC in awake, behaving animals (Hafting et al. 2005; Newman et al. 2012) have identified distinct functional cell types, such as grid cells and head direction cells, that respond to environmental or behavioural parameters (Hafting et al. 2005; Moser & Moser, 2008; Brandon et al. 2011; Koenig et al. 2011), and in vitro recordings using brain slice preparations of EC have characterized the cellular properties of different morphologically defined cell types, including stellate cells in layers II/III and pyramidal neurons (Klink & Alonso, 1997b; Egorov et al. 2002; Erchova et al. 2004; Giocomo et al. 2007; Nolan et al. 2007; Garden et al. 2008; Heys et al. 2010; Heys & Hasselmo, 2012). However, only a few previous studies have addressed the link between the dynamics of MEC neurons in vitro and in vivo (Quilichini et al. 2010; Hahn et al. 2012; Domnisoru et al. 2013; Schmidt-Hieber & Häusser, 2013). In the present study, we focused on layer II/III cells in MEC and performed intracellular recordings in urethane-anaesthetized rats to characterize cellular properties in vivo that have been proposed, based on in vitro studies, to contribute to neural function in behaving animals.
Acetylcholine is critical for performance in memory tasks (for review see Hasselmo, 2006; Heys et al. 2012; Newman et al. 2012) and modulates many of the neurophysiological properties in EC thought to underlie spatial processing (Klink & Alonso, 1997b; Egorov et al. 2002; Heys et al. 2010). Neurons in MEC exhibit subthreshold resonance (Erchova et al. 2004; Giocomo et al. 2007; Hu et al. 2009; Heys et al. 2010) in the theta frequency range (4–12 Hz), reflecting bandpass filtering (Hutcheon & Yarom, 2000) imparted by cellular properties including the hyperpolarization-activated cation current, Ih (Dickson et al. 2000; Heys et al. 2010; Heys & Hasselmo, 2012). We delivered hyperpolarizing current steps and sweeps of increasing frequency stimulation (chirps) before and after systemic administration of the acetylcholinesterase inhibitor, physostigmine, to test in vivo the robustness of h-current activation and whether resonance frequency decreases during cholinergic activation as it does in vitro (Heys et al. 2010).
Persistent spiking is defined here as a phenomenon of sustained spiking that can be elicited from quiescence by a brief depolarizing input (Klink & Alonso, 1997b; Egorov et al. 2002; Fransén et al. 2006; Heys et al. 2012). This phenomenon is also dependent on cholinergic modulation in vitro and has been proposed as a cellular substrate for working memory encoding (Schon et al. 2004, 2005; Hasselmo & Stern, 2006). We delivered strong, transient current steps to drive high spike rates briefly in our recorded neurons and assessed spiking after the input for evidence of self-sustained spiking or persistently elevated spike rates. Finally, we also used slow, linearly increasing ramp stimuli to test the relationship of spike frequency response to current input and to determine in vivo how cholinergic modulation might affect the gain of input–output relationships for MEC neurons.
Methods
Animal surgery
Experiments were performed on 30 adult male Long–Evans rats (250–400 g; Charles River Laboratories, Wilmington, MA, USA), from which 14 successful neuronal recordings were used for analysis. An additional eight rats were used for six successful neuronal recordings for saline control experiments. Animals were anaesthetized with an intraperitoneal injection of urethane (1.2–1.5 g (kg bwt)−1), and additional doses of urethane were given as needed (0.3–0.4 g (kg bwt)−1). Body temperature was maintained with a disposable heating pad. The depth of anaesthesia and the rhythm of breathing were monitored during the entire experiment. Before surgery, bupivacaine was injected subcutaneously at the site of the incision, and an injection cannula was placed intraperitoneally to allow drug administration during the experiment. Each rat was placed in a stereotaxic apparatus (SR-8N; Narishige, Setagaya-ku, Tokyo, Japan) and prepared for acute electrophysiological recording from the MEC. Two holes were drilled for the hippocampus (posterior 4.2 mm, lateral 3.0 mm from bregma) and MEC (lateral 4.5 mm from lambda and the intersection with the lambdoidal suture). For recording the hippocampal EEG, twisted Teflon-insulated stainless-steel electrodes (#790900; A-M Systems, Sequim, WA, USA) were implanted in the stratum radiatum of the dorsal CA1 hippocampus (posterior 4.2 mm, lateral 3.0 mm from bregma; depth 2.4 mm from the surface) and secured in place using dental cement. Agarose was put on the surface of the brain above the MEC to reduce pulsation, drying and instability. An Ag–AgCl pellet was placed in the neck muscles as a reference electrode for intracellular recording.
Ethical approval
All experiments were performed in accordance with experimental guidelines approved by the Institutional Animal Care and Use Committee at Boston University and The Animal Welfare Act.
Electrophysiology
Intracellular recordings were performed with sharp intracellular recording electrodes created from glass capillaries (#BF150-86-10; Sutter Instruments, Novato, CA, USA) on an electrode puller (P-87 or P-97; Sutter Instruments). The internal solution for the micropipette electrode included 2 m potassium acetate, 2% biotin dextran amine (Dextran, Biotin, 3000 MW, Lysine Fixable, BDA-3000, #D-7135; Life Technologies, Grand Island, NY, USA) or 2% neurobiotin (#SP-1120; Vector Laboratories, Burlingame, CA, USA) or 2% biocytin (#B4261; Sigma, St. Louis, MO, USA). Micropipette impedance was 40–70 MΩ. The electrode was lowered to the MEC (lateral 4.5 mm, 0.35–1.00 mm anterior from the sinus on the cerebellum, angled 12 degrees to anterior, depth 2.0–4.5 mm from the surface) by a micromanipulator (MO-8-W; Narishige). An electronic buzz was performed to make the micropipette penetrate the cell membrane. The intracellular membrane potential and hippocampal EEG signal were amplified (Multiclamp 700B; Molecular Devices, Sunnyvale, CA, USA) and stored in a computer via an analog-to-digital (A/D) converter (Digidata 1440A; Molecular Devices) with pCLAMP software (sampling rates 10 kHz). Mechanical noise was reduced by use of an air table, and an audio monitor and oscilloscope were used to assist in searching for cells. In some experiments, the internal solution of the recording micropipette included 1 mm picrotoxin (#1128; Tocris, Bristol, England, UK) diluted in DMSO. In order to allow the picrotoxin to diffuse fully within the recorded cell, we waited 30 min after the initiation of intracellular recording (Yazaki-Sugiyama et al. 2009) before gathering data on persistent spiking in these conditions. During recording, the acetylcholinesterase inhibitor, physostigmine (0.4 mg (kg bwt)−1, #E8375 Eserine; Sigma), was administered intraperitoneally via the injection cannula. Although this is a systemic injection, it will increase the response to the endogenous local release of acetylcholine. In some cases, scopolamine methylbromide (0.1 mg (kg bwt)−1; Sigma), which does not cross the blood–brain barrier, was injected intraperitoneally beforehand to prevent excessive salivation caused by physostigmine administration. Only recordings with resting membrane potentials more hyperpolarized than −60 mV and spike peaks higher than −20 mV were used for analysis. All spike amplitudes were greater than 55 mV. At the end of each recording, current pulses were applied for biotin labelling (±1–2 nA, 500 ms at 1 Hz for 10–25 min).
Histology
After the completion of each experiment, the depth of anaesthesia was increased with an intraperitoneal injection of urethane (0.3 − 1.5 g (kg bwt)−1), and the animals were perfused with saline and 10% formalin transcardially. The brains were removed and postfixed overnight, and then put into 30% sucrose for cryoprotection. The brains were sliced into 50- or 100-μm-thick parasagittal sections using a cryostat (Leica, Buffalo Grove, IL, USA). Biotin-loaded cells were visualized using avidin–biotin complex [Vectastain Elite ABC kit (Standard), #PK-6100; Vector Laboratories] and DAB reactions (DAB Peroxidase Substrate Kit, #SK-4100; Vector Laboratories). The sections were mounted on gelatin-coated slides and counterstained with Neutral Red or Cresyl Violet.
