Abstract
Breathing in mammals depends on an inspiratory-related rhythm that is generated by glutamatergic neurons in the pre-Bötzinger complex (preBötC) of the lower brainstem. A substantial subset of putative rhythm-generating preBötC neurons derive from a single genetic line that expresses the transcription factor Dbx1, but the cellular mechanisms of rhythmogenesis remain incompletely understood. To elucidate these mechanisms, we carried out a comparative analysis of Dbx1-expressing neurons (Dbx1+) and non-Dbx1-derived (Dbx1−) neurons in the preBötC. Whole-cell recordings in rhythmically active newborn mouse slice preparations showed that Dbx1+ neurons activate earlier in the respiratory cycle and discharge greater magnitude inspiratory bursts compared with Dbx1− neurons. Furthermore, Dbx1+ neurons required less input current to discharge spikes (rheobase) in the context of network activity. The expression of intrinsic membrane properties indicative of A-current (IA) and hyperpolarization-activated current (Ih) tended to be mutually exclusive in Dbx1+ neurons. In contrast, there was no such relationship in the expression of currents IA and Ih in Dbx1− neurons. Confocal imaging and digital morphological reconstruction of recorded neurons revealed dendritic spines on Dbx1− neurons, but Dbx1+ neurons were spineless. The morphology of Dbx1+ neurons was largely confined to the transverse plane, whereas Dbx1− neurons projected dendrites to a greater extent in the parasagittal plane. The putative rhythmogenic nature of Dbx1+ neurons may be attributable, in part, to a higher level of intrinsic excitability in the context of network synaptic activity. Furthermore, Dbx1+ neuronal morphology may facilitate temporal summation and integration of local synaptic inputs from other Dbx1+ neurons, taking place largely in the dendrites, which could be important for initiating and maintaining bursts and synchronizing activity during the inspiratory phase.
Key points
The transcription factor Dbx1 gives rise to putatively respiratory rhythm-generating neurons in the pre-Bötzinger complex. Comparative analysis of Dbx1-derived (Dbx1+) and non-Dbx1- derived (Dbx1−) neurons can help elucidate the cellular bases of respiratory rhythm generation.
In vitro, Dbx1+ neurons activate earlier in the respiratory cycle, discharge larger magnitude inspiratory bursts and exhibit a lower rheobase compared with Dbx1− neurons.
The Dbx1+ neurons tend to express the intrinsic currents IA (transient outward A-current) and Ih (hyperpolarization-activated current) in diametric opposition, which may facilitate temporal summation of excitatory synaptic inputs, whereas the Dbx1− neurons show no significant pattern of expression regarding IA and Ih.
The Dbx1+ neurons exhibit smooth, spineless dendrites that project in the transverse plane, whereas the Dbx1− neurons are confined to the transverse plane to a lesser extent and sometimes exhibit spines.
The properties of Dbx1+ neurons that may contribute to respiratory rhythmogenesis include a high level of excitability linked to ongoing network activity and dendritic properties that may facilitate synaptic integration.
Introduction
The pre-Bötzinger complex (preBötC) of the ventrolateral medulla contains the neurons that generate the inspiratory phase of the respiratory rhythm (Smith et al. 1991; Feldman & Del Negro, 2006). Rhythm generation depends on glutamatergic neurons, as well as neurons that express neuropeptides and peptide receptors (Gray et al. 1999, 2001; Wallen-Mackenzie et al. 2006; Tan et al. 2008). Neurons with glutamatergic and peptidergic transmitter phenotypes that also express peptide receptors form a superset of neurons from the same genetic lineage. In the mouse embryo, the homeobox gene Dbx1 controls the fate of glutamatergic commissural interneurons in the hindbrain, including rhythm-generating preBötC neurons (Bouvier et al. 2010; Gray et al. 2010). In the absence of Dbx1, the preBötC does not form, and mouse pups die at birth without taking a breath or making any respiratory movements whatsoever (Pierani et al. 2001; Bouvier et al. 2010; Gray et al. 2010). We hypothesize that the intrinsic and morphological features that differentiate Dbx1-derived (Dbx1+) neurons from non-Dbx1-derived (Dbx1−) neurons, which are probably GABAergic or glycinergic preBötC neurons (Kuwana et al. 2006; Winter et al. 2009; Morgado-Valle et al. 2010), may help to elucidate the cellular mechanisms of respiratory rhythmogenesis in the preBötC.
Brainstem slices containing the preBötC spontaneously generate inspiratory motor patterns in vitro that can be monitored via the hypoglossal (XII) cranial nerve (Smith et al. 1991; Feldman & Del Negro, 2006; Feldman et al. 2013). Previous studies have investigated the membrane properties implicated in rhythm generation, although without regard to the genotype or transmitter phenotype of the preBötC neurons studied. During a respiratory cycle in vitro that generally lasts 5–10 s, putatively rhythmogenic neurons are the earliest to activate, depolarizing and starting to discharge spikes approximately 400 ms prior to the inspiratory phase marked by XII motor output (Rekling et al. 1996a; Thoby-Brisson & Ramirez, 2001). The presence of a transient outward current, i.e. A-current (IA), and the lack of a hyperpolarization-activated mixed cationic current, i.e. h-current (Ih), have also been hypothesized to influence inspiratory burst generation by promoting orderly recruitment of constituent rhythmogenic neurons and preventing spurious discharge (Rekling et al. 1996a; Hayes et al. 2008). Neurons distributed throughout the ventral respiratory brainstem networks, including the preBötC, express persistent Na+ current (INaP), which gives rise to voltage-dependent membrane behaviours that are hypothesized to influence rhythmogenesis as well (Koshiya & Smith, 1999; Del Negro et al. 2002a; Ptak et al. 2005; Koizumi & Smith, 2008; but see Del Negro et al. 2002b, 2005; Pace et al. 2007a). Finally, since 2001, it has been recognized that inspiratory bursts in preBötC neurons also involve the Ca2+-activated non-specific cation current (ICAN), which can, in some cases, give rise to large-magnitude drive potentials that depolarize preBötC neurons to such an extent that spike-generating currents inactivate, i.e. ICAN can cause intraburst depolarization block (Thoby-Brisson & Ramirez, 2001; Pena et al. 2004; Crowder et al. 2007; Pace et al. 2007b; Rubin et al. 2009).
Here, we analyse the expression of the membrane properties described above in preBötC neurons with a known genetic background. Given that Dbx1 expression gives rise to glutamatergic as well as peptidergic and peptide receptor-expressing rhythmogenic neurons in the preBötC, the differences between Dbx1+ and Dbx1− neurons may help to reveal the cellular and ionic bases for respiratory rhythmogenesis.
Methods
Neonatal mouse slice preparations
The following protocols have been approved by The College of William & Mary Institutional Animal Care and Use Committee, which operates in accordance to the US National Institutes of Health Office of Laboratory Animal Welfare, and were performed to conform with the ethical standards as outlined by Drummond (2009). We used transgenic mice that express Cre recombinase fused to the tamoxifen-sensitive estrogen receptor Dbx1+/CreERT2 (Hirata et al. 2009), floxed reporter mice with inducible expression of the red fluorescent protein variant tdTomato (B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J or Rosa26tdTomato, Jax no. 007905; Madisen et al. 2010) and floxed reporter mice with inducible expression of enhanced YFP (Gt(ROSA)26Sortm1(Smo/EYFP)Amc/J or Rosa26EYFP, Jax no. 005130). Both the Dbx1+/CreERT2 and Rosa26EYFP strains were bred in-house using a CD-1 background strain. The Rosa26tdTomato was maintained as a homozygous line with C57BL/6J background. We verified animal genotype via real-time PCR using primers specific for Cre, EYFP and tdRFP (Transnetyx, Cordova, TN, USA).
Neonatal mice (n= 83) were obtained from timed matings of Dbx1+/CreERT2 with either Rosa26tdTomato (n= 69) or Rosa26EYFP reporter mice (n= 14). We visually inspected vaginal plugs to confirm successful mating and induced Cre recombination by administering tamoxifen to Dbx1+/CreERT2 dams at embryonic day 10.5 (E10.5). If no vaginal plug was detected after 4 days, but the female was judged pregnant by gain of mass and visual signs of abdominal distension, then we utilized a ‘48 h rule’, where we counted 48 h from the start of mating and designated that as E0. In this case, tamoxifen induction of the Cre recombinase falls within E9.5–E12.5, during which time Dbx1 is expressed in hindbrain (Pierani et al. 2001; Hirata et al. 2009; Gray et al. 2010). Tamoxifen (T5648; Sigma Aldrich, St Louis, MO, USA) was dissolved at a concentration of 10 mg ml−1 in corn oil and administered by oral gavage to pregnant dams at a concentration of 1 mg (40 g body mass)-1.
