Abstract
Rationale: Current clinical prediction scores for acute lung injury (ALI) have limited positive predictive value. No studies have evaluated predictive plasma biomarkers in a broad population of critically ill patients or as an adjunct to clinical prediction scores.
Objectives: To determine whether plasma angiopoietin-2 (Ang-2), von Willebrand factor (vWF), interleukin-8 (IL-8), and/or receptor for advanced glycation end products (sRAGE) predict ALI in critically ill patients.
Methods: Plasma samples were drawn from critically ill patients (n = 230) identified in the emergency department. Patients who had ALI at baseline or in the subsequent 6 hours were excluded, and the remaining patients were followed for development of ALI.
Measurements and Main Results: Nineteen patients developed ALI at least 6 hours after the sample draw. Higher levels of Ang-2 and IL-8 were significantly associated with increased development of ALI (P = 0.0008, 0.004, respectively). The association between Ang-2 and subsequent development of ALI was robust to adjustment for sepsis and vasopressor use. Ang-2 and the Lung Injury Prediction Score each independently discriminated well between those who developed ALI and those who did not (area under the receiver operating characteristic curve, 0.74 for each), and using the two together improved the area under the curve to 0.84 (vs. 0.74, P = 0.05). In contrast, plasma levels of sRAGE and vWF were not predictive of ALI.
Conclusions: Plasma biomarkers such as Ang-2 can improve clinical prediction scores and identify patients at high risk for ALI. In addition, the early rise of Ang-2 emphasizes the importance of endothelial injury in the early pathogenesis of ALI.
Keywords: acute respiratory distress syndrome, acute lung injury, receptor for advanced glycation end products, angiopoietin-2, Lung Injury Prediction Score
At a Glance Commentary
Scientific Knowledge on the Subject
Clinical scores are useful but imperfect methods of predicting the onset of acute lung injury (ALI). It is unknown whether the addition of plasma biomarkers can improve these clinical scores.
What This Study Adds to the Field
We found that angiopoietin-2 (Ang-2) can be used to predict the onset of ALI in a critically ill population and can improve the discrimination of the Lung Injury Prediction Score. The prediction capability of Ang-2 was robust to multivariate adjustment. Accurate prediction of ALI may allow for early treatment or prevention.
Despite many large randomized clinical trials for the treatment of acute lung injury (ALI), effective therapies initiated after the development of ALI remain limited (1–6). Thus, the National Heart, Lung, and Blood Institute recently announced their intention to assemble a multicenter network to study methods for the prevention and early treatment of ALI (7). However, one major challenge in targeting these interventions is the inability to accurately predict which patients will develop ALI.
Clinical prediction scores such as the Lung Injury Prediction Score (LIPS) have been developed with the goal of identifying patients at high risk for the development of ALI (8–10). However, although the LIPS has been reported in some studies to have an excellent negative predictive value (NPV) (0.96–0.98 at LIPS cutoffs between 4–6 points), its positive predictive value (PPV) is more limited (range of 0.14–0.23) (8, 9). One method to improve prediction scores may be to combine clinical data with plasma biomarkers, because these biomarkers may measure unique aspects of ALI. This method has been used in models that predict mortality among patients with ALI, wherein plasma biomarkers contribute additional information over clinical information alone (11, 12).
In the prediction of ALI, the most promising predictive plasma biomarkers are likely to be those that reflect the pathogenesis. Angiopoietin-2 (Ang-2) is an endothelial growth factor that is a potent regulator of vascular permeability and a key mediator of ALI in animal models (13–16). Higher levels of plasma Ang-2 have been associated with increased pulmonary leak index, severity of illness, and mortality in patients with ALI (13, 17–19). Ang-2 as part of a panel of biomarkers was able to differentiate patients with ALI among patients with traumatic injury (20). Furthermore, genetic studies have identified polymorphisms in the ANGPT2 gene that are associated with increased risk of developing ALI (21). Angiopoietin-1 (Ang-1), an antagonist to Ang-2, can be combined with Ang-2 to generate an Ang-2/Ang-1 ratio that predicts mortality among a broad population of patients with ALI (22).