Data analysis
Under urethane anaesthesia, there are two brain states, a fast-wave state (prominent 2–4 Hz) and a slow-wave state (prominent 0.5–1 Hz), which can be distinguished by EEG (Murakami et al. 2005; Wolansky et al. 2006; Tsuno et al. 2008; Schall & Dickson, 2010). Cholinergic neurons are active in the fast-wave state (Manns et al. 2000). In this experiment, we used a relatively high dose of urethane to maintain a deeply anaesthetized slow-wave state to ensure that the cholinergic system was not active before drug (physostigmine) injection. Physostigmine was then used to increase the levels of acetylcholine in the brain. All statistical comparisons were made with Student's two-tailed t tests.
Power spectra of hippocampal EEG
Power spectra were calculated from the downsampled hippocampal EEG (1 kHz) during a 50 s period from 60 s before and 15 min after physostigmine administration. The resolution of the power spectra was 0.122 Hz. The calculation was performed by pCLAMP.
Sag analysis
As a result of the rectifying nature of Ih, its time course and amplitude could be measured by applying hyperpolarizing current steps (−1000 pA for 2 s) and analysing the resulting ‘sag’ in the membrane potential back towards resting potential (Alonso & Klink, 1993; Giocomo & Hasselmo, 2008). Hyperpolarizing current steps were initiated from a membrane potential of approximately −70 mV, which was achieved, when necessary, using tonic applied current. We measured the sag potential amplitude as the difference between the peak hyperpolarization elicited by negative current steps and the steady-state voltage during the 2 s stimulus. In order to minimize variability in sag measurements across cells resulting from differences in input resistance, we normalized the raw sag potential amplitude by the peak hyperpolarization elicited by the stimulus. Only neurons which showed a raw sag potential larger than 3 mV were tested for cholinergic effects on sag potential. The membrane potential during the sag was fitted to a single exponential function instead of a double exponential, because the slow component of sag was not clear in vivo and the single exponential was better for fitting. The amplitude and time constant of the sag were calculated using the Clampfit utility of pCLAMP software.
Analysis of subthreshold resonance
Chirp stimuli (frequency sweeps) consisted of a constant-amplitude (100 or 200 pA) sinusoidal current, which increased in frequency linearly from 0 to 50 Hz over a 50 s period (Giocomo et al. 2007; Heys et al. 2010). These stimuli were generated with the MATLAB chirp function and used to obtain the impedance amplitude profile of recorded neurons. In order to maintain the membrane potential of each cell low enough to prevent spiking during chirp stimulation (between –69 and –77 mV), a steady hyperpolarizing current was injected when necessary (between 0 and –500 pA). Only the data for which the difference of the membrane potential during current injection was less than 3 mV were used for comparison between control and physostigmine conditions. During the frequency sweep, the resulting voltage response, V(t), was measured, and the frequency-dependent impedance profile, Z(f), was calculated by taking the fast Fourier transform (FFT) of the membrane potential with chirp stimulation [Vf(t)] minus the FFT of the membrane potential without chirp stimulation [Vcontrol,f(t)], and the difference between these values was divided by the FFT of the injected sinusoidal current [If(t)]. Subtraction was performed to reduce the effect of in vivo oscillatory dynamics, such as network slow-wave oscillations, which are independent from the chirp stimulation, as follows:
The resonance frequency, fres, was defined as the stimulus frequency that maximized Z(f). Fitting for Z(f) was done using a 10th-order polynomial, and the resonance frequency, fres, was detected as the peak of the fitting. Cholinergic modulation of resonance frequency was tested for those cells that showed a clear peak in resonance frequency.
Spike frequency during injected ramp current (f–I curve)
In order to analyse the frequency–current (f–I) relationship of each cell with a single stimulus application, we delivered slow, linearly increasing and decreasing current ramps, starting from a tonic current injection level that held each cell below the threshold for tonic spiking. Ramp stimuli have been used previously in studies of motoneurons, wherein hysteresis was categorized as type 1–4 (Button et al. 2006) or clockwise/counterclockwise (Iglesias et al. 2011). We used this framework to characterize the spiking responses of MEC neurons to ramp stimuli and to test the effects of cholinergic modulation on intrinsic properties in MEC neurons. A 45 s upward ramp (increasing to 1000 pA) followed by a 45 s downward ramp current stimulation was used. After each ramp stimulation, a negative current pulse (–1000 pA for 2 s) was applied to eliminate potential effects of the ramp stimulus on persistent spiking. After detection of all of the spikes during the stimulus, instantaneous frequencies of spiking were calculated from the interspike intervals. The f–I curve was obtained for each recorded neuron by smoothing instantaneous spike frequencies during ramp stimulation with a sliding window spanning 10 spike cycles and then anchoring to the respective instantaneous injected current values. The initial up-ramp slope, terminal up-ramp slope, initial down-ramp slope and terminal down-ramp slope of the f–I curve were measured with linear fitting to segments of the f–I curve containing at least 20 interspike intervals during at least 10 s of stimulation. This approach allowed reasonable estimates of the f–I curve slope despite local variations in spike rate caused by slow-wave activity. The averaged slopes were calculated across one to four stimulus repetitions for each condition. Analysis was done using MATLAB. Spike threshold was determined for the first spike during up-ramp stimulation and the last spike during down-ramp stimulation. Thresholds of spikes were determined by the highest slope of the change of the membrane potential immediately before the action potentials. Average values across one to five stimuli were used for analysis. If spikes occurred before up-ramp stimulation or after down-ramp stimulation, the spike threshold was not determined.
Results
Effects of physostigmine confirmed by the change of hippocampal EEG
We used systemic injections of physostigmine, an acetylcholinesterase inhibitor, to enhance cholinergic modulation during the experiments. In our recordings, the hippocampal EEG, which is highly modulated by cholinergic input, transitioned from a slow-wave pattern during the deeply anaesthetized state before physostigmine application (Fig. 1A and C), to a fast-wave pattern (Fig. 1B and D) after physostigmine application. By observing the change of hippocampal EEG from slow wave to fast wave, we confirmed that physostigmine application was effective in increasing cholinergic modulation.
Figure 1. Hippocampal EEG and membrane potentials change after physostigmine (Phys) application.
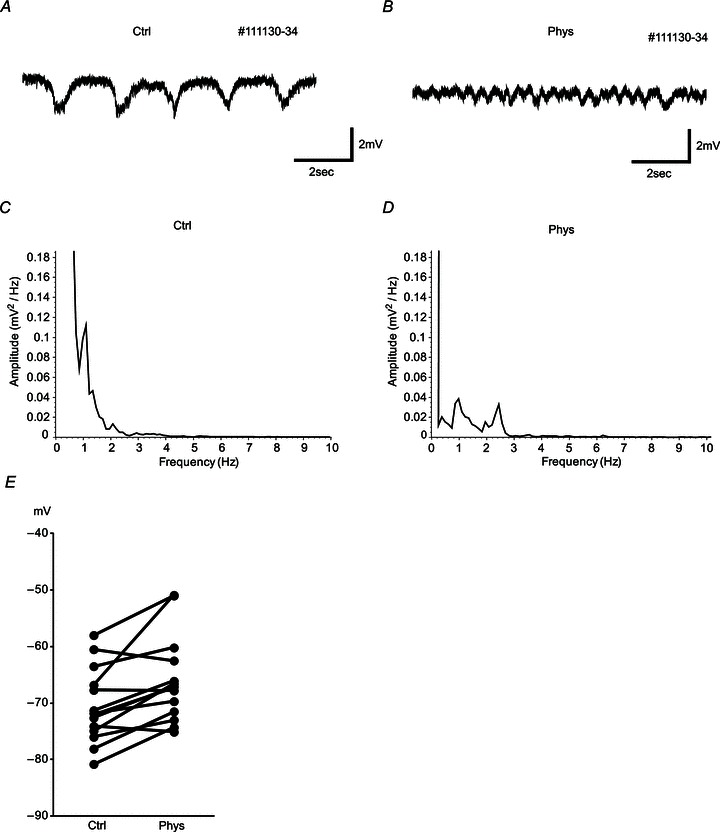
A and B, examples of hippocampal EEG are shown 30 s before (A) and 15 min after the physostigmine application intraperitoneally (B). Hippocampal EEG changed from a 0.5–1 Hz slow oscillation to a 2–4 Hz faster oscillation. C and D, power spectra of the hippocampal EEG in control condition (C) and in the presence of physostigmine (D). Relatively large power is observed in the 0.5–1 Hz band in control conditions (C) and in the 2–4 Hz band in the presence of physostigmine (D). E, averages of membrane potentials before (Ctrl) and after physostigmine application with no current injection. Ten of 14 cells showed a significant increase of membrane potentials. Averages were −70.6 (Ctrl) and −65.9 mV (Phys).