Neonatal Dbx1+/CreERT2; Rosa26tdTomato and Dbx1+/CreERT2; Rosa26EYFP mice at postnatal day 0–5 (P0–5) were killed following 4 min of immersion in crushed ice until there was no longer a pinch response from the animals (in compliance with the 2011 guidelines of the Animal Research Advisory Committee, Office of Animal Care and Use, National Institutes of Health, Bethesda, MD, USA). This method renders the animals insentient to the same degree as would occur with gaseous anaesthetics (Danneman & Mandrell, 1997; Fox et al. 2007) and facilitates the rapid isolation of the intact brainstem and spinal cord, which would otherwise be damaged if cervical dislocation were used to kill the animals. After the animals were anaesthetized via immersion hypothermia, they were transferred to a dissecting dish and two transections were performed at bregma and the thorax. The neuraxis, from the pons to the lower thoracic spinal cord, was then rapidly removed and further dissected in dish filled with standard artificial cerebrospinal fluid (ACSF) containing (mm): 124 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 25 NaHCO3, 0.5 NaH2PO4 and 30 dextrose, equilibrated with 95% O2 and 5% CO2 (pH 7.4). The neuraxis was isolated and pinned onto a paraffin-coated paddle, with its rostral side up and ventral surface facing out. The paddle was fixed into the vice of a vibrating microtome. We cut transverse 550-μm-thick brainstem slices with the preBötC exposed at the rostral face (Ruangkittisakul et al. 2011). Slices were then perfused with ACSF at 26–28°C in a recording chamber on a fixed-stage Zeiss Axioskop (Thornwood, NY, USA) with infrared-enhanced differential interference contrast (IR-DIC) imaging and epifluorescence, which enables visual identification and selective recording of target neurons. The K+ concentration in the ACSF was elevated to 9 mm (standard 9 mm K+ ACSF) to maintain long-term stability of the preBötC rhythm.
Electrophysiology
Inspiratory-related motor output was recorded from the XII nerve rootlets, which are captured with the preBötC in transverse slices, using suction electrodes and a differential amplifier (Dagan Instruments, Minneapolis, MN, USA). The amplifier gain was set at 2000 and the bandpass filter was set at 300–1000 Hz. The XII discharge was full-wave rectified and smoothed for display. Whole-cell patch-clamp recordings were obtained using patch pipettes with resistance of 4–6 MΩ and a Dagan IX2-700 current-clamp amplifier. The IR-DIC imaging was used to target patch pipettes after fluorescent identification of Dbx1+ neurons. All recordings were digitally acquired at 10 kHz using a PowerLab 16-bit A/D converter after 1 kHz low-pass filtering (AD Instruments, Colorado Springs, CO, USA).
The patch solution contained (mm): 140 potassium gluconate, 10 Hepes, 5 NaCl, 1 MgCl2, 0.1 EGTA, 2 Mg-ATP, 0.3 Na3-GTP and 2 mg ml−1 biocytin (B4261; Sigma Aldrich). We added 50 μm of an Alexa hydrazide dye to the patch solution for fluorescent visualization of the neurons recorded in the whole-cell configuration. Alexa 568 (A10436; Invitrogen, Carlsbad, CA, USA) was used in Rosa26EYFP-labelled neurons and Alexa 488 (A10437; Invitrogen) in Rosa26tdTomato-labelled neurons.
Current-clamp protocols were performed first in standard 9 mm K+ ACSF, and then in the presence of a cocktail of ionotropic neurotransmitter receptor antagonists, hereafter called ‘blockers’ (5 μm picrotoxin, 5 μm strychnine, 10 μm 6-cyano-7-nitroquinoxaline-2, 3-dione and 20 μM DL-2-amino-5-phosphonovalerate) or in a modified low-Ca2+, high-Mg2+ ACSF, hereafter called ‘low Ca2+’. The low-Ca2+ ACSF contained 0.5 mm Ca2+ and 2 mm MgSO4. Equimolar substitution of Mg2+ for Ca2+ ensured that the total concentration of divalent cations remained fixed. We used standard 9 mm K+ ACSF to record membrane properties of Dbx1+ neurons in conditions where the respiratory network functions in vitro and the synaptic and intrinsic activity can be measured in the context of respiratory rhythm. Then, we added blockers to stop the rhythm and thus measure intrinsic membrane properties in the absence of several common types of ionotropic EPSPs and IPSPs. However, metabotropic receptor-mediated synaptic transmission may still persist in the presence of blockers. Therefore, we used the low-Ca2+ ACSF to attain a more complete synaptic isolation of Dbx1+ neurons and remeasured intrinsic membrane properties, albeit with the caveat that other biophysical membrane properties, such as Ca2+-dependent K+ currents, could be affected (Onimaru et al. 2003; Zavala-Tecuapetla et al. 2008). Nevertheless, low-Ca2+ ACSF does not directly affect Na+-dependent outward currents in preBötC neurons (Del Negro et al. 2009; Krey et al. 2010). Membrane potential values were not corrected for the liquid junction potential, which measured 1 mV.
We analysed inspiratory drive potentials using the peak parameters plugin in Chart version 7.0 software (AD Instruments). Amplitude and area were measured by digitally smoothing the traces to minimize spikes but preserve the underlying envelope of depolarization of the inspiratory drive potential (Pace et al. 2007a). Inspiratory drive latency, i.e. burst latency, quantifies the time interval during which a neuron receives temporally summating excitatory synaptic input and may begin to depolarize and discharge spikes, prior to the onset of the XII motor output, which defines the onset of the inspiratory phase in vitro. We measured the time interval from the point of first depolarization due to summating EPSPs above baseline membrane potential to the maximal slope of the XII output (Rekling et al. 1996a; Hayes & Del Negro, 2007). In order to determine the burst latency for any given preBötC neuron, we averaged this interval from at least five consecutive cycles.
Three intrinsic properties (described below) were measured from a baseline membrane potential of −60 mV to provide a uniform standard of comparison among Dbx1+ and Dbx1− preBötC neurons. Direct current bias was applied to hold baseline voltage at −60 mV. The membrane time constant (τM) was fitted by regression to an exponential function based on the membrane voltage response to a 500 ms hyperpolarizing current pulse. To obtain input resistance (RN), we applied 1 s current steps in a 10-step sequence. We plotted the resulting voltage–current relationship (V–I) and measured its slope in the linear region between (approximately) −95 and −55 mV. To measure the rheobase at a baseline of −60 mV (rheobase-60), we applied 12 ms current pulses and manually adjusted the step-current magnitude until it evoked a single spike on the termination of the current pulse at least 50% of the time. The whole-cell capacitance (CM) was calculated using RN and τM.
We tested for membrane properties indicative of the transient outward current IA (Rekling et al. 1996a). We applied suprathreshold 500 ms depolarizing current pulses from baseline membrane potentials of approximately −80 and −50 mV, maintained using direct current bias. The presence of IA caused a delay of 100–250 ms in spiking activity when the depolarizing current pulse was applied from a holding potential of −80 mV, but no delay in the onset of spiking activity from a holding potential of −50 mV.
We tested for Ih, a mixed cationic current activated by hyperpolarization. We applied 500 ms hyperpolarizing current pulses from a holding potential of −55 mV. The presence of Ih causes a voltage-dependent ‘sag’ response, wherein the membrane potential trajectory depolarizes back towards the holding potential during the step command (Biel et al. 2009).
We compared each parameter measured in Dbx1+ and Dbx1− neurons at postnatal ages P0–5. Data were analysed and reported as means ± SEM. Significance was assessed using a two-way ANOVA for data with multiple independent variables. The F values from the ANOVAs were reported with the degrees of freedom in parentheses (between groups, within groups). For data with binary classification, we used Fisher's exact test, which is a type of χ2 test used when a particular category has five or fewer samples. Significance was set at a minimum of P < 0.05. Statistical significance is indicated as *P < 0.05 and **P < 0.01.
Morphological reconstruction
Slices containing biocytin-loaded neurons were fixed in 4% paraformaldehyde in 0.1 m sodium phosphate buffer for at least 16 h at 4°C. Then, the slices were treated with Scale solution containing 4 m urea, 10% (w/v) glycerol and 0.1% (w/v) Triton X-100, for 10 days to clear the tissue and remove opaque background staining (Hama et al. 2011). Slices were washed three times for 15 min each in PBS + 1% Triton X-100 and then blocked in 10% heat-inactivated fetal bovine sera (F4135; Sigma Aldrich) for 45 min. Finally, the slices were incubated in fluorescein isothiocyanate-conjugated ExtrAvidin (E2761; Sigma Aldrich) for 2–4 h, rinsed with PBS and coverslipped in Vectashield (H-1500; Vector Laboratories, Burlingame, CA, USA). We visualized recorded neurons using a laser-scanning confocal microscope (Zeiss LSM 510). Images were contrast enhanced and pseudocoloured using the free ImageJ software (National Institutes of Health, Bethesda, MD, USA), and then digitally reconstructed using the freeware Neuromantic reconstruction tool (Myatt et al. 2012). The three-dimensional digital reconstructions functioned as tree-like digital objects that we analysed further using the freeware L-Measure (Scorcioni et al. 2008). We compared Dbx1+ and Dbx1− neuron morphologies according to 39 different measurements and used Student's unpaired t tests to compare morphological features between Dbx1+ and Dbx1− neurons with significance set to a minimum value of P < 0.05.
