Abstract
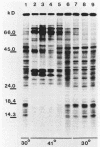
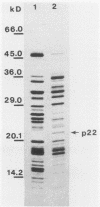
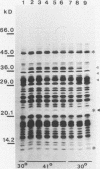
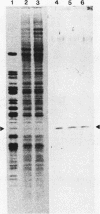
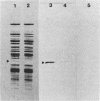
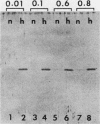
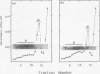
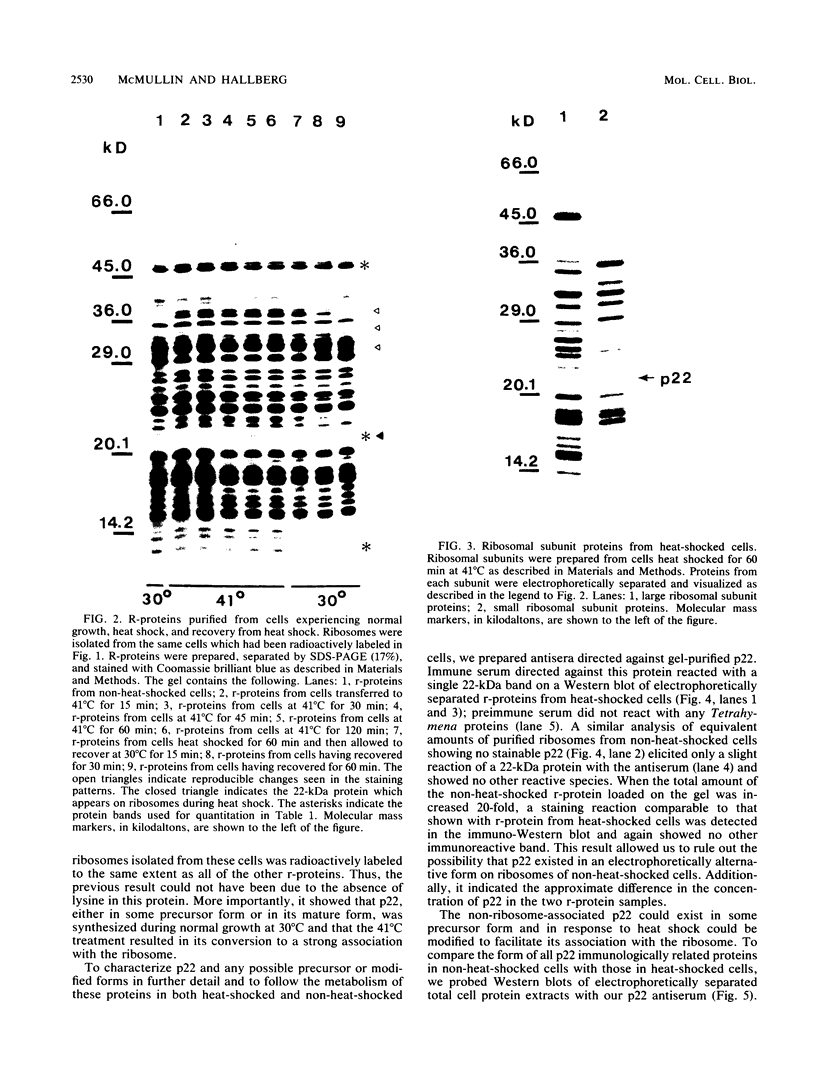
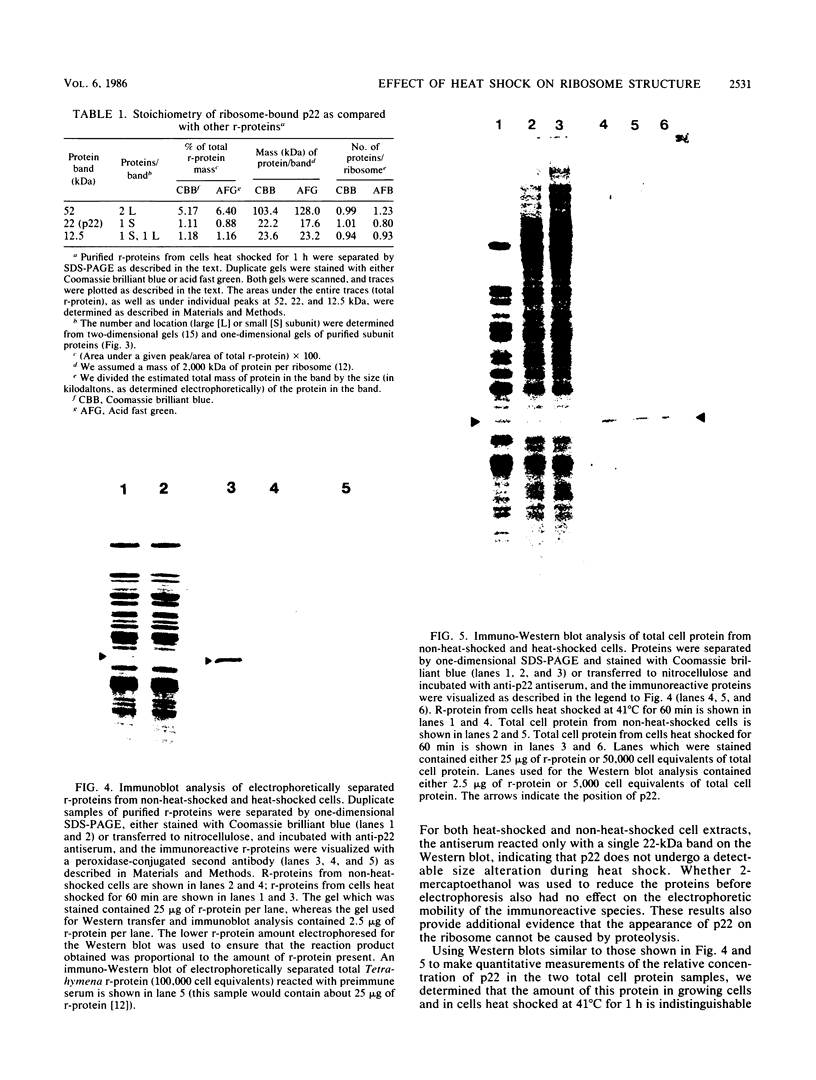
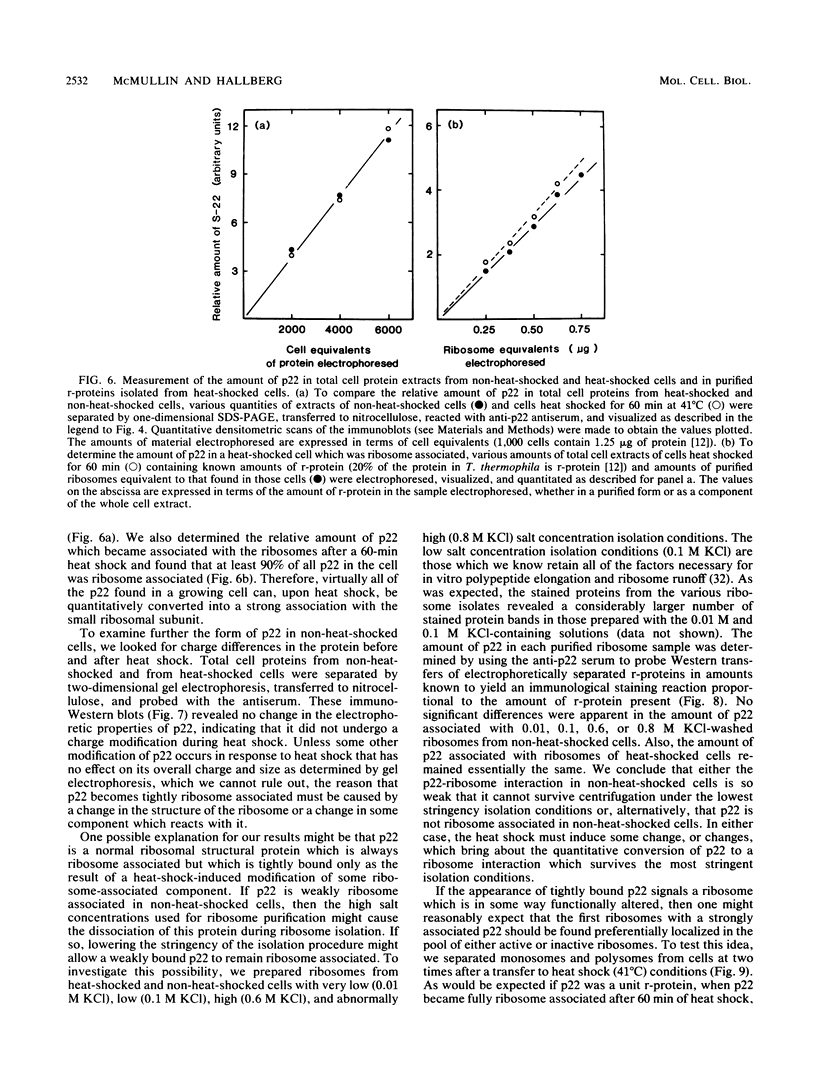
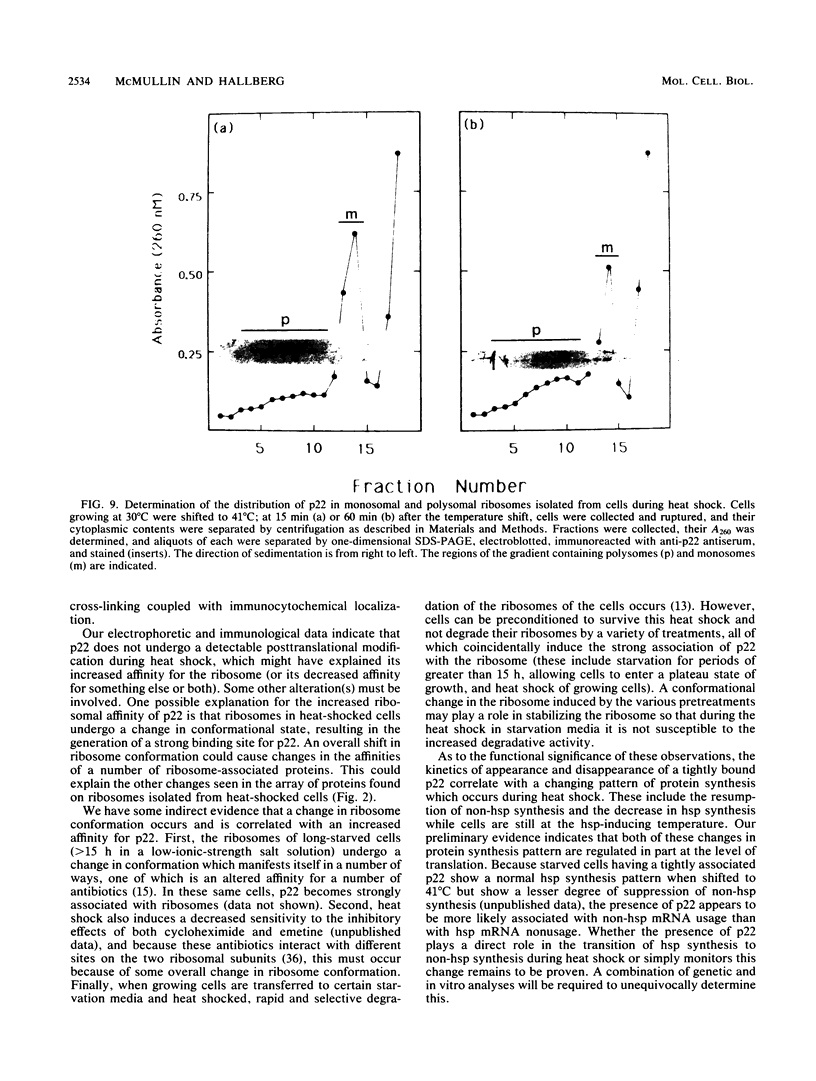
After a nonlethal but heat shock protein-inducing hyperthermic treatment, ribosomes isolated from Tetrahymena thermophila contained an additional 22-kilodalton protein (p22). When maximally ribosome associated, this protein was found to be on the small subunit in a 1:1 stoichiometric ratio with other ribosomal proteins. Using an antiserum directed against the purified 22-kilodalton protein, we found that non-heat-shocked and heat-shocked cells contain identical amounts of this protein, the only difference being that in the stressed cells p22 is entirely ribosome bound, whereas in the unstressed cells p22 has little or no detectable ribosome association. Because the two-dimensional electrophoretic properties of p22 showed no alterations after heat shock, this change in state of ribosome-p22 interaction does not appear to be caused by a chemical modification of p22. When not strongly ribosome associated, p22 is not found free in the cytoplasm. During that time in