Change of membrane potential after physostigmine application
We analysed the data to see whether physostigmine application changed the membrane potential of the recorded cells (n= 14; Fig. 1E). Ten of 14 neurons showed a significant increase of membrane potential (P < 0.001, Student's two-tailed unpaired t test). Averages of membrane potentials of cells were −70.6 ± 1.69 mV (mean ± SEM) in the control conditions and −65.9 ± 1.96 mV in the physostigmine conditions with no current injection (14 cells, P < 0.01, Student's two-tailed paired t test). The value of resting membrane potential in the control conditions is consistent with a previous report (Quilichini et al. 2010). For the experiment on sag potentials and resonance frequency, if the membrane potential changed after physostigmine application, the membrane potentials were matched between the two conditions by using current injection. If the membrane potentials differed by more than 7 mV between the two conditions, the data were eliminated.
Cholinergic modulation of h-current in vivo
We focused on recordings from neurons in MEC layers II (Klink & Alonso, 1997a; Quilichini et al. 2010) and III (Dickson et al. 1997; Gloveli et al. 1997; Quilichini et al. 2010) as verified with histological staining (Fig. 2) to elucidate the effects of cholinergic modulation on cellular physiology in vivo. Of the 14 cells recorded in this study, 11 cells were putative stellate cells in layer II, two cells were in layer III, and one cell was in layer II or III. Stellate cells of layer II/III of the MEC possess a strong hyperpolarization-activated, mixed cation current (h-current) mediated by HCN channels (Ludwig et al. 1998; Dickson et al. 2000). The h-current is inwardly rectifying and underlies the prominent sag potential exhibited by entorhinal stellate cells, which consists of a slower depolarization of the membrane potential during negative applied current steps after the initial hyperpolarization at the onset of the stimulus. We applied 2 s current steps of −1000 pA to characterize the sag potential and input resistance of layer II/III MEC neurons in vivo in conditions with and without increased cholinergic modulation due to physostigmine administration (Fig. 3A and B). With physostigmine, the sag potential amplitude decreased significantly from 5.32 ± 0.45 to 4.34 ± 0.34 mV (mean ± SEM; Fig. 3C; P < 0.05, Student's two-tailed paired t test, n= 5). The time constant of the sag showed an increase from 12.3 ± 0.9 to 18.0 ± 0.9 ms (Fig. 3D; P < 0.05, n= 4), and input resistance increased from 22.7 ± 2.7 to 28.9 ± 3.0 MΩ (Fig. 3E; P < 0.05, n= 5). The normalized amplitude of the sag potential was reduced significantly from 0.20 ± 0.03 to 0.14 ± 0.02 (Fig. 3F; P < 0.01, n= 5). These data in vivo replicate the effects of bath application of the acetylcholine receptor agonist, carbachol, in brain slice preparations (Heys et al. 2010). None of these changes was observed after intraperitoneal injection of saline instead of physostigmine (P > 0.05, n= 5).
Figure 2. Examples of the recorded cells.
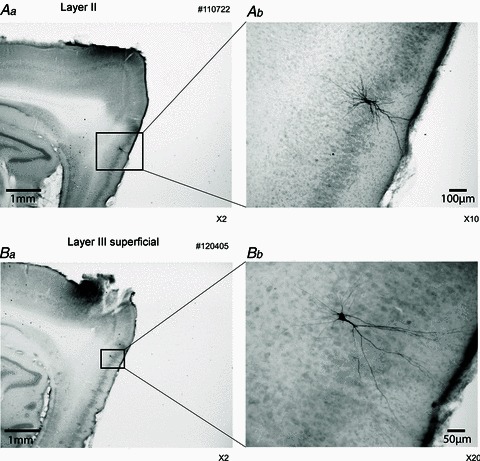
Aa, layer II cell (parasagittal section). Ab, higher magnification of Aa. The biotin-labelled cell is a stellate cell in layer II of the medial entorhinal cortex (MEC). Ba, layer III superficial cell (parasagittal section). Bb, higher magnification of Ba. This neuron shows non-spiny dendrites, and the initial bifurcation is close to the cell body, which indicates a type 2 projection neuron as described by Gloveli et al. (1997).
Figure 3. Sag potential amplitude and time constant during physostigmine application.
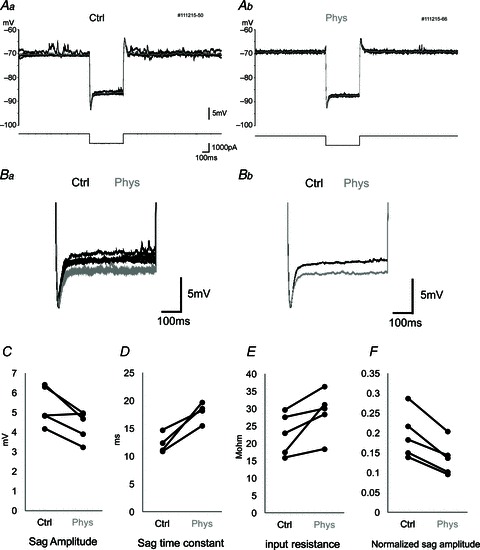
A, membrane potential response (upper traces) to current square pulse (−1000 pA) stimulation (lower traces) in control conditions (Aa) and after physostigmine application (Ab). A large sag potential is observed immediately after the initiation of the current pulse. Five traces are overlaid, and the grey trace is the average. B, higher magnification of sag potentials. Five raw traces (Ba) and averaged traces (Bb) are overlaid in control conditions (black) and after physostigmine application (grey). Traces were aligned with the trough immediately after the onset of negative current stimulation. C–F, averaged values of sag amplitude (C), sag time constant (D), input resistance (E) and normalized sag amplitude (F) in individual neurons in control conditions (Ctrl) and in the presence of physostigmine (Phys). Each connected pair indicates the data from a single cell. The normalized sag amplitude (F) is calculated as sag amplitude divided by the amplitude of the initial negative potential immediately after the negative current pulse to cancel out the effect of input resistance change on sag amplitude. There is a significant increase in the sag time constant during physostigmine application.