Results
Properties of Dbx1+ and Dbx1− preBötC neurons measured in the context of respiratory rhythm
To study Dbx1+ neurons, we used brainstem slice preparations from neonatal Dbx1+/CreERT2; Rosa26tdTomato (n= 69) and Dbx1+/CreERT2; Rosa26EYFP (n= 14) mice. We visually selected and recorded fluorescent Dbx1+ neurons (n= 106) and non-fluorescent Dbx1− neurons (n= 42) in the preBötC, which were dialysed with fluorescent dye to confirm the recording of the intended neuron in each case (Fig. 1A–C). Inspiratory neurons were identified by depolarizing drive potentials occurring in phase with the XII output. We measured the magnitude of the inspiratory drive potential and the inspiratory drive latency (or burst latency), which quantifies pre-inspiratory activity prior to XII output (see Methods). Baseline membrane potential was −46 ± 1 mV (n= 76) for Dbx1+ neurons and −47 ± 1 mV (n= 42) for Dbx1− neurons (P= 0.6). Figure 1 displays the rhythmic behaviour of Dbx1+ neurons from EYFP (Fig. 1A) and tdTomato (Fig. 1B) reporter strains, and a Dbx1− neuron from the tdTomato strain (Fig. 1C), which suggests differences in drive potential characteristics and burst latency. From a baseline membrane potential of −47 mV, the Dbx1+ neuron is active during the interinspiratory burst interval, including during the pre-inspiratory phase, which was observed as early as ∼1 s prior to XII output (Fig. 1A, red arrows). Inspiratory bursts all exceed 10 mV in amplitude in this Dbx1+ neuron, and there is some voltage-dependent spike inactivation (i.e. depolarization block) during the inspiratory burst (Fig. 1A). In contrast, the Dbx1− neuron, at a baseline of −49 mV shows a flat voltage trajectory during the interinspiratory burst interval, becomes active immediately prior to XII output, and its inspiratory bursts rarely exceed 10 mV (Fig. 1C). Even when a Dbx1+ neuron is held at a hyperpolarized baseline membrane potential (−60 mV) using DC bias, summating synaptic activity is evident during the pre-inspiratory phase several hundred milliseconds prior to XII output. In this case, the lower baseline membrane potential compared with control (not shown) augments the driving force, and the amplitude of the inspiratory drive potential exceeds 20 mV (Fig. 1B). Figure 2A and B illustrates the measurement of the drive potential characteristics and burst latency from a baseline of approximately −50 mV, with XII output, in a Dbx1+ and a Dbx1− neuron. Given that long burst latency (e.g. Fig. 2A) accentuates the drive potential area, we additionally assessed drive potential amplitude, which is insensitive to pre-inspiratory timing.
Figure 1. Dbx1+ (A and B) and Dbx1− inspiratory neurons (C) within the pre-Bötzinger complex (preBötC) of Dbx1+/CreERT2; Rosa26EYFP (A) or Dbx1+/CreERT2; Rosa26tdTomato neonatal mouse slice preparations (B and C).
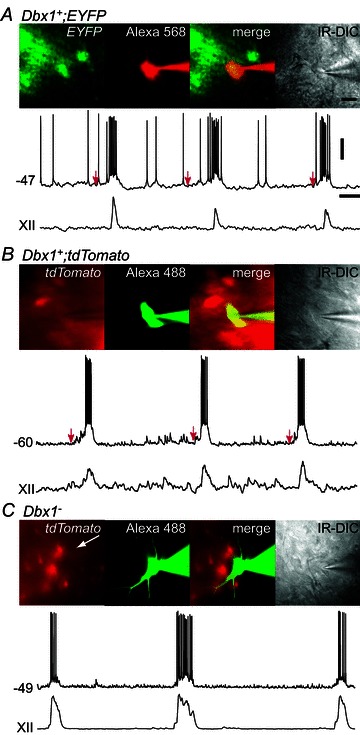
A, EYFP-labelled Dbx1+ neuron, Alexa 568-filled, merged fluorescence and infrared-enhanced differential interference contrast (IR-DIC) images with whole-cell recording (top trace) and integrated hypoglossal cranial nerve (XII) output (bottom trace). Red arrows mark the start of the pre-inspiratory phases. B, tdTomato-labelled Dbx1+ neuron in a Dbx1+/CreERT2; Rosa26tdTomato slice, Alexa 488-filled, merged fluorescence and IR-DIC images with whole-cell recording (top trace) and XII output (bottom trace). C, unlabelled Dbx1− neuron, Alexa 488-filled, merged fluorescence and IR-DIC images with whole-cell recording (top trace) and XII output (bottom trace). Voltage (20 mV) and time (1 s) scale bars apply to all traces. The scale bar (20 μm) shown in the IR-DIC image in A applies to all photographs.
Figure 2. Properties of Dbx1+ and Dbx1− neurons measured in the context of respiratory rhythm.
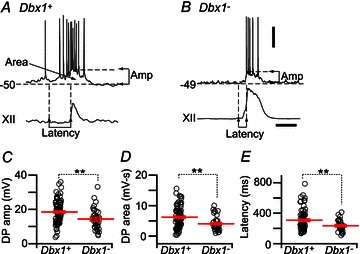
A and B, top traces, single inspiratory burst in a Dbx1+ (A) and a Dbx1− neuron (B) and the corresponding XII output (bottom traces). Horizontal dashed lines indicate the baseline membrane potential and the amplitude of the depolarization envelope in the burst. Vertical dashed lines indicate: (1) the onset of subthreshold pre-inspiratory depolarization; and (2) the onsets of the inspiratory burst and the XII output. The time interval denotes latency. C–E, individual measurements (open circles) of drive potential (DP) amplitude (C), drive potential area (D) and latency (E) of Dbx1+ (n= 82 for drive potential amplitude and area, n= 70 for latency) and Dbx1− neurons (n= 33 for drive potential amplitude and area, n= 28 for latency). Long red horizontal lines show mean values. Short red lines display SEM. **P < 0.01 (two-way repeated measures ANOVA). Voltage (20 mV) and time (0.5 s) scale bars apply to traces in A and B.
We measured Dbx1+ and Dbx1− neurons at P0–5 to determine whether early postnatal development affects rhythmic properties. We used a two-way repeated-measures ANOVA to compare the effect of age on the drive potential characteristics and burst latency between the two groups. There was no significant main effect of age on the drive potential amplitude (F5,100= 1.6, P= 0.17), drive potential area (F5,100= 0.6, P= 0.69) or burst latency (F5,83= 2.2, P= 0.057), so we pooled the data for all postnatal ages (Fig. 2C–E).
The main effect of group on the drive amplitude was significant (F1,100= 9.4, P= 0.0028). The Dbx1+ neurons showed larger drive potential amplitude (18.5 ± 0.8 mV, n= 82) than Dbx1− neurons (14.4 ± 1.1 mV, n= 33). There was also a significant main effect of group on drive potential area (F1,100= 10.6, P= 0.0015), where Dbx1+ neurons showed larger drive potential area (6168 ± 385 mV ms, n= 82) than Dbx1− neurons (4036 ± 438 mV ms, n= 33). Drive latency was also significantly different between the two groups (F1,83= 7.6, P= 0.0073), where Dbx1+ neurons activated earlier (308 ± 16 ms, n= 70) than the Dbx1− neurons (236 ± 15 ms, n= 28).
Intrinsic membrane properties
We measured τM, RN, CM and rheobase-60 in standard 9 mm K+ ACSF in the context of respiratory network activity, and then after silencing the network using a 3 mm K+ ACSF containing a cocktail of ionotropic receptor antagonists (i.e. blockers) or low-Ca2+ ACSF. In each set of conditions, we compared τM, RN and CM measurements between the Dbx1+ and Dbx1− neurons and at the different ages (Fig. 3 and Table 1).
Figure 3. Intrinsic properties of Dbx1+ (open circles) and Dbx1− neurons (open triangles) at the different postnatal ages (P0–5).
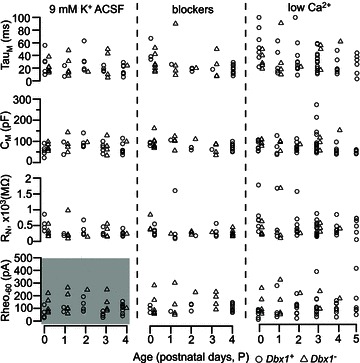
Membrane time constant (τM), input resistance (RN) and rheobase at −60 mV baseline (Rheo-60) were measured in standard 9 mm K+ artificial cerebrospinal fluid (ACSF; left panel), in the presence of ionotropic receptor antagonists/blockers (middle panel), and in low-Ca2+ ACSF (right panel). Whole-cell capacitance (CM) was computed from τM and RN measurements. Rheobase-60 in 9 mm K+ ACSF was significantly different between Dbx1+ and Dbx1− neurons (shaded region in lower left panel). No other measure was statistically significant.
Table 1.