Similarly, IL-8, von-Willebrand factor (vWF), and soluble receptor for advanced glycation end products (sRAGE) have been linked to both the pathogenesis and clinical outcomes of ALI. IL-8 is a cytokine and marker of inflammation that has been associated with increased mortality and decreased ventilator-free and organ failure–free days and has been used as part of a panel of biomarkers to diagnose ALI (11, 12, 20, 23–25). vWF is a glycoprotein that is involved with hemostasis and is present in vascular endothelial cells (14). Increased vWF is associated with increased mortality and fewer organ failure–free days in patients with ALI (23, 26–28). sRAGE is a multiligand receptor that is expressed at high levels in type 1 epithelial cells of the lung and has been studied as a marker of lung epithelial injury (14, 29). It has been associated with increased duration of mechanical ventilation and intensive care unit (ICU) stay in patients after lung transplantation and with mortality among those patients treated with high Vt ventilation (30, 31). sRAGE has also been used as part of a diagnostic biomarker panel for ALI (20). Both vWF and sRAGE have previously been shown in small, single-center studies to predict ALI in specific populations: vWF in patients with nonpulmonary sepsis (32) and sRAGE in children after cardiac surgery (33). However, IL-8, Ang-2, and the Ang-2/Ang-1 ratio have never been studied in this capacity, and no study to date has explored any predictive biomarkers for ALI in a broad critically ill population. We hypothesized that plasma Ang-2, Ang-2/Ang-1 ratio, sRAGE, IL-8, and vWF measured early in the course of critical illness would predict the subsequent development of ALI and improve the LIPS. Some of these data have been previously reported in the form of an abstract (34–36).
Methods
See the online supplement for a more detailed description of the study procedures.
Participants
We studied 230 prospectively enrolled critically ill adult patients admitted to a tertiary care hospital ICU from the emergency department (as part of the Early Assessment of Renal and Lung Injury study). Patients qualified as “critically ill” when the emergency physician requested an ICU admission and the patient was subsequently accepted and admitted to the ICU. Patients were excluded if they were admitted for an isolated neurological or neurosurgical diagnosis without any significant medical comorbidities or if they were admitted to the trauma service. The study was approved by the Institutional Review Board of the University of California, San Francisco.
Primary Outcome and Additional Variables
Plasma specimens were obtained as soon as possible after presentation to the emergency department, and patients with samples obtained more than 24 hours after admission to the ICU were excluded. The primary outcome, as adjudicated by two board-certified critical care physicians who were blinded to the ELISA measurements, was the development of ALI at least 6 hours after the time of the sample draw. Patients were followed for 2 weeks for ALI ascertainment and 60 days for mortality. The time of onset of ALI was defined as the time the patient met the last of the 1994 American-European Consensus Conference criteria (37); the recently developed Berlin definition of ARDS was also used for a sensitivity analysis (38). Patients who met criteria for ALI at initial assessment, before the sample draw, or within the subsequent 6 hours were excluded from this analysis to ensure removal of ALI that was present at baseline (n = 49). Patients with bilateral infiltrates on chest radiograph who lacked an arterial blood gas within 24 hours of the chest radiograph were also excluded because we could not adjudicate the outcome (n = 14).
Biologic Sample Collection, Processing, and Measurements
Ang-2, Ang-1, sRAGE, IL-8 (all R&D Systems, Minneapolis, MN), and vWF (Diagnostica Stago, Parsippany, NJ) were measured in duplicate using commercially available two-antibody sandwich ELISA.
Statistical Methods
Discrimination of each biomarker was tested by calculating the area under a receiver operating characteristic curve (AUROC). We also calculated sensitivity and specificity at the cutpoint that minimized the distance to perfect sensitivity and specificity (coordinates [0,1] on the graph). Confidence intervals (CIs) for the AUROC were obtained using a bootstrap method with 1,000 repeated replacement samples from the cohort. Discrimination between two models was compared with a test of equality for the AUROC and the integrated discrimination improvement as previously described (12, 39). The discrimination of the models was validated using 10-fold cross-validation techniques (repeated 10 times and averaged).
Multivariate logistic regression models were used to adjust for potential confounding (sepsis, infection-related risk factor) and to illustrate that a biomarker contains additional predictive information compared with common markers of severity of illness (vasopressor use, Acute Physiology and Chronic Health Evaluation [APACHE] II). An a priori multivariate model adjusted for both severe sepsis and APACHE II score. Biomarkers were included after a natural log transformation to better fit the linearity assumption with the log-odds of the outcome. All models were additionally checked with a Hosmer-Lemeshow goodness-of-fit test. A two-sided P value less than 0.05 was considered statistically significant. Statistical analysis was performed with STATA/IC 12 (College Station, TX).
Results
Baseline Characteristics
The baseline characteristics of the study sample are described in Table 1. Patients who went on to develop ALI were more severely ill, with a higher APACHE II score and increased vasopressor use in the emergency department, compared with patients who never developed ALI. Patients with ALI were more likely to be intubated than those who never developed ALI. Patients who were excluded because they were already diagnosed with ALI at the time that their sample was drawn were similar to those who went on to develop ALI both in severity of disease and in distribution of primary ALI risk factors (see Table E1 in the online data supplement).
TABLE 1.