heat shock when p22 is first becoming ribosome associated, it is found preferentially on polysomal ribosomes. Subsequently, all ribosomes, whether polysome bound or not, obtain a bound p22. The functional significance of this association is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Allen R. L., Wiggins J. C., Chicoine L. G., Richman R. Proteolytic processing of h1-like histones in chromatin: a physiologically and developmentally regulated event in Tetrahymena micronuclei. J Cell Biol. 1984 Nov;99(5):1669–1677. doi: 10.1083/jcb.99.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Banerji S. S., Theodorakis N. G., Morimoto R. I. Heat shock-induced translational control of HSP70 and globin synthesis in chicken reticulocytes. Mol Cell Biol. 1984 Nov;4(11):2437–2448. doi: 10.1128/mcb.4.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Heat shock-induced translational alterations in HeLa cells. Initiation factor modifications and the inhibition of translation. J Biol Chem. 1984 Oct 10;259(19):11882–11889. [PubMed] [Google Scholar]
- Fink K., Zeuthen E. Heat shock proteins in Tetrahymena studied under growth conditions. Exp Cell Res. 1980 Jul;128(1):23–30. doi: 10.1016/0014-4827(80)90382-1. [DOI] [PubMed] [Google Scholar]
- Glover C. V. Heat shock induces rapid dephosphorylation of a ribosomal protein in Drosophila. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1781–1785. doi: 10.1073/pnas.79.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Hallberg R. L., Bruns P. J. Ribosome biosynthesis in Tetrahymena pyriformis. Regulation in response to nutritional changes. J Cell Biol. 1976 Nov;71(2):383–394. doi: 10.1083/jcb.71.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg R. L., Kraus K. W., Findly R. C. Starved Tetrahymena thermophila cells that are unable to mount an effective heat shock response selectively degrade their rRNA. Mol Cell Biol. 1984 Oct;4(10):2170–2179. doi: 10.1128/mcb.4.10.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg R. L., Kraus K. W., Hallberg E. M. Induction of acquired thermotolerance in Tetrahymena thermophila: effects of protein synthesis inhibitors. Mol Cell Biol. 1985 Aug;5(8):2061–2069. doi: 10.1128/mcb.5.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg R. L., Wilson P. G., Sutton C. Regulation of ribosome phosphorylation and antibiotic sensitivity in Tetrahymena thermophila: A correlation. Cell. 1981 Oct;26(1 Pt 1):47–56. doi: 10.1016/0092-8674(81)90032-5. [DOI] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A. Modulation of heat-shock polypeptide synthesis in HeLa cells during hyperthermia and recovery. Biochemistry. 1982 Mar 30;21(7):1513–1521. doi: 10.1021/bi00536a008. [DOI] [PubMed] [Google Scholar]