Intrinsic membrane properties of Dbx1+ and Dbx1− neurons
| Treatment | Genotype | τM (ms) | RN (MΩ) | CM (pF) | Rheo-60 (pA) |
|---|---|---|---|---|---|
| 9 mm K+ | Dbx1+ | 23 ± 3 | 340 ± 34 | 69 ± 5 | 87 ± 8* |
| ACSF | (26) | (27) | (26) | (26) | |
| Dbx1- | 23 ± 3 | 295 ± 54 | 82 ± 7 | 138 ± 18* | |
| (17) | (17) | (17) | (16) | ||
| Blockers | Dbx1+ | 24 ± 3 | 308 ± 38 | 75 ± 4 | 101 ± 7 |
| (17) | (17) | (17) | (16) | ||
| Dbx1- | 28 ± 5 | 346 ± 90 | 88 ± 8 | 134 ± 21 | |
| (16) | (16) | (16) | (16) | ||
| Low Ca2+ | Dbx1+ | 33 ± 3 | 499 ± 50 | 76 ± 6 | 107 ± 12 |
| (54) | (51) | (51) | (46) | ||
| Dbx1- | 37 ± 6 | 428 ± 105 | 95 ± 7 | 146 ± 23 | |
| (14) | (14) | (14) | (14) |
Abbreviations: ACSF, artificial cerebrospinal fluid; CM, whole-cell capacitance; Rheo-60, rheobase at a baseline of −60 mV; RN, input resistance; τM, membrane time constant. Values are means ± SEM. The number of neurons is indicated in parentheses. *P < 0.05 (two-way repeated measures ANOVA).
Results of a two-way ANOVA revealed that there was no significant main effect of age or group on the membrane properties τM, RN or CM over all conditions (Fig. 3; P > 0.05), so we pooled the data for all postnatal ages (Table 1). In 9 mm K+ ACSF, τM measured 23 ± 3 ms in both Dbx1+ (n= 26) and Dbx1− neurons (n= 17; F1,32= 0.02, P= 0.89). The RN measured 340 ± 34 MΩ (n= 27) for Dbx1+ and 295 ± 54 MΩ (n= 17) for Dbx1− neurons (F1,33= 0.3, P= 0.59). In Dbx1+ neurons, CM was calculated to be 69 ± 5 pF (n= 26), and in Dbx1− neurons CM was calculated to be 82 ± 7 pF (n= 17; F1,32= 2.0, P= 0.17).
The two-way ANOVA revealed no main effect of age or group on the rheobase-60 measured in blockers and low Ca2+. However, there was a significant main effect of group, but not age, for rheobase-60 in 9 mm K+ ACSF. The Dbx1+ neurons had a lower rheobase-60 (87 ± 8 pA, n= 26) than Dbx1− neurons (138 ± 18 pA, n= 16; F1,27= 7.3, P= 0.012; Fig. 3, grey bottom left panel, and Table 1). Rheobase-60 was lower for Dbx1+ neurons in standard 9 mm K+ ACSF in the context of network activity, but not when synaptically isolated.
Depolarization block during inspiratory bursts
Large-magnitude drive potentials, which cause depolarization block of spiking during inspiratory bursts, are hypothesized to be a signature feature of the mechanism of rhythmogenesis that amplifies synaptic input in preBötC neurons (Rubin et al. 2009). Figure 4 displays Dbx1+ (Fig. 4A and B) and Dbx1− neurons (Fig. 4C) that exhibit depolarization block to emphasize that this phenotype was expressed in both groups. Depolarization block during inspiratory bursts may be attributable to ICAN. To test this putative ionic mechanism, we selectively recorded from four Dbx1+ neurons showing drive potential ≥20 mV, three of which exhibited depolarization block. We previously demonstrated that flufenamic acid (FFA), which reduces but does not completely block ICAN at 100 μm, attenuates inspiratory drive potentials by ∼40% in preBötC neurons (Pace et al. 2007b). Likewise, in Dbx1+ neurons, bath application of FFA (100 μm) reduced the mean drive potential amplitude by 42%, from 30.3 ± 4.0 to 17.6 ± 4.0 mV (n= 4; Fig. 4D, all symbols). In the subset of Dbx1+ neurons showing depolarization block (n= 3), 100 μm FFA abolished the depolarization block in two cases (Fig. 4A and D, filled circles). In the third neuron, FFA did not abolish the depolarization block, although the magnitude of the drive potential was attenuated by 22% (Fig. 4B and D, open triangles). In the Dbx1+ neuron that did not show depolarization block, the drive potential was attenuated by 48% (Fig. 4D, open squares). These data reaffirm that ICAN contributes to inspiratory burst generation and provide experimental evidence suggesting that ICAN contributes substantially to depolarization block. However, ICAN is not the only ionic mechanism underlying depolarizing block; non-ICAN mechanisms may contribute measurably, especially in the case of extremely large-magnitude inspiratory bursts (e.g. Fig. 4B). We observed a total of 41 neurons (both Dbx1+ and Dbx1−) that exhibited depolarization block; however, depolarization block was more common in Dbx1+ neurons. Thirty-five of 95 (37%) Dbx1+ neurons and six of 42 Dbx1− neurons (14%) exhibited depolarization block in more than half of their inspiratory burst cycles, which was a statistically significant difference (Fig. 4E; Fisher's exact test, P= 0.0085).
Figure 4. Intraburst depolarization block in Dbx1+ and Dbx1− neurons.
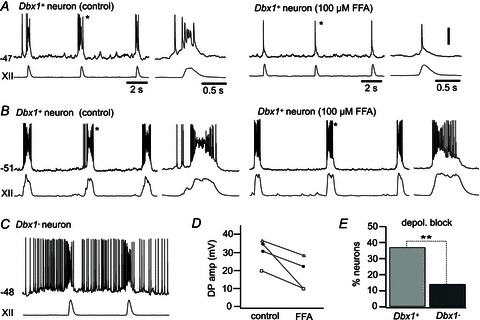
A and B, two example Dbx1+ neurons exhibiting depolarization block during inspiratory bursts in standard 9 mm K+ ACSF (control) and their bursts after bath application of 100 μm flufenamic acid (FFA). Baseline membrane potential reflects zero applied current. C, Dbx1− neuron exhibiting depolarization block during an inspiratory burst. D, drive potential amplitudes in four Dbx1+ neurons in control conditions and in the presence of 100 μm FFA. All four neurons exhibited a decrease in the drive potential amplitude upon bath application of 100 μM FFA. Depolarization block was eliminated by FFA application in two neurons (filled circles), whereas depolarization block persisted after FFA application in one neuron (open triangles, neuron in B). A Dbx1+ neuron with no depolarization block in control conditions also showed a reduction in drive potential amplitude in the presence of FFA (open squares). E, percentage of Dbx1+ (n= 95) and Dbx1− neurons (n= 42) exhibiting intraburst depolarization. Vertical scale bar in A (20 mV) applies to all traces. Separate time scale bars are given for main traces in A–C as well as the single-burst insets in A and B only. **P < 0.01 (Fisher's exact test).
Voltage-dependent bursting properties
We performed rudimentary tests for voltage-dependent bursting properties attributable to coexpression of INaP and non-gated leak K+ current in the appropriate ratio (Del Negro et al. 2002a; Koizumi & Smith, 2008). We adjusted the baseline membrane potential to between −55 and −45 mV and then observed whether ‘ectopic’ bursting emerged in the intervals between XII bursts. This rudimentary test for voltage-dependent pacemaker properties is unsophisticated but nonetheless quite reliable (Del Negro et al. 2001, 2002a,b, 2005; Thoby-Brisson & Ramirez, 2001; Pena et al. 2004). Example traces show inspiratory and ‘ectopic’ bursts of Dbx1+ (Fig. 5A) and Dbx1− neurons (Fig. 5B). Ectopic bursts that occur between inspiratory cycles of XII output, when the baseline membrane potential is biased to the activation threshold of INaP (approximately −55 mV), are a reliable indicator of voltage-dependent bursting properties (Smith et al. 1991; Thoby-Brisson & Ramirez, 2001; Del Negro et al. 2002b, 2005). At baseline voltages below its activation threshold, INaP remains deactivated, and ectopic bursts do not occur. Seventeen of 91 (19%) Dbx1+ neurons and seven of 42 Dbx1− neurons (17%) exhibited ectopic bursting at membrane potentials above −55 mV, whereas burst activity for both groups was confined to the inspiratory cycles at membrane potentials less than −60 mV. We confirmed the voltage-dependent bursting properties by synaptically isolating 12 Dbx1+ neurons that showed ectopic bursts. All continued bursting after application of blockers or low-Ca2+ ACSF (Fig. 5A, right). The relative expression of voltage-dependent bursting activity in Dbx1+ versus Dbx1− neurons was not significantly different (Fisher's exact test, P= 1; Fig. 5C).
Figure 5. Voltage-dependent bursting properties in Dbx1+ and Dbx1− neurons.