BASELINE CHARACTERISTICS
| No ALI (n = 148) | Develop ALI (n = 19) | P Value | |
| Age, yr | 65 ± 18 | 69 ± 16 | 0.4 |
| Male sex | 71 (48) | 8 (42) | 0.6 |
| Race | 1 | ||
| Caucasian | 59 (40) | 9 (47) | |
| African American | 20 (14) | 3 (16) | |
| Asian | 39 (26) | 4 (21) | |
| Hispanic | 26 (18) | 3 (16) | |
| Other | 4 (3) | 0 (0) | |
| Admitting service | 0.9 | ||
| Medicine | 93 (63) | 13 (68) | |
| Cardiology | 29 (20) | 3 (16) | |
| Surgery | 7 (5) | 0 (0) | |
| Other | 18 (12) | 3 (16) | |
| Primary admission diagnosis | 0.005 | ||
| Cardiac | 34 (23) | 2 (11) | |
| Respiratory | 24 (16) | 6 (32) | |
| Gastrointestinal | 14 (9) | 1 (5) | |
| Infectious | 24 (16) | 9 (47) | |
| Neurological | 23 (16) | 0 (0) | |
| Other | 29 (20) | 1 (5) | |
| Primary ALI risk factor | <0.001 | ||
| None | 60 (41) | 0 (0) | |
| Sepsis | 39 (26) | 7 (37) | |
| Pneumonia | 12 (8) | 8 (42) | |
| Transfusion | 20 (14) | 3 (16) | |
| Aspiration | 13 (9) | 1 (5) | |
| Pancreatitis | 1 (1) | 0 (0) | |
| Drug overdose | 3 (2) | 0 (0) | |
| Other | 0 (0) | 0 (0) | |
| APACHE II score | 21.4 ± 8.4 | 28.4 ± 8.5 | 0.003 |
| Vasopressor use in ED | 20 (14) | 7 (37) | 0.01 |
| Intubated during stay | 51 (34) | 13 (68) | 0.004 |
| 60-d mortality | 35 (24) | 6 (32) | 0.45 |
Definition of abbreviations: ALI = Acute Lung Injury; APACHE = Acute Physiology and Chronic Health Evaluation; ED = emergency department.
Data are presented as mean ± SD or n (%). Analysis performed using Wilcoxon rank-sum, chi-square test, or Fisher exact test as appropriate.
Plasma samples were drawn a median of 9.9 hours after presentation to the emergency department (range, 0.2–33 h) and a median of 2.5 hours after admission to the ICU (range, −14 to 24 h). Patients who developed ALI at least 6 hours after the sample draw had a median of 22 hours between the sample draw and the time they met criteria for ALI (range, 6–110 h, with a single outlier at 195 h). A sensitivity analysis excluding the patient at 195 hours did not change the results presented below.
Predictive Value of Plasma Biomarkers for Subsequent ALI
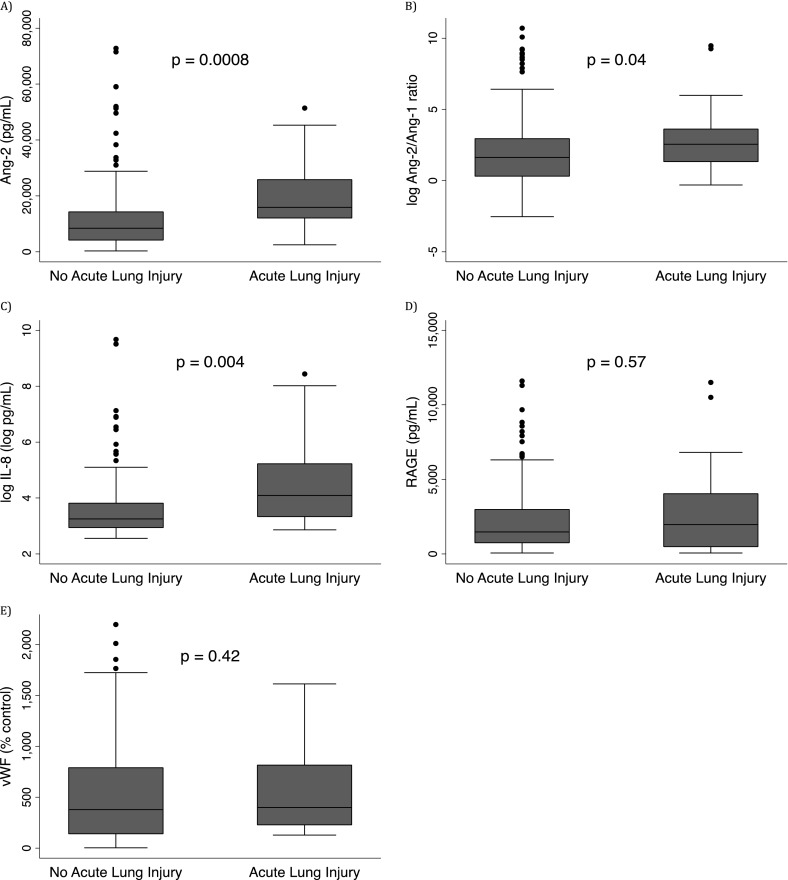
Ang-2, the Ang-2/Ang-1 ratio, and IL-8 were significantly higher among those who developed ALI compared with those who did not (Figures 1A–1C; P = 0.0008, 0.04, and 0.004, respectively). In contrast, plasma levels of sRAGE (P = 0.57) and vWF (P = 0.42) were similar in each group (Figures 1D and 1E). Because sRAGE and vWF were not predictive of ALI, they were not analyzed further.