Cholinergic modulation of subthreshold resonance in vivo
Subthreshold resonance is a reflection of the preferential responsiveness that a neuron may possess to inputs with particular frequency composition. Stellate cells in the MEC exhibit subthreshold resonance in the theta frequency range as a consequence, in part, of the time constants of h-current activation, which acts as a high-pass filter by opposing slow changes in voltage by inward rectification. It was recently reported that cholinergic modulation with carbachol reduces the peak resonance frequency of MEC stellate cells recorded in vitro during stimulation with frequency sweeps of sinusoidal current, referred to as chirp or ZAP stimuli (Heys et al. 2010). Heys and colleagues also reported that co-administration of carbachol and the selective h-current blocker, ZD7288, yielded no additional effect on the impedance profile and sag potential of MEC stellate cells, suggesting that the effect on subthreshold resonance is also mediated by the modulation of h-current. During our intracellular recordings, we applied chirp stimuli composed of linearly increasing frequencies (from 0 to 50 Hz) of sinusoidal current injection and derived the impedance profiles of MEC neurons in vivo, taking the peak of the impedance profile as the resonance frequency (Fig. 4A–D). The average resonance frequency was significantly reduced from 11.4 ± 0.9 to 8.7 ± 1.1 Hz (mean ± SEM) after physostigmine application (Fig. 4E; P < 0.05, Student's two-tailed paired t test, n= 4), confirming in vivo the reduction of resonance frequency by cholinergic modulation observed in vitro (Heys et al. 2010). No significant reduction of resonance frequency was observed in experiments in which a saline injection was administered instead of physostigmine (P > 0.05, n= 5).
Figure 4. Resonance frequency becomes lower after physostigmine application.
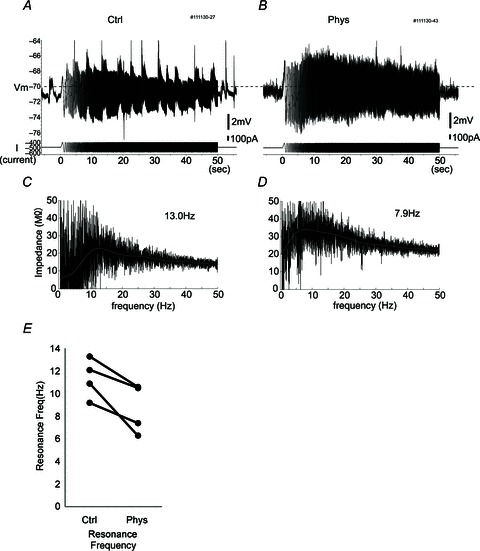
A and B, raw traces of the response to frequency sweep (chirp) stimulation in control conditions (A) and in the presence of physostigmine (B). Top traces are membrane potential; bottom traces are injected current. Chirp stimulation consists of a sine wave that increases in frequency from 0 to 50 Hz linearly over 50 s. The dashed line indicates −70 mV. C and D, impedance profile in each frequency calculated by the response amplitude (A and B) of chirp stimulation in control conditions (C) and in the presence of physostigmine (D). Grey trace indicates the fitted trace using a 10th-order polynomial. Note that the resonance (peak) frequency shifted from 13.0 to 7.9 Hz after physostigmine administration. E, averaged values of resonance frequencies in individual neurons (n= 4, P < 0.05).
Absence of bistable persistent spiking in vivo
Bistable persistent spiking in the MEC entails a self-sustained spiking state mediated by a non-specific calcium-sensitive cationic current (ICAN; Fransén et al. 2006) via TRPC channels (Zhang et al. 2011), which can be elicited in vitro from quiescence with a brief depolarizing current step. Bistable persistent spiking has been proposed to underlie the elevated spiking observed in EC of behaving monkeys and rats during the delay period of delayed match to sample tasks (Suzuki et al. 1997; Young et al. 1997), and therefore as a cellular substrate for information held in working memory (Camperi & Wang, 1998). However, there is no experimental evidence to date that distinguishes whether bistable persistent spiking and/or network mechanisms, such as recurrent excitation (Major & Tank, 2004), underlie delay activity in behaving animals.
In order to examine whether bistable persistent spiking is robust in the intact entorhinal network in vivo, during our intracellular recordings we delivered positive current pulses to MEC neurons before and after physostigmine application (Fig. 5), mimicking as closely as possible the in vitro protocols for eliciting bistable persistent spiking with cholinergic modulation (Egorov et al. 2002; Tahvildari et al. 2007; Schultheiss & Hasselmo, 2011). We started the protocol by changing the basal injection current amplitude to set the membrane potential slightly below the spike threshold, and we then presented a positive current pulse (1000 pA for 2 s) to drive spiking briefly at a high rate. Spiking subsequent to the current pulse was analysed to determine whether a persistent spiking state was elicited by the inputs or whether there was a consistent effect on mean spike frequency. Contrary to expectations, persistent spiking could not be elicited during cholinergic activation in any of our recordings (Fig. 5Ab, Bb and Bc), and there was no consistent effect of current pulse stimuli on the subsequent spike rate; seven of nine neurons showed no effect whatsoever. Although the remaining two neurons showed a slight increase in spiking after the first current pulse delivered following physostigmine administration, this change could not be elicited consistently, and firing frequency was not reduced by delivery of a subsequent negative current pulse as expected based on in vitro studies (Egorov et al. 2002; Tahvildari et al. 2007). In an additional two experiments, the GABAA receptor antagonist, picrotoxin, was included in the recording micropipette to block inhibitory input to the recorded neurons selectively. These two neurons showed a slight increase of firing frequency after one current pulse, but this also did not occur consistently (Fig. 5Ba–c). In both cases, we were unable to elicit persistent spiking even in the absence of synaptic inhibition.
Figure 5. Persistent spiking did not appear in vivo under urethane anaesthesia.
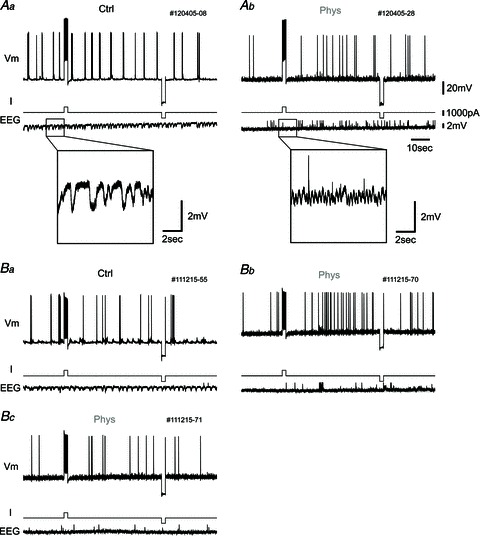
A, one example of the response to current pulse injection. Top traces, membrane potential (Vm); middle traces, injected current (I); and bottom traces, hippocampal EEG (EEG). Aa, in this protocol, membrane potential is first set slightly below the level of constant spike firing by changing the injected current. Then, a 1000 pA, 2 s current pulse is injected to elicit persistent firing after the cessation of current stimulation. After the waiting period (50 s), a 2 s negative current pulse (−1000 pA) is injected to eliminate any remaining effect of the positive current pulse. The hippocampal EEG shows slow-wave activity in the control condition (inset, higher magnification). Ab, in the presence of physostigmine, persistent spiking does not occur after current pulse injection. The hippocampal EEG shows fast-wave activity due to physostigmine administration (inset, higher magnification). B, another example of the response to current pulse injection with picrotoxin in the recording pipette. Ba, there is no change of spiking in control condition. Bb, after physostigmine application, the cell tends to increase firing rate after the positive current pulse in this case, but does not show continuous persistent spiking. Bc, this is the response of the same neuron with the same current injection protocol as shown in Bb, but the neuron does not show an increase in firing frequency again.