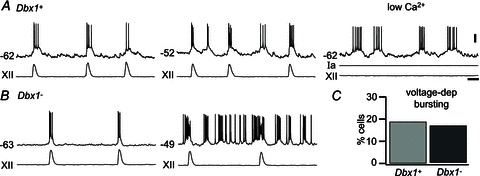
A, a Dbx1+ neuron showing inspiratory bursts at a baseline membrane potential of −62 mV (left), ectopic voltage-dependent bursts at a more depolarized membrane potential of −52 mV (centre) and in low-Ca2+ conditions (right). Ia refers to applied current to demonstrate that bursting is intrinsic after the network activity stops (i.e. no XII output). B, inspiratory bursts and XII output in a Dbx1− neuron (left) and ectopic voltage-dependent bursts at more depolarized membrane potential (right). C, percentage of neurons with voltage-dependent bursting properties in a sample size of 91 Dbx1+ and 42 Dbx1− neurons in the preBötC. Voltage (20 mV) and time (1 s) scale bars apply to all traces.
Relative expression of delayed excitation and ‘sag’ potentials
It has been proposed that preBötC neurons that express IA, but lack Ih, are of primary importance for rhythmogenesis (Rekling et al. 1996a; Gray et al. 1999). These peptide-sensitive neurons were dubbed ‘type 1’ to indicate their putative importance. Neurons that express Ih but not IA, and were shown to be less sensitive to neuropeptides, were proposed to activate downstream from type 1 neurons; hence, this second phenotype was dubbed ‘type 2’ (Rekling et al. 1996a,b; Gray et al. 1999). We evaluated this classification scheme in Dbx1+ and Dbx1− neurons in 9 mm K+ ACSF and in low-Ca2+ conditions.
We performed basic tests for delayed excitation indicative of IA. Twenty-seven of 48 Dbx1+ neurons tested exhibited a 100–250 ms delay in the onset of spiking activity in response to a suprathreshold current step from a holding potential of approximately −80 mV (e.g. Fig. 6A). During this delay, the neurons exhibited a depolarizing ramp-like voltage trajectory, which suggests the slow inactivation of IA (Hayes & Del Negro, 2007). After adjusting DC bias, we injected the same net current during the 500 ms current step, but observed no delay in the onset of spiking from a baseline of −50 mV. In contrast, 21 of 48 Dbx1+ (Fig. 6B) and 12 of 16 Dbx1− neurons (Fig. 6C) showed no delay in the onset of spiking activity in response to 500 ms depolarizing current steps from a holding potential of −80 mV. The spike rate in these neurons (Fig. 6B and C) peaked at the onset of the current step and tended to accommodate thereafter for the remainder of the current stimulus; nor was there a delay in the onset of spiking in these Dbx1+ and Dbx1− neurons in response to the same net current from a holding potential of −50 mV (Fig. 6B and C).
Figure 6. Delayed excitation and ‘sag’ potentials in Dbx1+ (A and B) and Dbx1− neurons (C).
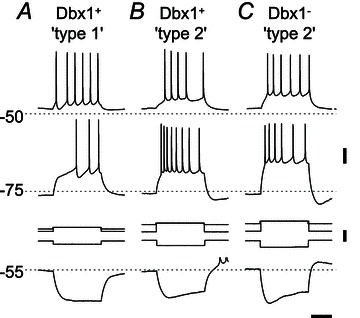
Depolarizing pulses (500 ms) were injected from a depolarized potential (above −50 mV) and from a hyperpolarized potential of −75 mV. Hyperpolarizing pulses were injected from −55 mV. A, a type 1 Dbx1+ neuron exhibiting delayed excitation from −75 mV, but not from −50 mV, and no sag potential in response to the hyperpolarizing pulse. B and C, a type 2 Dbx1+ (B) and a Dbx1− neuron (C) exhibiting sag potentials but no delayed excitation. Spikes are truncated above −50 mV in B (bottom trace). Voltage (20 mV), current (0.2 nA) and time (0.2 s) scale bars apply to all traces.
The presence of Ih causes a depolarizing ‘sag’ in the membrane potential trajectory in response to a sustained hyperpolarizing step current (Biel et al. 2009). Twenty of 27 Dbx1+ neurons that expressed IA did not exhibit such a sag response (Fig. 6A). In contrast, there was a >10 mV sag response in 17 of 21 Dbx1+ (Fig. 6B) and eight of 12 Dbx1− neurons (Fig. 6C) that did not express IA. Neurons that expressed sag potentials often exhibited postinhibitory rebound at the end of the current pulse (Fig. 6B), which could be attributed to slow deactivation of Ih or possibly the de-inactivation of a low-voltage-activated Ca2+ current (Elsen & Ramirez, 2005).
The expression of IA and Ih showed a statistically significant tendency to be mutually exclusive in Dbx1+ neurons, as suggested by the type 1 versus type 2 classification scheme (Rekling et al. 1996a,b; Gray et al. 1999). The Dbx1+ neurons exhibited delayed excitation without sag (type 1; Fig. 6A) or sag without delayed excitation (type 2; Fig. 6B) in comparable proportions (42%, 20 of 48 tested, and 35%, 17 of 48 tested; Table 2). Type 1 and type 2 Dbx1+ phenotypes, as defined by mutually exclusive expression of IA and Ih, occur at significantly higher frequency than the two other possible ‘types’, namely delayed excitation and sag both present (15%, 7 of 48 tested; Table 2) or delayed excitation and sag both absent (8%, 4 of 48 tested; Table 2; Fisher's exact test, P= 0.0004). The Dbx1− neurons (Fig. 6C) generally expressed the type 2 phenotype (50%, 8 of 16 tested; Table 2). However, the other combinations, including type 1 (12.5%, 2 of 16 tested; Table 2), delayed excitation and sag both present (12.5%, 2 of 16 tested; Table 2) and finally delayed excitation and sag both absent (25%, 4 of 16 tested; Table 2), were observed at commensurate frequencies, so the relative prevalence of type 2 among Dbx1− neurons did not rise to statistical significance (Fisher's exact test, P= 0.24).
Table 2.
Expression of delayed excitation and sag potential in Dbx1+ and Dbx1− neurons
| Dbx1+ (n= 48) | Dbx1- (n= 16) | |||
|---|---|---|---|---|
| Characteristic | Sag | No sag | Sag | No sag |
| Delayed excitation | 7 (15%) | 20 (42%) | 2 (12.5%) | 2 (12.5%) |
| No delayed excitation | 17 (35%) | 4 (8%) | 8 (50%) | 4 (25%) |
Morphological properties
We imaged 23 Dbx1+ and 14 Dbx1− biocytin-filled inspiratory neurons (Fig. 7A, B), and then performed three-dimensional digital reconstruction in 14 Dbx1+ and 12 Dbx1− neurons (Figs 8A–F and 9A–F). Digital reconstructions have been added to the public domain via the NeuroMorpho.org database. There were no noticeable differences in the soma shape of the Dbx1+ and Dbx1− neurons, which appeared fusiform or triangular in contour. The Dbx1+ and Dbx1− neurons were not significantly different in surface area measurements. The Dbx1+ neurons measured 561 ± 48 μm2 in soma surface area and 4008 ± 634 μm2 in total somatodendritic surface area, whereas Dbx1− neurons measured 672 ± 59 and 4769 ± 452 μm2, respectively (Student's unpaired t test, P= 0.16 and P= 0.34, respectively).
Figure 7. Morphological characteristics of Dbx1+ and Dbx1− neurons.
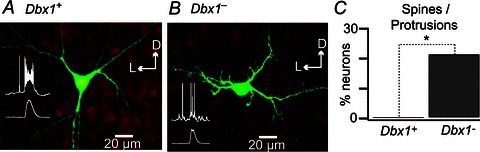
A and B, confocal images of biocytin-filled neurons processed with fluorescein isothiocyanate-conjugated ExtrAvidin. Dbx1+ (A) and Dbx1− neuron (B) in a Dbx1+/CreERT2; Rosa26tdTomato and a representative inspiratory burst and XII output (insets). C, bar graph showing the percentage of Dbx1+ (n= 23) and Dbx1− neurons (n= 14) with dendritic spines/protrusions. *P < 0.05 (Student's unpaired t test). Dorsal and lateral orientation are indicated via arrows labeled D and L.
Figure 8. Digital reconstruction of Dbx1+ (A–C) and Dbx1− neurons (D–F) in the transverse plane, with axons indicated.
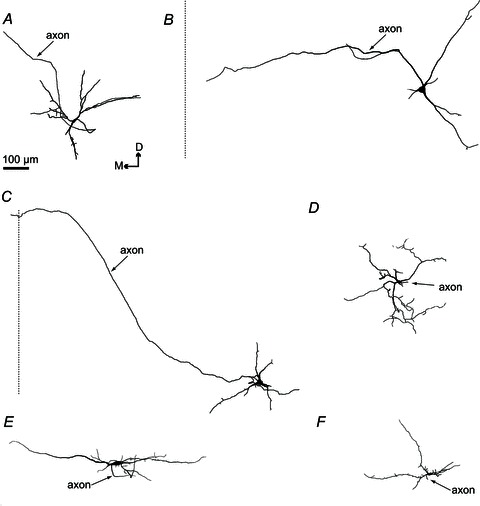
A–C, Dbx1+ neurons (n= 14) with axons that project towards the mid-line of the slice preparation (shown with a dotted line). The axon of one Dbx1+ neuron (C) crossed the mid-line of the slice. D–F, Dbx1− neurons (n= 12) with axons that project short distances before truncation. Dorsomedial orientation is indicated. Scale bar applies to A–F.