Figure 1.
Plasma biomarker levels by newly developed acute lung injury (ALI). Patients who went on to develop ALI at least 6 hours after the sample draw (n = 19) had statistically significant increased levels of angiopoietin (Ang)-2 (A), Ang-2/Ang-1 ratio (B), and IL-8 (C) compared with those who did not develop ALI (n = 148). Receptor for advanced glycation end products (sRAGE) (D) and von Willebrand factor (vWF) (E) levels were equivalent between the two groups.
Multivariate Adjustment of Predictor Models
In a logistic regression model, Ang-2 alone significantly predicted the development of ALI (odds ratio [OR], 2.4 [95% CI, 1.3–4.2] for each log increase in Ang-2; Table 2). Similarly, IL-8 also predicted the development of ALI (OR, 1.5 [95% CI, 1.1–2.0] for each log increase in IL-8).
TABLE 2.
THE ASSOCIATION BETWEEN ANGIOPOIETIN-2 AND PREDICTION OF ACUTE LUNG INJURY IN MULTIVARIATE ANALYSES
| Models | OR | 95% CI | P Value |
| Ang-2 | 2.4 | 1.3–4.2 | <0.01 |
| Ang-2 + sepsis Day 1 | 2.2 | 1.2–4.0 | <0.01 |
| Ang-2 + severe sepsis Day 1 | 2.2 | 1.2–3.9 | 0.01 |
| Ang-2 + ED vasopressors | 2.5 | 1.4–4.6 | <0.01 |
| Ang-2 + APACHE II | 1.9 | 1.0–3.5 | 0.06 |
| Ang-2 + infection-related ALI risk | 2.1 | 1.1–4.0 | 0.03 |
| Ang-2 + APACHE II + severe sepsis Day 1 | 1.8 | 1.0–3.4 | 0.06 |
Definition of abbreviations: ALI = acute lung injury; Ang-2 = angiopoietin-2; APACHE = Acute Physiology and Chronic Health Evaluation; ED = emergency department; OR = odds ratio.
Infection-related ALI risk includes all patients with sepsis or pneumonia as a risk factor for ALI. Ang-2 is natural log–transformed in the logistic regression model to meet assumption of linearity with log-odds of outcome. The ORs presented here are for each log increase in the level of plasma Ang-2.
Sepsis is a potential confounder of the relationship between Ang-2 and ALI, because sepsis is a risk factor for ALI and has previously been associated with elevated Ang-2 (40). Even after adjustment for sepsis and severe sepsis in separate models, plasma Ang-2 remained statistically significantly associated with developing ALI (OR, 2.2 [95% CI, 1.2–4.0] and 2.2 [95% CI, 1.2–3.9], respectively; Table 2). The association between Ang-2 and ALI was similarly robust to adjustment for an infection-related ALI risk factor (OR, 2.1 [95% CI, 1.1–4.0]; Table 2). There was no qualitative interaction between patients with and without sepsis, as both strata of patients had similar increased odds of ALI at higher Ang-2 levels.
To ensure that Ang-2 had predictive value that went beyond general ability to identify patients who were sick and thus likely to develop ALI, we also adjusted for markers of severity of illness in multivariate models (Table 2). These analyses showed that the association between Ang-2 and ALI was robust to adjustment for vasopressor use, as a surrogate marker for severe shock. Likewise, the association between Ang-2 and ALI was only mildly attenuated by adjustment for APACHE II score, despite the fact that the APACHE II score incorporates data from a later time point in the patient’s critical illness (the first 24 h in ICU). A model that adjusted for both APACHE II score and the presence of severe sepsis on day 1 similarly showed only a mild attenuation.
Although IL-8 was significantly associated with ALI alone or when adjusted for emergency department vasopressor use (OR, 1.5 [95% CI, 1.1–2.0]), it was less robust to adjustment for sepsis (OR, 1.4 [95% CI, 0.99–1.9]), APACHE II (OR, 1.3 [95% CI, 0.97–1.8]), or infection-related ALI risk (OR, 1.3 [95% CI, 0.94–1.9]).