Slope of spike frequency to input current (f–I curve) increased by cholinergic modulation
In order to characterize the effect of cholinergic modulation on the relationship of firing frequency to injected current, we delivered slow, linearly increasing and decreasing current ramp stimuli (up- and down-ramps) before and after physostigmine application (Fig. 6). Recordings showed that firing frequency increased during up-ramp stimulation and decreased during down-ramp stimulation. Quantitative analysis showed that the initial up-ramp slope of the f–I curve became steeper after physostigmine application than the slope observed before physostigmine application (P < 0.05, Student's two-tailed paired t test, n= 10), suggesting that the sensitivity to input increased during cholinergic modulation. For five of 10 neurons, the average slope of the initial up-ramp f–I curve became more than 2.0 times steeper after physostigmine injection than prior to physostigmine injection (Fig. 7A), and for three of the remaining neurons, the initial f–I slope was increased by 1.2–2.0 times (Fig. 7B). The final two neurons showed a ratio less than 1.2 (Fig. 7C). Thus, five neurons showed large modulation and three neurons showed moderate modulation by physostigmine application of the initial slope during the up-ramp. Further analysis of the in vivo f–I curves showed that some neurons exhibited clockwise hysteresis (categorized as a type 2 f–I relationship by Button et al. 2006), i.e. firing frequency increased rapidly at first (large initial f–I slope), reaching a plateau frequency of approximately 15–25 Hz during the up-ramp (flat terminal slope), before subsequently decreasing with an intermediate slope during the down-ramp. As a measure of this hysteresis, we compared the initial down-ramp slope with the terminal up-ramp slope. Both in control conditions and in the presence of physostigmine, the average slope of the initial down-ramp was steeper than that of the terminal up-ramp (P < 0.05 and P < 0.01, respectively, Student's two-tailed paired t test, n= 10). In order to examine the change of the hysteresis property by physostigmine application, the ratio of the initial down-ramp slope to the last up-ramp slope was compared between control and physostigmine conditions. Two of 10 neurons showed that the ratio in the presence of physostigmine was more than 2 times larger than that in control conditions. Overall, however, there was no significant difference between the ratio in control conditions and in the presence of physostigmine (P= 0.24, Student's two-tailed paired t test, n= 10). In these experiments, persistent spiking was never observed after ramp stimulation, which differs from results in our in vitro studies (N. W. Schultheiss and M. E. Hasselmo, unpublished observations). All parameters of ramp stimulation data are summarized in Table 1.
Figure 6. The frequency–current (f–I) curve becomes steeper after physostigmine application.
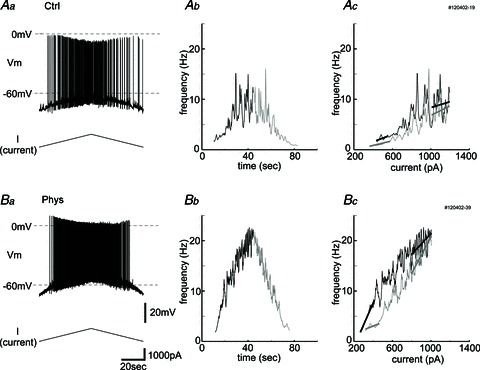
Increasing and decreasing ramp stimulation (1000 pA) is used to examine the f–I curve properties in control conditions (Aa–c) and in the presence of physostigmine (Ba–c). Aa and Ba, top traces, membrane potential and bottom traces, injected current. Ab and Bb, the relationships between time and frequency of spikes. Black traces are during the up-ramp, and grey traces are during the down-ramp. The traces are smoothed by averaging 10 interspike intervals. Ac and Bc, f–I plot. Black traces are during the up-ramp, and grey traces are during the down-ramp. Initial up-ramp slope, terminal up-ramp slope (black lines), initial down-ramp slope and terminal down-ramp slope (grey lines) of the f–I curve were calculated by linear fitting. Note that the initial up-ramp slope in the presence of physostigmine (Bc) is steeper than that in control conditions (Ac). Recordings show a slight hysteresis, which means a steeper initial slope during the down-ramp than the terminal slope during the up-ramp. This was observed more prominently after physostigmine injection (Bc).
Figure 7. Most neurons show a steeper slope of the f–I curve during ramp stimulation after physostigmine application.
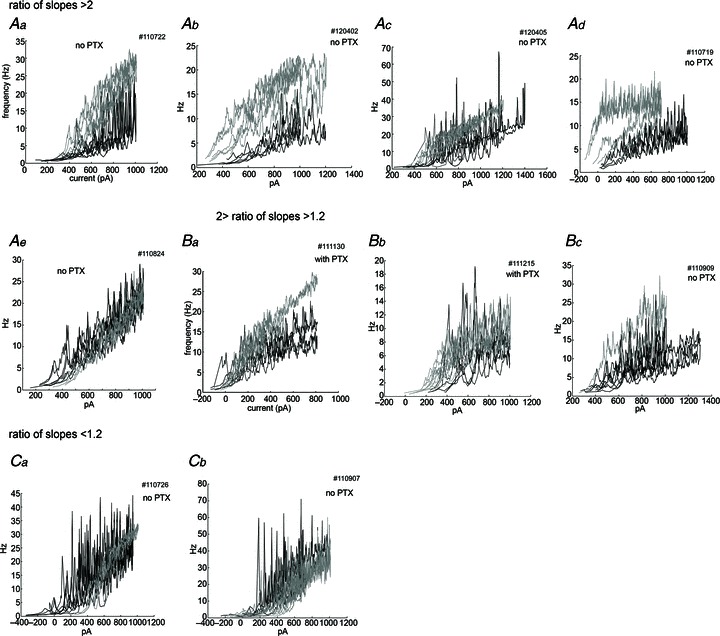
Control traces (black) and physostigmine traces (grey) are overlaid for each cell. These data are aligned by the ratio of the averaged initial up-ramp slope in the presence of physostigmine to that in the control conditions. The ratio was larger than 2.0 in 5 neurons (Aa–e), between 1.2 and 2.0 in 3 neurons (Ba–c), and less than 1.2 in 2 neurons (Ca–b). Note that Ad and Bc showed clear hysteresis in the presence of physostigmine. Traces were smoothed by averaging 10 interspike intervals.
Table 1.
All parameters of ramp stimulation data
| Ramp no. | PTX | Ratio of slopes | Layer | Sag | Rin (Ctrl; MΩ) | Rin (Phys; MΩ) |
|---|---|---|---|---|---|---|
| 110719 | — | 2.40 | III | No | 50 | 40 |
| 110722 | — | 8.30 | II | Sag | 7 | 9 |
| 110726 | — | 0.99 | II | Sag | 20 | 20 |
| 110824 | — | 2.10 | II | Sag | 34 | 34 |
| 110907 | — | 0.20 | II | No | 40 | 10 |
| 110909 | — | 1.13 | II | Sag | 14 | 25 |
| 111130 | PTX | 1.47 | II | Sag | 28 | 32 |
| 111215 | PTX | 1.34 | II | Sag | 22 | 23 |
| 120402 | — | 3.80 | II | Sag | 14 | 19 |
| 120405 | — | 2.47 | III | Sag | 35 | 40 |
Columns are as follows, from the left: the cell number; the use of picrotoxin (PTX) inside the recording pipette; the ratio of the averaged initial up-ramp slope in the presence of physostigmine to that in control conditions; layer; sag potential; and input resistance (Rin) in control conditions and after physostigmine (Phys).
Change of spike threshold by cholinergic modulation
We examined the threshold of the first spike during up-ramp stimulation and the threshold of the last spike during down-ramp stimulation. There were no differences between the thresholds of the first spike during up-ramp and the thresholds of the last spike during the down-ramp either without or with physostigmine (P= 0.94 and P= 0.36, respectively, n= 7). However, spike threshold was increased after physostigmine administration relative to control conditions from −55.4 ± 2.3 to −48.0 ± 2.8 mV (mean ± SEM) for the first spike during the up-ramp (Fig. 8A; P < 0.01, Student's two-tailed paired t test, n= 8) and from −55.4 ± 2.6 to −49.0 ± 3.3 mV (Fig. 8B; P < 0.05, n= 7) for the last spiking during the down-ramp. No significant differences of spike threshold were observed in experiments in which a saline injection was administered instead of physostigmine (P > 0.05, respectively, n= 4).
Figure 8. Spike thresholds are increased after physostigmine application.
