Abstract
Cronkhite-Canada syndrome (CCS) is a rare nonfamilial polyposis syndrome characterized by epithelial disturbances both in the gastrointestinal tract and in the epidermis. The pathologic finding of the polyp is usually a hamartomatous polyp of the juvenile type; however, the possibility of serrated adenoma associated malignant neoplasm was reported in some Japanese cases. Up till now in South Korea, 13 CCS cases have been reported, but there was no case accompanied by the colon cancer. We report the first case of CCS associated with malignant colon polyp and serrated adenoma in Korea. A 72-year-old male patient who complained of diarrhea and weight loss was presented with both hands and feet nail dystrophy, hyperpigmentation, and alopecia. Endoscopic examination showed numerous hamartomatous polyps from the stomach to the colon. The pathologic results confirmed colon cancer and serrated adenoma. Helicobacter pylori eradication and prednisolone was used. Thus, the authors report this case along with a literature review.
Keywords: Intestinal polyposis, Colonic neoplasms, Serrated adenoma, Helicobacter pylori
INTRODUCTION
Cronkhite-Canada syndrome (CCS) is a rare nonfamilial polyposis syndrome characterized by two kinds of marked epithelial disturbances. The first is epidermal manifestations including alopecia, onychodystrophy, and hyperpigmentation, and the second is gastrointestinal tract expressions involving hamartomatous polyps of the juvenile type. Usually, CCS colon polyp has been known as a benign neoplasm, but the possibility of serrated adenoma associated with malignant neoplasm was reported in some Japanese cases. In South Korea, 13 CCS cases have been studied until now, yet there was no case accompanied by the colon cancer. The authors diagnosed a CCS patient who was accompanied with multiple polyps in the stomach, the small bowel and the whole colon. Throughout the endoscopic colon polypecotmy, adenocarcinoma in situ along with tubular adenoma and serrated adenoma were confirmed. This is the first case of CCS associated with colon cancer and serrated adenoma in South Korea, so we report here with a literature review.
CASE REPORT
A 72-year-old male complained of chronic diarrhea and weight loss of 10 kg in 1 month. He took a colonoscopy examination at a private clinic showing multiple colonic polyps of varying sizes. He was referred to the Gastrointestinal Department of Presbyterian Medical Center.
There were no abnormalities in his past medical and family history such as gastrointestinal polyposis or colorectal cancer. He had been drinking about 1.5 bottles of Soju (Korean liquor) every day for 20 years.
He was showing chronic signs of illness, but his vital signs were relatively stable. He was 164.3 cm tall and weighed 47 kg (body mass index, 17.4). His physical examination revealed mild hair loss and hair pull test showed that more than 10 hairs were pulled out (Fig. 1A). Black discolorations in both hands and feet were observed in limb examination (Fig. 1B). Also, all fingernails of both hands were dry, cracked, and transformed (Fig. 1C).
Fig. 1.
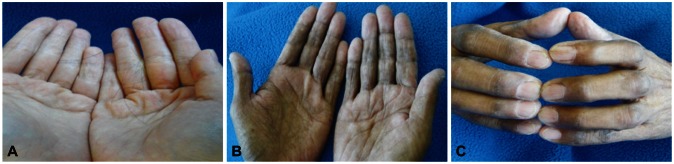
Patient appearance findings. (A) Hair pull test showed that more than 10 hairs were pricked out. (B) Black pigmentation and (C) nail dystrophy was observed in both hand.