Discrimination of Clinical and Biomarker Predictive Models
To evaluate discrimination, we calculated an AUROC. Ang-2 had good discrimination, with an AUROC of 0.74 (95% CI, 0.62–0.84; Figure 2). The addition of Ang-1 to generate the Ang-2/Ang-1 ratio did not add predictive value (AUROC, 0.65; 95% CI, 0.52–0.76) among all critically ill patients.
Figure 2.
Receiver operating characteristic (ROC) curves. Angiopoietin-2 (Ang-2) (area under receiver operating curve [AUROC], 0.74; 95% CI, 0.62–0.84) and the Lung Injury Prediction Score (LIPS) (AUROC, 0.74; 95% CI, 0.65–0.84) each show good discrimination between those who will develop acute lung injury and those who will not, but the combination of both LIPS and Ang-2 is superior to either alone (AUROC, 0.84; P = 0.05 for test of equality of AUROC compared with LIPS alone).
The LIPS alone also showed good discrimination between those who will develop ALI and those who will not, with an AUROC of 0.74 (95% CI, 0.65–0.84; Figure 2). The addition of Ang-2 to the LIPS score to generate a combined model improved the discrimination compared with the LIPS alone (AUROC, 0.84 [95% CI, 0.78–0.91]; P = 0.05 for comparison to LIPS alone using test of equality; Figure 2). The integrated discrimination improvement confirmed that the addition of Ang-2 improved the LIPS model (P = 0.04). In a 10-fold cross-validation averaged over 10 repetitions, Ang-2 (AUROC, 0.70) and the combined Ang-2/LIPS model (AUROC, 0.81) showed only minor decreases in discrimination.
Test Characteristics of Ang-2 and LIPS for Predicting ALI
Using conventional AUC methods, a cutpoint for Ang-2 was set at 12,100 pg/ml. At this cutpoint, Ang-2 had a sensitivity of 79%, a specificity of 70%, a PPV of 25%, and an NPV of 96% for development of ALI. In our cohort, Ang-2 outperformed LIPS (cutpoint > 4 points: PPV, 21%; NPV, 94%) (8). The combination of LIPS and Ang-2 (LIPS > 4 and Ang-2 ≥ 12,100 pg/ml) had a PPV of 40% (n = 20 patients), a substantial improvement over the 21 to 25% of each variable alone. Conversely, the NPV of the combination of LIPS and Ang-2, using the same cutoffs as above, was 100% (n = 72 patients).
Sensitivity Analysis: Restricting to Population at Risk for ALI
The main analyses were repeated on a population restricted only to those with a risk factor for ALI over the first 2 weeks of the study (n = 107, 19 of whom developed ALI at least 6 h after the sample draw). Ang-2 was still statistically significantly higher among those who developed ALI than those who did not (P = 0.003). Ang-2 alone (AUROC, 0.72 [95% CI, 0.57–0.83]) and LIPS alone (AUROC, 0.69 [95% CI, 0.56–0.76]) both had adequate discrimination in this subset, and the addition of Ang-2 to the LIPS score improved the discrimination compared with the LIPS alone (AUROC, 0.80 [95% CI, 0.70–0.88]; P = 0.05 for comparison to LIPS alone using test of equality). Restricting to patients at risk for ALI did not materially change the ORs or P values in the multivariate models as presented above.
Sensitivity Analysis: Association between Ang-2 and ALI Present at Baseline
To further test the biologic plausibility of the role of Ang-2 in early ALI, we compared Ang-2 levels in the patients with ALI at baseline or within the subsequent 6 hours (n = 49, excluded from the previous analyses as described in Methods) and individuals who never developed ALI. As in our prior analyses, Ang-2 was significantly higher among patients with ALI at baseline than in patients who never developed ALI.
Sensitivity Analysis: Berlin Definition for ARDS
The main analyses were repeated using the Berlin definition of ARDS as the outcome (n = 14 in our cohort instead of n = 19 with the 1994 consensus definition). Ang-2 was still statistically significantly higher among those who developed ALI than those who did not (P = 0.004). Analyses of discrimination and multivariate models were also not materially different from the main Ang-2 analyses presented above.
Discussion
In this sample of critically ill patients identified early in their hospital course, elevated levels of plasma Ang-2 predicted the subsequent development of ALI, even after adjustment for sepsis, and improved the discrimination, PPV, and NPV of a previously validated clinical prediction score. In contrast, plasma sRAGE, vWF, and the Ang-2/Ang-1 ratio do not contribute predictive value for ALI. IL-8 was similar to Ang-2 in predicting ALI and improving discrimination of LIPS in unadjusted models, but its predictive value was not as robust to adjustment in multivariable models, and further studies in additional cohorts will be needed to determine whether IL-8 is also a significant predictive marker. These biomarker findings are important not only because they provide a potential approach for ALI prediction but also because they provide important insight into the early pathogenesis of ALI.