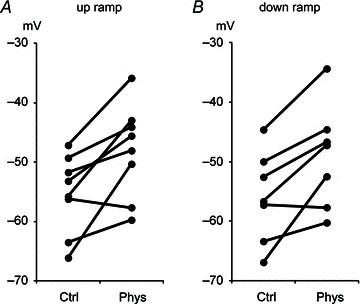
The thresholds of the first spike during up-ramp stimulation (A) and the thresholds of the last spike during down-ramp stimulation (B) before and after physostigmine application.
Discussion
In the present study, we explored cellular properties of MEC neurons that have been proposed to contribute to the spatial function of entorhinal cortex. We show that acetylcholine activation in vivo modulates sag potentials, subthreshold resonance and the f–I curve, as it does in vitro, supporting interpretations of the contributions of these properties to MEC function in behaving animals. However, the phenomenon of persistent spiking observed in vitro did not appear in our in vivo study, suggesting that intrinsic bistability of MEC neurons is not robust in the intact network and is not likely to correspond directly to delay activity underlying the storage of working memory. These results are a critical foundation for understanding the interactions of cellular properties of MEC neurons with network mechanisms of the functional medial temporal lobe circuit.
Decrease of resonance frequency by cholinergic modulation via h-current
The increase in cholinergic modulation induced by physostigmine administration in this experiment resulted in a decrease of sag amplitude, an increase of sag time constant and a decrease of resonance frequency in vivo, which is consistent with the data from in vitro slice preparations (Heys et al. 2010). The decrease of sag amplitude indicates a decrease of the Ih as shown in voltage-clamp studies in vitro (Heys & Hasselmo, 2012). The h-current (Ih) is a non-selective cation current, activated by hyperpolarization, meaning that it acts as a slow inward rectifier, mediated by HCN channels. Previous evidence suggests that the HCN channel has a critical role in resonance frequency (Nolan et al. 2004, 2007; Garden et al. 2008; Giocomo & Hasselmo, 2009), and differences in the composition of functional HCN channels by HCN1 and HCN2 subtypes appear to contribute to the dorsal–ventral gradient of resonance frequency in entorhinal neurons (Giocomo & Hasselmo, 2009). Knockout of the HCN1 subunit of the channel that mediates the h-current alters network theta rhythm amplitude (Nolan et al. 2004) and increases the size and spacing of the firing fields of grid cells in the MEC (Giocomo et al. 2011) and place cells in hippocampus (Hussaini et al. 2011). Given that Ih is the inductive element of resonance, the decrease of Ih exhibited by reduced sag can account for the decrease of resonance frequency with cholinergic modulation (Narayanan & Johnston, 2008). As the cholinergic system is active during arousal and attentional processes (Acquas et al. 1996; Parikh et al. 2007), the decrease of sag amplitude and decrease of resonance frequency might cause a change in the frequency of theta rhythm oscillations and thereby influence oscillatory interference (Hasselmo et al. 2007; Burgess, 2008; Hasselmo, 2008). These modulatory effects could underlie changes in the spacing of grid cells in novel environments (Barry et al. 2012a) and allow detection of a mismatch between grid cells with expanded spacing and other spatial inputs (Barry et al. 2012b).
Absence of intrinsic bistable persistent spiking in vivo
Bistable persistent spiking and mechanistically related after-depolarizations or plateau potentials have been reported throughout the extended medial temporal lobe memory system, including the MEC (Klink & Alonso, 1997b; Egorov et al. 2002), hippocampus (El-Hassar et al. 2011), subiculum (Kawasaki & Avoli, 1996; Kawasaki et al. 1999) and postsubiculum (Yoshida & Hasselmo, 2009). Seminal investigations of persistent spiking identified that the spiking state is dependent on a calcium-sensitive, non-selective cation current (ICAN; Shalinsky et al. 2002; Fransén et al. 2006). Accumulating evidence now suggests that TRPC (transient receptor potential canonical) channels are responsible for the ICAN that generates persistent spiking (Reboreda et al. 2011).
Perhaps the most dramatic difference between properties of neurons recorded in vivo versus in vitro was the absence of evidence for persistent spiking in our in vivo recordings, even though we used the same protocol which successfully induced persistent spiking in vitro (Egorov et al. 2002; Yoshida & Hasselmo, 2009; N. W. Schultheiss and M. E. Hasselmo, unpublished observations). We hypothesized that GABAergic input might prevent the appearance of persistent spiking. However, we did not observe persistent spiking in experiments with the GABAA receptor blocker, picrotoxin, in the pipette to cause selective blockade of inhibition on the recorded neuron, even though the firing frequency tended to be increased. One possible reason is that during in vivo recording, ICAN might not be strong enough to elicit persistent spiking. Bistable persistent spiking depends on the balance between after-hyperpolarization (AHP) currents, which account for spike frequency adaptation (Vergara et al. 1998), and after-depolarization currents, which account for reverse spike frequency adaptation (Wilson et al. 2004). It is possible that the AHP effect is too strong to elicit bistable persistent spiking in the in vivo conditions. The SK channel, which mediates a medium or slow AHP current (IAHP), is one of the candidates for inducing spike frequency adaptation (Vergara et al. 1998) in these neurons, but the slow IAHP of stellate cells in MEC layer II is apamin insensitive (Khawaja et al. 2007). The apamin-insensitive SK channel might be the mediator to inhibit the bistable persistent spiking. Decrease of Im (m-current), a slow voltage-sensitive K+ current, by cholinergic input also depolarizes the membrane potential (Halliwell & Adams, 1982; Hu et al. 2002), but it was recently reported that Im is not significantly present in stellate cells in layer II of the MEC (Heys & Hasselmo, 2012).
Another possible reason that persistent spiking might not appear in vivo is the relatively high membrane conductance compared with recordings in vitro (Fernandez & White, 2008). This high-conductance state might result in difficulties for neurons to maintain enough depolarization to induce persistent spiking.
Although we did not observe persistent spiking, in some cases a slight increase in firing was observed after the first depolarizing current injection, which might be the result of ICAN activation. Although current injections to individual neurons can elicit persistent spiking with cholinergic modulation in vitro, we do not have evidence that persistent spiking occurs due to an intrinsic mechanism in vivo. This result indicates that some other mechanisms are needed to support persistent activity in vivo. Recently, Gupta et al. (2012) reported that persistent spiking was not observed during the delay period of a spatial delayed response task in awake, behaving rats. In previous studies, persistent spiking during the delay period was observed in EC (Suzuki et al. 1997; Young et al. 1997), but the population which showed persistent spiking was small. These reports suggest that the persistent spiking is much more rare in vivo than in vitro, and this is consistent with our finding.
There is a debate about whether persistent spiking occurs due to intrinsic mechanisms in single cells or network mechanisms involving interactions of multiple neurons. It might be possible that network input with multiple neurons is needed to maintain persistent spiking in vivo. Network mechanisms may contribute to the in vivo persistent spiking observed during sleep in layer III of medial entorhinal cortex (Hahn et al. 2012). It has also been reported that potassium conductances were increased by urethane in a visual cortex slice experiment (Sceniak & MacIver, 2006). It is possible that application of urethane diminishes the ability to elicit persistent spiking via the increase of potassium conductances.
In this study, most of the recorded cells were layer II neurons. Although persistent spiking is evident in some layer II neurons in vitro (N. W. Schultheiss and M. E. Hasselmo, personal communication), layer III and layer V neurons exhibit persistent spiking more reliably, perhaps contributing to the difficulty of observing persistent spiking in this study.
Further studies are needed to investigate whether and how intrinsic bistable persistent spiking can be elicited in the intact entorhinal network. They will help to understand the mechanism and meaning of the persistent neuronal activity in vivo.