A peripheral blood test showed a hemoglobin level of 13.1 g/dL, hematocrit 38.2%, and evidence of megalocytes with mean corpuscular volume of 105.2 fL, mean corpuscular hemoglobin of 36.1 pg, and mean corpuscular hemoglobin concentration of 34.3%. The white blood cell count was 5,600/mm3 and platelet count was 209,000/mm3. Biochemical examinations showed aspartate aminotransferase of 35 IU/L, an alanine aminotransferase of 24 IU/L, total protein level of 5.5 g/dL, albumin level of 3.4 g/dL, total bilirubin level of 0.6 mg/dL, calcium level of 8.6 mg/dL, blood urea nitrogen (BUN) level of 10 mg/dL, creatinine level of 0.8 mg/dL, Na/K/Cl/Co2 of 139/4.3/104/27 mEq/L, and serum carcinoembryonic antigen level of 5.8 ng/mL.
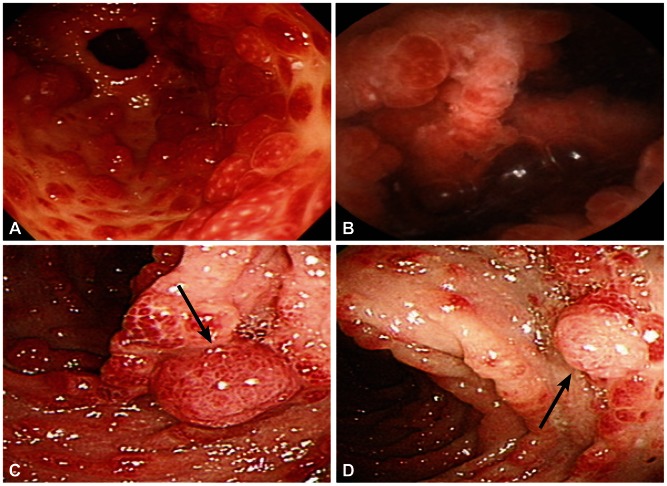
The esophagus was normal in gastroscopy, but several hundred strawberry-like polyps of varying sizes (0.5 to 2.5 cm) were observed in the stomach (Fig. 2A). The number and sizes of the polyps increased in the distal stomach than in the proximal stomach. They were diagnosed as hamartomatous polyps from the biopsy. Rapid urease test (Campylobacter-like organism, CLO test) showed positive result and the stool Helicobacter pylori Ag were also positive. The duodenum was covered with multiple small hyperemic polypoid lesions and numerous tiny polyps on whole small bowel were also observed by capsule endoscopy, but these were not confirmed histologically (Fig. 2B). Thousands of little grape-like polyps that were less than 10 mm in size and some colon polyps of more than 1 cm in size were observed during colonoscopy. Small scale-like hyperemic mucosa under the size of 1 mm was observed without normal mucosa in the terminal ileum. These colon polyps were resected by endoscopic method, and the pathologic results confirmed colon cancer (adenocarcinoma in situ) (Fig. 2C) and serrated adenoma (Fig. 2D).
Fig. 2.
Endoscopic findings. (A) Several hundred 0.5- to 2.5-cm-sized, strawberry-like polyps with normal mucosa in the gastric antrum. (B) Numerous tiny polyps in the small bowel on capsule endoscopy. (C) A 15-mm-sized, grape-shaped polyp (black arrow) with thousands of polyps in the transverse colon (pathologically confirmed adenocarcinoma in situ). (D) A 10-mm-sized, round-shaped polyp (black arrow) in the ascending colon (pathologically confirmed serrated adenoma).