Given an increased focus on the importance of early therapeutics and prevention of ALI (7, 41), the development of reliable methods to predict ALI is a high priority. Focused clinical prediction scores, such as the surgical lung injury prediction model (10) and the post-trauma prediction model (42), may have some value in specific risk populations. However, neither of these prediction models has ever been validated, and the models may not generalize to all patients at risk for ALI. The LIPS has been validated in a broad population and has excellent NPV for ALI (8, 9), although it has limited PPV. We had a priori reason to expect plasma biomarkers to improve the clinical-only LIPS score, because the addition of biomarkers has added value to prognostic scores that predict mortality in ALI (11, 12). Indeed, the addition of an Ang-2 cutoff to the LIPS cutoff resulted in substantial improvement in PPV over the LIPS score alone. Although the PPV was still relatively low at 40%, this finding has important implications for future clinical trial enrollment, because the LIPS is already in use to enrich these populations for ALI. Furthermore, the addition of an Ang-2 cutoff resulted in a perfect NPV, which, if confirmed in future studies, suggests that a combination of LIPS and Ang-2 could be clinically useful to exclude future development of ALI.
Both vWF and sRAGE were promising biomarkers that we had hypothesized would predict ALI and improve the LIPS score. Indeed, they were the only two biomarkers that had been previously associated with prediction of ALI: vWF was found to predict ALI in patients with nonpulmonary sepsis (32), and sRAGE was predictive in children after cardiac surgery (33). However, these two studies are the only reports of predictive biomarkers for ALI; neither study has yet been replicated, and each focuses on a specific population subset. In our population of all critically ill patients, neither biomarker predicted ALI. Because the previous findings on vWF were published in 1990 (32), it is likely that changing secular trends including new standardized definitions of sepsis and ALI as well as changes in emergency department or early hospital protocols make it difficult to compare results. There are a few reasons that our results may have differed from the other study that examined sRAGE (33). First, sRAGE may only be predictive among children undergoing cardiac surgery and may not generalize to adult critically ill patients. Second, the authors measured sRAGE immediately after surgery, but they do not address when after surgery the patients developed ALI. If ALI developed during surgery or soon after, then sRAGE may be useful as a diagnostic marker instead of a predictive marker.
The unique and novel prediction ability of Ang-2 over other important biomarkers, such as sRAGE and vWF, may enrich our understanding of the early pathogenesis of ALI. Previous studies have already linked Ang-2 with increased pulmonary leak index (a measure of increased lung vascular permeability to protein), severity of illness, and mortality in patients with ALI (13, 15, 17–19). Our novel finding that Ang-2 predicts ALI suggests that endothelial injury and vascular permeability may be among the first mechanisms of injury that begin early in the course of the disease. Laboratory data have demonstrated that Ang-2 is not just a marker of disease but also an important mediator of the biology of ALI. In vitro studies of Ang-2 have found that high levels disrupt the junctional architecture of cultured lung endothelial cells and that the barrier can be rescued with administration of Ang-1 (16, 17). Perhaps most interestingly, genetic studies have identified polymorphisms in the ANGPT2 gene that are associated with increased risk of developing ALI (21). These studies suggest that modification of the Ang-2/Ang-1 axis may be a useful therapeutic approach in ALI. Indeed, Ang-1 has already been shown to be a useful therapeutic in a murine model of sepsis, another disease that has been linked to high plasma levels of Ang-2 (43).
Of particular interest is the contrast between the prognostic value of Ang-2 and another endothelial marker, vWF. Several possible explanations could account for this discrepancy. First, differences in half-life or mechanisms of clearance, both of which are poorly understood for these markers, may contribute to their differing prognostic values. Second, although Ang-2 and vWF are initially both released from Weibel Palade bodies, it is unclear whether they are differentially produced once their initial stores are depleted. Third, vWF has other sources, such as α granules in megakaryocytes, and it is unknown whether other sources may affect plasma concentrations in ALI (44). However, despite the uncertainty in the exact mechanism of this dissociation, a similar pattern has been observed in other studies (18, 19). Further study is needed to better understand this process in ALI, especially early ALI.