Cholinergic modulation of the gain of spike frequency to input relationships
With cholinergic modulation, we observed that the initial slope of the measured f–I curves became considerably steeper in most cases than that in control conditions, and hysteresis was observed in several neurons. A steeper f–I curve indicates that neurons will change from a low-frequency firing state to a high-frequency firing state more rapidly with small increases in current application, which means that the sensitivity of the cell and the gain of the cell's input–output relationship are elevated. In cases where we observed hysteresis, firing frequency increased steeply to a plateau during the up-ramp and then decreased during the down-ramp, resulting in an f–I plot that appears to transition in a clockwise manner during the ramp stimulus. The increased initial slope of the f–I relationship can be understood as a consequence of activation of ICAN, which underlies persistent spiking in vitro, and reduced adaptation mediated by the decrease of KAHP current. However, increased input resistance, which is probably mediated by blockade of leak current (IK,leak; Cole & Nicoll, 1984) and/or inhibition of h-current observed during physostigmine administration, is likely also to contribute to the increase of the initial slope of the f–I curve. We did not observe any differences of ramp stimulation responses when comparing those experiments performed with picrotoxin versus those performed without picrotoxin application via the recording pipette. We noted the interesting observation that the spike threshold changes during cholinergic activation. This means that both the steeper f–I slope and the increase in spike threshold occur during cholinergic input. As a result, the cholinergic input makes a clearer contrast between the non-firing state and the firing state of the neuron. A possible mechanism for this threshold change is an influence of acetylcholine on the properties of voltage-dependent cation channels, including potassium channels and sodium channels (Klink & Alonso, 1997b). Another possibility is that modulation causes a difference in spike triggering sites, shifting from spike triggering near the soma to initiation of spikes in the dendrites at distal locations relative to the soma.
In summary, these results show that the cholinergic modulation of intrinsic resonance properties during in vivo intracellular recordings of MEC neurons are similar to data from in vitro recordings in brain slice preparations, but the recordings show differences in the cholinergic modulation of persistent spiking. These results provide insights concerning the modulation of intrinsic properties in vivo, and start to bridge the gap between the data from slice physiology and electrophysiological data from freely behaving animals. Further understanding of the effects of acetylcholine on intrinsic properties in vivo will help in understanding the role of acetylcholine in network theta rhythm oscillations and its role in behavioural mechanisms of attention and memory.
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) R01 grants MH61492, MH60013 and ONR MURI N00014-10-1-0936. This work was also supported by the Uehara memorial foundation (Y.T.). We would like to thank Dr James G. Heys, Dr Motoharu Yoshida, Dr Ehren Newman, Dr Erik Fransén, Tyler Ware, Jason Climer, Dr Eric Zilli and all Hasselmo laboratory members for useful comments. We also thank Professor Matt Wachowiak for assistance with the experimental set-up and Professors Chantal E. Stern and Howard Eichenbaum for the use of laboratory resources.
Glossary
- AHP
after-hyperpolarization
- Ctrl
control
- EC
entorhinal cortex
- FFT
fast Fourier transform
- f–I curve
frequency–current curve
- kg bwt
kilograms body weight
- ICAN
non-specific calcium-sensitive cationic current
- Ih
hyperpolarization-activated cation current
- MEC
medial entorhinal cortex
- Phys
physostigmine
- PTX
picrotoxin
- Rin
input resistance
- TRPC
transient receptor potential canonical
Author contributions
Y.T., N.W.S. and M.E.H. designed the experiments, interpreted data and wrote the article. Y.T. collected all data. Y.T. and N.W.S. performed analysis. All the authors approved the final version for publication.
References
- Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci. 1996;16:3089–3096. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol. 1993;70:128–143. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- Barry C, Ginzberg LL, O’Keefe J, Burgess N. Grid cell firing patterns signal environmental novelty by expansion. Proc Natl Acad Sci U S A. 2012a;109:17687–17692. doi: 10.1073/pnas.1209918109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C, Heys JG, Hasselmo ME. Possible role of acetylcholine in regulating spatial novelty effects on theta rhythm and grid cells. Front Neural Circuits. 2012b;6:1–13. doi: 10.3389/fncir.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus. 2008;18:1157–1174. doi: 10.1002/hipo.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button DC, Gardiner K, Marqueste T, Gardiner PF. Frequency–current relationships of rat hindlimb α-motoneurones. J Physiol. 2006;573:663–677. doi: 10.1113/jphysiol.2006.107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camperi M, Wang XJ. A model of visuospatial working memory in prefrontal cortex: recurrent network and cellular bistability. J Comp Neurosci. 1998;5:383–405. doi: 10.1023/a:1008837311948. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. The pharmacology of cholinergic excitatory responses in hippocampal pyramidal cells. Brain Res. 1984;305:283–290. doi: 10.1016/0006-8993(84)90434-7. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Magistretti J, Shalinsky MH, Fransén E, Hasselmo ME, Alonso A. Properties and role of Ih in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol. 2000;83:2562–2579. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Mena AR, Alonso A. Electroresponsiveness of medial entorhinal cortex layer III neurons in vitro. Neuroscience. 1997;81:937–950. doi: 10.1016/s0306-4522(97)00263-7. [DOI] [PubMed] [Google Scholar]
- Domnisoru C, Kinkhabwala AA, Tank DW. Membrane potential dynamics of grid cells. Nature. 2013;495:199–204. doi: 10.1038/nature11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- El-Hassar L, Hagenston AM, D’Angelo LB, Yeckel MF. Metabotropic glutamate receptors regulate hippocampal CA1 pyramidal neuron excitability via Ca2+ wave-dependent activation of SK and TRPC channels. J Physiol. 2011;589:3211–3229. doi: 10.1113/jphysiol.2011.209783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erchova I, Kreck G, Heinemann U, Herz AVM. Dynamics of rat entorhinal cortex layer II and III cells: characteristics of membrane potential resonance at rest predict oscillation properties near threshold. J Physiol. 2004;560:89–110. doi: 10.1113/jphysiol.2004.069930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez FR, White JA. Artificial synaptic conductances reduce subthreshold oscillations and periodic firing in stellate cells of the entorhinal cortex. J Neurosci. 2008;28:3790–3803. doi: 10.1523/JNEUROSCI.5658-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransén E, Tahvildari B, Egorov AV, Hasselmo ME, Alonso AA. Mechanism of graded persistent cellular activity of entorhinal cortex layer V neurons. Neuron. 2006;49:735–746. doi: 10.1016/j.neuron.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Garden DLF, Dodson PD, O’Donnell C, White MD, Nolan MF. Tuning of synaptic integration in the medial entorhinal cortex to the organization of grid cell firing fields. Neuron. 2008;60:875–889. doi: 10.1016/j.neuron.2008.10.044. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Time constants of h current in layer II stellate cells differ along the dorsal to ventral axis of medial entorhinal cortex. J Neurosci. 2008;28:9414–9425. doi: 10.1523/JNEUROSCI.3196-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Knock-out of HCN1 subunit flattens dorsal–ventral frequency gradient of medial entorhinal neurons in adult mice. J Neurosci. 2009;29:7625–7630. doi: 10.1523/JNEUROSCI.0609-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hussaini SA, Zheng F, Kandel ER, Moser M-B, Moser EI. Grid cells use HCN1 channels for spatial scaling. Cell. 2011;147:1159–1170. doi: 10.1016/j.cell.2011.08.051. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Zilli EA, Fransén E, Hasselmo ME. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science. 2007;315:1719–1722. doi: 10.1126/science.1139207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloveli T, Schmitz D, Empson RM, Dugladze T, Heinemann U. Morphological and electrophysiological characterization of layer III cells of the medial entorhinal cortex of the rat. Neuroscience. 1997;77:629–648. doi: 10.1016/s0306-4522(96)00494-0. [DOI] [PubMed] [Google Scholar]