- Kennedy I. M., Burdon R. H., Leader D. P. Heat shock causes diverse changes in the phosphorylation of the ribosomal proteins of mammalian cells. FEBS Lett. 1984 Apr 24;169(2):267–273. doi: 10.1016/0014-5793(84)80331-2. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem. 1976 May 25;251(10):2867–2875. [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Xuong N. H., Geiduschek E. P. A response of protein synthesis to temperature shift in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5222–5225. doi: 10.1073/pnas.76.10.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A. S., Triemer D. F., Sanders M. M. Dephosphorylation of S6 and expression of the heat shock response in Drosophila melanogaster. Mol Cell Biol. 1983 Nov;3(11):2017–2027. doi: 10.1128/mcb.3.11.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétridou B., Cuny M., Guérin M. F., Hayes F. Ribosomal subunits and ribosomal proteins of Tetrahymena thermophila. Effect of the presence of iodoacetamide during ribosome extraction on the properties of the subunits. Eur J Biochem. 1983 Oct 3;135(3):425–434. doi: 10.1111/j.1432-1033.1983.tb07669.x. [DOI] [PubMed] [Google Scholar]
- Scharf K. D., Nover L. Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell. 1982 Sep;30(2):427–437. doi: 10.1016/0092-8674(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Ares M., Jr, Hallberg R. L. Cycloheximide resistance can be mediated through either ribosomal subunit. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3158–3162. doi: 10.1073/pnas.75.7.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]