Our study has some limitations. First, although we obtained our plasma samples earlier than most other studies, 72% of the patients with ALI developed ALI before the sample draw or within the subsequent 6 hours. Although our overall rate of ALI (31%) matches that in other studies (21), our focus on prediction of ALI required excluding those patients with ALI on or soon after the sample draw. Thus, the number of patients with ALI in our models is modest (n = 19, of whom 13 are intubated), which limits our precision and our power to show a difference in mortality at Day 60. Further studies with larger numbers of outcomes are needed to confirm our findings. Second, it is possible that excluding patients with ALI present on admission may limit our generalizability, because individuals who presented to the hospital before developing ALI may have had less severe disease than those who presented already with ALI. Arguing against this possibility is the finding that demographic and severity measures were similar between the included patients with ALI and the excluded patients with ALI, as seen in Table E1. However, further studies in other populations, such as already-hospitalized patients or patients from the operating room, are needed before generalizing our findings to those patients. Third, there is no point-of-care test for Ang-2 at this time, which limits its current application in clinical medicine. If Ang-2 is confirmed to be a useful predictive marker, further research will be needed to make rapid assays more immediately available to clinicians. Fourth, due to the modest number of outcomes, we could not include a large number of variables simultaneously in logistic regression models. For this reason we could not perform large-scale predictive model generation and validation using both clinical and biological variables. Last, our study lacks a validation cohort in which to verify the use of Ang-2 or the combination of LIPS/Ang-2 for prediction of ALI. The use of 10-fold cross-validation partially mitigates this concern, because it shows that the AUROC for both Ang-2 and the combined LIPS/Ang-2 prediction model decreased only slightly, and thus it is unlikely that our findings are due to overfitting of the data.
Our study also has several unique strengths. First, our cohort was designed for early collection of plasma samples, allowing us to capture many patients before the development of ALI. This is a difficult population to recruit, because most patients present with ALI, but it is also the population for whom prediction will be the most important and likely to provide the most benefit. Second, we were able to determine a time of diagnosis of ALI based on the time at which the last diagnostic criteria for ALI were met. This data point was critically important in being able to distinguish patients who already had ALI or who developed ALI soon after the sample draw from those who truly developed ALI after the sample draw. Third, our study included patients with ALI who were not intubated, which increases our generalizability compared with most other studies of ALI, which focus exclusively on intubated patients. Last, our prospective cohort design with few exclusion criteria allowed for a broad range of critically ill patients who represent a clinically relevant population and increases the generalizability of our study.
In conclusion, our study is one of the few studies to examine predictive biomarkers in ALI and the first study to report the predictive value of Ang-2. The use of a combined model that incorporates clinical variables with biological biomarkers such as Ang-2 may play an important role in research stratification of patients in future clinical trials for early therapeutics or preventative approaches for ALI. In addition, the early rise of Ang-2 demonstrates the importance of early endothelial injury and vascular permeability in the pathogenesis of ALI and provides further evidence that the Ang-2/Ang-1 axis may be a useful therapeutic target in this syndrome.
Acknowledgments
The authors thank all the coordinators and nurses who participated in the Early Assessment of Renal and Lung Injury study as well as the patients and their families.
Footnotes
Supported by National Institutes of Health/National Heart, Lung, and Blood Institute grants HL090833 and HL110969 (C.S.C.), the Flight Attendant Medical Research Institute (C.S.C.), University of California, San Francisco Department of Medicine (C.S.C., K.D.L.), National Institutes of Health/National Center for Research Resources/Office of the Director UCSF-Clinical and Translational Science Institute grants TL1 RR024129 (A.A.) and HL51856 (M.A.M.).
Author Contributions: Project conception, design, analysis, and interpretation: A.A., M.A.M., K.N.K., J.S., K.D.L., C.S.C. Data acquisition and interpretation: J.C.C., B.M.I., A.C., J.A. Drafting of article: A.A. Critical revision of article: M.A.M., K.N.K., J.S., J.C.C., B.M.I., A.C., J.A., K.D.L., C.S.C. Final article approval: all authors.