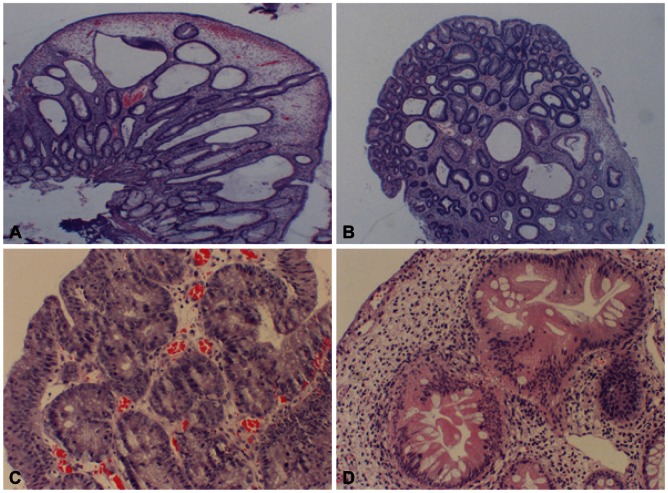
Colonoscopic polypectomy was performed on multiple polyps that were larger than 1 cm. Most of them were inflammatory polyps containing lamina propria, and proliferated tortuous and cystic dilated glands were observed (Fig. 3A). However, adenocarcinoma in situ with background low grade tubular adenomas was confirmed in one of them (Fig. 3B, C). Also, another polyp showed serrated architecture and cytological dysplasia of the crypts, corresponding to serrated adenoma (Fig. 3D).
Fig. 3.
Pathologic findings of colonoscopic polypectomy. (A) Inflamed lamina propria, proliferated tortuous and cystic dilated glands (H&E stain, ×200), (B) adenocarcinoma in situ with background low grade tubular adenomas (H&E stain, ×200), (C) adenocarcinoma in situ (H&E stain, ×400), and (D) serrated adenoma (H&E stain, ×400) were observed.
The patient was given a nutritional therapy under the diagnosis of CCS. Prednisolone of 40 mg/day was administered, and the dosage was reduced by 5 mg every week. H. pylori eradication (proton pump inhibitor, amoxicillin, and metronidazole combination therapy for 2 weeks) was performed and the follow-up CLO test was negative.
At 4 months follow-up, although the patient appeared to have improved, hundreds of 0.5 to 2.5 cm-sized hyperplastic gastric polyps were not improved compared to the previous endoscopic findings. In the follow-up colonoscopy, numerous colon polyps were still discovered in the whole colon. Additional colon polypectomy was performed and the pathologic results confirmed inflammatory polyps and tubular adenoma. The patient is under observation in the outpatient clinic.
DISCUSSION
Since Cronkhite and Canada described the first two cases in 1955, the number of reported cases has increased to more than 400 over the last decade in the world. An old estimate of incidence was about one case per 1 million population. The male-to-female ratio was 1.3:1 and 80% of the patients were over 50 years of age at the time of presentation.1
The characteristic two features of CCS are gastrointestinal polyposis and ectodermal changes that consist of alopecia, nail dystrophy, and hyperpigmentation. CCS usually evolves rapidly over several months and mild gastrointestinal and nutritional deficiency symptoms progress to substantial weight loss and general edema. Ectodermal changes are usually observed several weeks or months after gastrointestinal symptoms have begun and then two or more of the cutaneous triad appear in most patients.2
Until now, etiological studies have been limited because of very low incidence rate. Unlike many other polyposis syndromes, familial patterns of inheritance have not been identified in CCS. The Japanese author of the largest series described mental stress such as psychological suffering or family problems and physical fatigue as the important precipitating factors for their patients.3 The unique sequential involvement of two epithelial tissues suggests that potentially reversible derangements in epithelial cell-to-cell signaling or maturation may play a pivotal role in initiating the syndrome. Although no experimental evidence has validated this hypothesis, the observation that sulindac administration led to regression of CCS polyps may be interpreted as consistent with this mechanism.4
The efficacy of corticosteroids provides the strongest evidence to suggest an inflammatory cause for CCS. A recent provocative report described the infiltration of immunoglobulin G4 (IgG4)-producing plasma cells in half of the CCS polyps studied in seven affected individuals. The authors of this report speculated that CCS was an intestinal manifestation of IgG4-related autoimmune disease.5
The diagnosis of CCS depends on clinical manifestations. Important baseline blood results include serum electrolyte (e.g., calcium, magnesium, potassium, and zinc), BUN, creatinine, albumin, and total protein levels, as well as the prothrombin time and/or the activated partial thromboplastin time and complete blood count. Upper gastrointestinal series along with small bowel follow through is used to evaluate polyps in the stomach and small bowel. Gastroendoscopy and colonoscopy is also used to evaluate polyps in the gastrointestinal tract. Wireless capsule endoscopy has recently been used to visualize the abnormal mucosal appearance throughout most of the small bowel.