Figure 9. Digital reconstruction of Dbx1+ (A–C) and Dbx1− neurons (D–F) in the parasagittal plane, with axons indicated.
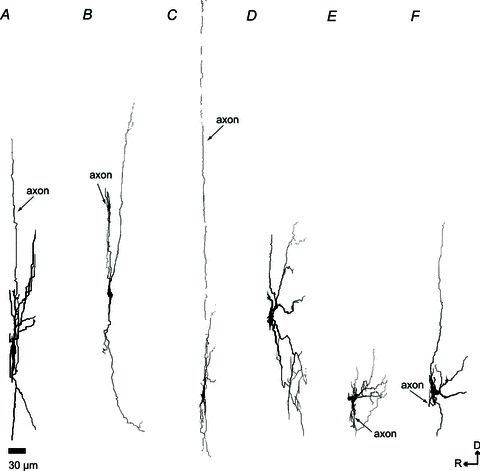
A–C, Dbx1+ neurons. D–F, Dbx1− neurons with projections that span the rostrocaudal axis to a greater extent than Dbx1+ neurons. Abbreviations: D, dorsal; and R, rostral. Scale bar applies to A–F.
The dorsoventral span of dendrites measured on average 269 ± 38 μm in Dbx1+ neurons and 247 ± 21 μm in Dbx1− neurons, which was not significantly different (t test, P= 0.61). The average mediolateral span was also comparable between the two groups (287 ± 38 μm in Dbx1+ versus 292 ± 43 μm in Dbx1−, t test, P= 0.94; Fig. 8). Although Dbx1+ and Dbx1− neurons did not appear to differ in size as measured in dorsoventral or mediolateral orientations, the same was not true for projection in the parasagittal plane. The dendrites of the Dbx1− neurons extended deeper in the rostrocaudal axis, with a total average depth of 54 ± 4 μm, compared with an average total depth of 37 ± 4 μm for Dbx1+ neurons (t test, P= 0.0071; Fig. 9).
The Dbx1− neurons appeared to have more branches (42 ± 8) than Dbx1+ neurons (26 ± 4). The maximal branch order from the soma (soma is order 0; a branch that originates from the soma is order 1; second bifurcation from the same branch is order 2, and so on) was 8 ± 2 in Dbx1− neurons compared with 4 ± 0.4 in Dbx1+ neurons. However, even though the number of branches and branch order differed by approximately 2-fold (Figs 7 and 8), these differences did not reach statistical significance (t tests, P= 0.08).
The dendrites of the 23 Dbx1+ neurons did not exhibit spines (Figs 7A and C and 8A–C) whereas three of 14 Dbx1− neurons (21%) showed spines and protrusions (Figs 7B and C and 8D–F). This difference in the expression (or lack) of dendritic spines and protrusions was statistically significant (Fig. 7C; Fisher's exact test, P= 0.047).
Eleven of 23 Dbx1+ neurons (48%) projected axons towards (n= 10) or across the mid-line (n= 1; Fig. 8A–C). Two of the 14 (14%) Dbx1− neurons had axons projecting towards, yet none crossing, the mid-line (Fig. 8D–F). In many cases, the axons of Dbx1− neurons appeared to be severed at the rostral face of the slice, suggesting a rostral projection path (n= 9 of 14 Dbx1− neurons reconstructed). The severed axon was identified by a bleb, significantly larger in diameter than its parent axon, at the immediate rostral surface of the slice preparation. Nonetheless, the relative fraction of neurons with contralateral projections did not rise to statistical significance (Fisher's exact test, P= 0.0740).
Discussion
The Dbx1+ neurons are putatively an essential respiratory rhythmogenic population and the predominant source of glutamatergic interneurons in the ventrolateral medulla, including the preBötC (Bouvier et al. 2010; Gray et al. 2010). A comparative analysis of inspiratory Dbx1+ and Dbx1− neurons in the preBötC may help to elucidate the cellular mechanisms of rhythm generation. The Dbx1+ neurons differ from Dbx1− neurons mainly on the basis of properties measurable in the context of respiratory network activity, including larger inspiratory burst magnitude and longer burst latency. We used current-clamp protocols to infer the expression of Ih and IA (Thoby-Brisson et al. 2000; Hayes et al. 2008). The Dbx1+ neurons tend to express IA with no detectable Ih, or Ih with no detectable IA, whereas the Dbx1− neurons show no consistent expression patterns. The Dbx1+ neuron dendrites are smooth (spineless) and largely confined to the transverse plane, whereas the Dbx1− neuron dendrites in some cases show spines and protrusions, and in general span a greater range of the parasagittal plane. We propose that intrinsic conductances evoked in the context of network activity and dendritic properties differentiate Dbx1+ and Dbx1− neurons in the preBötC and thus could explain, at least in part, the presumably rhythm-generating function of Dbx1+ neurons.
The Dbx1+ neurons show a longer inspiratory drive latency than the Dbx1− neurons and thus may provide the ‘on’ switch of inspiration. However, Dbx1− neurons also activate prior to XII motor output, and the difference in inspiratory drive latency, while statistically significant, is merely 72 ms. This property of early drive latency is consistent with the pre-inspiratory activity reported for putatively respiratory rhythmogenic neurons that were not identified by transmitter phenotype or genotype, but nonetheless showed drive latency of ∼400 ms and were characterized by expression of IA and neuropeptide sensitivity, but lack of Ih (Rekling et al. 1996a,b). Furthermore, pre-inspiratory activity due to recurrent synaptic excitation has been proposed to be a key rhythmogenic feature based on intracellular studies in vivo and in vitro for 30 years (Richter, 1982; Ezure, 1990; Bianchi et al. 1995; Schwarzacher et al. 1995; Richter & Spyer, 2001; Feldman & Del Negro, 2006).
The Dbx1+ neurons show significantly larger magnitude inspiratory drive potentials than the Dbx1− neurons. Moreover, a significantly greater number of Dbx1+ neurons exhibit intraburst depolarization block, which is hypothesized to be a signature feature of the rhythmogenic mechanism that amplifies excitatory synaptic input. The ionic mechanism underlying the larger inspiratory bursts and depolarization block, in particular, cannot be determined with certainty from the present study. Nevertheless, the recruitment of ICAN is proposed to be important for inspiratory burst generation (Thoby-Brisson & Ramirez, 2001; Pena et al. 2004; Del Negro et al. 2005; Ramirez & Viemari, 2005; Ramirez et al. 2011). Early drive latency and large burst magnitude may be attributable, at least in part, to recurrent synaptic excitation convolved with ICAN expression in Dbx1+ neurons, although other non-ICAN ionic mechanisms, which may include INaP as well as heretofore unrecognized intrinsic currents, cannot be ruled out (e.g. Fig. 4B). The interaction of synaptic input coupled to postsynaptic intrinsic conductances, including (but not limited to) ICAN, may be part of the principal rhythm-generating mechanism, because it is significantly different in Dbx1+ compared with Dbx1− neurons of the preBötC, and leads to greater magnitude inspiratory drive potentials. A critical area for future investigation is to measure the biophysical properties of the intrinsic currents (such as ICAN and INaP, as well as unrecognized novel non-ICAN currents) that amplify synaptic input, to understand the inception of inspiratory drive potential in Dbx1+ neurons of the preBötC.
Pre-Bötzinger complex neurons that express IA but lack Ih (dubbed type 1) were hypothesized to be primary rhythm generators based on their early drive latency (i.e. pre-inspiratory activity) and sensitivity to neuropeptide modulation. Pre-Bötzinger complex neurons that express Ih but not IA (dubbed type 2) were proposed to activate downstream from type 1 neurons (Rekling et al. 1996a,b; Gray et al. 1999; Hayes & Del Negro, 2007), and thus could serve a rhythmogenic or premotor role. A strict classification of type 2 preBötC neurons as premotor is questionable, because blockade of Ih modulates respiratory frequency, which suggests that a subpopulation of type 2 neurons are integrated into the mechanism of rhythm generation (Thoby-Brisson et al. 2000). A premotor role for type 2 neurons notwithstanding, it seems likely that type 1 and type 2 preBötC neurons both serve in a rhythmogenic capacity. The Dbx1+ neurons expressed IA and Ih with a significant trend towards mutual exclusivity, consistent with the classification scheme in the study by Rekling et al (1996). The Dbx1+ neurons belonged to either the type 1 or type 2 group in roughly equal numbers, and infrequently expressed IA and Ih together or lacked expression of both. In contrast, Dbx1− neurons showed no consistent relationship regarding IA and Ih expression, even though 50% were type 2.