This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201208-1460OC on January 17, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.ARDS Clinical Trials Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327–336 [DOI] [PubMed] [Google Scholar]
- 2.ARDS Clinical Trials Network Randomized, placebo-controlled clinical trial of an aerosolized beta-2 agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011;184:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, et al. Lung Open Ventilation Study I. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637–645 [DOI] [PubMed] [Google Scholar]
- 4.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, et al. Expiratory Pressure Study Group. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646–655 [DOI] [PubMed] [Google Scholar]
- 5.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. National Heart Lung Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 6.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 7.National Heart, Lung, and Blood Institute. Clinical Center for a Clinical Trials Research Network for the Prevention and Early Treatment of Acute Lung Injury (PETAL Network), NHLBI-HR-14-03. 2012 [accessed 2012 May 31]. Available from: http://www.fbo.gov/spg/HHS/NIH/NHLBI/NHLBI-HR-14-03/listing.html
- 8.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, III, Hoth JJ, Mikkelsen ME, Gentile NT, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 2011;183:462–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, Kojicic M, Kashyap R, Thakur S, Thakur L, Herasevich V, Malinchoc M, Gajic O. Acute lung injury prediction score: derivation and validation in a population-based sample. Eur Respir J 2011;37:604–609 [DOI] [PubMed] [Google Scholar]
- 10.Kor DJ, Warner DO, Alsara A, Fernandez-Perez ER, Malinchoc M, Kashyap R, Li G, Gajic O. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology 2011;115:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest 2010;137:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calfee CS, Ware LB, Glidden DV, Eisner MD, Parsons PE, Thompson BT, Matthay MA. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med 2011;39:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med 2006;12:1286–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett N, Ware LB. Biomarkers in acute lung injury–marking forward progress. Crit Care Clin 2011;27:661–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 2006;3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of tie2 signaling in the endothelium. Mol Cell Biol 2009;29:2011–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher DC, Parikh SM, Balonov K, Miller A, Gautam S, Talmor D, Sukhatme VP. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock 2008;29:656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 2008;63:903–909 [DOI] [PubMed] [Google Scholar]
- 19.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 2012;40:1731–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR, Matthay MA, et al. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma 2010;68:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, Fuchs BD, Lanken PN, Albelda SM, Rushefski M, et al. Angpt2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med 2011;183:1344–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong T, McClintock DE, Kallet RH, Ware LB, Matthay MA, Liu KD. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med 2010;38:1845–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cepkova M, Brady S, Sapru A, Matthay MA, Church G. Biological markers of lung injury before and after the institution of positive pressure ventilation in patients with acute lung injury. Crit Care 2006;10:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care 2008;12:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP, Network NARDSCT. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005;33:1–6, discussion 230–232 [DOI] [PubMed] [Google Scholar]
- 26.Moss M, Gillespie MK, Ackerson L, Moore FA, Moore EE, Parsons PE. Endothelial cell activity varies in patients at risk for the adult respiratory distress syndrome. Crit Care Med 1996;24:1782–1786 [DOI] [PubMed] [Google Scholar]
- 27.Ware LB, Conner ER, Matthay MA. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med 2001;29:2325–2331 [DOI] [PubMed] [Google Scholar]
- 28.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med 2004;170:766–772 [DOI] [PubMed] [Google Scholar]
- 29.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 2006;173:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, Ishizaka A, Lara A, Ranes JL, deCamp MM, et al. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant 2007;26:675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA, Network NA. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008;63:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin DB, Wiener-Kronish JP, Murray JF, Green DR, Turner J, Luce JM, Montgomery AB, Marks JD, Matthay MA. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest 1990;86:474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Chen Q, Shi S, Shi Z, Lin R, Tan L, Yu J, Shu Q, Fang X. Plasma sRAGE enables prediction of acute lung injury following cardiac surgery in children. Crit Care 2012;16:R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Presented at the National Clinical and Translational Sciences Predoctoral Programs Meeting. May 6–8, Rochester, MN.
- 35.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Presented at the UCSF Inter-School Research and Scholarly Activity Festival. May 3–4, San Francisco, CA. [DOI] [PMC free article] [PubMed]
- 36.Agrawal A, Matthay MA, Liu KD, Calfee CS. Plasma levels of the receptor for advanced glycation end products (RAGE) are elevated in early acute lung injury but not in severe sepsis [abstract]. Am J Respir Crit Care Med 2012;185:A6815 [Google Scholar]
- 37.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824 [DOI] [PubMed] [Google Scholar]
- 38.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 39.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 40.Ricciuto DR, dos Santos CC, Hawkes M, Toltl LJ, Conroy AL, Rajwans N, Lafferty EI, Cook DJ, Fox-Robichaud A, Kahnamoui K, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med 2011;39:702–710 [DOI] [PubMed] [Google Scholar]
- 41.Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, Hall J, Israel E, Jain M, Needham DM, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med 2010;181:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rainer TH, Lam PK, Wong EM, Cocks RA. Derivation of a prediction rule for post-traumatic acute lung injury. Resuscitation 1999;42:187–196 [DOI] [PubMed] [Google Scholar]
- 43.David S, Park JK, Meurs M, Zijlstra JG, Koenecke C, Schrimpf C, Shushakova N, Gueler F, Haller H, Kumpers P. Acute administration of recombinant angiopoietin-1 ameliorates multiple-organ dysfunction syndrome and improves survival in murine sepsis. Cytokine 2011;55:251–259 [DOI] [PubMed] [Google Scholar]
- 44.Harrison P, Cramer EM. Platelet alpha-granules. Blood Rev 1993;7:52–62 [DOI] [PubMed] [Google Scholar]