The universal histologic finding is hamartomatous polyps of the juvenile type throughout the gastrointestinal tract without typically involving the esophagus. Polyps are noted in the small bowel in approximately one half of all patients, most often in the duodenum and terminal ileum. Mucosal changes are characterized by intact surface epithelium, edematous chronically inflamed lamina propria, and proliferated tortuous glands. Some of glands are cystically dilated and filled with proteinaceous fluid or inspissated mucus.1
Adenomatous changes and carcinoma can occur from hamartomatous polyps in almost 15% of affected patients.6,7 With the increasing observation of gastric and colorectal cancer in patients with CCS, various hypothesizes about this association have been suggested.
In literature review, serrated adenoma has been reported in high prevalence in CCS associated with colorectal cancer. A Japanese group reported that 40% serrated adenomatous polyps in CCS were detected and it was significantly higher compared with about 1% incidence of non-CCS patient's gastrointestinal polyps. Therefore, they proposed the possibility of a serrated adenoma-carcinoma sequence underlying some CCS.7
Our present case is a CCS where serrated adenoma was discovered together with colon cancer. So we think that there is a possibility of a serrated adenoma-carcinoma sequence in this CCS patient. When the histological findings of 13 South Korean CCS cases were analyzed, most cases had hamartomatous polyps or inflammatory polyps and only one case showed tubular adenoma (Table 1).8-19 However, in our case, adenocarcinoma in situ accompanying by dysplasia and tubular adenomas were discovered. This is the first case in South Korea.
Table 1.
Cronkhite-Canada Syndrome in South Korea
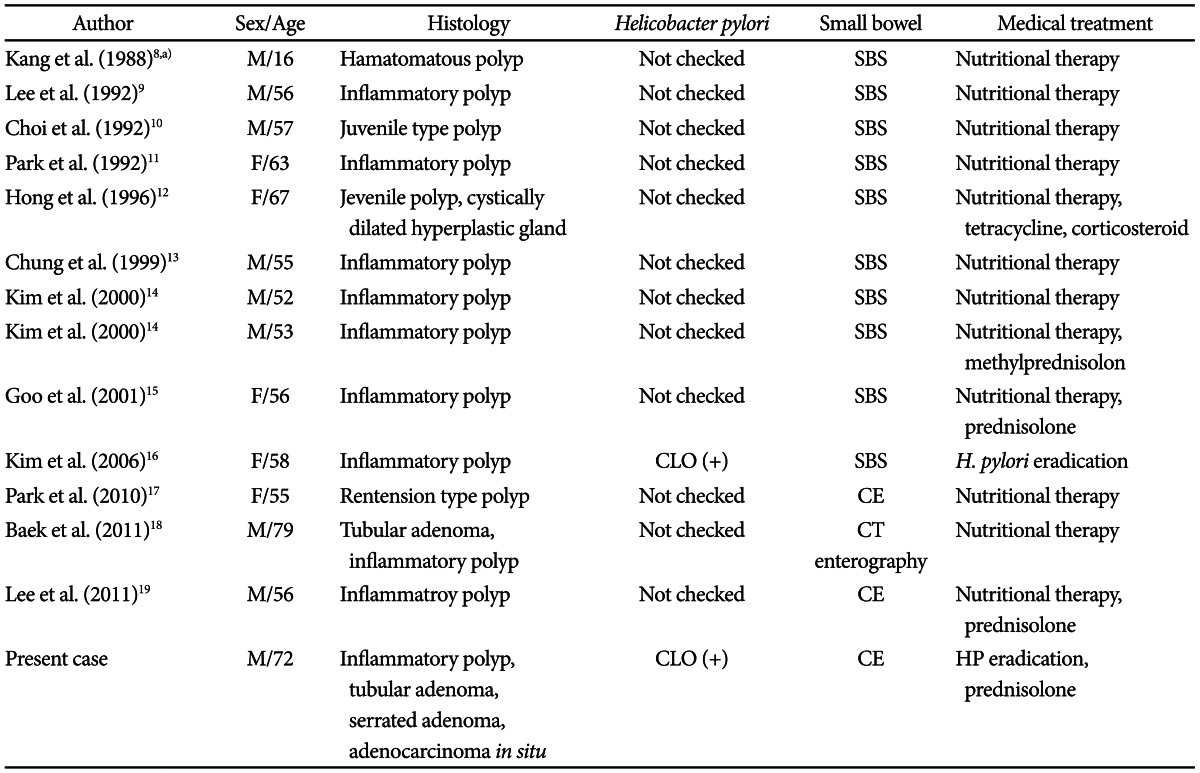
M, male; SBS, small bowel series; F, female; CLO, Campylobacter-like organism; CE, capsule endoscopy; CT, computed tomography.
a)Kang et al., Cronkhite-Canada syndrome in family with Peutz-Jeghers syndrome.
There was no evidence-based medicine or systematic investigations of medical and surgical interventions for CCS treatment. Usually, available management includes nutritional support, administration of zinc, acid suppression, cromolyn sodium, corticosteroid, and eradication of H. pylori. In one case, the successful use of nonsteroidal anti-inflammatory drugs to regress CCS polyps was described,4 but many anecdotal reports support the use of corticosteroid.20 However, the number of data supporting corticosteroid use are insufficient, so it is difficult to accept that as a recommendation.