Why might the Dbx1+ type 1 phenotype be rhythmogenic? The often complementary expression of IA without Ih could be important for rhythmogenesis if these expression patterns apply to dendrites. We previously measured somatic IA in outside-out patches and proposed that this intrinsic current prevents recurrent excitatory input from causing the membrane potential to cross spike threshold prematurely during the pre-inspiratory phase (Hayes et al. 2008). If IA is expressed in Dbx1+ neuron dendrites, then it could likewise activate at or above threshold to prevent spurious dendritic plateau depolarizations, as previously shown in CA1 pyramidal neuron dendrites (Hoffman et al. 1997; Acker & White, 2007). In contrast, in CA1 neuron dendrites, Ih limits subthreshold temporal summation, allowing proximal and distal inputs to produce the same temporal output patterns (Magee, 1999; Migliore et al. 2004). Therefore, it may be desirable to preclude Ih expression in Dbx1+ interneurons of the preBötC to promote temporal summation of subthreshold inputs in dendrites. We propose that the presence of IA and lack of Ih may prevent spurious burst discharge and yet promote temporal summation in type 1 Dbx1+ neurons during the pre-inspiratory interval. However, this remains to be tested fully by direct recordings in dendrites and via computer simulation.
Type 2 neurons were proposed to activate downstream from type 1 neurons (Rekling et al. 1996a), and in that regard may play a premotor role in respiratory network activity. However, blocking Ih in type 2 neurons significantly boosts collective activity in XII motoneurons and increases respiratory frequency, which indicates that type 2 neurons have a direct role in rhythm generation as well as premotor drive transmission (Thoby-Brisson et al. 2000). With respect to rhythmogenesis, type 2-like inspiratory neurons are proposed to modulate respiratory frequency. Whether Ih acts as a leak current or influences dendritic synaptic integration in a previously unrecognized manner remains to be determined (Thoby-Brisson et al. 2000). With respect to a premotor role, Dbx1+ neurons are found in a continuous dorsomedial-to-ventrolateral line in the transverse plane (Bouvier et al. 2010; Gray et al. 2010), which covers the intermediate region between the preBötC and the XII nucleus containing respiratory premotor neurons (Koizumi et al. 2008; Volgin et al. 2008). Therefore, type 2 Dbx1+ neurons may participate in an oligosynaptic premotor pathway from rhythmogenic preBötC neurons to XII motoneurons, but this remains to be demonstrated.
One-fifth (i.e. 20%) of all neurons in the preBötC, including Dbx1+ glutamatergic neurons (present study), glycinergic neurons (Morgado-Valle et al. 2010), and the neurokinin-1 receptor-expressing population (Pagliardini et al. 2005) that overlaps with Dbx1+ neurons, express voltage-dependent bursting properties. Although expression of INaP in the preBötC is higher than in adjacent regions of the medulla (Ptak et al. 2005; Koizumi & Smith, 2008), voltage-dependent bursting does not differentiate the critical Dbx1+ population from the Dbx1− population, and thus we conclude that voltage-dependent bursting properties are less likely to constitute a key rhythmogenic feature of Dbx1+ neurons. Our identification of voltage-dependent pacemaker properties in Dbx1+ and Dbx1− neurons in the preBötC is unlikely to detect ICAN-dependent pacemaker properties, which are far less voltage dependent (Pena et al. 2004; Del Negro et al. 2005; Ramirez et al. 2011), and thus unlikely to be recognized via ectopic bursting. Nevertheless, ICAN-dependent pacemaker neurons are extremely rare at P0–5 (Pena et al. 2004; Del Negro et al. 2005), so the deficiencies of the screening protocol are unlikely to affect the relative prevalence of pacemaker activity characterized in Dbx1+ versus Dbx1− neurons.
The Dbx1+ neurons are identical in basic membrane properties (τM, RN and CM) to Dbx1− neurons, but have lower rheobase-60 in the context of network activity. The Dbx1+ neurons may be ‘primed’ by ongoing synaptic activity acting via metabotropic receptors and intracellular signalling, which would enhance excitability and facilitate spike discharge, resulting in a lower threshold and rheobase. However, in type-1-like Dbx1+ neurons, the lower rheobase may be mitigated by expression of IA. If the role of IA is to provide an appropriate delay for the onset of plateau-like depolarization in support of inspiratory burst generation, then a lower rheobase may be important to ensure robust spiking at the onset of, and during, inspiratory bursts.
We identified axon projections of Dbx1+ neurons that either crossed or approached the mid-line, which is consistent with the previous finding that Dbx1+ interneurons are commissural and bilaterally synchronize the preBötC (Bouvier et al. 2010). Some Dbx1− neurons showed contralateral projections, but most did not. In many instances, the lack of contralateral projections reflects axotomy due to transverse slicing. A number of Dbx1− neurons appear to have axons projecting rostrally, which may indicate communication with rostral respiratory nuclei, such as the Bötzinger complex or the retrotrapezoid nucleus/parafacial respiratory group. In that regard, we speculate that the inspiratory bursts of Dbx1− neurons provide a faithful mimic of inspiratory burst activity in Dbx1+ neurons that is communicated via rostrally projecting axons to influence or possibly inhibit other respiratory-related nuclei outside the preBötC. If so, then Dbx1− neurons could play a role in co-ordinating inspiratory as well as expiratory motor outputs.
The Dbx1+ neurons have dendrites largely confined to the transverse plane, which may facilitate local synaptic integration among the bilaterally projecting Dbx1+ subpopulation. The Dbx1− neurons extend their dendritic arbor outside the plane of section, deeper in the rostrocaudal axis by ∼17 μm. This difference, while statistically significant, is rather small and suggests that Dbx1− neurons also project predominantly in the transverse plane. Therefore, Dbx1− neurons may also be optimized for synaptic integration locally in the preBötC, while simultaneously their slightly deeper dendritic projections may facilitate synaptic integration from other respiratory sites in the caudal medulla. Furthermore, because their axons were often severed in a rostral trajectory (n= 9 of 14 Dbx1− neurons reconstructed), we speculate that Dbx1− neurons may preferentially project to rostral respiratory circuits.
The Dbx1+ neurons have a tendency for fewer branches with no spines, whereas ∼20% of Dbx1− neurons show spines and protrusions. Smooth, unbranched dendrites in Dbx1+ neurons may promote signal conduction, which would help to retain the amplitude of synaptic input as it propagates from dendrite to soma (Rall & Agon-Snir, 1998). Spines and protrusions have been previously reported on inspiratory preBötC neurons (Koizumi et al. 2008), but our data suggest that these spiny neurons may not be Dbx1 derived.
Some Dbx1− neurons within the preBötC are Lmx1b-derived catecholaminergic neurons (of the C1 group), which are involved in modulating blood pressure and cardiovascular function, but are unlikely to be implicated in respiratory rhythm generation (Gray et al. 2010). The fraction of Dbx1− neurons in the present study that belongs to the C1 group is probably small, because the bulk of the C1 group lies ventral to the preBötC (Schreihofer & Guyenet, 1997).
Approximately half of the inspiratory neurons in the preBötC are reported to be inhibitory glycinergic neurons (Winter et al. 2009). It is therefore conceivable that Dbx1− neurons may be glycinergic neurons, presumably derived from transcription factor Lbx1, which is necessary for the development of glycinergic neurons in the ventral medulla (Pagliardini et al. 2008).
We also acknowledge the possibility of incomplete Cre recombination and that some of the recorded Dbx1− neurons were actually Dbx1-derived neurons that failed to express the reporter protein. We induce Cre recombination by administering tamoxifen at E10.5 to obtain robust reporter expression in hindbrain Dbx1+ neurons (Hirata et al. 2009; Gray et al. 2010). The appearance of a vaginal plug during breeding was sometimes in doubt, and thus it is possible that tamoxifen was administered as late as E12.5 in some cases. However, Dbx1 is expressed at E9.5 in the mouse brain and spinal cord (Lu et al. 1992; Shoji et al. 1996), and is observed even as early as E8.5 in small subsets of forebrain neurons (Causeret et al. 2011). Therefore, Dbx1+ cells that appear earlier than the tamoxifen administration date would remain undetectable via fluorescent protein expression, and could constitute a subset of Dbx1− neurons that exhibited rhythmic properties commensurate with Dbx1+ neurons. We contend that it is unlikely that our Dbx1− subpopulation is dominated by false-negative Dbx1+ neurons, given measurable differences in morphology. It is possible that the Dbx1− population in the present study reflects the GABAergic or glycinergic subpopulation in the preBötC (Kuwana et al. 2006; Winter et al. 2009; Morgado-Valle et al. 2010).
In summary, we evaluated the membrane properties of genetically identified, putatively rhythmogenic neurons in the preBötC to help unravel the cellular mechanisms of respiratory rhythmogenesis. The rhythmogenic nature of Dbx1+ neurons may be attributable, at least in part, to a higher level of intrinsic excitability in the context of network synaptic activity. Although it remains to be demonstrated, we propose that dendritic properties may facilitate temporal summation and integration of local synaptic inputs from other Dbx1+ neurons, which could be important for initiating and maintaining bursts and synchronizing activity during the inspiratory phase.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) grant R01-HL104127-01 (principal investigator: C. A. Del Negro) and National Institute of Neurological Disorders and Stroke (NINDS) grant 1-F31-NS071860-01 (M. C. D. Picardo).