- Gupta K, Keller LA, Hasselmo ME. Reduced spiking in entorhinal cortex during the delay period of a cued spatial response task. Learn Mem. 2012;19:219–230. doi: 10.1101/lm.025866.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hahn TTG, McFarland JM, Berberich S, Sakmann B, Mehta MR. Spontaneous persistent activity in entorhinal cortex modulates cortico-hippocampal interaction in vivo. Nat Neurosci. 2012;15:1531–1538. doi: 10.1038/nn.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus. 2008;18:1213–1229. doi: 10.1002/hipo.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM, Zilli EA. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus. 2007;17:1252–1271. doi: 10.1002/hipo.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends Cogn Sci. 2006;10:487–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys JG, Giocomo LM, Hasselmo ME. Cholinergic modulation of the resonance properties of stellate cells in layer II of medial entorhinal cortex. J Neurophysiol. 2010;104:258–270. doi: 10.1152/jn.00492.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys JG, Hasselmo ME. Neuromodulation of Ih in layer II medial entorhinal cortex stellate cells: a voltage-clamp study. J Neurosci. 2012;32:9066–9072. doi: 10.1523/JNEUROSCI.0868-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys JG, Schultheiss NW, Shay CF, Tsuno Y, Hasselmo ME. Effects of acetylcholine on neuronal properties in entorhinal cortex. Front Behav Neurosci. 2012;6:32. doi: 10.3389/fnbeh.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Graham LJ, Storm JF. Complementary theta resonance filtering by two spatially segregated mechanisms in CA1 hippocampal pyramidal neurons. J Neurosci. 2009;29:14472–14483. doi: 10.1523/JNEUROSCI.0187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussaini SA, Kempadoo KA, Thuault SJ, Siegelbaum SA, Kandel ER. Increased size and stability of CA1 and CA3 place fields in HCN1 knockout mice. Neuron. 2011;72:643–653. doi: 10.1016/j.neuron.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon B, Yarom Y. Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 2000;23:216–222. doi: 10.1016/s0166-2236(00)01547-2. [DOI] [PubMed] [Google Scholar]
- Iglesias C, Meunier C, Manuel M, Timofeeva Y, Delestrée N, Zytnicki D. Mixed mode oscillations in mouse spinal motoneurons arise from a low excitability state. J Neurosci. 2011;31:5829–5840. doi: 10.1523/JNEUROSCI.6363-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Avoli M. Excitatory effects induced by carbachol on bursting neurons of the rat subiculum. Neurosci Lett. 1996;219:1–4. doi: 10.1016/s0304-3940(96)13175-x. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Palmieri C, Avoli M. Muscarinic receptor activation induces depolarizing plateau potentials in bursting neurons of the rat subiculum. J Neurophysiol. 1999;82:2590–2601. doi: 10.1152/jn.1999.82.5.2590. [DOI] [PubMed] [Google Scholar]
- Khawaja FA, Alonso AA, Bourque CW. Ca2+-dependent K+ currents and spike-frequency adaptation in medial entorhinal cortex layer II stellate cells. Hippocampus. 2007;17:1143–1148. doi: 10.1002/hipo.20365. [DOI] [PubMed] [Google Scholar]
- Klink R, Alonso A. Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus. 1997a;7:571–583. doi: 10.1002/(SICI)1098-1063(1997)7:5<571::AID-HIPO12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Klink R, Alonso A. Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. J Neurophysiol. 1997b;77:1813–1828. doi: 10.1152/jn.1997.77.4.1813. [DOI] [PubMed] [Google Scholar]
- Koenig J, Linder AN, Leutgeb JK, Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science. 2011;332:592–595. doi: 10.1126/science.1201685. [DOI] [PubMed] [Google Scholar]
- Leonard BW, Amaral DG, Squire LR, Zola-Morgan S. Transient memory impairment in monkeys with bilateral lesions of the entorhinal cortex. J Neurosci. 1995;15:5637–5659. doi: 10.1523/JNEUROSCI.15-08-05637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol. 2004;14:675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000;20:1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Moser M-B. A metric for space. Hippocampus. 2008;18:1142–1156. doi: 10.1002/hipo.20483. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kashiwadani H, Kirino Y, Mori K. State-dependent sensory gating in olfactory cortex. Neuron. 2005;46:285–296. doi: 10.1016/j.neuron.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. The h channel mediates location dependence and plasticity of intrinsic phase response in rat hippocampal neurons. J Neurosci. 2008;28:5846–5860. doi: 10.1523/JNEUROSCI.0835-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Gupta K, Climer JR, Monaghan CK, Hasselmo ME. Cholinergic modulation of cognitive processing: insights drawn from computational models. Front Behav Neurosci. 2012;6:24. doi: 10.3389/fnbeh.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Dudman JT, Dodson PD, Santoro B. HCN1 channels control resting and active integrative properties of stellate cells from layer II of the entorhinal cortex. J Neurosci. 2007;27:12440–12451. doi: 10.1523/JNEUROSCI.2358-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsáki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilichini P, Sirota A, Buzsáki G. Intrinsic circuit organization and theta–gamma oscillation dynamics in the entorhinal cortex of the rat. J Neurosci. 2010;30:11128–11142. doi: 10.1523/JNEUROSCI.1327-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboreda A, Jiménez-Díaz L, Navarro-López JD. TRP channels and neural persistent activity. Adv Exp Med Biol. 2011;704:595–613. doi: 10.1007/978-94-007-0265-3_32. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, MacIver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol. 2006;95:3865–3874. doi: 10.1152/jn.01196.2005. [DOI] [PubMed] [Google Scholar]
- Schall KP, Dickson CT. Changes in hippocampal excitatory synaptic transmission during cholinergically induced theta and slow oscillation states. Hippocampus. 2010;20:279–292. doi: 10.1002/hipo.20632. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Häusser M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat Neurosci. 2013;16:325–331. doi: 10.1038/nn.3340. [DOI] [PubMed] [Google Scholar]
- Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25:9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss NW, Hasselmo ME. Washington, DC: Society for Neuroscience; 2011. Persistent spiking of medial entorhinal cortical neurons during theta frequency oscillations in vitro. Program No. 730.11. 2011 Neuroscience Meeting Planner. 2011. Online http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=b55b2d4a-c543-4ce7-8061-31814b571874&cKey=0c87867e-4912-4b6f-a387-0311cec0af97&mKey=%7b8334BE29-8911-4991-8C31-32B32DD5E6C8%7d Last accessed on April 9, 2013. [Google Scholar]
- Shalinsky MH, Magistretti J, Ma L, Alonso AA. Muscarinic activation of a cation current and associated current noise in entorhinal-cortex layer-II neurons. J Neurophysiol. 2002;88:1197–1211. doi: 10.1152/jn.2002.88.3.1197. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–13. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Tahvildari B, Fransén E, Alonso AA, Hasselmo ME. Switching between “On” and “Off” states of persistent activity in lateral entorhinal layer III neurons. Hippocampus. 2007;17:257–263. doi: 10.1002/hipo.20270. [DOI] [PubMed] [Google Scholar]
- Tsuno Y, Kashiwadani H, Mori K. Behavioral state regulation of dendrodendritic synaptic inhibition in the olfactory bulb. J Neurosci. 2008;28:9227–9238. doi: 10.1523/JNEUROSCI.1576-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NLM, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Weyrick A, Terman D, Hallworth NE, Bevan MD. A model of reverse spike frequency adaptation and repetitive firing of subthalamic nucleus neurons. J Neurophysiol. 2004;91:1963–1980. doi: 10.1152/jn.00924.2003. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J Neurosci. 2006;26:6213–6229. doi: 10.1523/JNEUROSCI.5594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Câteau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462:218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Hasselmo ME. Persistent firing supported by an intrinsic cellular mechanism in a component of the head direction system. J Neurosci. 2009;29:4945–4952. doi: 10.1523/JNEUROSCI.5154-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Reboreda A, Alonso A, Barker PA, Séguéla P. TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus. 2011;21:386–397. doi: 10.1002/hipo.20755. [DOI] [PubMed] [Google Scholar]