In the 13 CCS cases reported in South Korea, small bowel work-up was performed by small bowel series, computed tomography enterography or capsule endoscopy and examination of H. pylori infection was carried out by CLO test in only one case. Methylprednisolone and prednisolone were administered in four cases (30%) (Table 1).8-19 In one case, they reported antibiotic treatment (tetracycline) for diarrhea and another case was treated with H. pylori eradication (Table 1).8-19
In the present case, we tried medical treatment with endoscopic polypectomy. A 40 mg of prednisolone was started and was reduced by 5 mg every week. Additionally, H. pylori eradication was carried out.
The prognosis has not been good. A 5-year mortality rate of 55% was reported and malnutrition, hypoalbuminemia, repetitive infection, sepsis, heart failure, and gastrointestinal bleeding were considered as the causes of death.8 There is no guideline for follow-up and surgical treatment. However, we think that a follow-up endoscopic polypectomy can be considered as a follow-up method when there is the possibility of cancer, as in our case.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Daniel ES, Ludwig SL, Lewin KJ, Ruprecht RM, Rajacich GM, Schwabe AD. The Cronkhite-Canada Syndrome. An analysis of clinical and pathologic features and therapy in 55 patients. Medicine (Baltimore) 1982;61:293–309. [PubMed] [Google Scholar]
- 2.Dachman AH, Buck JL, Burke AP, Sobin LH. Cronkhite-Canada syndrome: radiologic features. Gastrointest Radiol. 1989;14:285–290. doi: 10.1007/BF01889219. [DOI] [PubMed] [Google Scholar]
- 3.Goto A. Cronkhite-Canada syndrome: epidemiological study of 110 cases reported in Japan. Nihon Geka Hokan. 1995;64:3–14. [PubMed] [Google Scholar]
- 4.Hizawa K, Nakamori M, Yao T, Matsumoto T, Iida M. A case of Cronkhite-Canada syndrome with colorectal adenomas: effect of the nonsteroidal anti-inflammatory drug sulindac. Am J Gastroenterol. 2007;102:1831–1832. doi: 10.1111/j.1572-0241.2007.01237.x. [DOI] [PubMed] [Google Scholar]
- 5.Riegert-Johnson DL, Osborn N, Smyrk T, Boardman LA. Cronkhite-Canada syndrome hamartomatous polyps are infiltrated with IgG4 plasma cells. Digestion. 2007;75:96–97. doi: 10.1159/000102963. [DOI] [PubMed] [Google Scholar]
- 6.Chadalavada R, Brown DK, Walker AN, Sedghi S. Cronkhite-Canada syndrome: sustained remission after corticosteroid treatment. Am J Gastroenterol. 2003;98:1444–1446. doi: 10.1111/j.1572-0241.2003.07509.x. [DOI] [PubMed] [Google Scholar]