Glossary
- ACSF
artificial cerebrospinal fluid
- CM
whole-cell capacitance
- E
embryonic day
- FFA
flufenamic acid
- IA
transient outward A-current
- ICAN
calcium-activated non-specific cation current
- Ih
hyperpolarization-activated current
- INaP
persistent sodium current
- IR-DIC
infrared-enhanced differential interference contrast
- P
postnatal day
- preBötC
pre-Bötzinger complex
- RN
input resistance
- τM
membrane time constant
- V–I
voltage–current
- XII
hypoglossal (12th) cranial nerve
Author contributions
C.A.D.N. and M.C.D.P. conceptualized and designed the experiments. M.C.D.P. collected and analysed the electrophysiological data. K.T.H.W. and V.T.A. performed reconstructions of the morphology. C.A.D.N. and M.C.D.P. interpreted the data, wrote and revised the article. All authors approved the final version of the manuscript.
References
- Acker CD, White JA. Roles of IA and morphology in action potential propagation in CA1 pyramidal cell dendrites. J Comput Neurosci. 2007;23:201–216. doi: 10.1007/s10827-007-0028-8. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- Causeret F, Ensini M, Teissier A, Kessaris N, Richardson WD, Lucas de Couville T, Pierani A. Dbx1-expressing cells are necessary for the survival of the mammalian anterior neural and craniofacial structures. PLoS One. 2011;6:e19367. doi: 10.1371/journal.pone.0019367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex. J Physiol. 2007;582:1047–1058. doi: 10.1113/jphysiol.2007.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danneman PJ, Mandrell TD. Evaluation of five agents/methods for anesthesia of neonatal rats. Lab Anim Sci. 1997;47:386–395. [PubMed] [Google Scholar]
- Del Negro CA, Johnson SM, Butera RJ, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. III. Experimental tests of model predictions. J Neurophysiol. 2001;86:59–74. doi: 10.1152/jn.2001.86.1.59. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Kam K, Hayes JA, Feldman JL. Asymmetric control of inspiratory and expiratory phases by excitability in the respiratory network of neonatal mice in vitro. J Physiol. 2009;587:1217–1231. doi: 10.1113/jphysiol.2008.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002a;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: an emergent network property. Neuron. 2002b;34:821–830. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen FP, Ramirez JM. Postnatal development differentially affects voltage-activated calcium currents in respiratory rhythmic versus nonrhythmic neurons of the pre-Bötzinger complex. J Neurophysiol. 2005;94:1423–1431. doi: 10.1152/jn.00237.2005. [DOI] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Barthold S, Davisson M, Newcomer C, Quimby F, Smith A, editors. The Mouse in Biomedical Research, 2nd edition, volume 3, Normative Biology, Husbandry, and Models. Burlington, MA, USA: Academic Press; 2007. [Google Scholar]
- Gray PA, Hayes JA, Ling G, Llona I, Tupai S, Picardo MC, Ross S, Hirata T, Corbin JG, Eugenin J, Del Negro CA. Developmental origin of preBötzinger complex respiratory neurons. J Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H, Fukami K, Sakaue-Sawano A, Miyawaki A. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 2011;14:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- Hayes JA, Del Negro CA. Neurokinin receptor-expressing pre-Bötzinger complex neurons in neonatal mice studied in vitro. J Neurophysiol. 2007;97:4215–4224. doi: 10.1152/jn.00228.2007. [DOI] [PubMed] [Google Scholar]
- Hayes JA, Mendenhall JL, Brush BR, Del Negro CA. 4-Aminopyridine-sensitive outward currents in preBötzinger complex neurons influence respiratory rhythm generation in neonatal mice. J Physiol. 2008;586:1921–1936. doi: 10.1113/jphysiol.2008.150946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141–149. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Smith JC. Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Bötzinger complex in vitro. J Neurosci. 2008;28:1773–1785. doi: 10.1523/JNEUROSCI.3916-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Wilson CG, Wong S, Yamanishi T, Koshiya N, Smith JC. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci. 2008;28:2353–2365. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Krey RA, Goodreau AM, Arnold TB, Del Negro CA. Outward currents contributing to inspiratory burst termination in prebotzinger complex neurons of neonatal mice studied in vitro. Front Neural Circuits. 2010;4:124. doi: 10.3389/fncir.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana S, Tsunekawa N, Yanagawa Y, Okada Y, Kuribayashi J, Obata K. Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Bötzinger complex. Eur J Neurosci. 2006;23:667–674. doi: 10.1111/j.1460-9568.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- Lu S, Bogarad LD, Murtha MT, Ruddle FH. Expression pattern of a murine homeobox gene, Dbx, displays extreme spatial restriction in embryonic forebrain and spinal cord. Proc Natl Acad Sci U S A. 1992;89:8053–8057. doi: 10.1073/pnas.89.17.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/12229. [DOI] [PubMed] [Google Scholar]
- Migliore M, Messineo L, Ferrante M. Dendritic Ih selectively blocks temporal summation of unsynchronized distal inputs in CA1 pyramidal neurons. J Comput Neurosci. 2004;16:5–13. doi: 10.1023/b:jcns.0000004837.81595.b0. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBötzinger complex of neonatal mouse. J Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt DR, Hadlington T, Ascoli GA, Nasuto SJ. Neuromantic – from semi-manual to semi-automatic reconstruction of neuron morphology. Front Neuroinform. 2012;6:4. doi: 10.3389/fninf.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ballanyi K, Homma I. Contribution of Ca2+-dependent conductances to membrane potential fluctuations of medullary respiratory neurons of newborn rats in vitro. J Physiol. 2003;552:727–741. doi: 10.1113/jphysiol.2003.049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Role of persistent sodium current in mouse preBotzinger Complex neurons and respiratory rhythm generation. J Physiol. 2007a;580:485–496. doi: 10.1113/jphysiol.2006.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cationic current linked to glutamate receptors. J Physiol. 2007b;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Adachi T, Ren J, Funk GD, Greer JJ. Fluorescent tagging of rhythmically active respiratory neurons within the pre-Bötzinger complex of rat medullary slice preparations. J Neurosci. 2005;25:2591–2596. doi: 10.1523/JNEUROSCI.4930-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Gray PA, Vandunk C, Gross M, Goulding M, Greer JJ. Central respiratory rhythmogenesis is abnormal in Lbx1-deficient mice. J Neurosci. 2008;28:11030–11041. doi: 10.1523/JNEUROSCI.1648-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Ptak K, Zummo GG, Alheid GF, Tkatch T, Surmeier DJ, McCrimmon DR. Sodium currents in medullary neurons isolated from the pre-Bötzinger complex region. J Neurosci. 2005;25:5159–5170. doi: 10.1523/JNEUROSCI.4238-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W, Agon-Snir H. Cable theory for dendritic neurons. In: Koch C, Segev I, editors. Methods in Neuronal Modeling: from Ions to Networks. 2nd edition. Cambridge, MA, USA: MIT Press; 1998. pp. 27–92. [Google Scholar]
- Ramirez JM, Koch H, Garcia AJ, 3rd, Doi A, Zanella S. The role of spiking and bursting pacemakers in the neuronal control of breathing. J Biol Phys. 2011;37:241–261. doi: 10.1007/s10867-011-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Viemari JC. Determinants of inspiratory activity. Respir Physiol Neurobiol. 2005;147:145–157. doi: 10.1016/j.resp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubié M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996a;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubié M. Thyrotropin-releasing hormone (TRH) depolarizes a subset of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996b;75:811–819. doi: 10.1152/jn.1996.75.2.811. [DOI] [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Panaitescu B, Ballanyi K. K+ and Ca2+ dependence of inspiratory-related rhythm in novel “calibrated” mouse brainstem slices. Respir Physiol Neurobiol. 2011;175:37–48. doi: 10.1016/j.resp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci U S A. 2009;106:2939–2944. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Scorcioni R, Polavaram S, Ascoli GA. L-Measure: a web-accessible tool for the analysis, comparison and search of digital reconstructions of neuronal morphologies. Nat Protoc. 2008;3:866–876. doi: 10.1038/nprot.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji H, Ito T, Wakamatsu Y, Hayasaka N, Ohsaki K, Oyanagi M, Kominami R, Kondoh H, Takahashi N. Regionalized expression of the Dbx family homeobox genes in the embryonic CNS of the mouse. Mech Dev. 1996;56:25–39. doi: 10.1016/0925-4773(96)00509-6. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger Complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Telgkamp P, Ramirez JM. The role of the hyperpolarization-activated current in modulating rhythmic activity in the isolated respiratory network of mice. J Neurosci. 2000;20:2994–3005. doi: 10.1523/JNEUROSCI.20-08-02994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgin DV, Rukhadze I, Kubin L. Hypoglossal premotor neurons of the intermediate medullary reticular region express cholinergic markers. J Appl Physiol. 2008;105:1576–1584. doi: 10.1152/japplphysiol.90670.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygård A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J, Hulsmann S. Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflugers Arch. 2009;458:459–469. doi: 10.1007/s00424-009-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Tecuapetla C, Aguileta MA, Lopez-Guerrero JJ, González-Marín MC, Peña F. Calcium-activated potassium currents differentially modulate respiratory rhythm generation. Eur J Neurosci. 2008;27:2871–2884. doi: 10.1111/j.1460-9568.2008.06214.x. [DOI] [PubMed] [Google Scholar]