- 7.Yashiro M, Kobayashi H, Kubo N, Nishiguchi Y, Wakasa K, Hirakawa K. Cronkhite-Canada syndrome containing colon cancer and serrated adenoma lesions. Digestion. 2004;69:57–62. doi: 10.1159/000076560. [DOI] [PubMed] [Google Scholar]
- 8.Kang YW, Park SK, Kim H, Bae OS, Chang ES. A case with some components of Cronkhite-Canada syndrome in a family with Peutz-Jeghers syndrome. Korean J Intern Med. 1988;3:136–141. doi: 10.3904/kjim.1988.3.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SK, Kim MY, Choi SH, et al. One case of Cronkhite-Canada syndrome. Korean J Intern Med. 1992;42:278–281. [Google Scholar]
- 10.Choi MS, Kim YT, Jung HC, et al. A case of Cronkhite-Canada syndrome. Korean J Gastroenterol. 1992;24:154–159. [PubMed] [Google Scholar]
- 11.Park UC, Oh MH, Park EU, Kim SY, Seo JM, Park JG. A case report of Cronkhite Canada syndrome in the entire gastrointestinal tract. J Korean Soc Coloproctol. 1992;8:173–180. [Google Scholar]
- 12.Hong SJ, Kwon SH, Kim HJ, et al. A case of Cronkhite-Canada syndrome. Korean J Med. 1996;51:825–831. [Google Scholar]
- 13.Chung ST, Chung EC, Choi JH, Sung KJ, Moon KC, Koh JK. A case of Cronkhite-Canada syndrome. Korean J Dermatol. 1999;37:381–385. [Google Scholar]
- 14.Kim HJ, Jeen YT, Chun HJ, et al. Two cases of Cronkhite-Canada syndrome with remission. Korean J Gastrointest Endosc. 2000;21:543–548. [Google Scholar]
- 15.Goo YS, Shin HJ, Park JY, et al. A case of Cronkhite-Canada syndrome with a remission to steroid therapy. Korean J Gastrointest Endosc. 2001;23:113–117. [Google Scholar]
- 16.Kim MS, Jung HK, Jung HS, et al. A case of Cronkhite-Canada syndrome showing resolution with Helicobactor pylori eradication and omeprazole. Korean J Gastroenterol. 2006;47:59–64. [PubMed] [Google Scholar]
- 17.Park W, Jeon WK, Lee JE, et al. A case of Cronkhite-Canada syndrome conducted with capsule endoscopy of small intestine. Korean J Gastrointest Endosc. 2010;40:126–129. [Google Scholar]
- 18.Baek JH, Kim TY. Cronkhite-Canada syndrome that developed in a patient taking levothyroxine sodium after total thyroidectomy. Korean J Dermatol. 2011;49:45–49. [Google Scholar]
- 19.Lee HJ, Park SJ, Choi HS, et al. A case of Cronkhite-Canada syndrome presenting with hematochezia. Intest Res. 2011;9:238–242. [Google Scholar]
- 20.Ward E, Wolfsen HC, Ng C. Medical management of Cronkhite-Canada syndrome. South Med J. 2002;95:272–274. [PubMed] [Google Scholar]